Introduction
Epithelial-mesenchymal transition (EMT) and
chemotaxis are critical processes in cancer metastasis (1–3), and
their interaction in cancer metastasis has been investigated in
recent years. Cancer cells acquire the capability to detach and
migrate away from primary tumors by EMT, involving loss of an
epithelial phenotype, including E-cadherin, and gain of a
mesenchymal phenotype, including Vimentin and Slug (1,2).
Chemokine and receptor interactions guide cancer cells to migrate
to distance favorite organs or lymph nodes (3).
Previous studies have identified that certain
chemokine receptors, including C-X-C chemokine receptor type 4
(CXCR4) and C-C chemokine receptor type 7 (CCR7), have been
detected in a variety of cancer cells and were driven by their
ligands to targeted metastasis of cancers (3,4).
CCR7 is shared by its ligands chemokine (C-C motif) ligand (CCL) 19
or CCL21, which are produced specifically in secondary lymphatic
tissues, including lymph nodes, Peyer's patches and splenic
follicles (5–8). Therefore, CCR7 is considered to
contribute to lymph node metastasis of cancers (9,10).
Many growth factors, including transforming growth factor (TGF)-β,
basic fibroblast growth factor and platelet-derived growth factor
have been identified as EMT inducers, and the molecular mechanisms
underlying promotion of cancer cell invasion have been investigated
(1). Notably, previous studies
have revealed that CXCR4 (11,12),
CCR2 (13) and CCR6 (14) binding with their chemokines may
induce EMT in cancer cells to stimulate cancer cell mobility.
Recently, CCL21/CCR7 were demonstrated to activate EMT in human
breast cancer cells (15), and
subsequently, other studies reported that CCL21/CCR7 may activate
the EMT process in gastric cancer cells (16).
Lung carcinoma is one of the most lethal
malignancies with poor prognosis due to its ability to metastasize,
causing ~84,590 mortalities in men and ~71,280 mortalities in women
in the United States in 2016 (17). However, the role of CCR7 and its
ligands in EMT of human lung cancer cells remains to be fully
clarified. The present study investigated the capacity of
CCL21/CCR7 for stimulating EMT, and associated extracellular
signal-regulated kinase (ERK) 1/2 signaling in lung cancer.
Materials and methods
Cell culture and reagents
The NCI-H157, A549, 973 and NCI-H520 human lung
carcinoma cell lines were obtained from the American Type Culture
Collection (Manassas, VA, USA). All cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in 5% CO2.
Protein expression levels of CCR7 and CCL21 in these cells were
detected by western blot analysis, and the results indicated that
CCR7 expression levels were differential in A549 and H520 cells;
therefore, they were selected for further analysis. A549 and
NCI-H520 cell lines were treated with or without CCL21 (cat. no.
300-35; PeproTech, Rocky Hill, NJ, USA) at designed concentrations
and times. PD98059 (cat. no. 9900; Cell Signaling Technology,
Danvers, MA, USA), a selective inhibitor of mitogen activated
protein kinase kinase (MEK), was used to estimate disruption to the
ERK signaling pathway, which is downstream of MEK.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was reverse transcribed from the mRNA
using a Revert Aid First Strand cDNA Synthesis kit (cat. no.
K01622; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. mRNA of EMT biomarkers including
E-cadherin, Vimentin and Slug, as well as GAPDH were detected by
RT-qPCR using Maxima SYBR®-Green qPCR Master Mix (cat.
no. K0252; Thermo Fisher Scientific, Inc.). The data were obtained
and analyzed using a Mx4000 Multiplex Quantitative PCR system
(version, 3.0; Stratagene; Agilent Technologies, Inc., Santa Clara,
CA, USA). Relative mRNA expression was calculated by the
2−∆∆Cq method (18). All values were normalized to an
endogenous GADPH control. The primers (BioSune, Shanghai, China)
used in RT-qPCR were as follows: Forward, 5′-CTGAGAACGAGGCTAACG-3′
and reverse, 5′-GTCCACCATCATCATTCAATAT-3′ for E-cadherin; forward,
5′-TCCGCCAGCAGTATGAAAG-3′ and reverse, 5′-TGGGTGTCAACCAGAGGAAG-3′
for Vimentin; forward, 5′-CAGCTCAGGAGCATACAG-3′ and reverse,
5′-GAGGAGGTGTCAGATGGA-3′ for Slug; and forward,
5′-GCCGCATCTTCTTTTGCGTCGC-3′ and reverse,
5′-TCCCGTTCTCAGCCTTGACGGT-3′ for GAPDH. The PCR conditions were:
Initial denaturation at 95°C for 30 sec followed by 40 cycles of
denaturation at 95°C for 5 sec, and annealing at 60°C for 30 sec.
Experiments were performed in triplicate.
Western blot analysis
The total protein was extracted from the cultured
cells using the Beyotime lysis buffer containing
radioimmunoprecipitation buffer and a protease inhibitor cocktail
(Beyotime Institute of Biotechnology, Haimen, China) followed by
centrifugation in 12,000 × g at 4°C for 30 min. A total of 50–60 µg
of protein lysates were separated by 10% SDS-PAGE and subsequently
transferred onto polyvinylidene fluoride membranes (PVDF; EMD
Millipore, Billerica, MA, USA), followed by blocking with 5% bovine
serum albumin (Amresco, LLC, Solon, OH, USA) in TBS with Tween-20
for 2 h. The PVDF membranes were incubated with the following
primary antibodies: Anti-β-actin (dilution, 1:2,000; cat. no.
8457P; Cell Signaling Technology, Inc.), anti-CCR7 (dilution,
1:5,000; cat. no. ab32527; Abcam, Cambridge, UK), anti-CCL21
(dilution, 1:400; cat. no. sc-5808; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), anti-Slug (dilution, 1:400; cat. no. sc-166476;
Santa Cruz Biotechnology, Inc.), anti-E-cadherin (dilution,
1:1,000; no. ab133597; Abcam), anti-Vimentin (dilution, 1:1,000;
cat. no. 5741P; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-ERK1/2 (dilution 1:1,000; cat. no. 4370;
Cell Signaling Technology, Inc.) and anti-ERK1/2 (dilution,
1:1,000; cat. no. 4695; Cell Signaling Technology, Inc.) overnight
at 4°C. Horseradish peroxidase-conjugated secondary antibodies,
including goat anti-mouse IgG (dilution, 1:10,000; cat. no.
ZB-5305; ZSGB-Bio, Beijing, China), goat anti-rabbit IgG (dilution,
1:10,000; cat. no. ZB-5301; ZSGB-Bio) and rabbit anti-goat IgG
(dilution, 1:10,000; cat. no. ZB-2306; ZSGB-Bio) were used at room
temperature for 1 h for the detection of immunoreactive proteins by
a luminochemiluminescence reagent (Santa Cruz Biotechnology, Inc.).
The semi-quantitative analyses of data were carried out using
ImageJ (version, 2.1; Windows 7; National Institutes of Health,
Bethesda, MD, USA). The experiment was performed three times.
Wound healing assay
A549 and NCI-H520 cells (3×105) were
seeded in 6-well culture dishes and cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), incubated with or without 50 µM
PD98059 (cat. no. 9900; Cell Signaling Technology, Inc.) at 37°C
for 1 h, and subsequently treated with 100 ng/ml CCL21 (cat. no.
300-35; PeproTech). When cells reached confluence, a scratch in the
central area was established by a sterile pipette tip, followed by
washing with PBS to remove cell debris and replenishing with fresh
medium. Following incubation for 48 h, the migrated cells in the
wound space were counted under a phase-contrast light microscope.
The experiment was repeated three times.
Matrigel invasion assay
A Transwell chamber system (24 wells; 8 mm pore
size; BD Biosciences, Franklin Lakes, NJ, USA) was used in A549 and
NCI-H520 cell lines. The Transwell filters (polycarbonate membrane)
were pre-coated with Matrigel (cat. no. 356234; BD Biosciences).
Tumor cells were pre-incubated with or without 50 µM PD98059 (cat.
no. 9900; Cell Signaling Technology, Inc.) at 37°C for 1 h,
following which 2×105 cells suspended in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 100 ng/ml CCL21 (cat.
no. 300-35; PeproTech) were loaded on Matrigel in the upper
chamber. RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) was
added to the lower chamber. Following 48 h incubation at 37°C,
noninvasive cells on the upper surface of Matrigel membrane (cat.
no. 356234; BD Biosciences) were removed with a cotton swab. The
invading cells on the underside of the Matrigel membrane (cat. no.
356234; BD Biosciences) were fixed with methanol for 30 min at
37°C, stained with 1% crystal violet for 30 min at 37°C and counted
in at least five random separate fields under a light microscope.
The assay was performed in triplicate.
Immunohistochemistry
Lung adenocarcinoma tissues were selected from 50
patients (29 females and 21 males) who underwent radical resection
of lung cancer and had been clinically and histopathologically
diagnosed in Shandong Tumor Hospital (Jinan, China). The tissues
were blocked by 10% formalin and embedded in paraffin. No patients
had received chemotherapy or radiation therapy prior to surgery.
The TNM stage system (19) was
applied to classify the specimens. Clinical pathological
information was obtained from patient records in hospital and is
summarized in Table I. All samples
used in the study were anonymized and informed consent was received
from all patients. The procedure in the present study was approved
by the Medical Ethical Committee of Shandong University (Jinan,
China).
 | Table I.Correlation of CCR7/Vimentin/Slug
expression with clinical pathological parameters in lung carcinoma
tissues. |
Table I.
Correlation of CCR7/Vimentin/Slug
expression with clinical pathological parameters in lung carcinoma
tissues.
|
|
| CCR7 | Slug | Vimentin |
|---|
|
|
|
|
|
|
|---|
| Parameter | n | Low | High | P | Low | High | P | Low | High | P |
|---|
| Age |
|
|
| 0.887 |
|
| 0.623 |
|
| 0.598 |
| ≤50 | 26 | 5 | 21 |
| 5 | 21 |
| 10 | 16 |
|
|
>50 | 24 | 5 | 19 |
| 6 | 18 |
| 11 | 13 |
|
| Sex |
|
|
| 0.254 |
|
| 0.793 |
|
| 0.917 |
|
Female | 29 | 4 | 24 |
| 6 | 23 |
| 12 | 17 |
|
| Male | 21 | 6 | 16 |
| 5 | 16 |
| 9 | 12 |
|
| Lymph node
status |
|
|
| 0.010a |
|
| 0.030a |
|
| 0.030a |
|
Positive | 28 | 2 | 26 |
| 3 | 25 |
| 8 | 20 |
|
|
Negative | 22 | 8 | 14 |
| 8 | 14 |
| 13 | 9 |
|
| Stage |
|
|
| 0.063 |
|
| 0.055 |
|
| 0.235 |
| I | 9 | 2 | 7 |
| 3 | 6 |
| 5 | 4 |
|
| II | 4 | 6 | 8 |
| 6 | 8 |
| 9 | 5 |
|
|
III | 12 | 1 | 11 |
| 1 | 11 |
| 4 | 8 |
|
| IV | 15 | 1 | 14 |
| 1 | 14 |
| 3 | 12 |
|
The paraffin-embedded tissues were cut into ~5
µm-thick sections, and immunohistochemical staining was performed
according to the manufacturer's protocol. Tissue sections were
de-waxed in xylene and rehydrated in a graded alcohol series.
Antigen retrieval was conducted. The endogenous peroxidase of
tissues was inactivated afterwards. Tissue slides were incubated
with the following primary antibodies at 4°C overnight:
anti-Vimentin (dilution, 1:200; cat. no. 5741P; Cell Signaling
Technology, Inc.), anti-Slug (dilution, 1:200; cat. no. sc-166476;
Santa Cruz Biotechnology, Inc.), anti-CCL21 (dilution, 1:200; cat.
no. sc-5808; Santa Cruz Biotechnology, Inc.) and anti-CCR7
(dilution, 1:500; cat. no. ab32527; Abcam). PBS was used as
negative control. Using the EnVision System (Dako; Agilent
Technologies, Inc.) primary antibodies were visualized, and nuclei
were counterstained by hematoxylin.
Immunostaining scoring for staining intensity and
positive percentage in five high-power fields was performed
according to a previous protocol under a light microscope
(magnification, ×400) (11). The
intensity score was obtained by background staining as was graded
as follows: None, 0; mild, 1; moderate, 2; intense, 3. The
percentage of positive tumor cells were assessed according to the
following patterns: 0% cells, 0; 0–25% cells, 1; 25–50% cells, 2;
>50% cells, 3. The final score was obtained by multiplying the
positive percentage score by the intensity score for each section,
which ranged from 0 to 9. If the final score was <4, the tissue
sections were classified as negative or low expression. In
contrast, if the score ≥4, they were regarded as positive or high
expression.
The same method was applied to assess the expression
of CCR7, Vimentin and Slug in interstitial stromal cells.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. A chi-squared test was used for
evaluating the association between CCR7 and EMT biomarkers in human
lung carcinoma and lymphoid tissues. Differences among groups were
compared by one-way analysis of the variance and a Tukey post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was carried out using
SPSS software (version, 18.0; SPSS Inc., Chicago, IL, USA).
Results
Differential expression of CCR7 and
CCL21 in human lung cancer cells
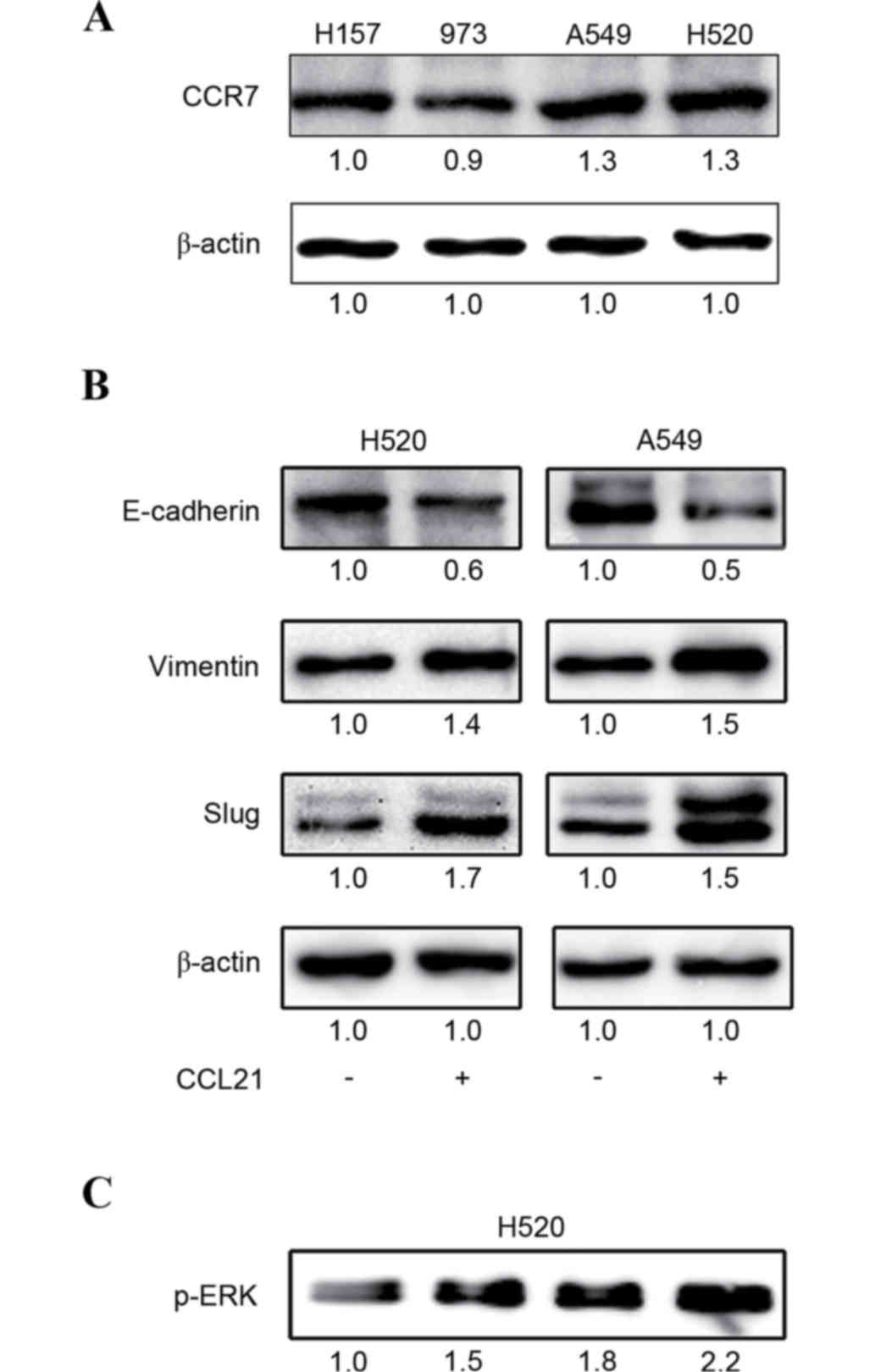
To detect CCR7 and CCL21 expression in different
lung cancer cell lines, western blot analysis was performed. CCR7
was expressed at different levels in H157, A549, 973 and H520 cell
lines, appearing visibly increased in H520 and A549 cells (Fig. 1A); therefore, H520 and A549 cell
lines were selected for the following assays. However, CCL21 was
not detected in all cell lines (data not shown).
EMT phenotype expression is induced by
CCL21 stimulation in lung cancer cells
To evaluate the roles of CCL21/CCR7 in activating
EMT processes in human lung cancer cells, H520 and A549 cells were
treated with or without 100 ng/ml CCL21 for 48 h. The results of
the western blot analysis demonstrated that CCL21 may promote EMT
biomarker protein expression in lung cancer cells, including
decrease of E-cadherin and increase of Vimentin and Slug (Fig. 1B).
ERK signaling is activated in response
to CCL21 stimulation in lung cancer cells
To investigate whether the ERK signaling pathway may
be activated by CCL21, H520 and A549 cells were treated with or
without CCL21 in different concentrations (0, 50, 100 or 200 ng/ml)
for 48 h, and the protein expression levels of p-ERK were detected
by western blot analysis. The results demonstrated that ERK
phosphorylation was positively increased in response to CCL21 in a
dose-dependent manner (Fig.
1C).
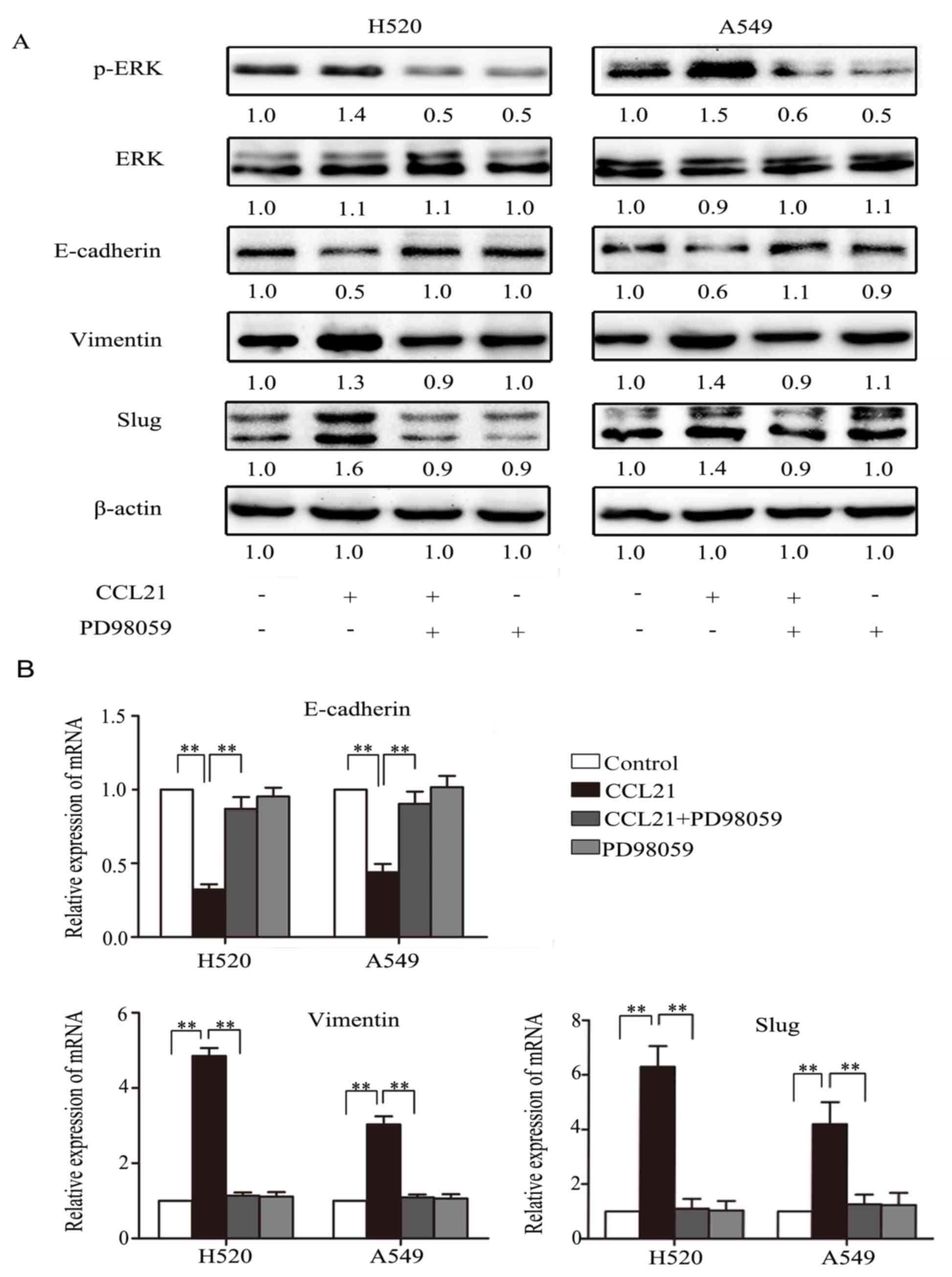
Inhibition of ERK phosphorylation
blocks EMT transformation in response to CCL21/CCR7 in lung cancer
cells
To investigate the potential roles of ERK1/2 in
CCL21/CCR7-mediated EMT activation in human lung cancer cells,
PD98059, a selective inhibitor of MEK, was used to disrupt
activation of its downstream target, ERK. H520 and A549 cells were
pre-treated with or without 50 µM PD98059 for 1 h, followed by
stimulation with or without 100 ng/ml CCL21 for 48 h. Western blot
analysis and RT-qPCR assays were performed. As presented in
Fig. 2A, the western blot assay
indicated that A549 and NCI-H520 cells presented a downregulation
of E-cadherin and upregulation of Vimentin and Slug, as well as
p-ERK, in response to CCL21 stimulation (P<0.01), but this
phenotype transformation was abolished when the cells were
pretreated with PD98059 (P<0.01). The results of RT-qPCR were
consistent with that of the western blot (Fig. 2B).
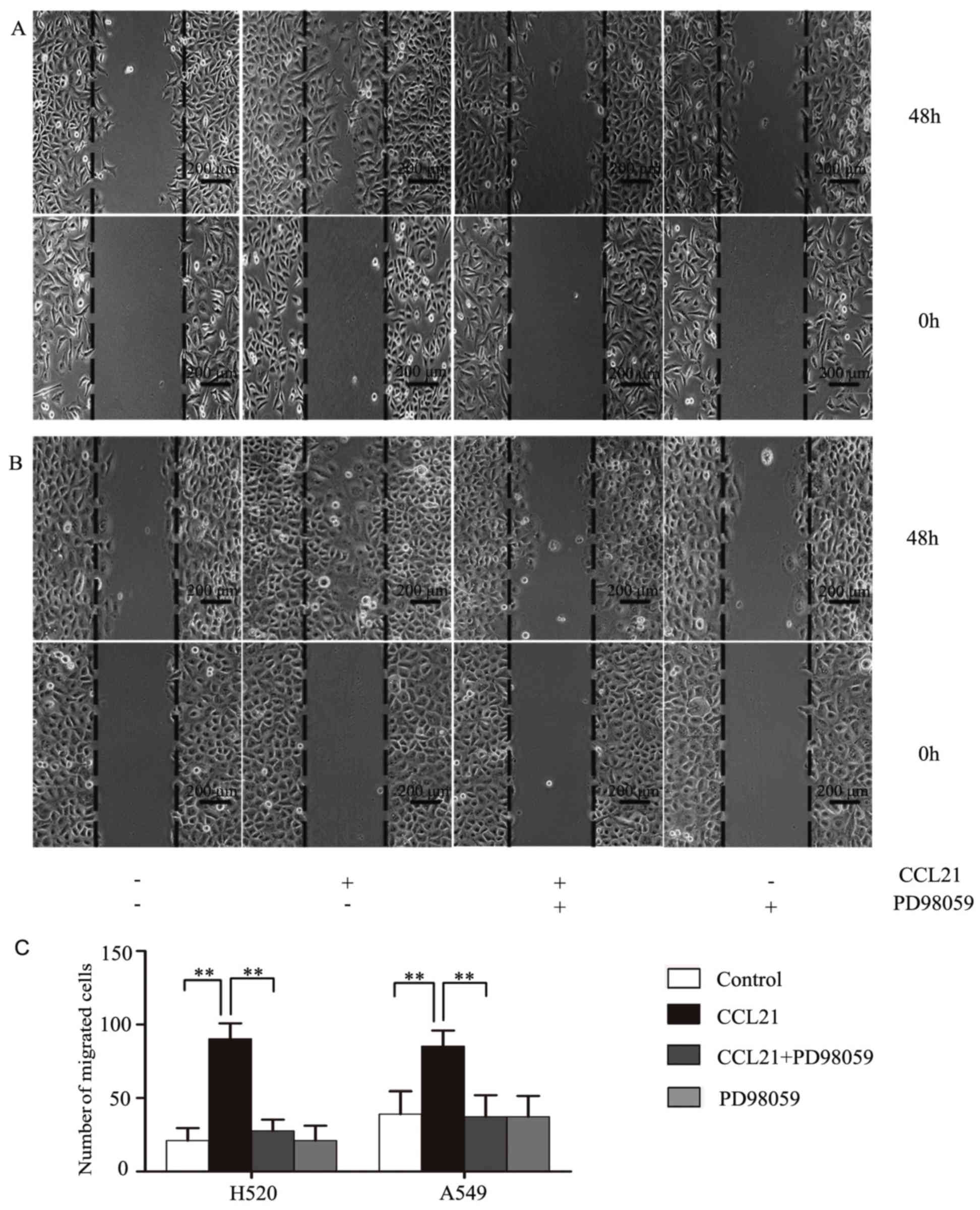
ERK1/2 promotes migration of human
lung cancer cells in response to CCL21/CCR7
The influence of ERK1/2 on cancer cell migration
behavior was investigated using a wound-healing assay. H520
(Fig. 3A) and A549 (Fig. 3B) cells were cultured with or
without PD98059, an inhibitor of MEK, followed by 100 ng/ml CCL21
stimulation for 48 h. The results revealed that there were more
cancer cells migrating to the wound area in response to CCL21
stimulation, whereas this was markedly inhibited when the cells
were pre-treated with PD98059 (P<0.01; Fig. 3C).
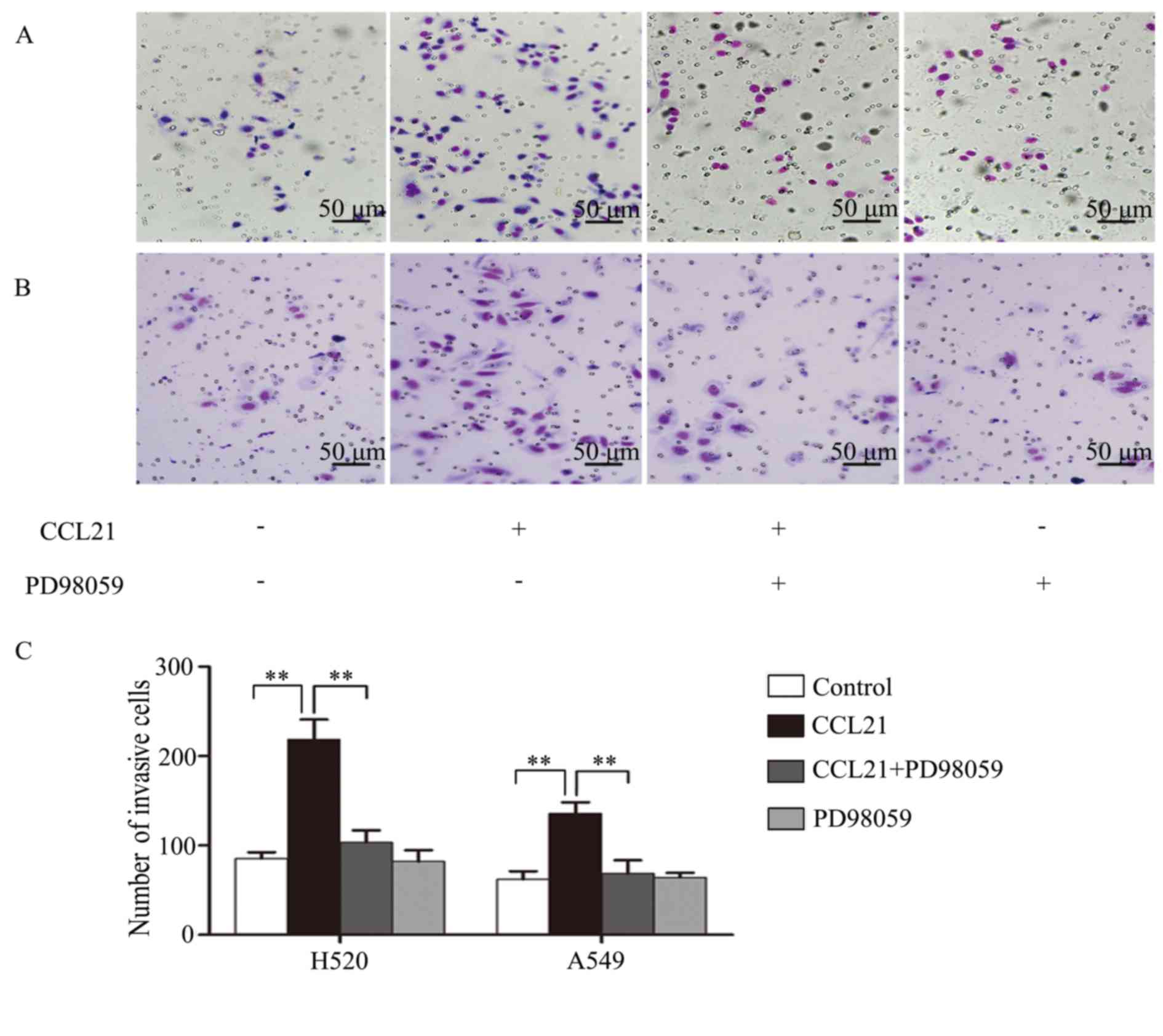
ERK1/2 increases invasion of human
lung cancer cells in response to CCL21/CCR7
The influence of ERK1/2 on invasiveness of human
lung cancer cells was tested using a Matrigel invasion assay.
Following pretreatment with PD98059 in H520 and A549 cells,
followed by 100 ng/ml CCL21 stimulation for 48 h, the invaded cells
travelling through the Matrigel were counted. The results indicated
that the number of invasive cells in H520 (Fig. 4A) and A549 (Fig. 4B) samples were increased in
response to CCL21 stimulation, whereas this was inhibited
remarkably when the cells were pretreated with PD98059 (P<0.01;
Fig. 4C).
CCR7 expression correlates with
clinicopathological parameters and Vimentin/Slug expression in
human lung cancer tissues
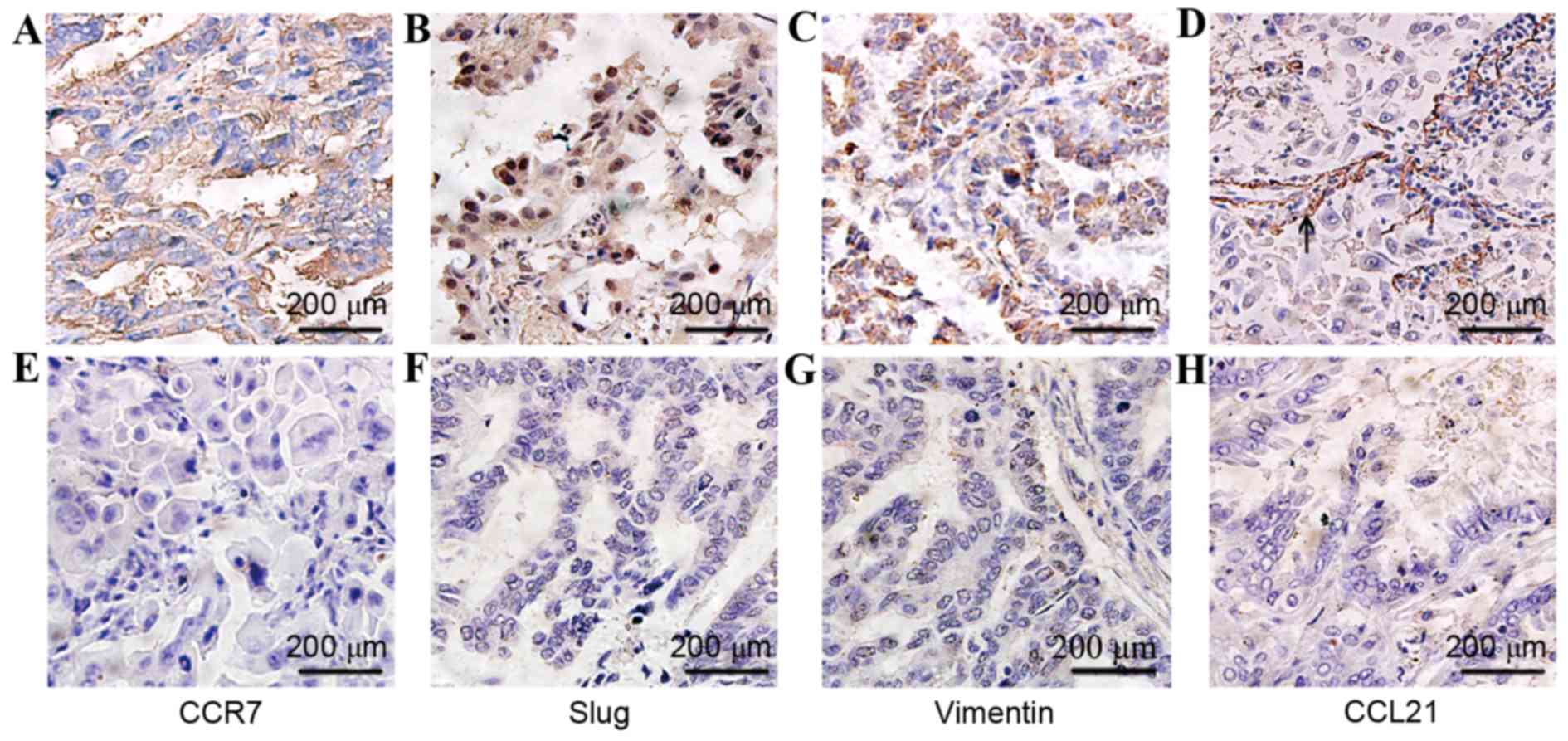
Immunohistochemical staining demonstrated that CCR7
and Vimentin were expressed in the cytoplasm and/or plasma
membranes of tumor cells, and Slug was expressed mainly in the
nucleus and partially in the cytoplasm of cancer cells (Fig. 5). Furthermore, CCR7, Vimentin and
Slug were additionally detected in 6 cases of interstitial stromal
cells in cancer regions and/or in non-neoplastic tissue adjacent to
carcinoma (data not shown), and the expression in interstitial
stromal cells was assessed as above. The final score consists of
tumor and interstitial stromal cells. CCR7, Slug and Vimentin were
all highly expressed in lung carcinoma tissues with positive rates
of 80% (40/50), 78% (39/50) and 58% (29/50), respectively.
Associations between expressions of CCR7/Slug/Vimentin and clinical
parameters were analyzed and are presented in Table I. The expression levels of CCR7,
Slug and Vimentin were significantly associated with lymph node
metastasis (P=0.010, P=0.030 and P=0.030, respectively).
Additionally, CCR7 expression was correlated positively with
expressions of Slug (R=0.459, P=0.001) and Vimentin
(R=0.385, P=0.006). However, CCL21, the CCR7 ligand, was
expressed mainly in the endothelium of lymphatic vessels adjacent
to cancer cells (Fig. 5), but only
weakly positive in cytoplasm of cancer cells in 34% (17/50) cases,
which includes 20% (10/50) cases in the lymph node metastasis group
and 14% (7/50) cases in the lymph node non-metastasis group. No
significant differences were observed between these two groups.
Discussion
In the present study, the expression levels of CCR7
and CCL21 in various human lung cancer cell lines was analyzed. The
results indicated that CCR7 was expressed in different cancer cell
lines, and was most highly expressed in H520, a lung squamous
cancer cell line, and A549, a lung non-small cell cancer cell line.
However, CCL21 was not detected in any cell lines in
vitro.
Based on the detection above, the expression levels
of CCR7 and CCL21 in human lung cancer tissues were assessed by
immunohistochemical staining. Similar to the findings in cancer
cell lines, CCR7 was highly expressed in cancer cells in 80% of
cancer tissues, and was associated with lymph node metastasis.
Nevertheless, CCL21 was primarily expressed in the endothelium of
lymphatic vessels surrounding the cancer foci, but hardly in cancer
cells. Therefore, CCL21, released by lymphatic endothelium, may
facilitate cancer cell migration to the lymphatic vessels. H520 and
A549 cells were selected to evaluate whether CCL21/CCR7 activated
EMT and the ERK signaling pathway in vitro.
The results demonstrated that CCL21/CCR7 effectively
induced EMT via the ERK signaling pathway in the H520 and A549 lung
cancer cell lines. Following treating with CCL21, the EMT phenotype
was accelerated in H520 and A549 cells. The epithelial marker
E-cadherin was downregulated, whereas the mesenchymal markers,
Vimentin and Slug, were upregulated. Immunohistochemical detection
revealed that expression of CCR7 was positively associated with EMT
Vimentin and Slug expression in human lung carcinoma tissues,
consistent with the in vitro findings. Additionally, these
results were consistent with previous studies in breast and gastric
cancer cells (15,16). EMT causes epithelial cells to
acquire invasion and migration capacities (1,2).
Therefore, the CCL21/CCR7 axis not only determines the targeted
migration, but also promotes cancer cell migration.
The effect of the ERK signaling pathway on
CCL21/CCR7 was investigated to identify whether it activates EMT in
human lung cancer cells in vitro. Following inhibition of
ERK phosphorylation, invasion and migration of cancer cells were
significantly decreased compared with cells without ERK inhibition.
In addition, the mRNA and protein expression levels of EMT
biomarkers were reduced in response to CCL21 stimulation. These
results indicated that CCL21/CCR7 activates the EMT procedure by
activating ERK phosphorylation. ERK1/2 belongs to the
mitogen-activated protein kinase family, working as the most common
signaling pathway in mediating EMT (20,21).
Therefore, ERK is a critical signal concerned with chemotaxis and
migration of cancer cells.
It has been demonstrated by immunohistochemical
staining that CCR7 is expressed in a variety of carcinomas
(4,22); however, its ligands CCL19 or CCL21
have rarely been investigated in previous reports. A report
previously demonstrated that a melanoma cell line and several
breast cancer cell lines secreted the CCR7 ligands, CCL21/19
(23), which may promote the
migrating capacity of cancer cells with high levels of CCR7.
However, in the present study, CCL21 was expressed at low levels in
lung cancer cells, although it was expressed strongly in the
endothelium of lymphatic vessels. Lymphatic vessels often exist
around the cancer foci, so certain cancer cells are situated far
from lymphatic vessels. Therefore, the present study aimed to
identify which factor may stimulate CCR7 expression when cancer
cells do not secrete CCR7 ligands. A previous study identified
that, under hypoxic conditions, hypoxia-inducible factor (HIF)-1α
and HIF-2α upregulate CCR7 expression in non-small lung cancer via
the ERK1/2 signaling pathway (24,25).
An additional study confirmed that TGFβ-1 may stimulate cancer
cells to express CCR7 (26).
TGFβ-1, a typical cytokine for EMT stimulation, may be released
widely by various cells including cancer cells in the tumor
microenvironment (27). Therefore,
these factors (except CCR7 ligands) may serve as more significant
stimulators for CCR7 expression of cancer cells in the cancer
microenvironment. This allows cancer cells rich in CCR7 to easily
initiate EMT and chemotaxis processes in response to CCL21/19
released by the capillary lymphatic endothelium.
In conclusion, CCL21/CCR7 may trigger EMT via the
ERK1/2 signaling pathway in human lung cancer cells. The present
study provided evidence that a close interaction exists between EMT
and CCL21/CCR7 chemotaxis processes in lung cancer metastasis.
Targeting the signal that connects CCR7 and EMT may become an
important strategy for oncotherapy. However, additional research is
required regarding the network and molecular interaction of signal
transduction pathways.
Acknowledgements
The present study was supported by the National
Science Foundation of Shandong Province (grant no.
ZR2013HM096).
References
|
1
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar
|
|
2
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar
|
|
3
|
Mukaida N and Baba T: Chemokines in tumor
development and progression. Exp Cell Res. 318:95–102. 2012.
View Article : Google Scholar
|
|
4
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View
Article : Google Scholar
|
|
5
|
Förster R, Davalos-Misslitz AC and Rot A:
CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev
Immunol. 8:362–371. 2008. View
Article : Google Scholar
|
|
6
|
Carlsen HS, Haraldsen G, Brandtzaeg P and
Baekkevold ES: Disparate lymphoid chemokine expression in mice and
men: No evidence of CCL21 synthesis by human high endothelial
venules. Blood. 106:444–446. 2005. View Article : Google Scholar
|
|
7
|
Baekkevold ES, Yamanaka T, Palframan RT,
Carlsen HS, Reinholt FP, von Andrian UH, Brandtzaeg P and Haraldsen
G: The CCR7 ligand ELC (CCL19) is transcytosed in high endothelial
venules and mediates T cell recruitment. J Exp Med. 193:1105–1112.
2001. View Article : Google Scholar :
|
|
8
|
Gunn MD, Tangemann K, Tam C, Cyster JG,
Rosen SD and Williams LT: A chemokine expressed in lymphoid high
endothelial venules promotes the adhesion and chemotaxis of naive T
lymphocytes. Proc Natl Acad Sci USA. 95:pp. 258–263. 1998;
View Article : Google Scholar :
|
|
9
|
Sperveslage J, Frank S, Heneweer C,
Egberts J, Schniewind B, Buchholz M, Bergmann F, Giese N, Munding
J, Hahn SA, et al: Lack of CCR7 expression is rate limiting for
lymphatic spread of pancreatic ductal adenocarcinoma. Int J Cancer.
131:E371–E381. 2012. View Article : Google Scholar
|
|
10
|
Legler DF, Uetz-von Allmen E and Hauser
MA: CCR7: Roles in cancer cell dissemination, migration and
metastasis formation. Int J Biochem Cell Biol. 54:78–82. 2014.
View Article : Google Scholar
|
|
11
|
Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W,
Bhat K, Wang F, Wu E and Wang Z: SDF-1/CXCR4 signaling induces
pancreatic cancer cell invasion and epithelial-mesenchymal
transition in vitro through non-canonical activation of Hedgehog
pathway. Cancer Lett. 322:169–176. 2012. View Article : Google Scholar :
|
|
12
|
Chen K, Li Z, Jiang P, Zhang X, Zhang Y,
Jiang Y, He Y and Li X: Co-expression of CD133, CD44v6 and human
tissue factor is associated with metastasis and poor prognosis in
pancreatic carcinoma. Oncol Rep. 32:755–763. 2014.
|
|
13
|
Lee SH, Kang HY, Kim KS, Nam BY, Paeng J,
Kim S, Li JJ, Park JT, Kim DK, Han SH, et al: The monocyte
chemoattractant protein-1 (MCP-1)/CCR2 system is involved in
peritoneal dialysis-related epithelial-mesenchymal transition of
peritoneal mesothelial cells. Lab Invest. 92:1698–1711. 2012.
View Article : Google Scholar
|
|
14
|
Marsigliante S, Vetrugno C and Muscella A:
Paracrine CCL20 loop induces epithelial-mesenchymal transition in
breast epithelial cells. Mol Carcinog. 55:1175–1186. 2016.
View Article : Google Scholar
|
|
15
|
Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong
G, Qu Y, Ntaka KS and Tian H: CCL21/CCR7 axis activating chemotaxis
accompanied with epithelial-mesenchymal transition in human breast
carcinoma. Med Oncol. 31:1802014. View Article : Google Scholar
|
|
16
|
Zhang J, Zhou Y and Yang Y: CCR7 pathway
induces epithelial-mesenchymal transition through up-regulation of
Snail signaling in gastric cancer. Med Oncol. 32:4672015.
|
|
17
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar
|
|
20
|
Chen X, Ye S, Xiao W, Wang W, Luo L and
Liu Y: ERK1/2 pathway mediates epithelial-mesenchymal transition by
cross-interacting with TGFβ/Smad and Jagged/Notch signaling
pathways in lens epithelial cells. Int J Mol Med. 33:1664–1670.
2014.
|
|
21
|
Buonato JM and Lazzara MJ: ERK1/2 blockade
prevents epithelial-mesenchymal transition in lung cancer cells and
promotes their sensitivity to EGFR inhibition. Cancer Res.
74:309–319. 2014. View Article : Google Scholar
|
|
22
|
Maekawa S, Iwasaki A, Shirakusa T,
Kawakami T, Yanagisawa J, Tanaka T, Shibaguchi H, Kinugasa T and
Kuroki M and Kuroki M: Association between the expression of
chemokine receptors CCR7 and CXCR3, and lymph node metastatic
potential in lung adenocarcinoma. Oncol Rep. 19:1461–1468.
2008.
|
|
23
|
Shields JD, Fleury ME, Yong C, Tomei AA,
Randolph GJ and Swartz MA: Autologous chemotaxis as a mechanism of
tumor cell homing to lymphatics via interstitial flow and autocrine
CCR7 signaling. Cancer Cell. 11:526–538. 2007. View Article : Google Scholar
|
|
24
|
Li Y, Zhang Q, Jiang L, Qiu X and Wang E:
Upregulation of the Chemokine Receptor CCR7 expression by
HIF-1alpha and HIF-2alpha in non-small cell lung cancer. Zhongguo
Fei Ai Za Zhi. 11:724–728. 2008.(In Chinese).
|
|
25
|
Li Y, Zhang Q, Wang Y, Qiu X and Wang E:
Effects of hypoxia on the expression of CCR7 and proliferation,
invasiveness of A549 cells. Zhongguo Fei Ai Za Zhi. 11:704–706.
2008.(In Chinese).
|
|
26
|
Pang MF, Georgoudaki AM, Lambut L,
Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS,
Nelson CM, et al: TGF-β1-induced EMT promotes targeted migration of
breast cancer cells through the lymphatic system by the activation
of CCR7/CCL21-mediated chemotaxis. Oncogene. 35:748–760. 2016.
View Article : Google Scholar
|
|
27
|
Papageorgis P: TGFβ signaling in tumor
initiation, epithelial-to-mesenchymal transition and metastasis. J
Oncol. 2015:5871932015. View Article : Google Scholar :
|