Introduction
Neuroglioma is the most common primary malignant
tumor of the adult central nervous system and is known for its
aggressive proliferation (1,2).
Despite advances in the diagnosis and therapeutic strategies for
neuroglioma, the average survival rate of patients with malignant
glioma has improved only marginally in previous decades, and
>70% of patients succumb to the mortality within 2 years of
diagnosis (3–5). Currently, the treatment of
neuroglioma primarily includes surgery, radiotherapy and
chemotherapy, however, there are a number of side effects and
tangible improvements in the final clinical outcome of patients are
minimal (6). In order to improve
clinical outcomes, understanding of the exact molecular mechanisms
in the development and progression of neuroglioma is considered
essential. Previous studies have demonstrated that epidermal growth
factor receptor, vascular endothelial growth factor, the
Akt-pathway and the nuclear factor-κB pathway are associated with
glioma invasion (7). However, the
underlying molecular mechanisms of microRNA (miRNA)-mediated
post-transcriptional regulation in glioma remain to be
elucidated.
miRNAs are a class of small non-coding (18–25
nucleotides), naturally occurring endogenous, RNA molecules, which
regulate the translation of messenger RNAs (mRNAs) by binding to
the 3′-untranslated regions (3′-UTRs) of target mRNAs (8). The key features of miRNAs involve
their regulation of cell proliferation, differentiation and
apoptosis in various cell types (9,10).
An increasing number of studies have demonstrated that the
pathogenic changes in various tissues are linked to miRNAs, and
abnormal miRNAs expression via various cell signaling pathways
regulates cancer development (11). A number of miRNAs have been
identified to be markedly upregulated or downregulated in
neuroglioma (3,7,12,13).
A previous study confirmed that low levels of miR-1271 in cancer
tissues are correlated with a low rate of patient survival, and the
overexpression of miR-1271 can inhibit the proliferation of cancer
cells, and post-transcriptional regulatory mechanisms via targeting
of cyclin G1 (14), forkhead box
Q1 (15) or homeobox A5 (HOXA5) by
miR-1271 have been reported in various types of cancer (16). However, the molecular mechanisms
underlying the involvement of miR-1271 in glioma by targeting
fibronectin 1 (FN1) remain to be elucidated.
FN1 is an extracellular matrix (ECM) glycoprotein,
which is present in blood plasma in its soluble form (plasma type)
and in its insoluble form (cellular type) as a component of the ECM
in almost every tissue within organisms (17). Previous studies have indicated that
overexpressed FN distorts the architecture of the liver, and leads
to hepatic cirrhosis and consequently to hepatocellular cancer
(18,19). The abundant ECM in the glioma
microenvironment is critical in the maintenance of glioma
morphology, cell differentiation and proliferation, and FNs are
expressed at high levels in the ECM of glioma spheroids in
vitro and glioma tissues in vivo (20). However, the feasibility of
post-transcriptional regulation via FN1 as a therapeutic target for
glioma therapy remains to be elucidated. The present study
investigated whether the expression of FN1 was regulated by miRNAs,
and whether the tumorigenic role of FN1 was affected by miRNAs
in vitro.
Materials and methods
Patient samples
A total of 36 pairs of glioma tumor tissues and
corresponding adjacent normal tissues (NC) were collected from
patients who had undergone surgical excision at Linyi People's
Hospital (Linyi, China) between June 2010 and June 2014. All
collected tissue samples were immediately stored in liquid nitrogen
until use. The human tissue samples were obtained with written
informed consent from all patients. The present study was approved
by the Ethics Committee of the Linyi People's Hospital. The
clinicopathological characteristics of the patients are shown in
Table I.
 | Table I.Correlation between
clinicopathological factors and expression levels of miRNA-1271 in
patients with glioma. |
Table I.
Correlation between
clinicopathological factors and expression levels of miRNA-1271 in
patients with glioma.
| Variable | Patients (n) | miRNA-1271 low
(n) | miRNA-1271 high
(n) | P-value |
|---|
| Sex |
|
|
| 0.385 |
|
Male | 20 | 9 | 11 |
|
Female | 16 | 9 | 7 |
| Age (years) |
|
|
| 0.517 |
|
<60 | 22 | 10 | 12 |
|
≥60 | 14 | 8 | 6 |
| Tumor size (cm) |
|
<3 | 12 | 7 | 5 | 0.274 |
|
≥3 | 24 | 11 | 13 |
| TNM stage |
|
|
| 0.001 |
|
I–II | 22 | 14 | 8 |
|
III–IV | 14 | 4 | 10 |
Cell culture
Glioma cell lines (H4, A172, U251 and U87-MG) and a
normal astrocyte cell line (HA-1800) were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc.), 10% L-glutamine, 0.5%
penicillin/streptomycin, 10% nonessential amino acids and 10%
pyruvate, in a 5% CO2 atmosphere at 37°C.
Analysis of proliferation using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell (2×105 cells/well) proliferation was
measured using an MTT Cell Proliferation/Viability Assay kit
(R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
Caspase-3 activity assay
Cells (2×106 cells/well) lysates were
prepared in NP-40 buffer at ice-bath condition for 15 min and
centrifuged at 900 × g for 10 min at 4°C, and the supernatant was
collected. In brief, 20 µl of cell lysate incubated with
anti-caspase-3 antibody (cat. no. sc-7272; dilution, 1:200) at 37°C
for 1 h. The immunocomplexes were then incubated with peptide
substrate (2 µl of 10 mM acetyl-Asp-Glu-Val-Asp-p-nitroanilide) in
assay buffer (100 mM Hepes, pH 7.5, 20% v/v glycerol, 5 mM
dithiothreitol, and 0.5 mM EDTA) for 2 h at 37°C. The release of
p-nitroaniline was measured at 405 nm using an ELISA reader (MD
SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA)
according to the manufacturer's protocol.
Quantification of apoptosis using flow
cytometry
The quantitative assessment of apoptotic cells was
assessed using the terminal deoxynucleotidyl transferase-mediated
deoxyuridine triphosphate nick end labeling (TUNEL) method, which
enabled examination of DNA-strand breaks during apoptosis using a
BD ApoAlertTM DNA Fragmentation Assay kit (BD Biosciences, Franklin
Lakes, NJ, USA). The cells were trypsinized, fixed with 4%
paraformaldehyde and permeabilized with 0.1% Triton-X-100 in 0.1%
sodium citrate. Subsequent to being washed with PBS three times,
the cells (2×105 cells/well) were incubated with the
reaction mixture for 60 min at 37°C. The cells were immediately
analyzed using a FACScan flow cytometer and the CellQuest version
5.1 (BD Biosciences).
Immunohistochemical staining
The paraffin-embedded tumor tissues were cut into ~4
µm sections and mounted on glass slides for staining with
immunoperoxidase. The paraffinized sections were heated in an oven
at 65°C for 24 h, dewaxed to water and rinsed with PBS three times.
The washed sections were placed in EDTA buffer for microwave
antigen retrieval, boiled, and then boiled at a low heat following
an interval of 10 min. Following natural cooling, the sections were
washed with PBS three times, and were then placed into 3% hydrogen
peroxide solution for incubation at room temperature for 10 min, to
block endogenous peroxidase. The sections were washed with PBS
three times, and 5% bovine serum albumin (BSA; cat. no. ST023;
Beyotime Institute of Biotechnology, Haimen, China) was added for
20 min at room temperature. Following removal of the BSA, each
section was incubated with 50 µl diluted FN1-antibody (cat. no.
sc-81769; dilution, 1:100; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) overnight at 4°C, and then washed with PBS three
times. Following the removal of PBS, each section was incubated
with 50–100 µl secondary (cat. no. sc-516102; dilution, 1:2,000;
Santa Cruz Biotechnology, Inc.) antibody at 4°C for 50 min. The
sections were washed again with PBS three times, and each section
was added to 50–100 µl freshly prepared DAB solution with the use
of a microscope for controlling color. Following washing, the
sections were counterstained with hematoxylin, rinsed with tap
water, dehydrated and mounted for visualization under a microscope
(Leica DM 2500; Leica Microsystems GmbH, Wetzlar, Germany).
Luciferase reporter gene activity
assay
The 3′-UTR of the FN1 gene containing the predicated
target sites for miRNA-1271 was obtained by online predict software
(miRanda-mirSVR; www.microrna.org), DIANA TOOLS
(diana.imis.athena-innovation.gr) and TargetScan (www.targetscan.org), and synthesized by GenePharma
Co., Ltd. (Shanghai, China). The fragment was inserted into
multiple cloning sites in the pMIR-REPORT luciferase microRNA
expression reporter vector (Ambion; Thermo Fisher Scientific,
Inc.). The H4 cells (5×105 cells/well) were
co-transfected with luciferase reporters containing the FN1 3′-UTR
and miRNA-1271 mimics (50 or 100 nM) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The luciferase
activity was measured using a dual luciferase reporter assay kit
(cat. no. RG027; Beyotime Institute of Biotechnology) according to
the manufacturer's protocol.
Transfection with miRNA-1271 mimics
and inhibitor
The FAM modified 2′-OMe-oligonucleotides were
chemically synthesized and purified using high-performance liquid
chromatography (GenePharma, Co., Ltd.). The 2′-OMe-miR-1271 mimics
were composed of RNA duplexes with the following sequence:
5′-CUUGGCACCUAGCAAGCACUCA-3′. The sequences of the 2′-OMe-miR-1271
inhibitor and 2′-Ome-scramble oligonucleotides were as follows:
5′-UGAGUGCUUGCUAGGUGCCAAG-3′ and 5′-UCAGGAGCGUUGCCUGGCUCGG-3′. The
H4 (5×105 cells/well) and U251 cells (5×105
cells/well) were transfected using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at a final concentration of 100 nM.
At 24 h post-transfection, the culture medium was replaced and,
cells were harvested at 48 h for analysis.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
RNA extraction was performed using TRIzol according
to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). The synthesis of cDNAs was performed by RT
reactions with 4 µg of total RNA using moloney murine leukemia
virus reverse transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.) with oligo dT (15) primers
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. PCR reaction mixtures (25 µl) were
prepared, including 12.5 µl SYBR-Green Supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), 1 µl cDNA, 300 nM of each
primer, and DEPC H2O to a final volume of 25 µl. The
levels of miRNA-1271 were quantified using the mirVana qRT-PCR
miRNA detection kit (Ambion; Thermo Fisher Scientific, Inc.) in
conjunction with qPCR with SYBR Green. Following the cycling
reactions: 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec, 58°C for 30 sec, and 72°C for 30 sec, the quantification cycle
(Cq) was determined and the relative miRNA-1271 level was
calculated based on the 2−ΔΔCq method (21) and normalized to the level of U6 in
each sample, and relative expression levels of FN1 were normalized
to GAPDH. PCR was performed using the following primers: FN1,
forward 5′-GCGCCGGCTGTGCTGCACAGG-3′ and reverse
5′-GCCTGGGGACAGCGGTGCCC-3′; GAPDH, forward
5′-GCACCGTCAAGCTGAGAAC-3′ and reverse
5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
The tumor tissues, corresponding adjacent normal
tissues and glioma cells were homogenized and extracted in NP-40
buffer, respectively, followed by 5–10 min boiling and
centrifugation at 12,000 × g for 15 min at 4°C to obtain the
supernatant. Protein concentrations were determined using the
bicinchoninic acid kit (cat. no. BCA1-1KT; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Samples containing 50 µg of protein were
separated on a 10% SDS-PAGE gel and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). Following saturation with
5% (w/v) non-fat dry milk in TBS and 0.1 % (w/v) Tween-20 (TBST),
the membranes were incubated at 37°C for 2 h with the following
primary antibodies: FN1 (cat. no. sc-81769; 1:1,000), caspase-3
(cat. no. sc-271028; 1:2,000), B-cell lymphoma-2 (Bcl-2; cat. no.
sc-56015; 1:1,000) and Bcl-2-associated X protein (Bax; cat. no.
sc6236; 1:1,000) from Santa Cruz Biotechnology, Inc. Following
three washes with TBST, the membranes were incubated with the
appropriate horseradish peroxidase-conjugated secondary antibody
(cat. no. sc-516102; 1:10,000) for 1 h at 37°C, following
visualized using chemiluminescence (Thermo Fisher Scientific,
Inc.). Densitometry was used to assess the signals with Quantity
One software version 4.5 (Bio Rad Laboratories, Inc., Hercules, CA,
USA) and normalized to β-actin (cat. no. sc-130065; 1:2,000).
Statistical analysis
The data from experiments are reported as the mean ±
standard deviation for each group. All statistical analyses were
performed using PRISM version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Statistical differences between two groups were
determined using Student's t test. The correlation between FN1 and
miR-1271 levels was analyzed using linear regression analysis.
Groups were compared using one-way analysis of variance, followed
by Tukey's multiple comparison as a post hoc test to compare the
mean values of each group. Survival rates were calculated using the
Kaplan-Meier method with the log-rank test applied for comparison.
Differences between the expression levels of miRNA-1271 and
different clinicopathological factors were calculated using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
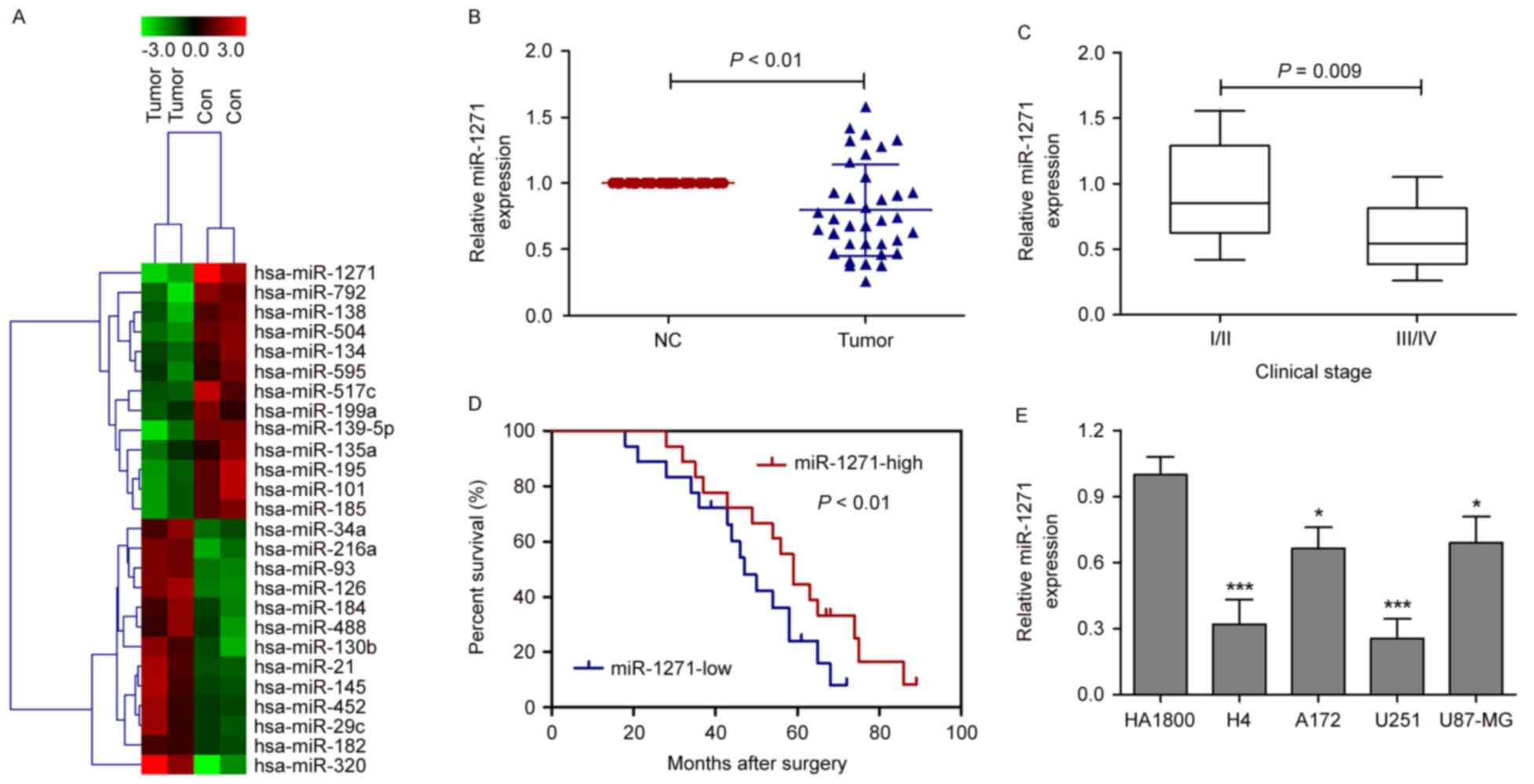
Profiles of miRNAs in glioma tissues
and corresponding adjacent normal tissues
To identify the critical miRNAs involved in glioma,
tumor tissues and corresponding adjacent normal tissues were
collected from 36 patients with neuroglioma, and miRNA microarray
chips were performed in two pairs of randomly selected tumor
tissues and corresponding adjacent normal tissues. A total of 13
miRNAs (miR-134, miR-1271, miR-139-5p, miR-517c, miR-195, miR-135a,
miR-101, miR-199a, miR-792, miR-138, miR-504, miR-595 and miR-185)
were downregulated and 13 miRNAs were upregulated in the tumor
tissues, compared with corresponding adjacent normal tissues
(Fig. 1A). Among these miRNAs, the
levels of miRNA-1271 were markedly lower, compared with those of
other miRNAs in the tumor tissues. Therefore, the present study
focused on miRNA-1271. To further validate the miRNA microarray
results, the levels of miRNA-1271 were measured using RT-qPCR
analysis in the 36 pairs of tumor tissues and corresponding
adjacent normal tissues. The results showed that the expression of
miRNA-1271 was markedly suppressed in tumor tissues, compared with
corresponding adjacent normal tissues (Fig. 1B). In addition, to validate the
clinical significance of miRNA-1271 in glioma, the association
between the expression of miRNA-1271 and clinicopathological
parameters, including tumor size and clinical stage, were assessed.
As shown in Fig. 1C, the
expression levels of miRNA-1271 were significantly lower in
patients with stage III/IV glioma, compared with patients with
stage I/II glioma, however, no significant difference was found in
the expression of miRNA-1271 with tumor size in the patients with
glioma (data not shown). A Kaplan-Meier survival curve was used to
evaluate whether the expression level of miRNA-1271 was associated
with overall survival rate. Patients were segregated into a
miRNA-1271-high group and miRNA-1271-low group according to the
median expression of miRNA-1271 in tumor tissues. Patients with low
levels of miRNA-1271 had a significantly poorer prognosis, compared
with those with high expression (Fig.
1D; P<0.01). Based on the above observations, analysis of
the expression of miRNA-1271 was performed among four glioma cell
lines (H4, A172, U251 and U87-MG) and a normal astrocyte cell line
(HA-1800). miRNA-1271 levels were significantly downregulated in
the four glioma cell lines, compared with the level in the HA-1800
cells, particularly in the H4 and U251 cell lines (Fig. 1E). Therefore, the H4 and U251 cell
lines were selected as representative glioma cells in the following
experiments.
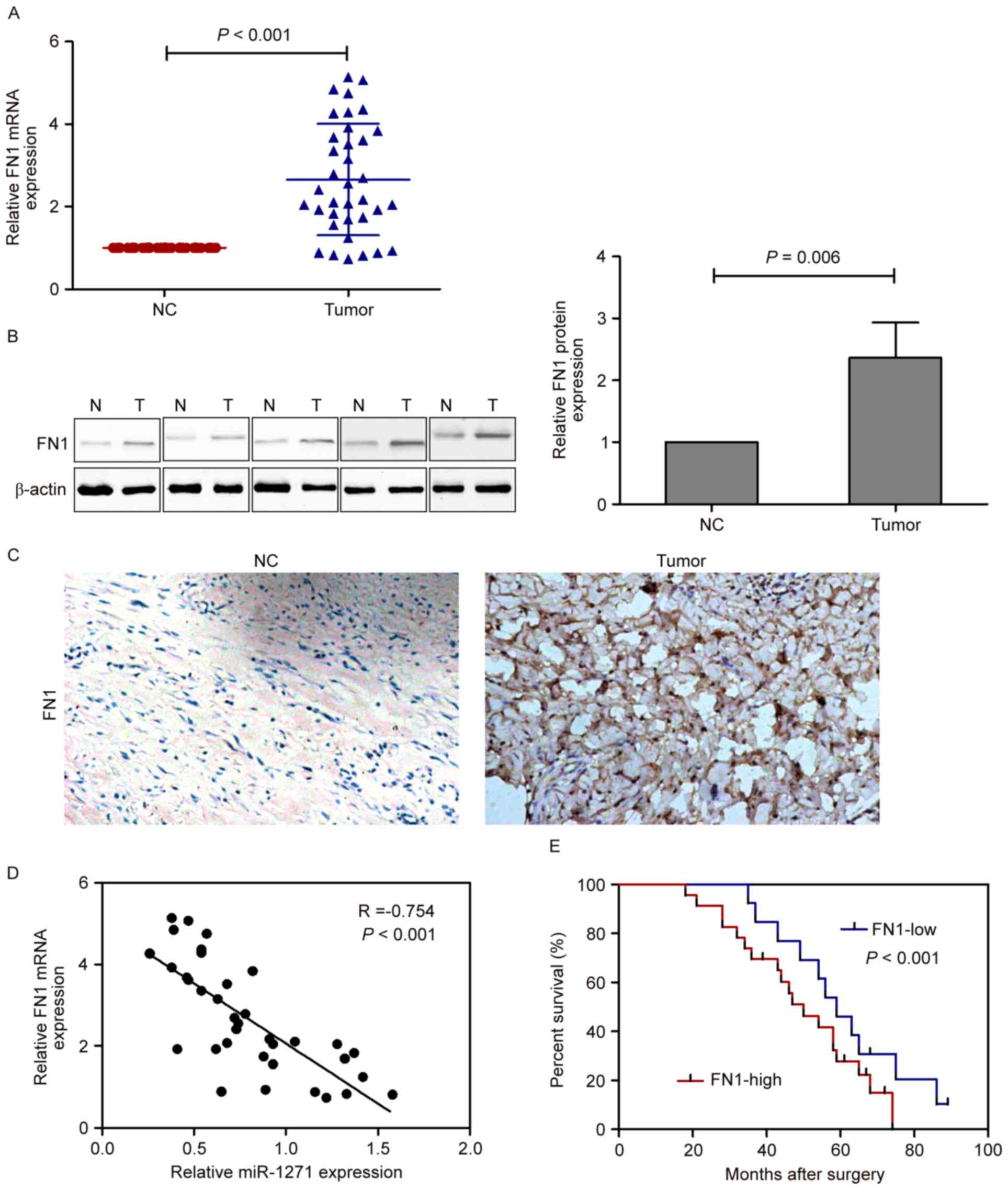
Expression of FN1 is upregulated in
glioma tissues
The results of the present study showed that the
expression of FN1 differed significantly between the glioma tissues
and the corresponding adjacent normal tissues, and the mRNA levels
of FN1 were significantly increased in the glioma tissues, compared
with the corresponding adjacent normal tissues (Fig. 2A). In addition, the results of the
western blot analysis (Fig. 2B)
and immunohistochemical staining (Fig.
2C) showed a marked increase in the levels of FN1 in the glioma
tissues, compared with those in the corresponding adjacent normal
tissues. Spearman's rank correlation analysis showed that the
expression levels of FN1 and miRNA-1271 were inversely correlated
in the 36 human glioma tissues (Spearman's R=−0.754; P<0.001;
Fig. 2D). Furthermore, a
Kaplan-Meier survival curve was used to evaluate whether the
expression level of FN1 was associated with overall survival rate.
Patients were segregated into an FN1-high group and FN1-low group
according to the median mRNA expression of FN1 in tumor tissues.
Patients with high mRNA levels of FN1 had a significantly poorer
prognosis, compared with those with a low levels (Fig. 2E; P<0.001).
FN1 is a direct target of
miRNA-1271
To investigate the mechanisms by which miRNA-1271
regulates the expression of FN1. Binding sites in the FN1 3′-UTR
were predicted using bioinformatics analysis (Fig. 3A). The pmirGLO-wild-FN1 and
pmirGLO-mut-FN1 plasmids were synthesized and the FN1 3′-UTR
luciferase reporter assay was performed in H4 cells. The luciferase
activity was markedly decreased in H4 cells transfected with the
miRNA-1271 mimics (50 or 100 nM) and pmirGLO-wild-FN1, however, no
significant difference in luciferase activity was observed between
H4 cells transfected with the pmirGLO-mut-FN1 and the control group
(Fig. 3B). These results suggested
that FN1 was a direct target of miRNA-1271. Based on the above, the
mRNA expression levels of FN1 were measured among the four glioma
cell lines (H4, A172, U251 and U87-MG) and the normal astrocyte
line (HA-1800). The results indicated that FN1 was significantly
upregulated in the H4 and U251 cell lines, compared with the
HA-1800 cell lines, however, there was no significant difference in
the mRNA expression of FN1 in the A172 and U87-MG cells, compared
with the HA-1800 cells (Fig. 3C).
To confirm the association between FN1 and miRNA-1271, RT-qPCR and
western blot analyses were performed to investigate the expression
levels of FN1 in the H4 and U251 cell lines following transfection
with miRNA-1271 mimics, miRNA-1271 inhibitor, or the corresponding
NC or scramble. As shown in Fig. 3D
and E, the mRNA and protein expression levels of FN1 in the H4
and U251 cell lines were significantly decreased following
transfection with the miRNA-1271 mimics and increased following
transfection with the miRNA-1271 inhibitor.
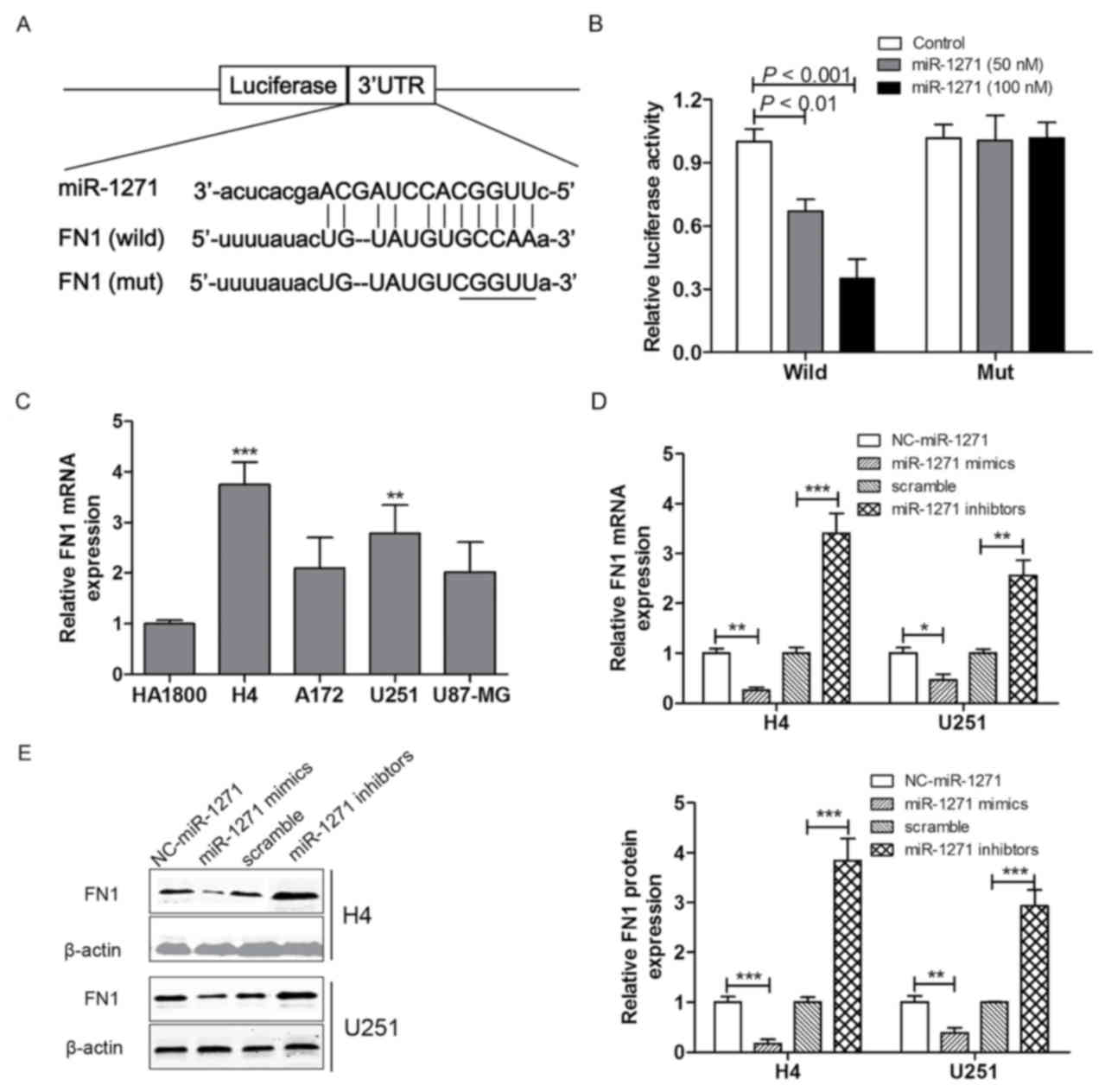 | Figure 3.FN1 is a direct target of miRNA-1271.
(A) Schematic representation of the putative miRNA-1271 binding
site in the FN1 3′UTR and (B) luciferase activity assay. (C) mRNA
expression levels of FN1 were analyzed using RT-qPCR analysis in
H4, A172, U251 and U87-MG glioma cell lines and the HA-1800 normal
astrocyte cell line. (D) mRNA and (E) protein expression levels of
FN1 were analyzed using RT-qPCR or western blot analysis,
respectively, in H4 and U251 cell lines transfected with miRNA-1271
mimics or inhibitor. Values were expressed as the mean ± standard
deviation (n=3 in each group). **P<0.01 and ***P<0.001, vs.
control group. FN1, fibronectin 1; miR, microRNA; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; NC, normal
tissue; 3′UTR, 3′untranslated region. |
Overexpression of miRNA-1271 induces
cell apoptosis
As described above, the present study found that the
expression of miRNA-1271 was downregulated in tumor tissues and
glioma cell lines. It was also demonstrated that miRNA-1271
directly regulated the expression of FN1 via binding sites within
the 3′-UTR of FN1. To examine the role of miRNA-1271 in glioma cell
apoptosis in vitro, cell viability was examined using an MTT
assay in cells transfected with the miRNA-1271 mimics or inhibitor
for 24, 48 and 72 h. The viabilities of the H4 and U251 cells were
significantly suppressed following transfection with the miRNA-1271
mimics and increased following transfection with the miRNA-1271
inhibitor (Fig. 4A). An apoptosis
assay, caspase-3 activity assay and TUNEL staining were performed
following transfection of the H4 and U251 cells with miRNA-1271
mimics or inhibitor for 48 h. The results indicated that the
activity of caspase-3 in the miRNA-1271 mimic group was
significantly higher, compared with that of the control group. This
was in contrast to the H4 and U251 cells transfected with
miRNA-1271 inhibitor, which suppressed the activity of caspase-3
(Fig. 4B). The TUNEL staining
showed that the miRNA-1271 mimics induced apoptosis, whereas the
miRNA-1271 inhibitor suppressed apoptosis of the H4 and U251 cells
(Fig. 4C). Furthermore,
apoptosis-associated proteins were measured using western blot
analysis in H4 and U251 cells transfected with miRNA-1271 mimics or
inhibitor for 48 h. As shown in Fig.
4D-F, the protein expression levels of caspase-3 and Bax were
markedly increased following transfection with the miRNA-1271
mimics and decreased following transfection with the miRNA-1271
inhibitor. By contrast, the protein expression of Bcl-2 was
significantly decreased following transfection with the miRNA-1271
mimics and increased following transfection with the miRNA-1271
inhibitor.
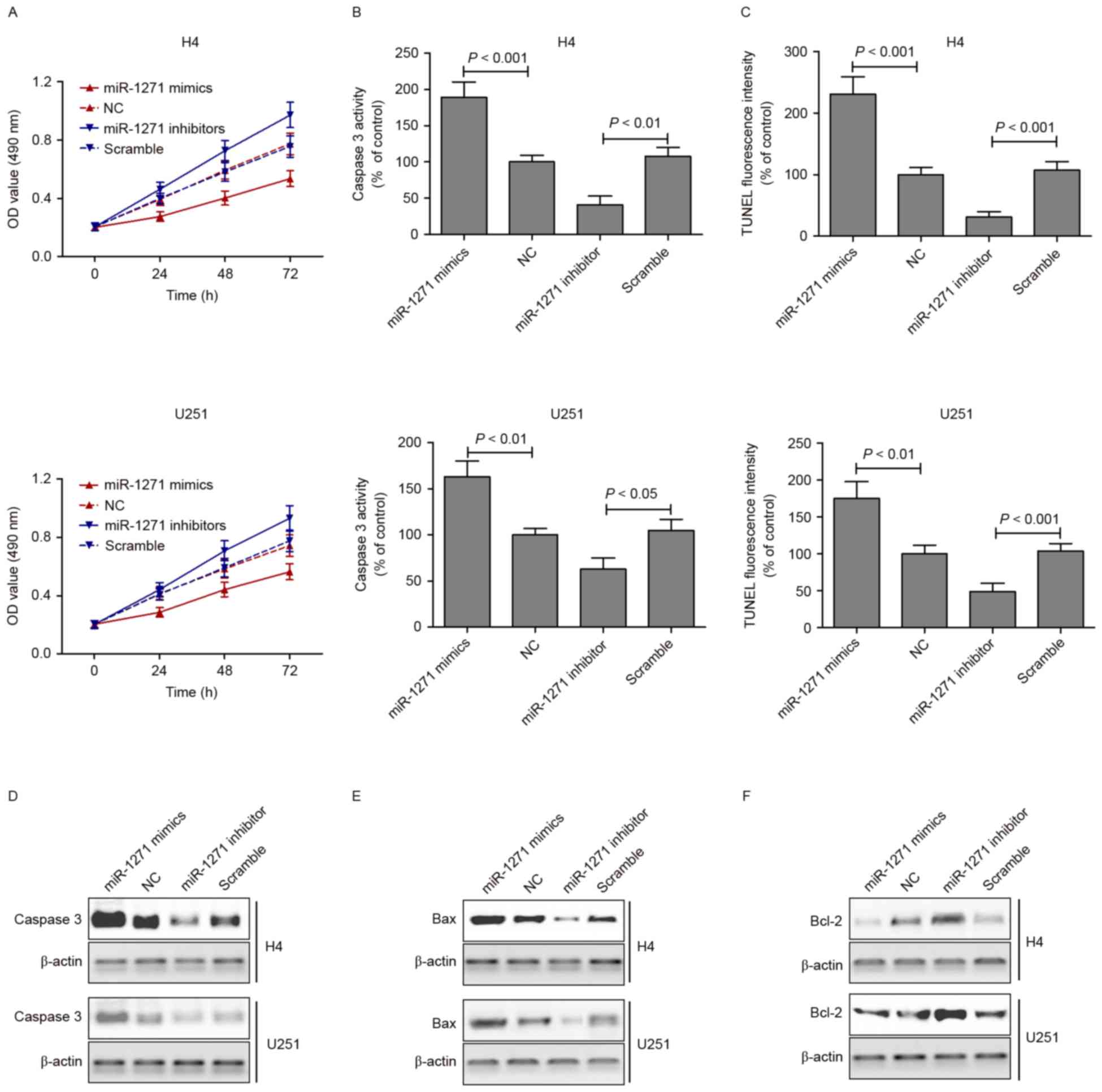 | Figure 4.Overexpression of miRNA-1271 induces
cell apoptosis. (A)
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays
were performed on H4 and U251 cell lines transfected with
miRNA-1271 mimics or inhibitor for 24, 48 and 72 h. (B) A Caspase-3
activity assay and (C) TUNEL staining were performed following
transfection of the H4 and U251 cell lines with miRNA-1271 mimics
or inhibitor for 48 h. The protein expression of (D) caspase-3, (E)
Bax and (F) Bcl-2 were measured using western blot analysis in H4
and U251 cell lines transfected with miRNA-1271 mimics or inhibitor
for 48 h. FN1, fibronectin 1; miR, microRNA; Bcl-2, B cell
lymphoma-2; Bax, Bcl-2-associated X protein; NC, normal tissue;
TUNEL, triphosphate nick-end labeling; OD, optical density. |
Discussion
Previous studies have demonstrated the essential
role of miRNAs in the progression of cancer (22,23),
however, the role of miRNA-1271 in glioma has not been determined.
In the present study, the clinical significance and underlying
molecular mechanisms of miRNA-1271 in the development of glioma
were investigated. The results showed that miRNA-1271 was
downregulated in glioma tumor tissues and cell lines. In addition,
it was demonstrated that low levels of miRNA-1271 in patients with
glioma were correlated with low survival rates. In vitro,
cell viability was significantly suppressed following transfection
with the miRNA-1271 mimics and increased following transfection
with the miRNA-1271 inhibitor. The miRNA-1271 mimics induced cell
apoptosis, whereas the miRNA-1271 inhibitor suppressed cell
apoptosis in the H4 and U251 cell lines. Furthermore, 3′-UTR of FN1
was bound by miRNA-1271. Therefore, it was concluded that
miRNA-1271 inhibited glioma cell growth by targeting FN1, and the
levels of miRNA-1271 in glioma tumor tissues were associated with
lower survival rates in patients with glioma.
Although it has been reported that miRNA-1271 is
markedly suppressed in various types of tumor, and is associated
with tumor proliferation and metastasis (14,15,24,25),
data concerning miRNA-1271 in human glioma remains limited. The
results of the present study revealed the antitumor role of
miRNA-1271 in human glioma and showed that miRNA-1271 offered
potential for use as a prognostic predictor. The present study
demonstrated for the first time, to the best of our knowledge, that
miRNA-1271 was downregulated in glioma tumor tissues, compared with
corresponding adjacent normal tissues, and was closely associated
with clinical stage. However, how miRNA-1271 contributes to the
malignant behavior of glioma cells remains to be fully elucidated.
Previous studies have indicated that miRNA-1271 inhibits ovarian
cancer growth by targeting cyclin G1 (14), regulates cisplatin resistance of
human gastric cancer cell lines by targeting insulin-like growth
factor 1 receptor, insulin receptor substrate 1, mammalian target
of rapamycin and Bcl-2 (26), and
promotes non-small-cell lung cancer cell proliferation and invasion
by targeting HOXA5 (16). In
addition, the downregulation of miRNA-1271 has been associated with
the concomitant upregulation of glypican-3, one of the most
abnormally expressed genes contributing to liver carcinogenesis
(27). In the present study,
bioinformatics analysis was performed, and it was found that
miRNA-1271 directly regulated the expression of FN by targeting its
3′-UTR in glioma cells. miRNA-1271 mimics induced apoptosis and
miRNA-1271 inhibitor suppressed apoptosis in the H4 and U251 cell
lines. In addition, the correlation between miRNA-1271 and FN1
showed that endogenous miRNA-1271 was negatively correlated with
the mRNA levels of FN1 in glioma tissues. These data provided
further evidence of a functional link between miRNA-1271 and FN1 in
human glioma.
In conclusion, the present study identified
miRNA-1271 as a novel tumor suppressor, which inhibited human
glioma cell proliferation and induced cell apoptosis through the
suppression of FN1. The findings that miRNA-1271 targeted FN1 as a
post-translational mechanism provides novel insight into the
underlying mechanism and therapeutic strategies for the treatment
of human glioma.
References
|
1
|
Tong YQ, Liu B, Zheng HY, Gu J, Liu H, Li
F, Tan BH, Hartman M, Song C and Li Y: MiR-215, an activator of the
CTNNBIP1/β-catenin pathway, is a marker of poor prognosis in human
glioma. Oncotarget. 6:25024–25033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi R, Wang PY, Li XY, Chen JX, Li Y,
Zhang XZ, Zhang CG, Jiang T, Li WB, Ding W and Cheng SJ: Exosomal
levels of miRNA-21 from cerebrospinal fluids associated with poor
prognosis and tumor recurrence of glioma patients. Oncotarget.
6:26971–26981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han L, Liu D, Li Z, Tian N, Han Z, Wang G,
Fu Y, Guo Z, Zhu Z, Du C and Tian Y: HOXB1 is a tumor suppressor
gene regulated by miR-3175 in glioma. PloS One. 10:e01423872015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson DR and Galanis E: Incorporation of
prognostic and predictive factors into glioma clinical trials. Curr
Oncol Rep. 15:56–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bageritz J, Puccio L, Piro RM, Hovestadt
V, Phillips E, Pankert T, Lohr J, Herold-Mende C, Lichter P and
Goidts V: Stem cell characteristics in glioblastoma are maintained
by the ecto-nucleotidase E-NPP1. Cell Death Differ. 21:929–940.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H and Wang Y: Five miRNAs considered
as molecular targets for predicting neuroglioma. Tumour Biol.
37:1051–1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabbri E, Brognara E, Montagner G,
Ghimenton C, Eccher A, Cantù C, Khalil S, Bezzerri V, Provezza L
and Bianchi N: Regulation of IL-8 gene expression in gliomas by
microRNA miR-93. BMC Cancer. 15:6612015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paranjape T, Heneghan H, Lindner R, Keane
FK, Hoffman A, Hollestelle A, Dorairaj J, Geyda K, Pelletier C,
Nallur S, et al: A 3′-untranslated region KRAS variant and
triple-negative breast cancer: A case-control and genetic analysis.
Lancet Oncol. 12:377–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujii T, Shimada K, Tatsumi Y, Hatakeyama
K, Obayashi C, Fujimoto K and Konishi N: microRNA-145 promotes
differentiation in human urothelial carcinoma through
down-regulation of syndecan-1. BMC Cancer. 15:8182015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re32015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim M, Kasinski AL and Slack FJ: MicroRNA
therapeutics in preclinical cancer models. Lancet Oncol.
12:319–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li R and Li X, Ning S, Ye J, Han L, Kang C
and Li X: Identification of a core miRNA-pathway regulatory network
in glioma by therapeutically targeting miR-181d, miR-21, miR-23b,
β-Catenin, CBP, and STAT3. PloS One. 9:e1019032014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kouri FM, Ritner C and Stegh AH: miRNA-182
and the regulation of the glioblastoma phenotype-toward miRNA-based
precision therapeutics. Cell Cycle. 14:3794–3800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Ma L, Rao Q, Mao Y, Xin Y, Xu H, Li
C and Wang X: MiR-1271 inhibits ovarian cancer growth by targeting
cyclin G1. Med Sci Monit. 21:3152–3158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang XJ, Deng J, Liu YW, Wan LY, Feng M,
Chen J and Xiong JP: MiR-1271 inhibits cell proliferation, invasion
and EMT in gastric cancer by targeting FOXQ1. Cell Physiol Biochem.
36:1382–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Xu L and Jiang L: miR-1271
promotes non-small-cell lung cancer cell proliferation and invasion
via targeting HOXA5. Biochem Biophys Res Commun. 458:714–719. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM,
Chien HF, Tsai JS, Lee CW, Yen FL and Chen YL: Thalidomide inhibits
fibronectin production in TGF-β1-treated normal and keloid
fibroblasts via inhibition of the p38/Smad3 pathway. Biochem
Pharmacol. 85:1594–1602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng C, Wang YL, Xie C, Sang Y, Li TJ,
Zhang M, Wang R, Zhang Q, Zheng L and Zhuang SM: Identification of
a novel TGF-β-miR-122-fibronectin 1/serum response factor signaling
cascade and its implication in hepatic fibrogenesis. Oncotarget.
6:12224–12233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Shen S, Liao Z, Shi W, Wang Y,
Zhao J, Hu Y, Yang J, Chen J, Mei H, et al: Targeting fibronectins
of glioma extracellular matrix by CLT1 peptide-conjugated
nanoparticles. Biomaterials. 35:4088–4098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang
Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al:
Microenvironment-induced PTEN loss by exosomal microRNA primes
brain metastasis outgrowth. Nature. 527:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kontomanolis EN and Koukourakis MI:
MicroRNA: The potential regulator of endometrial carcinogenesis.
Microrna. 4:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong D, Zhang G, Ma H and Jiang G:
miR-1271 inhibits OSCC cell growth and metastasis by targeting ALK.
Neoplasma. 62:559–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jensen KP and Covault J: Human miR-1271 is
a miR-96 paralog with distinct non-conserved brain expression
pattern. Nucleic Acids Res. 39:701–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding
Y, Shu Y and Liu P: miR-1271 regulates cisplatin resistance of
human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR, and
BCL2. Anticancer Agents Med Chem. 14:884–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maurel M, Jalvy S, Ladeiro Y, Combe C,
Vachet L, Sagliocco F, Bioulac-Sage P, Pitard V, Jacquemin-Sablon
H, Zucman-Rossi J, et al: A functional screening identifies five
microRNAs controlling glypican-3: Role of miR-1271 down-regulation
in hepatocellular carcinoma. Hepatology. 57:195–204. 2013.
View Article : Google Scholar : PubMed/NCBI
|