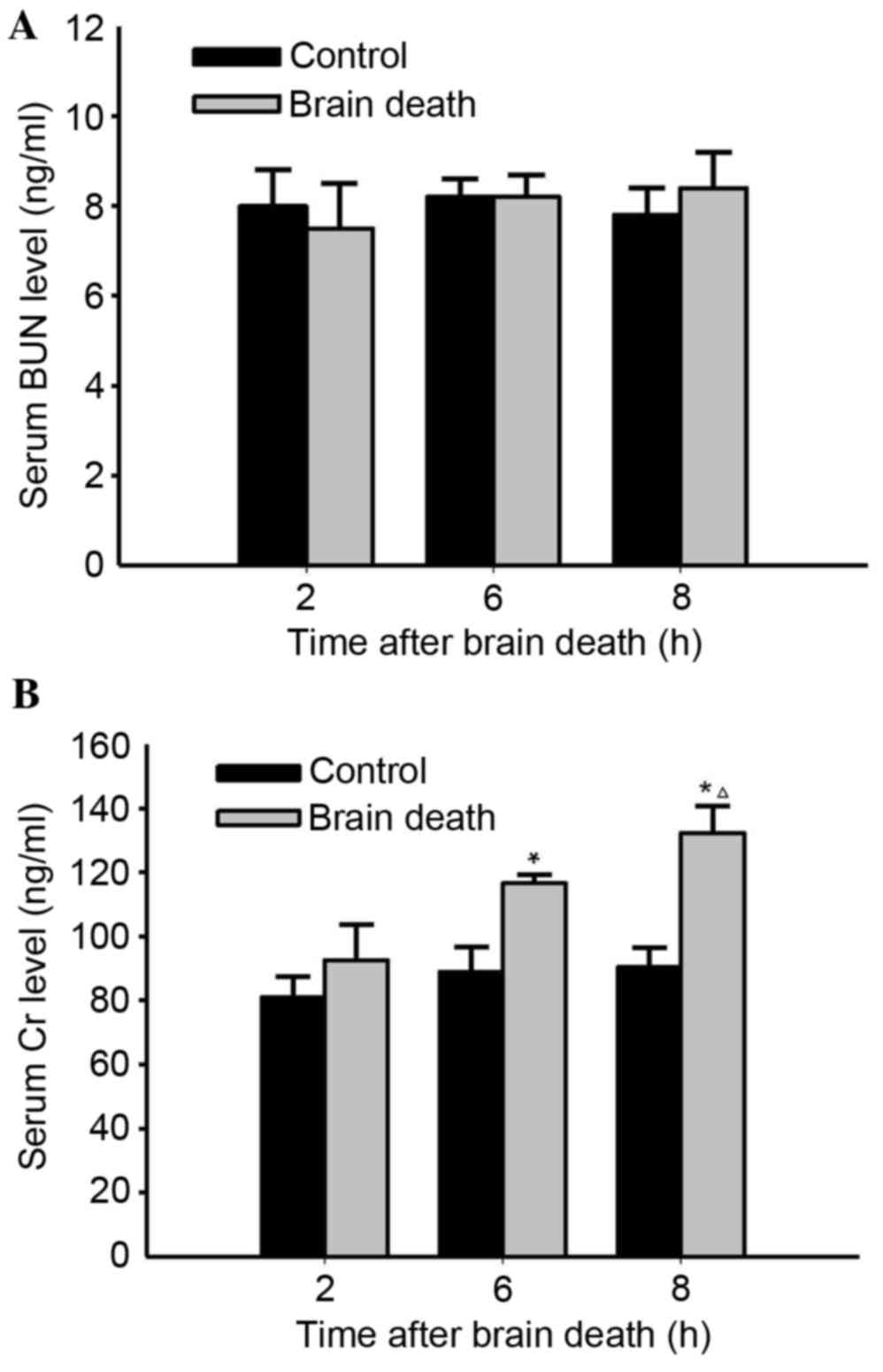
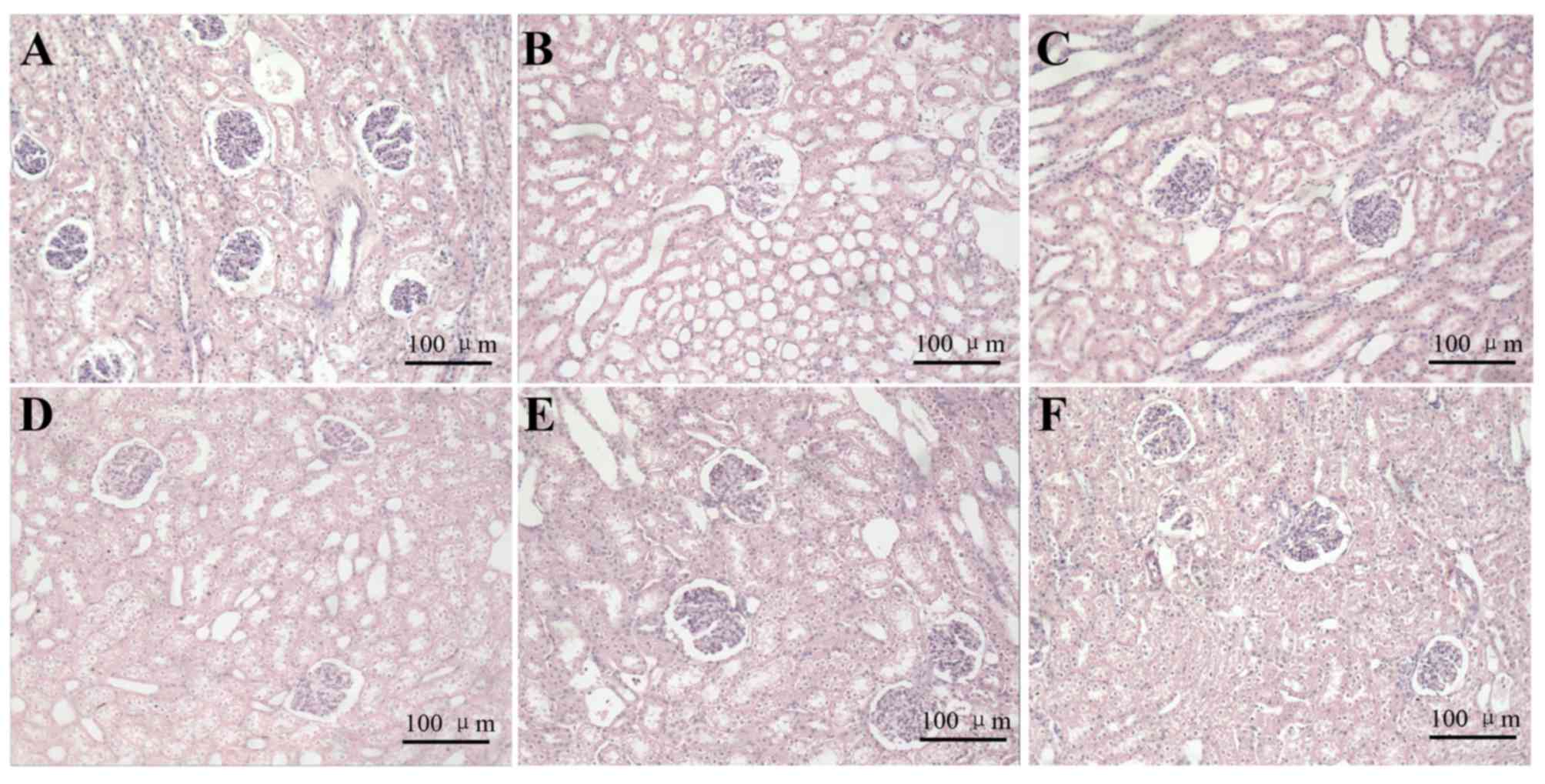
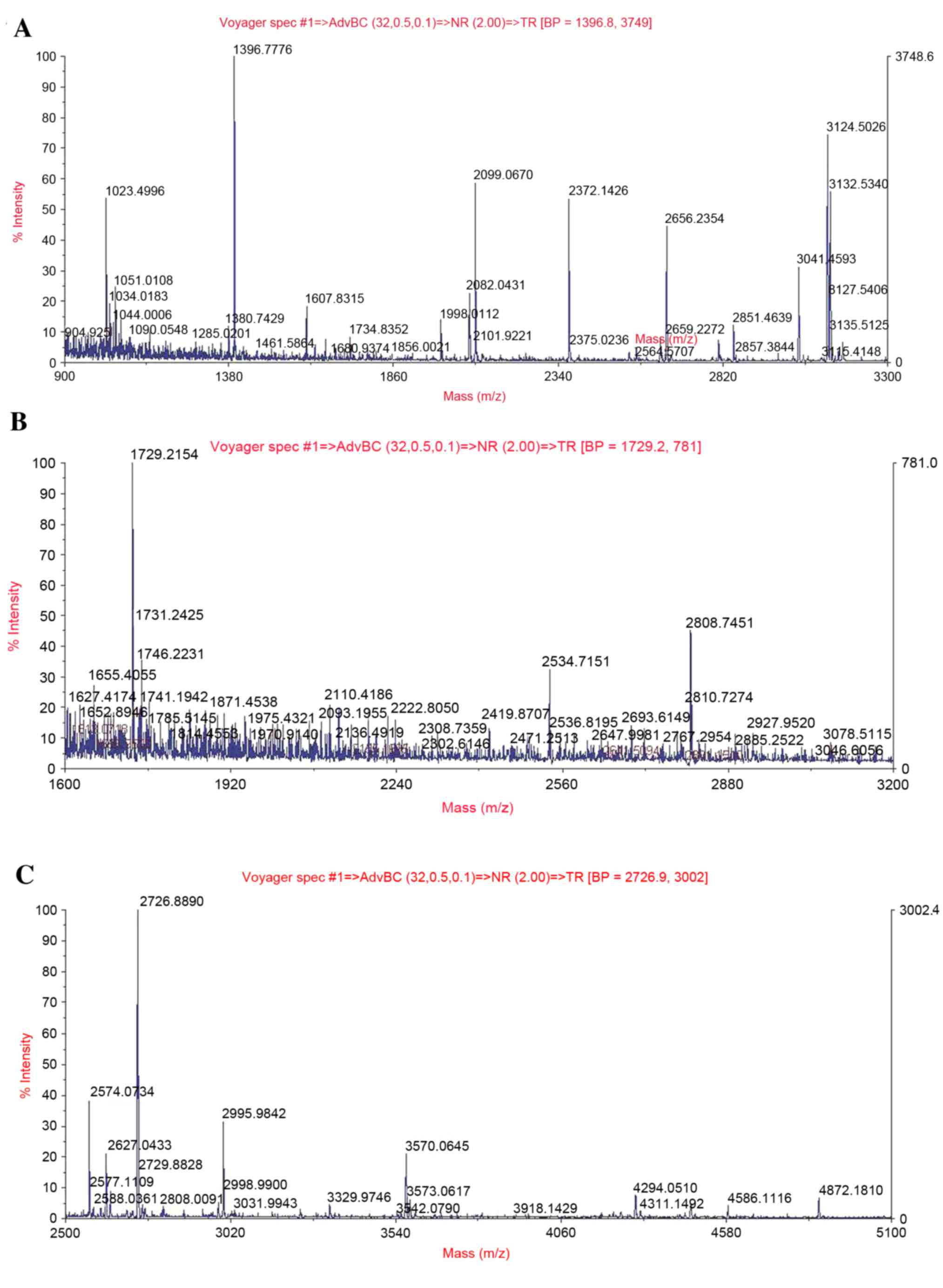
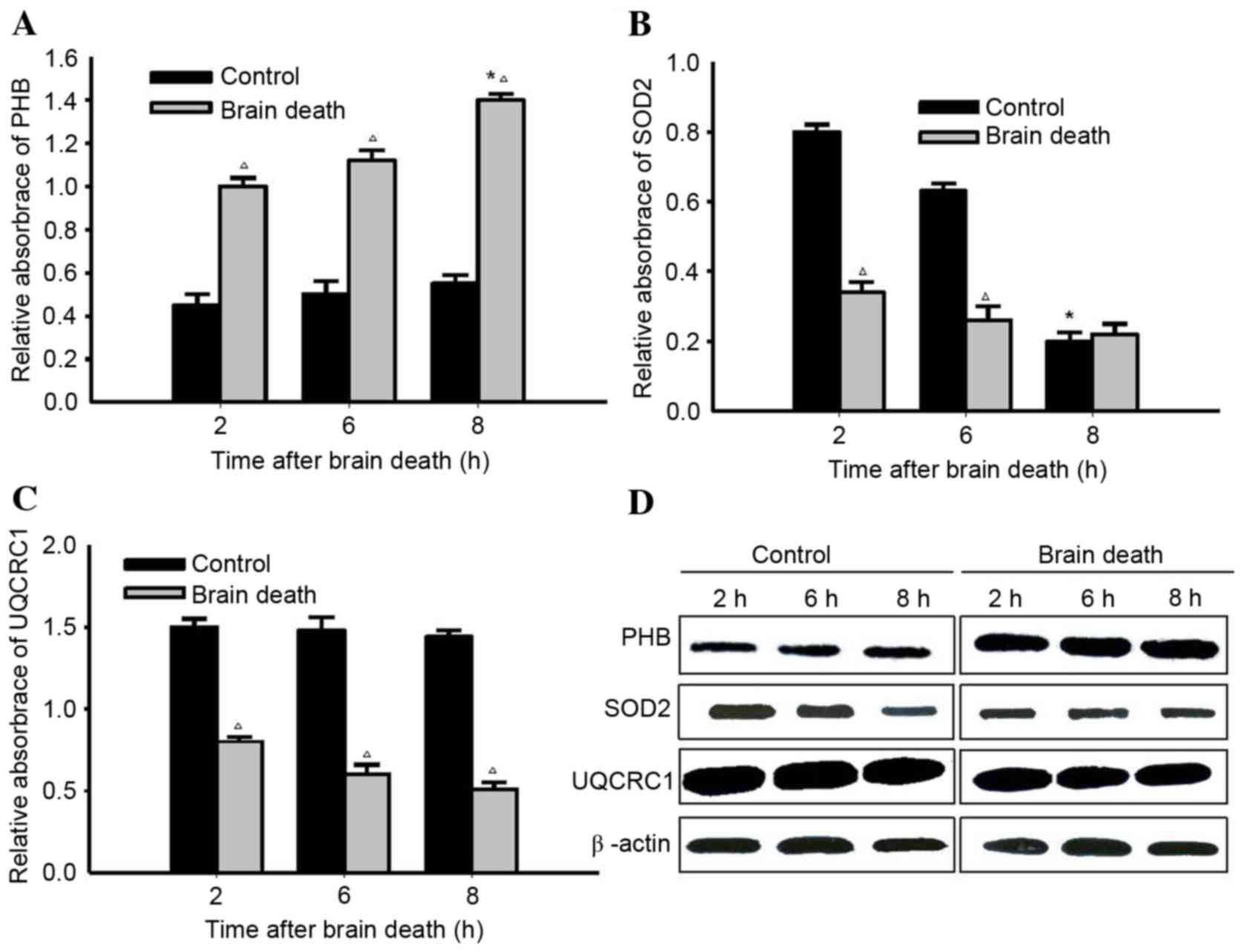
|
1
|
Li Y, Li J, Fu Q, Chen L, Fei J, Deng S,
Qiu J, Chen G, Huang G and Wang C: Kidney transplantation from
brain-dead donors: Initial experience in China. Transplant Proc.
48:2592–2595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sui WG, Yan Q, Xie SP, Chen HZ, Li D, Hu
CX, Peng WJ and Dai Y: Successful organ donation from brain dead
donors in a Chinese organ transplantation center. Am J Transplant.
11:2247–2249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Liu L, Fu Q, Meng F, Li J, Deng S,
Fei J, Yuan X, Han M, Chen L, et al: Kidney transplantation from
donors after brain or cardiac death in China-a clinical analysis of
94 cases. Transplant Proc. 45:1323–1326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Zeng L, Gao X, Wang H and Zhu Y:
Transformation of organ donation in China. Transpl Int. 28:410–415.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Q, Gao X, Ko DS and Li XC: Organ
transplantation in China-not yet a new era-Authors' reply. Lancet.
384:741–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kosmoliaptsis V, Salji M, Bardsley V, Chen
Y, Thiru S, Griffiths MH, Copley HC, Saeb-Parsy K, Bradley JA,
Torpey N and Pettigrew GJ: Baseline donor chronic renal injury
confers the same transplant survival disadvantage for DCD and DBD
kidneys. Am J Transplant. 15:754–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatamizadeh P, Molnar MZ, Streja E,
Lertdumrongluk P, Krishnan M, Kovesdy CP and Kalantar-Zadeh K:
Recipient-related predictors of kidney transplantation outcomes in
the elderly. Clin Transplant. 27:436–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mertes PM, el Abassi K, Jaboin Y, Burtin
P, Pinelli G, Carteaux JP, Burlet C, Boulange M and Villemot JP:
Changes in hemodynamic and metabolic parameters following induced
brain death in the pig. Transplantation. 58:414–418. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barklin A, Larsson A, Vestergaard C,
Kjaergaard A, Wogensen L, Schmitz O and Tønnesen E: Insulin alters
cytokine content in two pivotal organs after brain death: A porcine
model. Acta Anaesthesiol Scand. 52:628–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lisman T, Leuvenink HG, Porte RJ and Ploeg
RJ: Activation of hemostasis in brain dead organ donors: An
observational study. J Thromb Haemost. 9:1959–1965. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang H, Zhang S, Guo W, Cao S, Yan B, Lu Y
and Li J: Cobalt protoporphyrin protects the liver against
apoptosis in rats of brain death. Clin Res Hepatol Gastroenterol.
39:475–481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oberhuber R, Ritschl P, Fabritius C,
Nguyen AV, Hermann M, Obrist P, Werner ER, Maglione M, Flörchinger
B, Ebner S, et al: Treatment With tetrahydrobiopterin overcomes
brain death-associated injury in a murine model of pancreas
transplantation. Am J Transplant. 15:2865–2876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qureshi MS, Callaghan CJ, Bradley JA,
Watson CJ and Pettigrew GJ: Outcomes of simultaneous
pancreas-kidney transplantation from brain-dead and controlled
circulatory death donors. Br J Surg. 99:831–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pratschke J, Wilhelm MJ, Kusaka M, Basker
M, Cooper DK, Hancock WW and Tilney NL: Brain death and its
influence on donor organ quality and outcome after transplantation.
Transplantation. 67:343–348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tezel G: A proteomics view of the
molecular mechanisms and biomarkers of glaucomatous
neurodegeneration. Prog Retin Eye Res. 35:18–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du B, Li L, Zhong Z, Fan X, Qiao B, He C,
Fu Z, Wang Y and Ye Q: Brain death induces the alteration of liver
protein expression profiles in rabbits. Int J Mol Med. 34:578–584.
2014.PubMed/NCBI
|
|
17
|
Pratschke J, Wilhelm MJ, Kusaka M,
Laskowski I and Tilney NL: A model of gradual onset brain death for
transplant-associated studies in rats. Transplantation. 69:427–430.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shevchenko A, Wilm M, Vorm O and Mann M:
Mass spectrometric sequencing of proteins silver-stained
polyacrylamide gels. Anal Chem. 68:850–858. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jou YC, Fang CY, Chen SY, Chen FH, Cheng
MC, Shen CH, Liao LW and Tsai YS: Proteomic study of renal uric
acid stone. Urology. 80:260–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sade RM: Brain death, cardiac death, and
the dead donor rule. J S C Med Assoc. 107:146–149. 2011.PubMed/NCBI
|
|
21
|
Avlonitis VS, Wigfield CH, Kirby JA and
Dark JH: The hemodynamic mechanisms of lung injury and systemic
inflammatory response following brain death in the transplant
donor. Am J Transplant. 5:684–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rostron AJ, Avlonitis VS, Cork DM, Grenade
DS, Kirby JA and Dark JH: Hemodynamic resuscitation with arginine
vasopressin reduces lung injury after brain death in the transplant
donor. Transplantation. 85:597–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuecuek O, Mantouvalou L, Klemz R, Kotsch
K, Volk HD, Jonas S, Wesslau C, Tullius S, Neuhaus P and Pratschke
J: Significant reduction of proinflammatory cytokines by treatment
of the brain-dead donor. Transplant Proc. 37:387–388. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koudstaal LG, t Hart NA, Ottens PJ, van
den Berg A, Ploeg RJ, van Goor H and Leuvenink HG: Brain death
induces inflammation in the donor intestine. Transplantation.
86:148–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Auraen H, Mollnes TE, Bjørtuft Ø, Bakkan
PA, Geiran O, Kongerud J, Fiane A and Holm AM: Multiorgan
procurement increases systemic inflammation in brain dead donors.
Clin Transplant. 27:613–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hvas CL, Fenger-Eriksen C, Høyer S,
Sørensen B and Tønnesen E: Hypercoagulation following brain death
cannot be reversed by the neutralization of systemic tissue factor.
Thromb Res. 132:300–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu X, Du Z, Yu J, Sun Y, Pei B, Lu X, Tang
Z, Yin M, Zhou L and Hu J: Activity of factor VII in patients with
isolated blunt traumatic brain injury: Association with
coagulopathy and progressive hemorrhagic injury. J Trauma Acute
Care Surg. 76:114–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leber B, Stadlbauer V, Stiegler P, Stanzer
S, Mayrhauser U, Koestenbauer S, Leopold B, Sereinigg M, Puntschart
A, Stojakovic T, et al: Effect of oxidative stress and endotoxin on
human serum albumin in brain-dead organ donors. Transl Res.
159:487–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vespa PM: Hormonal dysfunction in
neurocritical patients. Curr Opin Crit Care. 19:107–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ranasinghe AM and Bonser RS: Endocrine
changes in brain death and transplantation. Best Pract Res Clin
Endocrinol Metab. 25:799–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mikhova KM, Don CW, Laflamme M, Kellum JA,
Mulligan MS, Verrier ED and Rabkin DG: Effect of cytokine
hemoadsorption on brain death-induced ventricular dysfunction in a
porcine model. J Thorac Cardiovasc Surg. 145:215–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vazquez A, Flammini A, Maritan A and
Vespignani A: Global protein function prediction from
protein-protein interaction networks. Nat Biotechnol. 21:697–700.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chowdhury I, Branch A, Olatinwo M, Thomas
K, Matthews R and Thompson WE: Prohibitin (PHB) acts as a potent
survival factor against ceramide induced apoptosis in rat granulosa
cells. Life Sci. 89:295–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|