Introduction
Colorectal cancer (CRC) is currently the third most
common type of cancer, with >1 million people developing it
every year (1,2), and >0.5 million mortalities
annually (3). CRC is treatable if
detected and removed at an early stage, prior to metastasis, with
95% of patients surviving beyond 5 years (4). However, ~20% patients with primary
CRC have distant metastasis at the time of diagnosis, and only
10–30% of these patients are able to receive the potentially
curative resection of the primary tumor and the distant metastatic
focus (5–7). For the past 20 years, the early
diagnosis of CRC was through the widely used methods of fecal
occult blood test (FOBT) and colonoscopy. These tests have improved
OS rates for CRC, although FOBT has a low sensitivity and certain
foods and medications may lead to false-positive results, and
colonoscopy is expensive and invasive. Therefore, the
identification of novel markers and the development of accurate and
non-invasive tests for the diagnosis and prognosis of CRC are
essential (8).
Cyclin-dependent kinases (CDKs) serve important
roles in cell cycle progression, transcription and differentiation.
Previous studies have demonstrated that overexpression of the cell
division cycle (Cdc) 2-related serine/threonine protein kinase
PFTAIRE 1 (PFTK1; also known as CDK14) can promote cell migration
and is associated with the motile phenotype of cancer cells
(9,10). In addition, PFTK1 may be involved
in cancer progression (11,12).
PFTK1 is a novel member of the Cdc2-related serine/threonine
protein kinases family, which can regulate the expression of
cyclins and the cell cycle (13).
Previous studies have demonstrated high expression of PFTK1 in
several types of malignant tumor, including hepatocellular
carcinoma (14), esophageal
(11), breast (15) and gastric (16) cancers, as well as glioma (17). It is involved in cell cycle
regulation, tumor proliferation, migration and invasion (18). Several studies have reported that
high expression levels of PFTK1 were significantly correlated with
a poorer prognosis in certain cancers, such as esophageal squamous
cell, breast and gastric cancers (11,15,16).
However, the expression of PFTK1 in CRC and its correlation with
clinical characteristics have not been evaluated to date.
The present study aimed to investigate the
expression and prognostic role of PFTK1 in CRC. PFTK1 mRNA
expression in fresh-frozen tissues was examined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Subsequently, tissue microarrays (TMAs) were prepared to determine
the expression of PFTK1 protein, by immunohistochemical (IHC)
staining, in 179 specimens of CRC tissue and 47 normal control
samples. In addition, the association between PFTK1 expression and
clinicopathological characteristics of patients with CRC was
analyzed. The findings suggested that PFTK1 expression may
represent a novel indicator of poor prognosis and may provide a
potential anticancer target for CRC therapy.
Materials and methods
Clinical information and preparation
of TMAs
A total of 179 postoperative CRC tissue samples,
confirmed by histopathological examination, were obtained from the
Department of Pathology, Affiliate Hospital of Nantong University
(Jiangsu, China), between January 2009 and May 2014. All patients
were diagnosed with CRC in accordance with the 7th edition of the
Union for International Cancer Control and the American Joint
Committee on Cancer tumor node metastasis (TNM) classification for
CRC (19). An additional 10
fresh-frozen CRC tissues and 10 normal tissues as controls were
collected for mRNA isolation to determine expression levels by
RT-qPCR. The 179 CRC tissue samples and the 47 control samples of
benign colorectal lesions collected from the Department of
Pathology, Affiliated Hospital of Nantong University, were prepared
as TMAs for PFTK1 protein determination by IHC. None of the
patients had received preoperative radiotherapy or chemotherapy,
and written informed consent was obtained from each patient.
Ethical approval to perform the present study was obtained from the
Human Research Ethics Committee of the Affiliated Hospital of
Nantong University (Jiangsu, China).
RNA extraction and RT-qPCR
Total RNA was isolated from 20 fresh-frozen tissues
(including 10 fresh CRC and 10 surrounding normal tissues) using
RNeasy Plus Mini kit (cat. no. 74134; Qiagen GmbH, Hilden, Germany)
and converted to cDNA using a High Capacity RNA-to-cDNA kit (cat.
no. 4387406; Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RT-qPCR was performed using Power SYBR-Green PCR
Master Mix (cat. no. 4367659; Life Technologies; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, and an
ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and amplified with target gene-specific primers.
The primer sequences were: PFTK1, forward
5′-CTTGACATGTGGGGAGTAGGTT-3′, reverse 5′-CCATGTGTCCTCATTTGGTG-3′;
and the internal control GAPDH, forward 5′-AGTTGTCATGGATGACCTTGG-3,
and reverse 5′-GGCATGGACTGTGGTCATGAG-3′. PCR conditions consisted
of 10 min at 95°C for Taq activation, followed by 40 cycles
of 95°C for 15 sec and 60°C (58°C for GAPDH) for 1 min, 72°C for 2
min, and the final elongation at 72°C for 5 min.
All experiments were performed in triplicate with
three technical replicates (20).
TMA construction and IHC analysis
To explore PFTK1 protein expression in CRC tissues,
IHC analysis was performed as previously described (21). Briefly, a total of 179 CRC tissues
and 47 normal tissues were used for TMA construction. A Quick-Ray
Manual Tissue Microarray System (cat. no. UT06; Unitma Co., Ltd.,
Seoul, Korea) was used to produce 2 mm thick, paraffin-embedded CRC
TMA sections. A total of 47 normal tissues were sectioned to use as
control. Core tissue biopsies (diameter, 2 mm) were obtained from
individual paraffin-embedded sections and arranged in the new
recipient paraffin blocks. TMA blocks were cut into 2 mm sections
and placed on uperfrost glass microscope slides. Following this,
the slides were firstly placed in the baking box at a constant
temperature of 60°C for 30 min, and then soaked in xylene and
xylene II for 10 min. The slides were then placed in turn, in a 95,
85, and 75% alcohol solution for 5 min and flushed with distilled
water for 3 min. This process was repeated twice. Next, the slides
were arranged in the high temperature resistant plastic slice
frame, immersed in citrate buffer (pH=6.0; TA501031; ZSGB-BIO,
Beijing, China), placed in a microwave box, mid-range microwave
heated for 10 min, microwave box water-cooled and then rinsed with
distilled water and washed with phosphate buffered saline (PBS;
ZLI-9062; ZSGB-BIO) for 3 min. This process was repeated three
times. Incubation then occurred with 3% H2O2
at room temperature for 10 min to eliminate endogenous peroxidase
activity, and slides were washed with PBS three times each for 5
min. The slides were then incubated with a primary anti-PFTK1
antibody (cat. no. sc-50475; 1:100; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) in buffer containing 1% bovine serum albumin (BSA;
A7906; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), at 4°C
overnight. Slides were washed with phosphate-buffered saline (PBS)
and incubated with a horseradish peroxidase-conjugated anti-rabbit
IgG polymer as a secondary antibody (catalog no. A0210; 1:1,000;
EnVision kit; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) for 20 min at room temperature and then washed with PBS. The
color was developed by 15 min incubation with 3,3′-diaminobenzidine
solution (Kem-En-Tec Diagnostics, Taastrup, Denmark), and the
sections were weakly counterstained with hematoxylin. For negative
controls, PBS was used instead of the primary antibody.
IHC staining results were evaluated under an optical
microscope by two independent, trained pathologists in a
double-blind manner. PFTK1 protein expression levels were analyzed
as previously described (22).
Briefly, the percentage of PFTK1-positive cells was scored as
follows: 0, 0% staining; 1, 1–33%; 2, 34–66%; and 3, 67–100%. The
intensity of PFTK1 staining was also scored as follows: 0, no
staining; 1, weak staining; 2, light/moderate staining; and 3,
strong staining. The final staining score was calculated as the
product of the intensity score and percentage scores. The cutoff
value for the PFTK1 protein expression score that was statistically
significant in terms of overall survival (OS) rate was determined
using the X-tile software program (Version 3.6.1; The Rimm Lab,
Yale University; http://medicine.yale.edu/lab/rimm/research/software.aspx),
as previously described (21).
Statistical analyses
SPSS software version 22.0 (IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. A paired Student's t-test
was performed to compare PFTK1 mRNA expression between CRC and
normal tissues. Pearson's χ2 test was used to analyze
differences in PFTK1 protein expression between CRC and normal
tissues, and for the correlation between PFTK1 and
clinicopathological characteristics. OS rate curves were calculated
using the Kaplan-Meier method. Univariate and multivariate analyses
were performed using the Cox proportional hazards model; the risk
ratio and 95% confidence interval were recorded for each marker.
P<0.05 was considered to indicate a statistically significant
difference for all analyses.
Results
Analysis of PFTK1 mRNA expression in
CRC by RT-qPCR
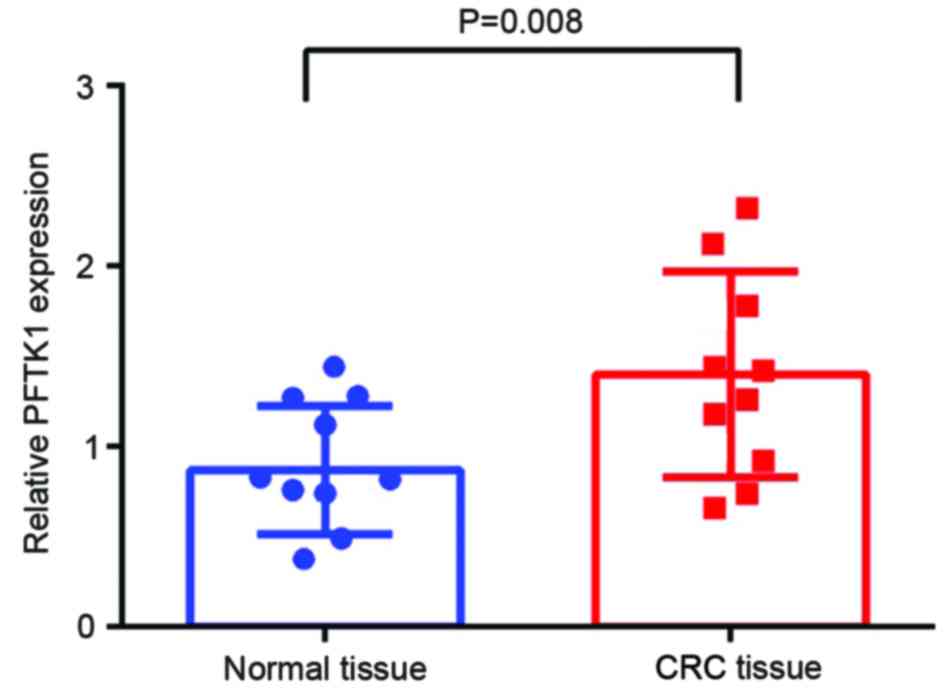
RT-qPCR was performed to detect the expression of
PFTK1 mRNA in CRC and corresponding normal tissues. The expression
of PFTK1 mRNA relative to the expression of the internal control
GAPDH was higher in CRC tissues compared with normal tissues
(1.433±0.168 vs. 0.853±0.107, respectively; t=1.97; P=0.008;
Fig. 1).
Detection of PFTK1 protein expression
in CRC by IHC
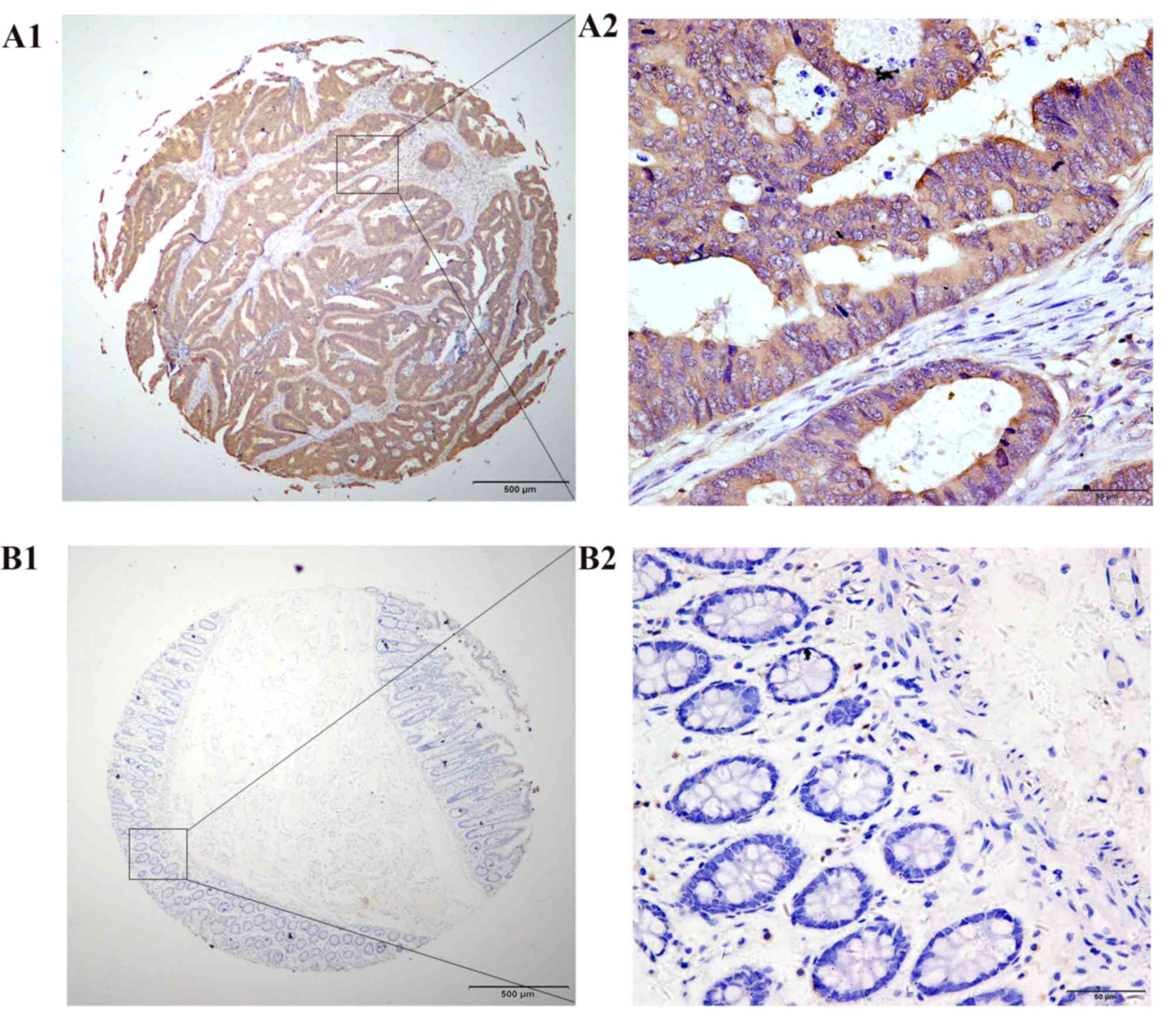
To detect the expression levels and location of
PFTK1 protein in cancer tissues, IHC was performed on a TMA
consisting of 179 CRC tissues and 74 matched non-cancerous
specimens. PFTK1 protein was primarily expressed in the cytoplasm
and membranes of the cancer cells, with low or no positive signals
detected in the nuclei of cancer cells and stromal cells (Fig. 2A1 and A2); no positive signals were
detected in normal colorectal glandular cells (Fig. 2B1 and B2). High levels of PFTK1
protein expression was detected in 51.40% (92/179) of the cancer
cells in the CRC samples (Table
I).
 | Table I.Relationship between the expression of
PFTK1 and clinicopathological characteristics in colorectal
cancer. |
Table I.
Relationship between the expression of
PFTK1 and clinicopathological characteristics in colorectal
cancer.
| Characteristic | n | Low expression
(%) | High expression
(%) | Pearson's
χ2 | P-value |
|---|
| Total | 179 | 87 (48.60) | 92 (51.40) |
|
|
| Sex |
|
|
| 0.561 | 0.454 |
| Male | 114 | 53 (46.49) | 61 (53.51) |
|
|
|
Female | 65 | 34 (52.31) | 31 (47.69) |
|
|
| Age |
|
|
| 1.892 | 0.169 |
|
<60 | 59 | 33 (55.93) | 26 (44.07) |
|
|
| ≥60 | 120 | 54 (45.00) | 66 (55.00) |
|
|
| Tumor location |
|
|
| 0.012 | 0.911 |
|
Colon | 131 | 64 (48.85) | 67 (51.15) |
|
|
|
Rectum | 48 | 23 (47.92) | 25 (52.08) |
|
|
| Histological
type |
|
|
| 0.012 | 0.911 |
| Tubular
and papillary | 131 | 64 (48.85) | 67 (51.15) |
|
|
|
Othera | 48 | 23 (47.92) | 25 (52.08) |
|
|
|
Differentiation |
|
|
| 3.154 | 0.076 |
| Low
grade | 15 | 4 (26.67) | 11 (73.33) |
|
|
|
Middle/high grade | 164 | 83 (50.61) | 81 (49.39) |
|
|
| TNM stage |
|
|
| 6.358 | 0.042 |
|
0-I | 34 | 23 (67.65) | 11 (32.35) |
|
|
| II | 70 | 32 (46.38) | 37 (53.62) |
|
|
|
III+IV | 75 | 32 (42.11) | 44 (57.89) |
|
|
| Tumor
classification |
|
|
| 5.278 | 0.022 |
|
Tis+T1+T2 | 44 | 28 (63.64) | 16 (36.36) |
|
|
| T3,
4b | 135 | 59 (43.70) | 76 (56.30) |
|
|
| Node
classification |
|
|
| 1.168 | 0.558 |
| N0 | 108 | 56 (51.85) | 52 (48.15) |
|
|
|
N1a | 36 | 16 (44.44) | 20 (55.56) |
|
|
| N1b,
N2a,b | 35 | 15 (42.86) | 20 (57.14) |
|
|
| Preoperative
CEA |
|
|
| 14.249 |
<0.001 |
| ≤15
ng/ml | 144 | 80 (55.56) | 64 (44.44) |
|
|
| >15
ng/ml | 35 | 7 (20.00) | 28 (80.00) |
|
|
Association between PFTK1 protein
expression and clinical characteristics
Associations between PFTK1 protein expression levels
and clinicopathological characteristics of CRC are demonstrated in
Table I. Pearson χ2
analysis revealed a significant correlation between positive PFTK1
expression in cancer cells and the TNM stage (P=0.042), tumor
classification (P=0.022) and preoperative CEA level (P<0.001).
By contrast, no significant correlation was identified for sex,
age, degree of differentiation, tumor location, histological type
or lymphatic metastasis.
Overexpression of PFTK1 in CRC is
associated with poor prognosis
Univariate analysis demonstrated a correlation
between the OS rates of patients with CRC and the degree of
differentiation (P<0.001), tumor classification (P<0.001),
lymph node metastasis (P<0.001), TNM stage (P<0.001),
preoperative CEA level (P<0.001) and positive PFTK1 expression
(P<0.001) (Table II).
Multivariate Cox regression analysis further demonstrated that high
PFTK1 expression [hazard ratio (HR)=1.999; P=0.019], degree of
differentiation (HR=0.368; P=0.003), TNM stage (HR=2.118; P=0.001),
and preoperative CEA level (HR=2.302; P=0.003) were independent
prognostic factors for OS rate (Table
II).
 | Table II.Univariate and multivariable analysis
of prognostic factors for 5-year survival in colorectal cancer. |
Table II.
Univariate and multivariable analysis
of prognostic factors for 5-year survival in colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Prognostic
factor | HR | P-value | 95% | CI | HR | P-value | 95% | CI |
|---|
| PFTK1 expression:
High vs. low and none | 2.813 |
<0.001 | 1.621 | 4.881 | 1.999 | 0.019 | 1.122 | 3.561 |
| Age (years): ≤60
vs. >60 | 1.007 | 0.981 | 0.590 | 1.718 |
|
|
|
|
| Sex: Male vs.
female | 1.434 | 0.199 | 0.827 | 2.487 |
|
|
|
|
| Tumor location:
Colon vs. rectum | 1.266 | 0.394 | 0.736 | 2.177 |
|
|
|
|
| Histological type:
Tubular and papillary vs. othersa | 0.925 | 0.855 | 0.398 | 2.148 |
|
|
|
|
| Differentiation:
High and middle vs. low | 0.218 |
<0.001 | 0.115 | 0.412 | 0.368 | 0.003 | 0.190 | 0.713 |
| TNM stage: 0 and I
vs. II vs. III and IV | 2.659 |
<0.001 | 1.744 | 4.052 | 2.118 | 0.001 | 1.358 | 3.302 |
| Tumor
classification: Tis+T1 vs. T2 vs. T3 and 4a | 12.367 |
<0.001 | 3.020 | 50.641 |
|
|
|
|
| Node
classification: N0 vs. N1a vs. N1b vs. N2a and N2b | 1.888 |
<0.001 | 1.418 | 2.514 |
|
|
|
|
| Preoperative CEA
(ng/ml): ≤5 vs. >5 | 4.078 |
<0.001 | 2.428 | 6.847 | 2.302 | 0.003 | 1.316 | 4.026 |
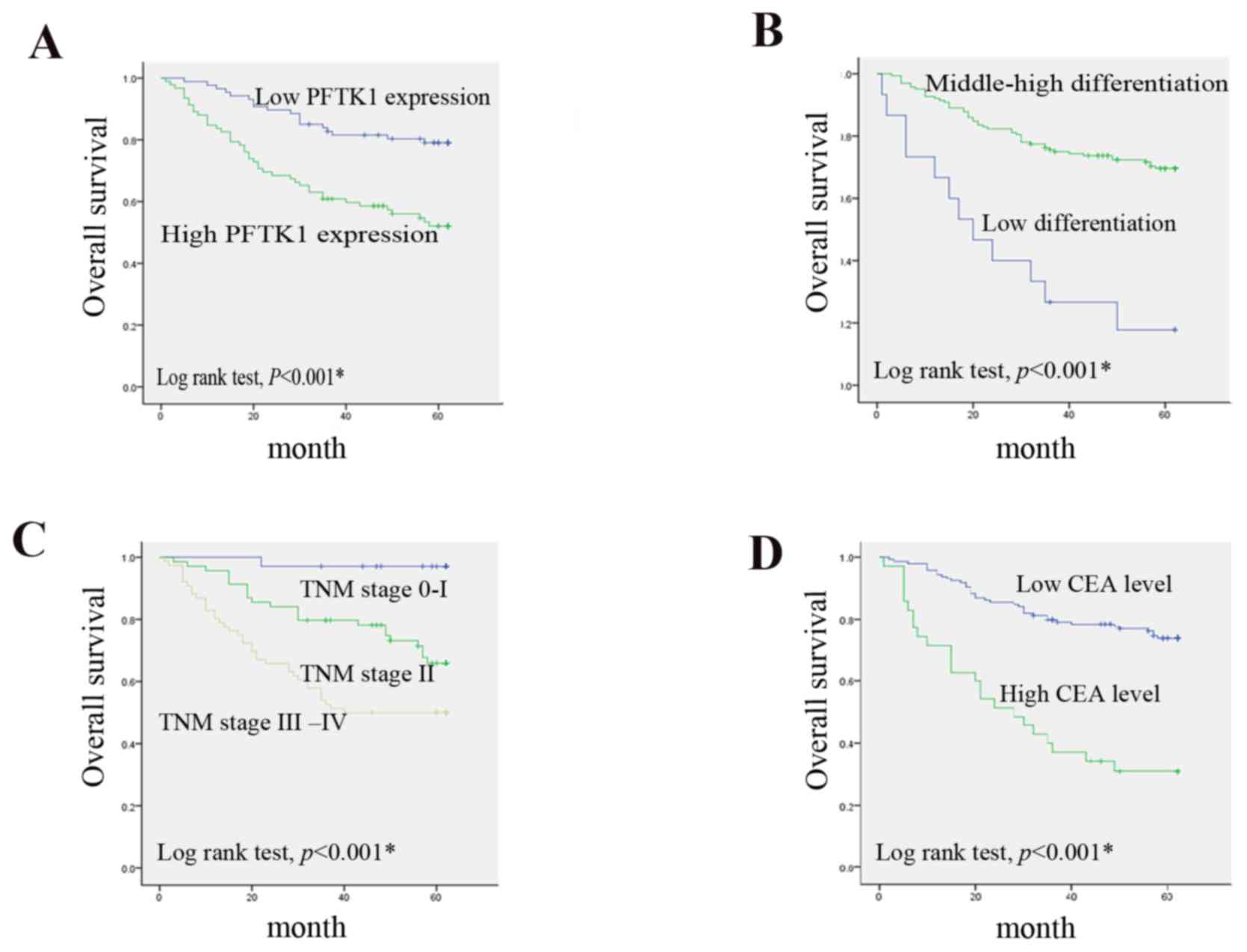
Kaplan-Meier OS rate curves demonstrated that
patients with CRC that have positive PFTK1 expression in the
cytoplasm exhibited significantly lower OS rates compared with
those that were negative for PFTK1 expression (Fig. 3A; P<0.001). A high degree of
differentiation was also associated with an unfavorable OS rate
(Fig. 3B; P<0.001). In
addition, the OS rate in patients with advanced stages of TNM
(stage III–IV) was significantly lower compared with patients with
early-stage disease (stage 0-I and stage II; Fig. 3C; P<0.001). Additionally,
patients with a high preoperative CEA level had a significantly
poorer OS rate compared with those with low levels of preoperative
CEA (Fig. 3D; P<0.001).
Discussion
Previous studies have reported that CDKs may be
involved in human cancers, and CDK1, CDK4 and CDK6 expression may
be diagnostic in a subset of cancers (23–28).
In addition, CDK2 expression or activity may be able to predict the
prognosis of breast (25), ovarian
(29) and oral (30) cancers. As a novel member of the CDK
family, PFTK1 was previously reported to influence tumorigenesis
and tumor progression (31).
Although cell cycle proteins have long been considered to be unable
to influence the migration of cells, previous studies have
demonstrated that PFTK1 protein either activated or was involved in
Wnt signaling and promoted migration and invasion (16). A study has previously indicated
that PFTK1 can modulate oligodendrocyte differentiation via the
PI3K/AKT pathway (32), whereas
downregulation of PFTK1 expression inhibited glioma cell migration
(17). Based on these results,
PFTK1 was considered to be a potent target for cancer therapy. It
has been demonstrated that overexpression of PFTK1 predicts
resistance to chemotherapy in esophageal squamous cell carcinoma,
and that PFTK1 may also be a potential target of molecular-targeted
therapy (11).
However, the expression of PFTK1 and its association
with the clinical parameters of patients with CRC remains to be
elucidated. The present study explored the potential role of PFTK1
in CRC development. PFTK1 mRNA expression in small samples of
cancerous and normal colorectal tissues revealed a significantly
higher level of expression in cancerous tissues, consistent with
the results in other cancers (11,12).
IHC staining for PFTK1 protein expression in CRC on 179 CRC and 47
matched non-cancerous specimens confirmed the RT-qPCR results;
higher PFTK1 protein expression was identified in CRC compared with
noncancerous tissues, which suggests that CRC may result from
increased PFTK1 expression. Furthermore, the correlation between
PFTK1 expression and OS rate in patients with CRC was investigated.
In the present study, the Kaplan-Meier analysis demonstrated that
the OS rates were lower in patients with positive PFTK1 expression
compared with patients exhibiting no PFTK1 expression. Univariate
and multivariate analyses demonstrated that PFTK1 expression,
degree of differentiation, TNM stage, tumor classification, lymph
node metastasis and preoperative CEA level were correlated with the
OS rates of patients with CRC. Among these factors, positive PFTK1
expression, degree of differentiation, TNM classification, and
preoperative CEA level were identified as independent prognostic
factors affecting CRC. These findings demonstrated that positive
PFTK1 expression significantly influenced the poor prognosis in
patients with CRC.
As a genetic disease, cancer is primarily caused by
mutations in oncogenes and tumor suppressors, which serve to
control tissue homeostasis (33).
Altered function, in turn, leads to deregulated mitogenic survival
and the growth of tumors that frequently exhibit
oncogene-activating genomic alterations, including gene
amplification or gain-of-function point mutations. Tumors may also
exhibit gene deletions, loss-of-function point mutations or
epigenetic silencing that may inactivate tumor-suppressor genes
(33). Irrespective of gene
mutation or cell cycle disorder in cancer, it is to be hoped that
there is an attractive candidate for cancer therapy and a great
number of studies is urgently required for precision medicine
therapy.
In conclusion, and to our best knowledge, the
present study provides the first evidence that PFTK1 expression is
significantly higher in CRC tissues compared with matched adjacent
non-cancerous colorectal tissues. The associations between PFTK1
expression and clinicopathological characters, and PFTK1 expression
and the OS rate following resection suggested that PFTK1 may
represent a novel biomarker of poor prognosis in CRC and may be a
potential anticancer target for gene therapy. However, further
studies are required to elucidate the precise mechanisms of action
of PFTK1 in affecting proliferation, migration and invasion ability
of CRC cells.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics: CA Cancer. J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Gansler T, Ganz PA, Grant M, Greene FL,
Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR Jr, Thun MJ, et
al: Sixty years of CA: A cancer journal for clinicians. CA Cancer J
Clin. 60:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang CW, Tsai HL, Chen YT, Huang CM, Ma
CJ, Lu CY, Kuo CH, Wu DC, Chai CY and Wang JY: The prognostic
values of EGFR expression and KRAS mutation in patients with
synchronous or metachronous metastatic colorectal cancer. BMC
Cancer. 13:5992013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Pyrhönen S, James RD, Punt
CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham
CA, Awad L, et al: Randomised trial of irinotecan plus supportive
care versus supportive care alone after fluorouracil failure for
patients with metastatic colorectal cancer. Lancet. 352:1413–1418.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karoui M, Roudot-Thoraval F, Mesli F,
Mitry E, Aparicio T, Des Guetz G, Louvet C, Landi B, Tiret E and
Sobhani I: Primary colectomy in patients with stage IV colon cancer
and unresectable distant metastases improves overall survival:
Results of a multicentric study. Dis Colon Rectum. 54:930–938.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Platell C, Ng S, O'Bichere A and Tebbutt
N: Changing management and survival in patients with stage IV
colorectal cancer. Dis Colon Rectum. 54:214–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kleespies A, Füessl KE, Seeliger H,
Eichhorn ME, Müller MH, Rentsch M, Thasler WE, Angele MK, Kreis ME
and Jauch KW: Determinants of morbidity and survival after elective
non-curative resection of stage IV colon and rectal cancer. Int J
Colorectal Dis. 24:1097–1109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hollis M, Nair K, Vyas A, Chaturvedi LS,
Gambhir S and Vyas D: MicroRNAs potential utility in colon cancer:
Early detection, prognosis, and chemosensitivity. World J
Gastroenterol. 21:8284–8292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Juliano R: Movin' on through with Cdc2.
Nat Cell Biol. 5:589–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manes T, Zheng DQ, Tognin S, Woodard AS,
Marchisio PC and Languino LR: Alpha(v)beta3 integrin expression
up-regulates cdc2, which modulates cell migration. J Cell Biol.
161:817–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyagaki H, Yamasaki M, Miyata H,
Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Fujiwara Y, Ishii
H, Tanaka F, et al: Overexpression of PFTK1 predicts resistance to
chemotherapy in patients with oesophageal squamous cell carcinoma.
Br J Cancer. 106:947–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pang EY, Bai AH, To KF, Sy SM, Wong NL,
Lai PB, Squire JA and Wong N: Identification of PFTAIRE protein
kinase 1, a novel cell division cycle-2 related gene, in the motile
phenotype of hepatocellular carcinoma cells. Hepatology.
46:436–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang T and Chen JY: Identification and
cellular localization of human PFTAIRE1. Gene. 267:165–172. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leung WK, Ching AK, Chan AW, Poon TC, Mian
H, Wong AS, To KF and Wong N: A novel interplay between oncogenic
PFTK1 protein kinase and tumor suppressor TAGLN2 in the control of
liver cancer cell motility. Oncogene. 30:4464–4475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu X, Wang Y, Wang H, Ni Q, Zhang C, Zhu
J, Huang W, Xu P, Mao G and Yang S: Upregulated PFTK1 promotes
tumor cell proliferation, migration, and invasion in breast cancer.
Med Oncol. 32:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Zhu J, Huang H, Yang Q, Cai J,
Wang Q, Zhu J, Shao M, Xiao J, Cao J, et al: PFTK1 promotes gastric
cancer progression by regulating proliferation, migration and
invasion. PLoS One. 10:e01404512015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan S, Zhao C, Zhang L, Dai S, Ren J,
Zhang X, Ban N, He X, Yang L, Bao Z, et al: Knockdown of PFTK1
inhibits the migration of glioma cells. J Mol Neurosci. 57:257–264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai M, Al-Odaini AA, Fils-Aimé N,
Villatoro MA, Guo J, Arakelian A, Rabbani SA, Ali S and Lebrun JJ:
Cyclin D1 cooperates with p21 to regulate TGFβ-mediated breast
cancer cell migration and tumor local invasion. Breast Cancer Res.
15:R492013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Novak J and Fabian P: Comments on the TNM
classification of malignant tumours-7th edition. Klin Onkol.
24:149–150. 2011.(In Czech). PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun R, Wang X, Zhu H, Mei H, Wang W, Zhang
S and Huang J: Prognostic value of LAMP3 and TP53 overexpression in
benign and malignant gastrointestinal tissues. Oncotarget.
5:12398–12409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang J, Zhang X, Tang Q, Zhang F, Li Y,
Feng Z and Zhu J: Prognostic significance and potential therapeutic
target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol.
64:343–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poomsawat S, Buajeeb W, Khovidhunkit SO
and Punyasingh J: Alteration in the expression of cdk4 and cdk6
proteins in oral cancer and premalignant lesions. J Oral Pathol
Med. 39:793–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakayama S, Torikoshi Y, Takahashi T,
Yoshida T, Sudo T, Matsushima T, Kawasaki Y, Katayama A, Gohda K,
Hortobagyi GN, et al: Prediction of paclitaxel sensitivity by CDK1
and CDK2 activity in human breast cancer cells. Breast Cancer Res.
11:R122009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SJ, Nakayama S, Miyoshi Y, Taguchi T,
Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara
H and Noguchi S: Determination of the specific activity of CDK1 and
CDK2 as a novel prognostic indicator for early breast cancer. Ann
Oncol. 19:68–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hansel DE, Dhara S, Huang RC, Ashfaq R,
Deasel M, Shimada Y, Bernstein HS, Harmon J, Brock M, Forastiere A,
et al: CDC2/CDK1 expression in esophageal adenocarcinoma and
precursor lesions serves as a diagnostic and cancer progression
marker and potential novel drug target. Am J Surg Pathol.
29:390–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Semczuk A and Jakowicki JA: Alterations of
pRb1-cyclin D1-cdk4/6-p16(INK4A) pathway in endometrial
carcinogenesis. Cancer Lett. 203:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simon R, Struckmann K, Schraml P, Wagner
U, Forster T, Moch H, Fijan A, Bruderer J, Wilber K, Mihatsch MJ,
et al: Amplification pattern of 12q13-q15 genes (MDM2, CDK4, GLI)
in urinary bladder cancer. Oncogene. 21:2476–2483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marone M, Scambia G, Giannitelli C,
Ferrandina G, Masciullo V, Bellacosa A, Benedetti-Panici P and
Mancuso S: Analysis of cyclin E and CDK2 in ovarian cancer: Gene
amplification and RNA overexpression. Int J Cancer. 75:34–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mihara M, Shintani S, Nakahara Y, Kiyota
A, Ueyama Y, Matsumura T and Wong DT: Overexpression of CDK2 is a
prognostic indicator of oral cancer progression. Jpn J Cancer Res.
92:352–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue W, Zhao X, Zhang L, Xu S, Liu Z, Ma L,
Jia W, Qian Z, Zhang C, Wang Y and Yang X: Cell cycle protein
cyclin Y is associated with human non-small-cell lung cancer
proliferation and tumorigenesis. Clin Lung Cancer. 12:43–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang HJ, Wang L, Wang M, Ma SP, Cheng BF,
Li ZC and Feng ZW: Serine/threonine-protein kinase PFTK1 modulates
oligodendrocyte differentiation via PI3K/AKT pathway. J Mol
Neurosci. 55:977–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mullen AR and DeBerardinis RJ:
Genetically-defined metabolic reprogramming in cancer. Trends
Endocrinol Metab. 23:552–559. 2012. View Article : Google Scholar : PubMed/NCBI
|