Introduction
Breast cancer (BC) is the most frequently diagnosed
malignancy and is one of the leading causes of mortality in women,
with nearly a quarter of million new cases occurring in the United
States in 2016 (1,2). The heterogeneity of breast cancer is
a primary factor that contributes to the intractable clinical
treatments. For example, hormone therapy is available for luminal A
or B breast cancers that are estrogen (ER)/progesterone (PR)
positive. However, there are limited available treatments for
breast cancer that is triple-negative for ER, PR and human
epidermal growth factor receptor 2 (HER2). Numerous molecular
biomarkers and signaling pathways have been investigated, including
aberrant activation of Akt and Wnt signaling pathways, in addition
to changes in microRNAs and oncogenes (3–6).
However, the mechanisms underlying the development of breast cancer
require further understanding, particularly the development of
malignant phenotypes.
The deubiquitinating enzyme RPN11, alternatively
known as proteasome 26S subunit non-ATPase 14 or POH1, is a
component of the 26S proteasome and reverses the effects of the
ubiquitin-proteasome degradation system. RPN11 functions in diverse
biological processes, including DNA repair, embryonic cell
development and differentiation, programmed cell death and drug
resistance (7–9). RPN11 has been reported to modulate
the proliferation of tumor cells by regulating the phosphorylation
of retinoblastoma protein and cyclin-dependent kinases (10). In addition, RPN11 was significantly
upregulated in hepatocellular carcinoma and promoted tumorigenesis
by stabilizing E2F transcription factor 1 (E2F1) and its target
genes (11). Gallery et al
(12) reported that the
JAB1/MPN/Mov34 metalloenzyme motif of RPN11 was essential for cell
viability. A previous study has suggested that RPN11 may regulate
the ErbB2 receptor and c-jun ubiquitination, and enhance the
transcriptional activity of microphthalmia-associated transcription
factor (13–15). However, there is currently no
evidence for the potential role of RPN11 in breast cancer
development and treatment.
The present study reports that mRNA and protein
expression levels of RPN11 were overexpressed in breast cancer
tissue. RPN11 significantly promoted tumor cell proliferation and
inhibited apoptosis. In addition, knockdown of RPN11 inhibited cell
migration by suppressing epithelial-mesenchymal transition (EMT).
Therefore, the results of the present study demonstrate that
inhibition of RPN11 may serve as a treatment for breast cancer.
Materials and methods
Tissue specimens
A total of 59 breast cancer tissues and paired
noncancerous tissues were obtained from the First Zone of
Cardiothoracic Department, Qingyuan People's Hospital (Qingyuan,
China). The study was approved by the Ethical Committee of Qingyuan
People's Hospital and the written informed consent was obtained.
The patients did not receive radiotherapy or chemotherapy prior to
tissue collection. In addition, six pairs of fresh breast cancer
tissues and adjacent noncancerous tissues were utilized for western
blot analysis. Tissues were stored at −80°C prior to processing. A
tissue microarray containing 142 cases of breast cancer specimens
with complete clinicopathological features was utilized in this
study and was obtained from Shanghai Outdo Biotech Co. Ltd (cat.
no. HBre-Duc150Sur-02; Shanghai, China). The remaining patients
with breast cancer were obtained from GEO database (www.ncbi.nlm.nih.gov/).
Cell lines
MCF7, MDA-MB-231, Hs578T and T47D human cancer cell
lines were obtained from the American Type Culture Collection
(Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum, 100 µg/ml
streptomycin and 100 U/ml penicillin at 37°C and 5%
CO2.
Plasmid and siRNA transfection
The pcDNA3.1-RPN11 plasmid was utilized for
overexpression of RPN11 and was obtained from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). Cells at a density of
106 cells/well were plated into the 6-well plate and
transfected with pcDNA3.1-RPN11 or vector control using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). For siRNA transfection, cells
were transfected with a pool of three RPN11 siRNAs or a
negative control siRNA. siRNAs targeting RPN11 were obtained
from Chang Jing Bio-Tech, Ltd. (Changsha, China). The sequences
were as follows: Negative control siRNA,
5′-UUCUCCGAACGUGUCACGUTT-3′; RPN11 siRNA-1,
5′-CAAGTTAAATCTAGCTCAA-3′; RPN11 siRNA-2,
5′-GCAAGACAAGGGTCCATAT-3′ and RPN11 siRNA-3,
5′-TAAGACATCTGGCATCATT-3′. The cells were cultured for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized using the PrimeScript
RT Master kit (Takara Biotechnology Co., Ltd., Dalian, China).
RT-qPCR was performed on a 7500 Real-Time PCR system (Applied
Biosystems, Thermo Fisher Scientific, Inc.) using
SYBR®-Green. Primers were as follows: Forward,
5′-TGCTATGCCACAGTCAGGAA-3′ and reverse, 5′-ACAACCATCTCCGGCCTTC-3′
for human RPN11; forward, 5′-GTCTCCTCTTGGCTCTGCC-3′ and
reverse, 5′-AAATTCACTCTGCCCAGGACG-3′ for human E-cadherin; forward,
5′-GAGGCTTCTGGTGAAATCGC-3′ and reverse,
5′-TGCAGTTGCTAAACTTCACATT-3′ for human N-cadherin; forward,
5′-AATCCAGAGTTTACCTTCCAGCA-3′ and reverse,
5′-TCCCAGATGAGCATTGGCAG-3′ for human Snail; forward,
5′-GAACTGGACACACATACAGTGATT-3′ and reverse,
5′-AGTGATGGGGCTGTATGCTC-3′ for human Slug; forward,
5′-CGGGAGAAATTGCAGGAGGA-3′ and reverse, 5′-AAGGTCAAGACGTGCCAGAG-3′
for human vimentin; forward, 5′-ACAAACACTAATGTTAATTGCCCA-3′ and
reverse, 5′-TCTTGGCAGAGAGACATGCTT-3′ for human fibronectin 1. Human
GAPDH served as an internal control: GAPDH forward,
5′-CATGAGAAGTATGACAACAGCCT-3′ and reverse,
5′-AGTCCTTCCACGATACCAAAGT-3′. The PCR conditions were as follows:
95°C 30 sec, 95°C 30 sec, 60°C 30 sec for 40 cycles. The expression
levels of indicated genes were normalized using the
2−∆∆Cq method relative to the human GAPDH gene (16).
Western blot analysis
Tissue samples and cells were lysed in
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing protease and phosphatase inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland). Protein concentrations were
determined by the bicinchoninic acid assay. A total of 60 µg lysate
was loaded onto 12% gels, subjected to SDS-PAGE and transferred
onto polyvinylidene fluoride membranes. Membranes were blocked with
5% BSA (Sangon Biotech Co., Ltd., Shanghai, China) were incubated
with primary antibody overnight at 4°C, washed and probed with
HRP-labeled goat-anti-rabbit secondary antibody (dilution, 1:1,000;
cat. no. ab6721; Abcam, Cambridge, MA, USA) at room temperature for
2 h. Proteins were visualized using ECL chemiluminescent detection
substrate (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Primary antibodies, diluted at 1:1,000
were as follows: rabbit anti-RPN11 (catalog no. 12059-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA), mouse anti-tubulin
(catalog no. sc-5286; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit anti-cleaved PARP (catalog no. 5625) and mouse
anti-snail (catalog no. 3895; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-E-cadherin (catalog no. 1702),
rabbit anti-N-cadherin (catalog no. 2447), rabbit anti-cyclin D1
(catalog no. 2261) and rabbit anti-vimentin (catalog no. 2707;
Epitomics, Burlingame, CA, USA).
Cell viability assay
The rate of cell proliferation was measured by MTT
assay. The cells were transfected with RPN11 cDNA and vector as
control, or siRNA and NC as control. Subsequently, the cells were
seeded in 96-well plates to a density of 1,000 cells/well. MTT
reagent (5 mg/ml) was added to the medium at various time points.
Following 3 h incubation, the medium was discarded and DMSO was
added to form a precipitate. The absorbance was measured at a
wavelength of 540 nm.
Flow cytometric assay
Cells were seeded at 105 fixed with 70%
ethanol and stained with propidium iodide (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and RNase (Takara Biotechnology Co.,
Ltd.) for 30 min. Subsequently, the distribution of the cell cycle
was measured by flow cytometry and analyzed using ModFit LT™
software version 3.2 (Verity Software House, Topsham, ME, USA). In
addition, cell apoptosis was analyzed by flow cytometry following
Annexin V/7-aminoactinomycin D (AAD) staining kit (Thermo Fisher
Scientific, Inc.). Cells at a density of 105 were
cultured in 6-well plates, harvested and resuspended in binding
buffer. Following staining with Annexin V and 7-AAD for 20 min,
cells were analyzed using a flow cytometer.
Transwell assay
Cells were starved in serum free medium for 24 h and
seeded on the top chamber of a Transwell plate at 1×104
cells/well. The bottom chambers were filled with medium
supplemented with 20% FBS. After 48 h, cells remaining in the top
chamber were carefully removed and the lower surface was fixed in
4% paraformaldehyde and stained with 1% crystal violet. Cells were
counted and imaged in five random fields using a phase contrast
microscope. Experiments were repeated at least three times.
Immunohistochemical staining
(IHC)
Tissue microarray slides were deparaffinized,
rehydrated in ethanol and subjected to antigen retrieval by heating
in 0.01 M citrate buffer (Beyotime Institute of Biotechnology,
Haimen, China), to block endogenous peroxidases. Following washing
in PBS and blocking with 5% bovine serum albumin (Sangon Biotech
Co., Ltd.), the slides were incubated with a primary RPN11 antibody
(cat. no. 12059-1-AP; ProteinTech Group, Inc.; 1:500 dilution)
overnight at 4°C. Following three washes in PBS, slides were
incubated with a horseradish peroxidase-conjugated secondary
antibody (cat. no. AR1022; 1:500; Boster Biological Technology,
Ltd., Wuhan, China) for 1 h at room temperature and subsequently
washed three times in PBS. Tissues were stained with
diaminobenzidine and counterstained with hematoxylin and observed
using a light microscope.
Tissues were evaluated by two pathologists and
scored according to the percentage and intensity of staining, as
previously described (17).
Briefly, the percentage score was as followed: i) 0–5%; ii) 6–50%;
iii) 51–75% and iv) 76–100%. The intensity score was as followed:
i) No staining; ii) weak; iii) moderate and iv) strong. A final
score from 1 to 16 was calculated by multiplying the percentage
score by the intensity score. For each sample, ≤8=low expression
and >8=high expression.
Statistical analysis
Statistical calculations were performed using SPSS
version 21.0 software (IBM SPSS, Armonk, NY, USA). PROGgene version
2.0 software (Indiana University, Indianapolis, IN, USA) was used
to evaluate the prognosis of indicated types of cancer. Clinical
parameters were compared with RPN11 expression in tissue samples by
the χ2 test. Kaplan-Meier curves were utilized to
determine the overall survival distribution. Student's t-test was
performed to compare the significance between the control group and
experimental groups. Data are expressed as the mean ± standard
error of three independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
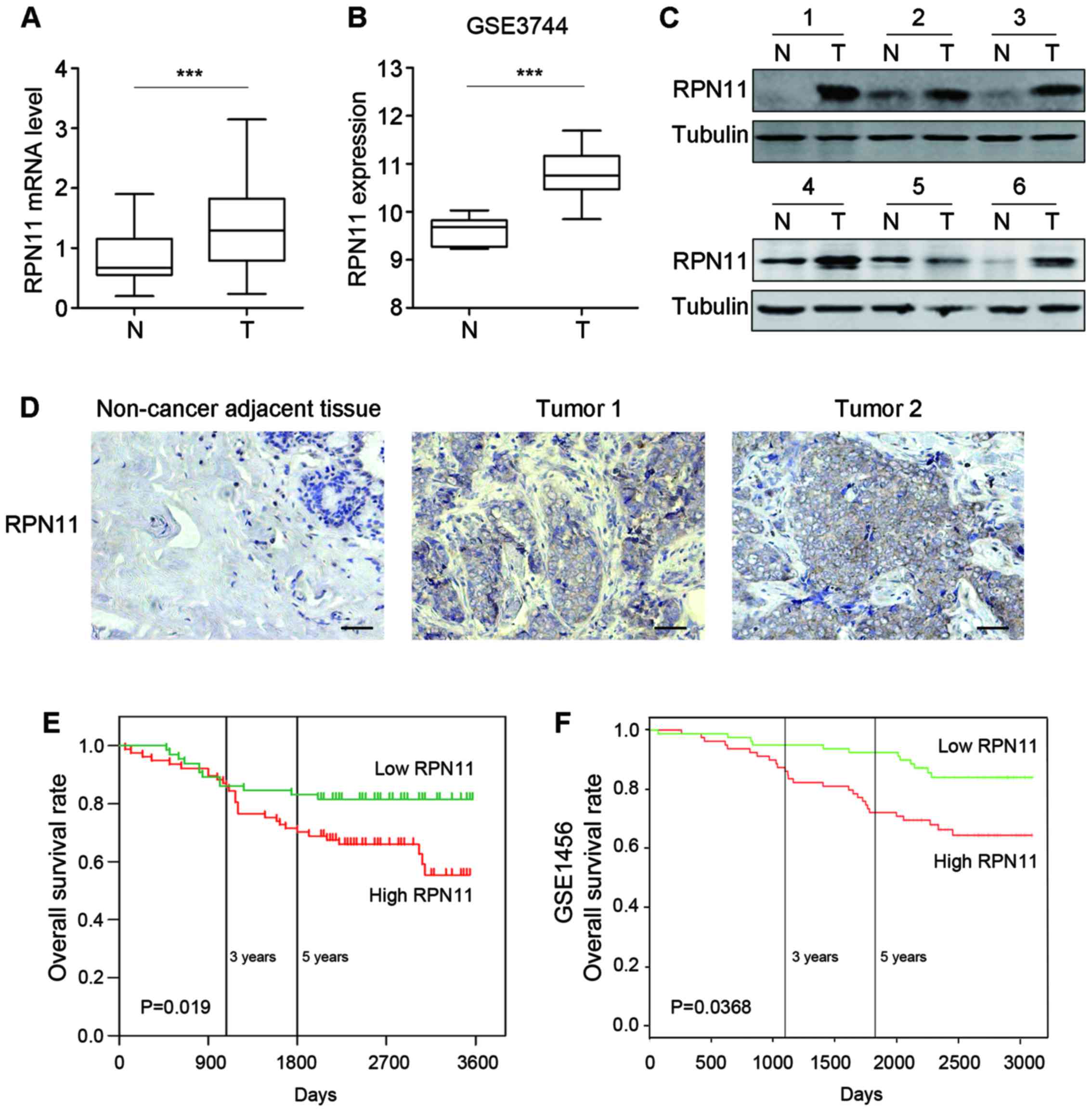
Elevated RPN11 expression in breast
cancer is associated with poor prognosis
To determine the potential role of RPN11 in breast
cancer pathogenesis, the mRNA expression levels of RPN11 was
determined in 59 cases of paired tumor and adjacent non-tumorous
tissues. Greater expression levels of RPN11 were detected in
breast cancer tissues compared with non-cancer adjacent tissue
(P<0.001; Fig. 1A).
Subsequently, the GSE3744 dataset was analyzed, which contained 47
human breast tumor cases and 7 cases of non-cancerous tissues. The
expression levels of RPN11 were greater in tumor tissues
compared with healthy tissues (P<0.001; Fig. 1B). Western blot analysis
demonstrated elevated RPN11 expression levels in six breast cancer
tissues compared with adjacent non-cancerous tissues (Fig. 1C).
IHC was performed on tissue microarrays to measure
RPN11 expression in 142 tissues derived from breast cancer
patients, whose 5-year follow-up data were available. Low levels of
RPN11 were detected in adjacent non-cancerous tissues, whereas high
levels of RPN11 staining were observed in breast cancer tissues
(Fig. 1D). In addition, RPN11
expression was associated with the clinical tumor stage (P<0.05;
Table I). However, there was no
association between RPN11 expression and age, tumor size, lymphatic
vessel invasion, T classification or N classification.
 | Table I.RPN11 expression status and
clinicopathological features of breast cancer tissue samples. |
Table I.
RPN11 expression status and
clinicopathological features of breast cancer tissue samples.
|
|
| RPN11 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | No. of cases | Negative | Positive | χ2 | P-value |
|---|
| Age |
|
|
| 0.333 | 0.564 |
| ≤55 | 64 | 31 | 33 |
|
|
|
>55 | 78 | 34 | 44 |
|
|
| Tumor size (cm) |
|
|
| 2.849 | 0.241 |
|
<2 | 19 | 12 | 7 |
|
|
| ≥2 and
<5 | 100 | 44 | 56 |
|
|
| ≥5 | 23 | 9 | 14 |
|
|
| Lymphatic vessel
invasion |
|
|
| 2.886 | 0.089 |
| Yes | 70 | 27 | 43 |
|
|
| No | 72 | 38 | 34 |
|
|
| T classification |
|
|
| 3.071 | 0.080 |
|
T0-1 | 38 | 22 | 16 |
|
|
|
T2-4 | 104 | 43 | 61 |
|
|
| N classification |
|
|
| 3.511 | 0.061 |
|
N0-1 | 110 | 55 | 55 |
|
|
|
N2-3 | 32 | 10 | 22 |
|
|
| Tumor stage |
|
|
| 6.435 | 0.040a |
| I | 24 | 14 | 10 |
|
|
| II | 80 | 40 | 40 |
|
|
|
III+IV | 38 | 11 | 27 |
|
|
Patients with high RPN11 expression exhibited
significantly poorer overall survival, as determined by
Kaplan-Meier analysis with log-rank test (log-rank=5.475; P=0.019;
Fig. 1E). This was supported by
the GSE1456 dataset, which was analyzed using PROGgene version 2.0
software (Indiana University, Indianapolis, IN, USA) (18,19).
High expression of RPN11 was associated with poorer survival
compared with tissues with low RPN11 expression (Fig. 1F; P=0.0368). In conclusion, mRNA
and protein expression levels of RPN11 were upregulated in breast
cancer tissues and this was associated with advanced cancer stage
and poor patient prognosis.
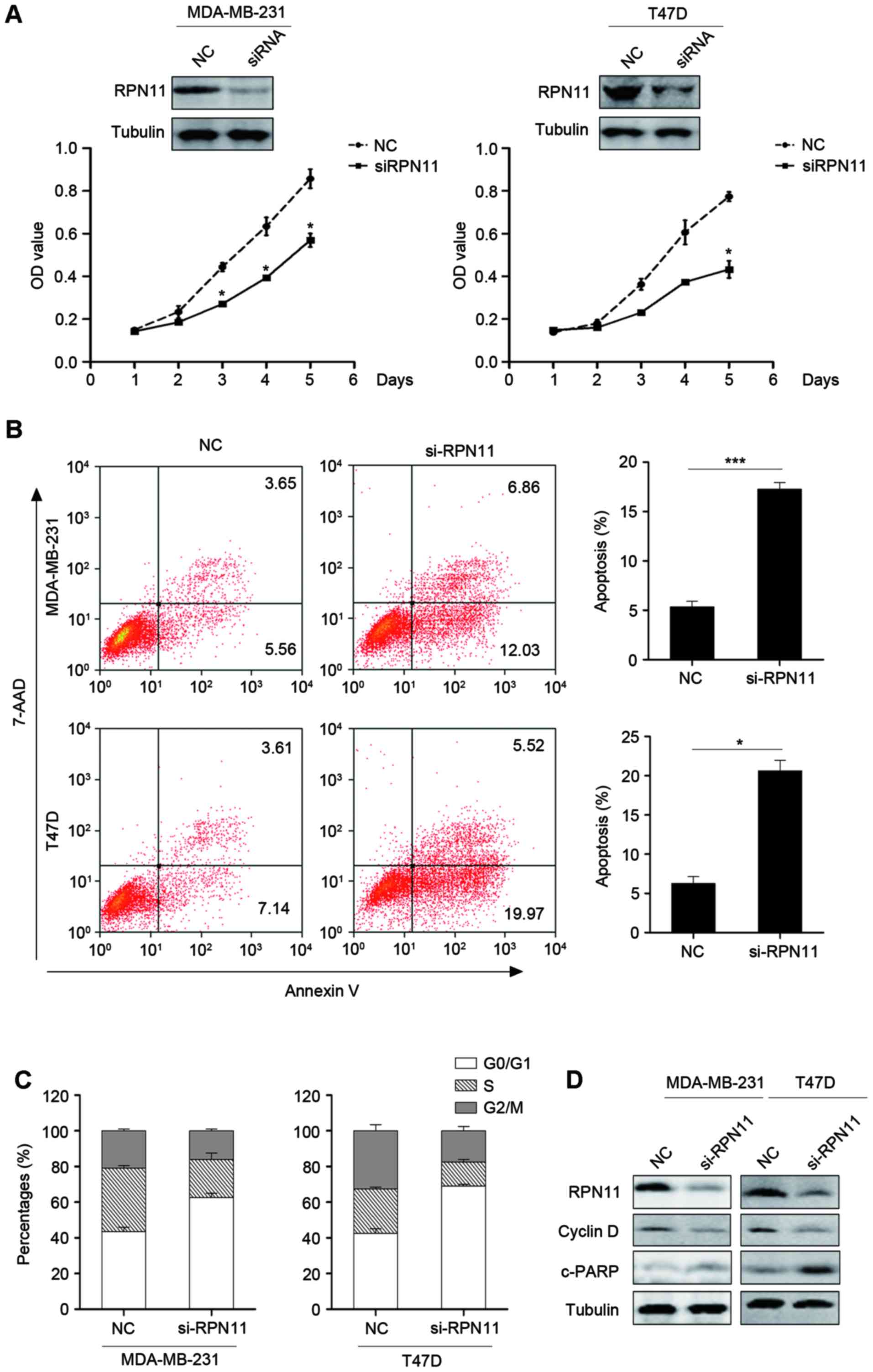
RPN11 promotes cell growth and
inhibits apoptosis in breast cancer cells
The effect of RPN11 inhibition on the MDA-MB-231 and
T47D breast cancer cell lines was investigated. Knockdown of RPN11
reduced the proliferation of MDA-MB-231 and T47D cells compared
with cells transfected with negative control siRNA, suggesting that
RPN11 may serve a role in oncogenesis (Fig. 2A). In addition, the effect of RPN11
knockdown on apoptosis and the cell cycle was investigated. Flow
cytometric analysis suggested that knockdown of RPN11 led to an
enhanced degree of apoptosis (Fig.
2B) and suppression of the cell cycle, compared with cells
transfected with negative control siRNA (Fig. 2C). Knockdown of RPN11 induced G0/G1
arrest (Fig. 2C). In addition,
cyclin D1, a marker of the cell cycle, and cleaved PARP expression,
a marker of apoptosis, were measured by western blot analysis.
Knockdown of RPN11 enhanced cleaved-PARP and reduced cyclin D1
expression levels (Fig. 2D). This
suggested that knockdown of RPN11 inhibited cell proliferation and
induced apoptosis.
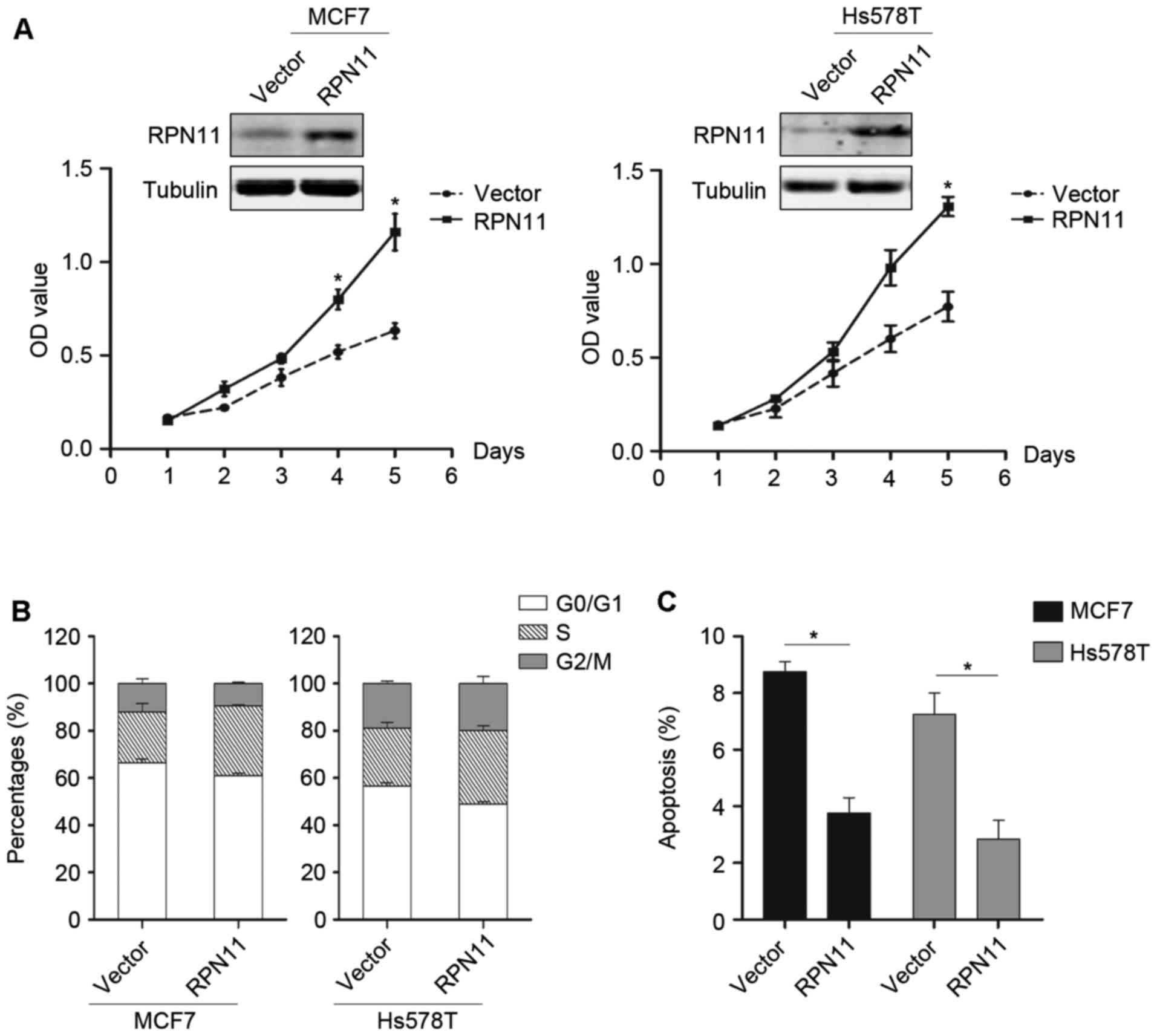
To further evaluate the role of RPN11 in breast
cancer, RPN11 was exogenously overexpressed in MCF7 and Hs578T
cells. Overexpression of RPN11 promoted cell growth (P<0.05;
Fig. 3A) and cell cycle
progression (Fig. 3B); however,
apoptosis was reduced compared with the vector control (P<0.05).
This data suggested that RPN11 may have oncogenic potential and may
be a therapeutic target.
RPN11 knockdown reduces breast cancer
cell migration
Knockdown of RPN11 resulted in reduced cell
migration in breast cancer cells compared with the negative
control, as determined by a Transwell assay (Fig. 4A). EMT is a key step in tumor cell
migration (20). Therefore, genes
associated with EMT were measured by RT-qPCR following RPN11
knockdown. The level of mRNA encoding the epithelial marker
E-cadherin was increased, whereas the levels of mRNA encoding the
mesenchymal markers N-cadherin and vimentin were reduced following
knockdown of RPN11, compared with the negative control (Fig. 4B). The mRNA expression levels of
EMT-associated transcriptional regulators, Snail and Slug, were
reduced following RPN11 knockdown. In addition, western blot
analysis corresponded with the RT-qPCR data (Fig. 4C). Collectively, the results
suggested that overexpression of RPN11 in breast cancer may promote
cancer cell migration.
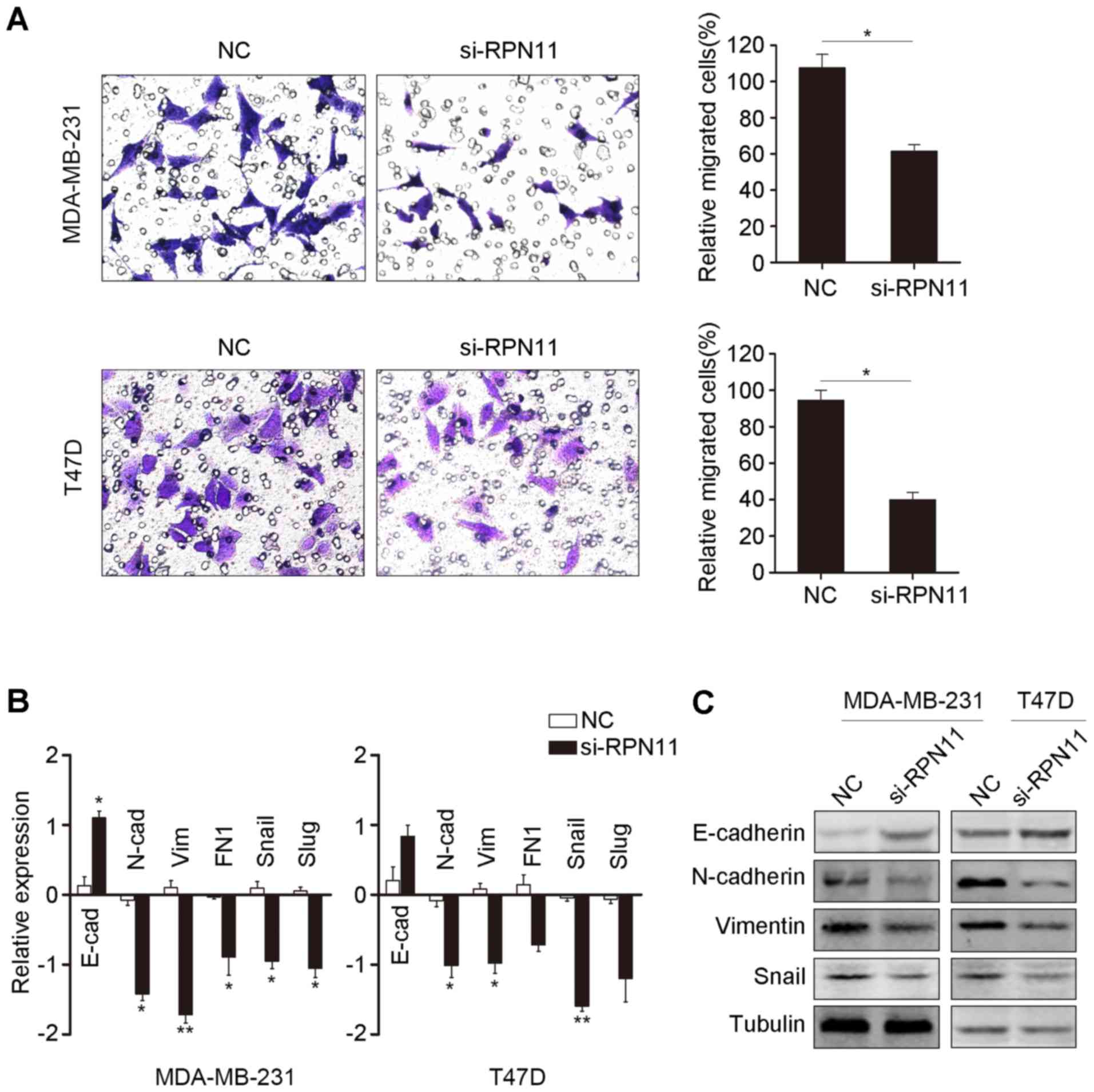 | Figure 4.Knockdown of RPN11 induces
mesenchymal-epithelial transition in breast cancer cells. (A)
Knockdown of RPN11 inhibited cell migration ability, as determined
by the Transwell assay. (B) EMT markers and EMT-associated
transcriptional factors, including E-cad, N-cad, vim, Fn1 Snail and
Slug, were measured by reverse transcription-quantitative
polymerase chain reaction following RPN11 knockdown in MDA-MB-231
and T47D cells. *P<0.05, **P<0.01. (C) Protein expression
levels of E-cad, N-cad, vim, Snail and tubulin were measured by
western blot analysis following transfection with the NC or
si-RPN11 in MDA-MB-231 and T47D cells. Data are expressed as the
mean ± standard error of three independent experiments. *P<0.05.
NC, negative control; siRPN11, RPN11-targeting small interfering
siRNA; E-cad, E-cadherin; N-cad, N-cadherin; Vim, vimentin; FN1,
fibronectin 1. |
Discussion
The mortality rate of breast cancer has declined due
to improvements in earlier diagnosis and adjuvant therapies. Novel
prognostic markers remain to be identified. carcinoembryonic
antigen and carcinoma antigen 15-3 are widely recognized as tumor
markers of breast cancer; however, the sensitivity and precision of
the assays utilized for their detection requires further
investigation (21–23). Therefore, it is necessary to
identify novel markers for the diagnosis and treatment of patients
with breast cancer.
The results of the present study indicated that
RPN11 was significantly upregulated in breast cancer tissues
compared with adjacent non-tumor tissues, and high expression of
RPN11 was associated with the clinical tumor stage. Patients with
high RPN11 expression levels had significantly poorer outcomes,
which suggested that RPN11 may be a prognostic factor for patients
with breast cancer. To validate these findings, the GSE1456 breast
cancer cohort was analyzed. Results revealed that patients with
high RPN11 expression had poor survival outcomes. In addition,
in vitro experiments demonstrated that knockdown of RPN11
reduced proliferation and induced cell cycle arrest and apoptosis
in breast cancer cells. By contrast, overexpression of RPN11 in
breast cancer cells promoted cell growth and inhibited
apoptosis.
Recently, RPN11 has been reported to function as an
oncogene in a number of cancer types, including hepatocellular
carcinoma and ovarian cancer (11). Wang et al (11) reported that RPN11 acts as a
deubiquitinase enzyme and stabilizes the expression of E2F1 by
removal of the polyubiquitin chain, resulting in abnormal cell
proliferation and tumorigenesis. In addition, it was demonstrated
that expression of RPN11 was associated with the expression of
survivin and forkhead box M1, both of which are considered to be
oncogenes. Byrne et al (10) suggested that knockdown of RPN11 in
HeLa cells enhanced cell cycle arrest and senescence by
downregulation of cyclin B1-cell division cycle 25C and cyclin D1,
and upregulation of p21, which was consistent with the findings of
the present study. Furthermore, RPN11 promoted the double-strand
DNA break response by processing of the polyubiquitin chain and
recruiting p53 binding protein 1 to the site of DNA damage
(24). RPN11 may specifically
cleave lysine 63-linked polyubiquitin chains, which are critical
for the regulation of substrates (25). Therefore, the downstream targets of
RPN11 require further investigation.
The results of the present study suggested that
RPN11 promotes cell migration by inducing EMT. Despite there being
no clinical association between RPN11 expression and N
classification, knockdown of RPN11 reduced cell migration by
inducing mesenchymal-epithelial transition (MET). In EMT, cells
lose their adhesion and epithelial features and switch to a
mesenchymal phenotype, which is an indicator of cancer metastasis
(26). In the present study, as
the relative expression of RPN11 was higher in MCF-7 and Hs578T
compared with MDA-MB-231 and T47D (data not shown), MCF-7 and H578T
were used for the overexpression of RPN11 and MDA-MB-231/T47D cells
were used for the knockdown. The Transwell assay suggested that
knockdown of RPN11 partially abrogates the migration of breast
cancer cells. To verify this, the expression levels of
EMT-associated genes were measured, including E-cadherin, FN1,
Snail and Slug. Results suggested that inhibition of RPN11 induced
MET.
In conclusion, overexpression of RPN11 in breast
cancer tissues was associated with an advanced clinical stage.
Patients with tumors with high expression of RPN11 had worse
prognosis. These findings suggest a role of RPN11 in breast cancer
development, progression, cell proliferation and migration by
inducing EMT. The data suggest that RPN11 may be a therapeutic
target for breast cancer.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan
J, Wu J and Li M: MicroRNA-374a activates Wnt/β-catenin signaling
to promote breast cancer metastasis. J Clin Invest. 123:566–579.
2013.PubMed/NCBI
|
|
6
|
Dvinge H, Git A, Gräf S, Salmon-Divon M,
Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA, et al:
The shaping and functional consequences of the microRNA landscape
in breast cancer. Nature. 497:378–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakarougkas A, Ismail A, Katsuki Y, Freire
R, Shibata A and Jeggo PA: Co-operation of BRCA1 and POH1 relieves
the barriers posed by 53BP1 and RAP80 to resection. Nucleic Acids
Res. 41:10298–10311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stitzel ML, Durso R and Reese JC: The
proteasome regulates the UV-induced activation of the AP-1-like
transcription factor Gcn4. Genes Dev. 15:128–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Butler LR, Densham RM, Jia J, Garvin AJ,
Stone HR, Shah V, Weekes D, Festy F, Beesley J and Morris JR: The
proteasomal de-ubiquitinating enzyme POH1 promotes the
double-strand DNA break response. EMBO J. 31:3918–3934. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Byrne A, McLaren RP, Mason P, Chai L,
Dufault MR, Huang Y, Liang B, Gans JD, Zhang M, Carter K, et al:
Knockdown of human deubiquitinase PSMD14 induces cell cycle arrest
and senescence. Exp Cell Res. 316:258–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang B, Ma A, Zhang L, Jin WL, Qian Y, Xu
G, Qiu B, Yang Z and Liu Y, Xia Q and Liu Y: POH1 deubiquitylates
and stabilizes E2F1 to promote tumour formation. Nat Commun.
6:878042015. View Article : Google Scholar
|
|
12
|
Gallery M, Blank JL, Lin Y, Gutierrez JA,
Pulido JC, Rappoli D, Badola S, Rolfe M and Macbeth KJ: The JAMM
motif of human deubiquitinase Poh1 is essential for cell viability.
Mol Cancer Ther. 6:262–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Buus R, Clague MJ and Urbé S:
Regulation of ErbB2 receptor status by the proteasomal DUB POH1.
PLoS One. 4:e55442009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nabhan JF and Ribeiro P: The 19 S
proteasomal subunit POH1 contributes to the regulation of c-Jun
ubiquitination, stability, and subcellular localization. J Biol
Chem. 281:16099–16107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwarz T, Sohn C, Kaiser B, Jensen ED and
Mansky KC: The 19S proteasomal lid subunit POH1 enhances the
transcriptional activation by Mitf in osteoclasts. J Cell Biochem.
109:967–974. 2010.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang J, Yu C, Chen M, Tian S and Sun C:
Over-expression of TRIM37 promotes cell migration and metastasis in
hepatocellular carcinoma by activating Wnt/β-catenin signaling.
Biochem Biophys Res Commun. 464:1120–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goswami CP and Nakshatri H: PROGgeneV2:
Enhancements on the existing database. BMC Cancer. 14:9702014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goswami CP and Nakshatri H: PROGgene: Gene
expression based survival analysis web application for multiple
cancers. J Clin Bioinforma. 3:222013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu SG, He ZY, Zhou J, Sun JY, Li FY, Lin
Q, Guo L and Lin HX: Serum levels of CEA and CA15-3 in different
molecular subtypes and prognostic value in Chinese breast cancer.
Breast. 23:88–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JS, Park S, Park JM, Cho JH, Kim SI
and Park BW: Elevated levels of preoperative CA 15-3 and CEA serum
levels have independently poor prognostic significance in breast
cancer. Annals of oncology. 24:1225–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Poznak C, Somerfield MR, Bast RC,
Cristofanilli M, Goetz MP, Gonzalez-Angulo AM, Hicks DG, Hill EG,
Liu MC, Lucas W, et al: Use of biomarkers to guide decisions on
systemic therapy for women with metastatic breast cancer: American
Society of Clinical Oncology Clinical Practice Guideline. J Clin
Oncol. 2015:2695–2704. 2015. View Article : Google Scholar
|
|
24
|
Morris JR: Attenuation of the ubiquitin
conjugate DNA damage signal by the proteasomal DUB POH1. Cell
Cycle. 11:4103–4104. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper EM, Cutcliffe C, Kristiansen TZ,
Pandey A, Pickart CM and Cohen RE: K63-specific deubiquitination by
two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal
Poh1. EMBO J. 28:621–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|