Introduction
Arising from either a functional defect in bile
formation at the hepatocyte or from impaired secretion and flow
capability of the bile duct, cholestasis is the primary cause of
hospitalization in children with liver diseases (1). In the rat model of cholestasis, bile
duct epithelial cells are synchronized with hepatic stellate cells
with respect to activation and proliferation, inducing
extracellular matrix deposition and hepatic fibrosis development
(2,3). Pathological examination of liver
tissue is the primary method of diagnosing hepatic fibrosis;
however, due to its invasiveness, the use of it is significantly
limited in infants. Therefore, developing a non-invasive method for
detecting hepatic fibrosis may provide an important clinical
advancement.
Known to cause acute cholestasis,
α-naphthylisothiocyanate (ANIT), a highly toxic substance (4), has been used to induce swelling and
necrosis of the bile ducts, resulting in hyperplasia and an
inflammatory reaction around the ductus biliferi interlobulares.
ANIT obstructs the bile ducts causing cholestasis, hepatic
parenchymal cell damage and hepatic fibrosis, and has been
identified to be a useful agent for studying cholestasis. In the
present study, ANIT was used to induce cholestatic hepatic injury
in neonatal rats in order to analyze four suspected biomarkers of
the disease. The present study was designed to assess the
correlation between the four biomarkers, and enzymes indicative of
liver function and hepatic fibrosis. An effective, simple, and
non-invasive way of diagnosing hepatic fibrosis in clinical
settings may become available in the future.
Materials and methods
Rodent model and rearing
conditions
A total of 38 healthy Sprague-Dawley rats (weight,
50–70 g; age, 3 weeks; male, 19; female, 19) were obtained from the
Laboratory Animal Center of Hebei Medical University (Shijiazhuang,
China). Animals were kept at an ambient temperature of 25±2°C, with
a 12:12 h light/dark cycle, and were given free access to standard
laboratory chow and tap water. All rats were allowed to acclimate
for 24 h prior to experimentation. All procedures involving animals
were reviewed and approved by the Institutional Animal Care and Use
Committee of Hebei Medical University. The animal protocol was
designed to minimize pain and discomfort to the animals.
Group designations and treatment
Animals were assigned at random to one of three
groups: The experimental model group (EG); the vehicle control
group (CG); and the blank control group (BCG). The EG rats were
administered 1% ANIT dissolved in corn oil (75 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) intragastrically, in
order to induce disease development. Rats in the CG were treated
with an equivalent volume of corn oil compared with the total
volume administered to the EG rats; the BCG rats were untreated,
and fasted for 12 h prior to and following treatment. Treatments
were administered daily for 2 days until sacrifice and all
procedures were conducted with approval of the Council on Animal
Care of Academia Sinica (Hebei Medical University, Shijiazhuang,
China).
Sample collection and preparation
Following 48 h of treatment, all animals were
sacrificed, and blood and liver samples were collected. Blood
samples from the heart were evaluated for markers indicative of
liver function and liver fibrosis, while liver samples were
preserved for histological and immunohistochemical examination in
10% (w/v) neutral formaldehyde. Histological surgery and evaluation
were performed by a certified veterinary pathologist.
Detection of biomarkers of hepatic
fibrosis
Radioimmuno-assays from Beijing North Institute of
Biotechnology (Beijing, China) were used in conjunction with a
γ-radioimmunoassay counter (XH-6020; Xi'an Nuclear Instrument
Factory, Shaanxi, China) in accordance with the manufacturers'
protocol. Rats fasted overnight and had 2–3 ml of venous blood
collected in pro-coagulant tubes, which were centrifuged at 1,006 ×
g for 3 min to isolate the serum. All serum samples were stored at
−20°C, and measurements of hyaluronic acid (HA), procollagen type
III (PCIII), laminin (LN) and collagen type IV (cIV) were
taken.
Measurement of liver function in the
serum
Detection kits specific for liver function
biomarkers [alanine aminotransferase (ALT); aspartate
aminotransferase (AST); total bilirubin (TBIL); direct bilirubin
(DBIL); indirect bilirubin (IBIL); γ-glutamyl transferase (γ-GT);
cholinesterase (CHE); and total bile acids (TBA)] were purchased
from Johnson & Johnson (New Brunswick, NJ, USA) and used in
conjunction with an automatic biochemical analyzer (AU5400TM;
Olympus Corporation, Tokyo, Japan). Blood samples were collected
from each mouse and processed as previously described (5). Measurements of liver function
biomarkers were based on assays using the initial rate method
(5), and measurements of TBIL,
DBIL and IBIL were based on colorimetric assays.
Hematoxylin and eosin staining
Formaldehyde-fixed paraffin embedded (FFPE) liver
specimens were processed into 4-µm-thick sections mounted onto
glass slides, stained with hematoxylin and eosin and examined with
light microscopy (6). This
procedure was performed by a certified veterinary pathologist.
Digital images were quantitatively and semi-quantitatively graded
for hepatic fibrosis utilizing the scoring criteria established by
Farleigh et al (7). Score
0, no fibrosis and no hyperplasia of collagen in liver; score 1,
few collagen fibrils extended from the central vein to the portal
tract (<25%); score 2, apparent collagen fibril extension
without encompassing the whole lobule (25–50%); score 3, collagen
fibrils extended into and encompassing the whole lobule (50–75%);
and score 4, diffuse extension of collagen fibrils and formation of
pseudo-lobule (>75%).
Masson staining
This procedure was performed by a certified
veterinary pathologist. FFPE liver samples of 6 µm thickness were
mounted onto glass slides and stained with masson (Shanghai, China)
(8).
Picric acid-sirius red staining
This procedure was performed by a certified
veterinary pathologist. FFPE liver samples were sliced into
6-µm-thick sections, mounted onto glass slides, and stained with
picric acid-sirius red (0.1% sirius red in saturated aqueous picric
acid for 10–15 min at room temperature of 20–25°C) to detect
hepatic fibrosis. Stained sections were examined using a polarizing
microscope (BX53-p; Olympus Corporation; magnification, ×400).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM SPSS, Armonk, NY, USA) and the results
are expressed as the mean ± the standard deviation. A Student's
t-test was used for normally distributed data, while the Wilcoxon
rank sum test was used for non-normally distributed data;
additionally, the rank sum test was used for disease grade data.
Partial correlation analysis was used to evaluate the association
between each liver function indicator and the four biomarkers. Data
from the pathological grading of tissue samples were analyzed using
Spearman's rank correlation coefficient. P<0.05 was considered
to indicate a statistically significant difference.
Results
Serum levels of enzymes indicative of
liver function were elevated in the EG
Serum levels of ALT, AST, TBIL, DBIL, IBIL, γ-GT and
TBA in neonatal rats with cholestasis were significantly increased,
compared with rats in the CG and BCG (P<0.01; Table I). CHE levels in neonatal rats with
cholestasis were unaltered compared with the control groups.
 | Table I.Variation of liver function test in
neonatal cholestatic rats. |
Table I.
Variation of liver function test in
neonatal cholestatic rats.
|
| Group |
|---|
|
|
|
|---|
| Factor | Vehicle control
(n=8) | Negative control
(n=7) | Cholestasis
(n=18) |
|---|
| ALT, U/l | 48.88±14.04 | 36.14±11.34 |
633.17±248.80a,b |
| AST, U/l | 349.50±114.41 | 320.71±148.83 |
2080.17±704.39a,b |
| TBIL, µmol/l | 3.28±0.87 | 2.43±0.67 |
144.81±24.90a,b |
| DBIL, µmol/l | 2.85±0.91 | 1.93±0.69 |
132.79±23.43a,b |
| IBIL, µmol/l | 0.43±0.29 | 0.50±0.33 |
12.02±5.60a,b |
| γ-GT, U/l | 0.75±1.04 | 0.14±1.57 |
19.94±10.37a,b |
| CHE, KU/l | 0.26±0.04 | 0.27±0.03 | 0.26±0.04 |
| TBA, µmol/l | 198.01±120.47 | 113.03±87.37 |
675.66±84.74a,b |
Serum levels of HA, LN, and cIV were
significantly elevated in the EG
Among the four biomarkers of hepatic fibrosis, serum
levels of HA, LN and cIV in neonatal rats with cholestasis were
significantly increased compared with the control groups
(P<0.01; Table II). PCIII
levels in neonatal rats with cholestasis were unaltered compared
with the control groups.
 | Table II.Variation of degrees of hepatic
fibrosis in neonatal cholestatic rats. |
Table II.
Variation of degrees of hepatic
fibrosis in neonatal cholestatic rats.
|
| Group |
|---|
|
|
|
|---|
| Factor | Vehicle control
(n=7) | Negative control
(n=6) | Cholestasis
(n=18) |
|---|
| HA, ng/ml | 1030.07±541.19 | 1018.95±555.13 |
2004.18±479.86a,b |
| PCIII, ng/ml | 46.63±2.63 | 36.20±9.65 | 45.52±2.16 |
| LN, ng/ml | 34.41±10.81 | 27.57±6.25 |
48.51±6.51a,b |
| cIV, ng/ml | 20.08±2.70 | 18.33±5.59 |
30.28±10.82a,b |
An increased grade of hepatic fibrosis
was associated with animals in the EG
The grade of pathological hepatic fibrosis was
notably increased in the cholestasis group compared with the
control groups (P<0.01; Table
III).
 | Table III.Variation of pathological hepatic
fibrosis grade in neonatal cholestatic rats. |
Table III.
Variation of pathological hepatic
fibrosis grade in neonatal cholestatic rats.
|
| Group |
|---|
|
|
|
|---|
| Factor | Vehicle control
(n=10) | Negative control
(n=8) | Cholestasis
(n=20) |
|---|
| Hepatic fibrosis
grade | 0.44±0.53 | 0.13±0.35 |
2.05±0.62a,b |
Hepatic tissue pathological
staining
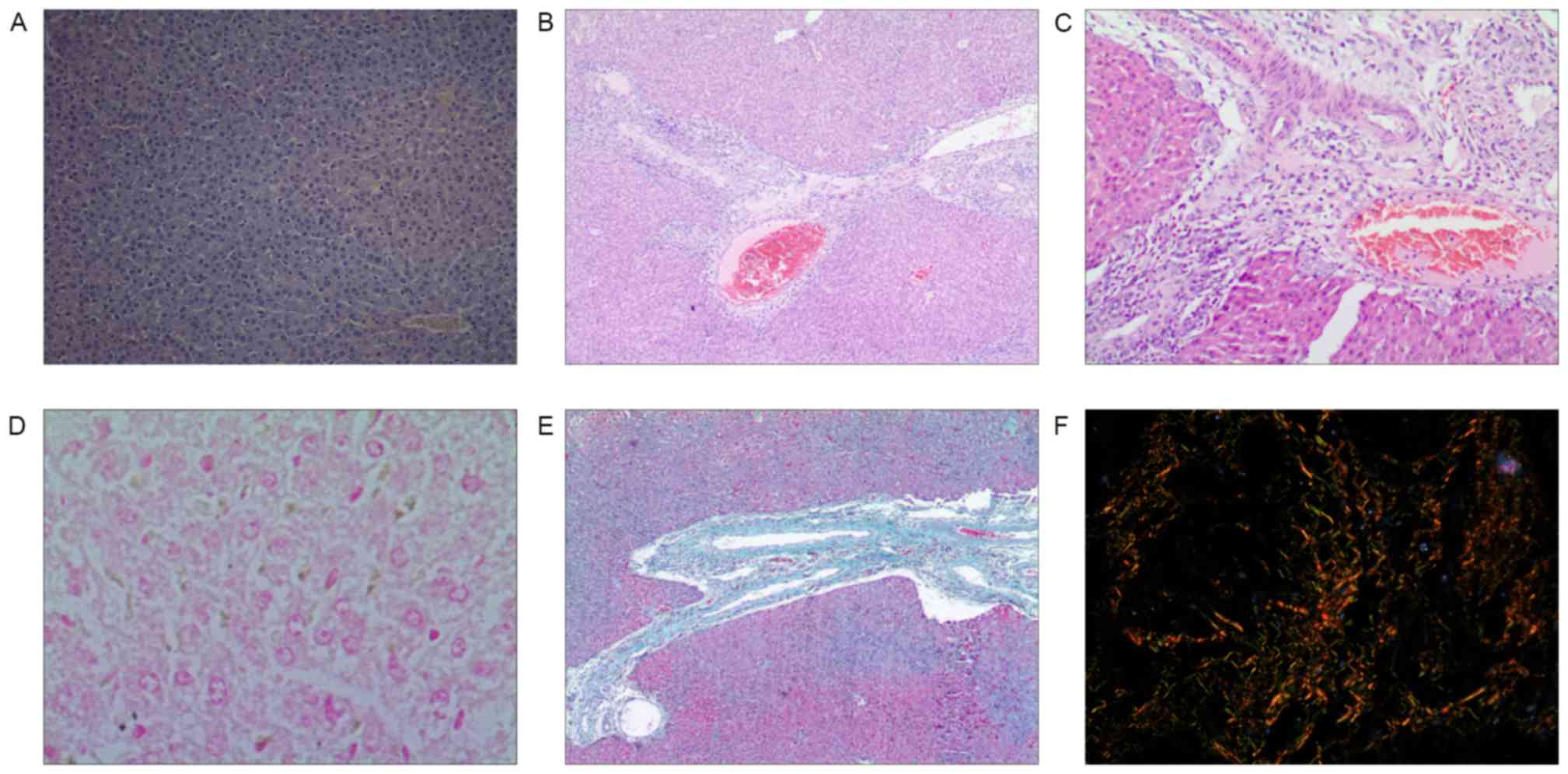
Hematoxylin and eosin staining
Hepatic lobules in the CG and BCG were holonomic and
clear, and the cells were arranged in a funicular form with no
infiltration in the portal area. Samples from the EG exhibited
fatty degeneration, edema, necrosis and apoptosis in 25–75% of each
sample, with damaged or absent hepatic lobules. Cholestasis was
observed in a majority of the hepatocytes and bile ducts, with few
normal hepatocytes. There was apparent fibrosis and necrosis in a
majority of the portal areas accompanied by severe infiltration of
neutrophilic granulocytes and eosinophilic granulocytes.
Additionally, a fibrous septum divided necrotic hepatic lobules
into pseudolobules (Fig.
1A-D).
Masson staining
There was a buildup of collagen in the hepatic
tissue of samples from the EG which was not observed in the control
groups (Fig. 1E).
Sirius red-saturated picric acid staining
Hepatic lobules of the experimental group were
observed to exhibit a large population of collagen fibers compared
with the control groups. Among the fibers in the EG, thick yellow
and red fibers had greater refractivity and appeared as circles
around the central vein and the clearance of hepatic sinus,
star-shaped around the portal area, or stretched into the lobule
forming interlobular separations. A few slender green fibers were
observed to be distributed in portal area, small blood vessels, and
central vein, and additionally were observed to be discontinuously
distributed around the clearance of the hepatic sinus (Fig. 1F).
Correlation analyses between liver function tests
and the four biomarkers of hepatic fibrosis in neonatal rats with
cholestasis
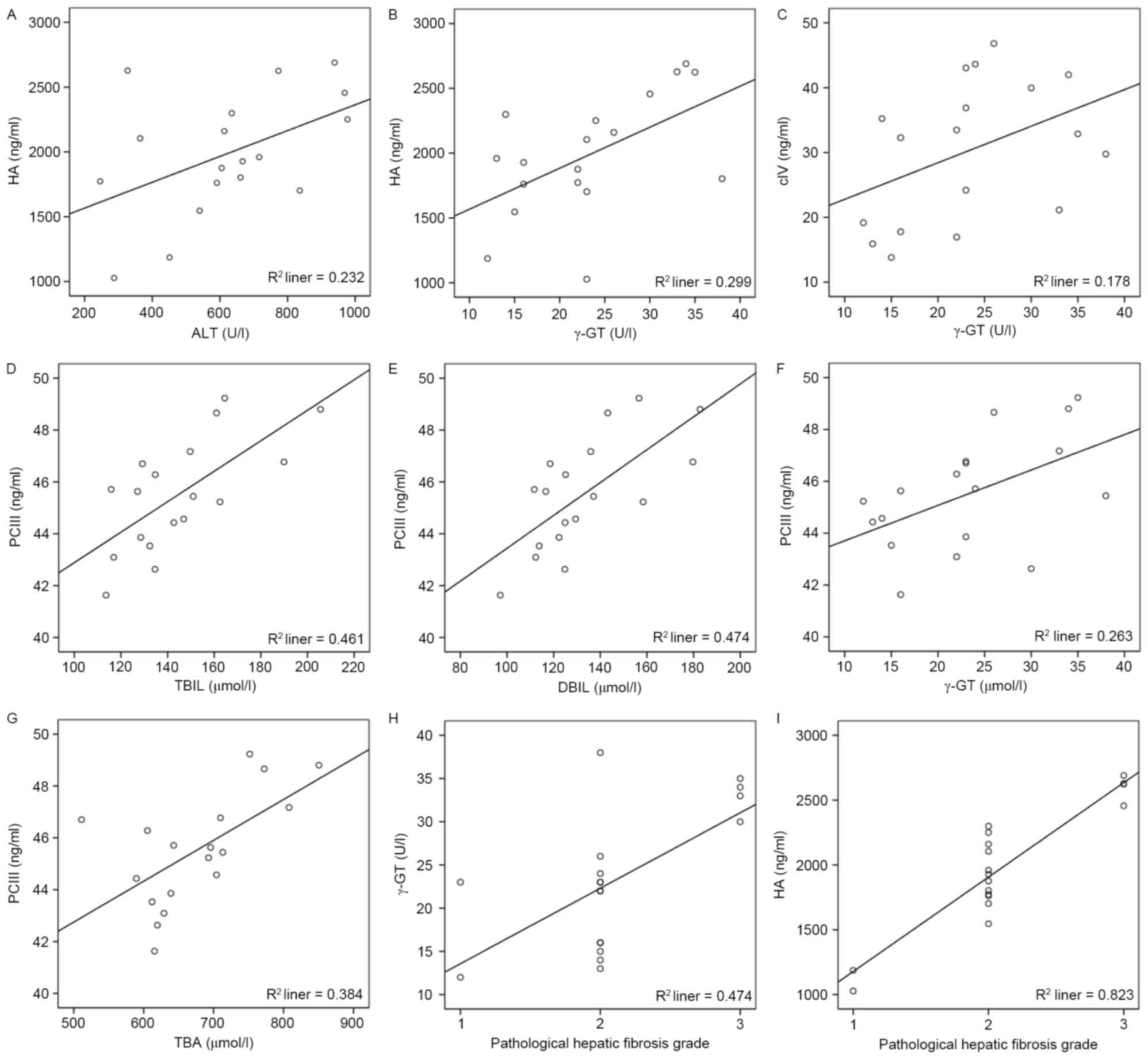
HA was positively correlated with ALT and γ-G
(r=0.47, P<0.05; r=0.53, P<0.05, respectively; Fig. 2A and B), while cIV was only
observed to positively correlate with γ-GT (r=0.50, P<0.05;
Fig. 2C; Table IV). Additionally, PCIII was
positively correlated with TBIL, DBIL, γ-GT and TBA (r=0.65,
P<0.01; r=0.65, P<0.01; r=0.54, P<0.05; r=0.62, P<0.01,
respectively; Fig. 2D-G).
 | Table IV.Correlation analysis of degrees of
hepatic fibrosis and liver function test in neonatal cholestatic
rats. |
Table IV.
Correlation analysis of degrees of
hepatic fibrosis and liver function test in neonatal cholestatic
rats.
|
| Factor |
|---|
|
|
|
|---|
|
| HA, ng/ml | PCIII, ng/ml | LN, ng/ml | cIV, ng/ml |
|---|
|
|
|
|
|
|
|---|
| Factor | r | P-value | r | P-value | r | P-value | r | P-value |
|---|
| ALT, U/l | 0.47 | <0.05 | 0.27 | N.S | 0.07 | N.S | 0.34 | N.S |
| AST, U/l | 0.39 | N.S | 0.10 | N.S | 0.19 | N.S | 0.38 | N.S |
| TBIL, µmol/l | 0.29 | N.S | 0.65 | <0.01 | −0.20 | N.S | 0.10 | N.S |
| DBIL, µmol/l | 0.26 | N.S | 0.65 | <0.01 | −0.17 | N.S | 0.13 | N.S |
| IBIL, µmol/l | 0.26 | N.S | 0.08 | N.S | −0.15 | N.S | −0.04 | N.S |
| γ-GT, U/l | 0.53 | <0.05 | 0.54 | <0.05 | 0.12 | N.S | 0.50 | <0.05 |
| CHE, KU/l | −0.27 | N.S | −0.17 | N.S | −0.01 | N.S | −0.10 | N.S |
| TBA, µmol/l | 0.43 | N.S | 0.62 | <0.01 | −0.17 | N.S | 0.32 | N.S |
Correlation analysis of liver function and
hepatic fibrosis grade
Pathological hepatic fibrosis grade was positively
correlated with γ-GT (r=0.62, P<0.01; Fig. 2H; Table V) among the biomarkers of liver
function of the experimental group.
 | Table V.Correlation analysis of liver
function test and pathological hepatic fibrosis grade in neonatal
cholestatic rats. |
Table V.
Correlation analysis of liver
function test and pathological hepatic fibrosis grade in neonatal
cholestatic rats.
|
| Statistical
analysis |
|---|
|
|
|
|---|
| Factor | r | P-value |
|---|
| ALT, U/l | 0.45 | N.S |
| AST, U/l | 0.41 | N.S |
| TBIL, µmol/l | 0.30 | N.S |
| DBIL, µmol/l | 0.23 | N.S |
| IBIL, µmol/l | 0.29 | N.S |
| γ-GT, U/l | 0.62 | <0.01 |
| CHE, KU/l | −0.20 | N.S |
| TBA, µmol/l | 0.37 | N.S |
Correlation analysis of serum biomarkers of liver
fibrosis and hepatic fibrosis grade
Pathological hepatic fibrosis grade was positively
correlated with HA (r=0.83, P<0.01; Fig. 2; Table VI) in the four biomarkers of
hepatic fibrosis of the experimental group.
 | Table VI.Correlation analysis of degrees of
hepatic fibrosis and pathological hepatic fibrosis grade in
neonatal cholestatic rats. |
Table VI.
Correlation analysis of degrees of
hepatic fibrosis and pathological hepatic fibrosis grade in
neonatal cholestatic rats.
|
| Factor |
|---|
|
|
|
|---|
| Statistical
analysis | HA, ng/ml | PCIII, ng/ml | LN, ng/ml | cIV, ng/ml |
|---|
| r | 0.83 | 0.38 | −0.01 | 0.26 |
| P-value | <0.01 | N.S | N.S | N.S |
Discussion
Non-invasive testing of liver fibrosis is among the
fastest evolving areas in the study of liver diseases (9). Liver biopsy has been the standard
method for the screening and surveillance of liver fibrosis.
However, this procedure is associated with certain limitations.
Liver biopsies are only able to sample a small portion of the liver
and, therefore, sampling errors may occur, particularly when
smaller-sized biopsies are analyzed. Histopathological diagnosis
may be subject to both intra- and inter-observer variability, even
with validated scoring systems (10). Liver biopsy is an invasive
procedure with associated adverse events, including pain, which
occurs in 20% of patients, and major complications, including
bleeding and liver laceration (11). Therefore, liver biopsy is a
particularly risky procedure in neonates. Further research is
required to identify non-invasive markers of liver fibrosis,
particularly in neonatal liver disease, where prompt, safe and
reliable diagnosis is required. In the present study, the utility
of four possible markers of fibrosis was assessed: HA, PCIII, LN,
cIV. Cholestatic jaundice and eventual fibrosis was induced using
ANIT.
Following its identification 30 years ago as a
chemical inducer of acute intrahepatic cholestasis, ANIT has been
utilized in several animal studies of cholestatic liver disease
(12). Although its mechanism of
action remains to be completely elucidated, the early stages of
liver injury induced by ANIT are characterized by increasing levels
of AST, ALT and TBIL (13). As the
damage progresses, epithelial cells in the bile ducts swell and
undergo necrosis, leading to capillary bile duct hyperplasia and an
inflammatory reaction which further obstructs the duct. The
application of ANIT induces cholestasis, liver parenchymal cell
damage and cholestatic jaundice. Previous studies have demonstrated
that, in the process of liver injury caused by treatment with ANIT,
serum levels of ALT, TBA and other markers increase until a peak is
reached at 48 h (14,15).
The results of the present study demonstrated the
successful generation of a model of acute cholestatic hepatic
fibrosis, using hematoxylin and eosin, masson and sirius
red-saturated picric acid staining. The histopathologic findings of
fibrosis demonstrated the association of the four markers of
fibrosis (HA, PCIII, LN, and cIV) with the onset of fibrosis.
HA is primarily absorbed and degraded by hepatic
sinusoidal endothelial cells. Following injury, cytokines mediate
the interaction of cells leading to the activation of hepatic
sinusoidal endothelial cells and the production of HA has been
observed (16). A previous report
demonstrated that hepatic fibrosis develops as disease progresses,
and a positive correlation has been identified between increases in
serum HA and the severity of histological lesions (17). Additionally, this rise in serum HA
may be used to accurately and sensitively interpret the damage
status of hepatocytes as well as the fiber accumulation in the
liver (18,19). The results of the present study are
consistent with previous observations that HA is a viable marker of
fibrosis, particularly in children with metabolic liver injury.
Levels of HA ≥2,100 ng/ml were observed to be correlated with F2,
F3 or F4 fibrosis (20).
An additional component of the hepatocyte basement
membrane is cIV, the expression levels of which have been observed
to increase in the early stages of hepatic fibrosis. It is during
this phase of early fibrosis that cIV is deposited, prior to the
onset of permanent liver damage (21). Therefore, early detection of cIV,
as demonstrated in the present study, is a viable marker of liver
fibrosis. In conjunction with cIV, LN is an additional potential
marker of liver fibrosis. Liver specimens obtained from patients
with alcoholic liver disease and chronic viral hepatitis have been
demonstrated to exhibit a strong reaction with antibodies in the
peri-sinusoidal space, including staining for LN (21). However, few studies have
investigated LN levels in neonatal cholestasis, leading to
fibrosis.
ALT, which is predominantly located in the hepatic
cytoplasm, is a sensitive indicator of damage to hepatocytes. An
additional marker, increased serum levels of γ-GT, an indicator of
bile duct obstruction, has been used to infer hepatocyte damage and
the degree of bile duct obstruction (22–24).
The results of the present study demonstrated a significant
increase in serum levels for all markers of liver function and
liver fibrosis, except CHE and PCIII, in the EG.
Du et al (25) observed that, in vitro, in
the initial S1 stage of liver tissue fibrosis, the level of PCIII
mRNA was increased compared with that of type I collagen; however,
with the development of hepatic fibrosis to the S2 stage, type I
collagen expression increased markedly. In the present study study,
subsequent to pathological grading using hematoxylin and eosin
staining, it was observed that the degree of fibrosis in the EG was
consistent with the S2 stage, and this was considered to be the
primary reason for the lack of a significant alteration in PCIII
expression. Increased CHE levels are associated with chronic, long
term hepatic cirrhosis; therefore, the decreased serum levels in
the present experimental model were expected.
Serum levels of HA, PCIII, and cIV were all observed
to be positively correlated with increased serum levels of the
markers of liver function, indicating their potential as indicators
of cholestatic hepatic injury.
Histopathological examination has been the primary
means of diagnosing hepatic fibrosis and cirrhosis. In the present
study, the grading results of hepatic fibrosis in hematoxylin and
eosin staining demonstrated that there were significant differences
when the EG was compared with the CG or BCG. The results of the
present study demonstrated that the pathological hepatic fibrosis
grade was positively correlated with the levels of HA and γ-GT in
serum. The results of the present study additionally indicated that
HA and γ-GT serum levels may serve as sensitive and accurate
biomarkers of hepatic fibrosis and degree of injury. The result of
the present study may provide a novel, non-invasive means of
diagnosis, thus leading to earlier diagnosis and treatment. The
results of the present study require validation in preclinical
studies prior to being used in human clinical trials, particularly
if applied to children and neonates.
Acknowledgements
The present study was supported by the Key Medical
Science Research Program of Hebei Province (grant no.
ZL20140195).
References
|
1
|
Zolner G and Trauner M: Mechanisms of
cholestasis. Clin Liver Dis. 12:1–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hines JE, Johnson SJ and Burt AD: In vivo
responses of macrophages and perisinusoidal cells to cholestatic
injury. Am J Pathol. 142:511–518. 1993.PubMed/NCBI
|
|
3
|
Zhang YP, Yao XX and Zhao X: Interleukin-1
beta up-regulates tissue inhibitor of matrix metalloproteinase-1
mRNA and phosphorylation of c-jun N-terminal kinase and p38 in
hepatic stellate cells. World J Gastroenterol. 12:1392–1396. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kossor DC, Meunier PC, Handler JA, Sozio
RS and Goldstein RS: Temporal relationship of changes in
hepatobiliary function and morphology in rats following
alpha-naphthylisothiocyanate (ANIT) administration. Toxico1 Appl
Pharmacol. 119:108–114. 1993. View Article : Google Scholar
|
|
5
|
Tang N, Zhang Y, Liu Z, Fu T, Liang Q and
Ai X: Correlation analysis between four serum biomarkers of liver
fibrosis and liver function in infants with cholestasis. Biomed
Rep. 5:107–112. 2016.PubMed/NCBI
|
|
6
|
Gao LN, Yan K, Cui YL, Fan GW and Wang YF:
Protective effect of Salvia miltiorrhiza and Carthamus
tinctorius extract against lipopolysaccharide-induced liver
injury. World J Gastroenterol. 21:9079–9092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farleigh RM, Knodell RG and Steele NM:
Characterization of a hepatic congestion model in the rat:
Application of pharmacological studies. Proc Soc Exp Biol Med.
166:134–140. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li GY, Gao HY, Huang J, Lu J, Gu JK and
Wang JH: Hepatoprotective effect of Cichorium intybus L., a
traditional Uighur medicine, against carbon tetrachloride-induced
hepatic fibrosis in rats. World J Gastroenterol. 20:4753–4760.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stasi C and Milani S: Non-invasive
assessment of liver fibrosis: Between prediction/prevention of
outcomes and cost-effectiveness. World J Gastroenterol.
22:1711–1720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bedossa P, Dargère D and Paradis V:
Sampling variability of liver fibrosis in chronic hepatitis C.
Hepatology. 38:1449–1457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cadranel JF, Rufat P and Degos F:
Practices of liver biopsy in France: Results of a prospective
nationwide survey. For the Group of Epidemiology of the French
Association for the Study of the Liver (AFEF). Hepatology.
32:477–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orsler DJ, Ahmed-Choudhury J, Chipman JK,
Hammond T and Coleman R: ANIT-induced disruption of biliary
function in rat hepatocyte couples. Toxicol Sci. 47:203–210. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Golbar HM, Izawa T, Yano R, Ichikawa C,
Sawamoto O, Kuwamura M, Lamarre J and Yamate J: Immunohistochemical
characterization of macrophages and myofibroblasts in
α-Naphthylisothiocyanate (ANIT)-induced bile duct injury and
subsequent fibrogenesis in rats. Toxicol Pathol. 39:795–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SK and Kim YC: Effects of betaine
supplementation on hepatic metabolism of sulfur-containing amino
acids in mice. J Hepatol. 42:907–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li SB, Xu FM, Xue C, Ding XJ, Li YC, QIian
LY, Zhang GL and Zeng F: Protective effects of ursodeoxycholic acid
on a-naphthylisothi-induced acute liver injury in rats. Chin J
Digestion. 32:325–329. 2012.
|
|
16
|
Lee HH, Seo YS, Um SH, Won NH, Yoo H, Jung
ES, Kwon YD, Park S, Keum B, Kim YS, et al: Usefulness of
non-invasive markers for predicting significant fibrosis in
patients with chronic liver disease. J Korean Med Sci. 25:67–74.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lijia S, Aifei T and Ruilong X: The
clinical value of serum markers of liver fibrosis in patients with
viral hepatitis. Jiangxi J Med Lab Sci. 25:453–454. 2007.
|
|
18
|
El-Shabrawi MH, El Zein Abedin MY, Omar N,
Kamal NM, Elmakarem SA, Khattab S, El-Sayed HM, El-Hennawy A and
Ali AS: Predictive accuracy of serum hyaluronic acid as a
non-invasive marker of fibrosis in a cohort of multi-transfused
Egyptian children with β-thalassaemia major. Arab J Gastroenterol.
13:45–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nath NC, Rahman MA, Khan MR, Hasan MS,
Bhuiyan TM, Hoque MN, Kabir MM, Raha AK and Jahan B: Serum
hyaluronic acid as a predictor of fibrosis in chronic hepatitis B
and C virus infection. Mymensingh Med J. 20:614–619.
2011.PubMed/NCBI
|
|
20
|
Nobili V, Alisi A, Torre G, De Vito R,
Pietrobattista A, Morino G, De Ville De Goyet J, Bedogni G and
Pinzani M: Hyaluronic acid predicts hepatic fibrosis in children
with nonalcoholic fatty liver disease. Transl Res. 156:229–234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teare JP, Sherman D, Greenfield SM,
Simpson J, Bray G, Catterall AP, Murray-Lyon IM, Peters TJ,
Williams R and Thompson RP: Comparison of serum procollagen III
peptide concentrations and PGA index for assessment of hepatic
fibrosis. Lancet. 342:895–898. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan L and Zhihua H: Significance of serum
5′-Nucleotidase and γ-glutamyltranspeptidase in differential
diagnosis for infantile hepafitis syndrome and biliary atresia. J
Clin Exper Med. 8:16–17. 2009.
|
|
23
|
Tarantino G, Finelli C, Colao A, Capone D,
Tarantino M, Grimaldi E, Chianese D, Gioia S, Pasanisi F, Contaldo
F, et al: Are hepatic steatosis and carotid intima media thickness
associated in obese patients with normal or slightly elevated
gamma-glutamyl-transferase? J Transl Med. 10:502012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tavian D, Degiorgio D, Roncaglia N,
Vergani P, Cameroni I, Colombo R and Coviello DA: A new splicing
site mutation of the ABCB4 gene in intrahepatic cholestasis of
pregnancy with raised serum gamma-GT. Dig Liver Dis. 41:671–675.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du W, Zhang Y and Zhai W: A study on type
I, II and IV collagen product ion in CCL4 induced rat liver
fibrosis. Zhonghua Bing Li Xue Za Zhi. 26:74–77. 1997.(In Chinese).
PubMed/NCBI
|