Introduction
How much medium should be used for cell culture?
Some companies provide a recommended media volume for culture
dishes, plates and flasks, however, there are few reports
investigating the ideal media volume for individual wells, dishes
and flasks. The optimal medium volume for the culture of embryos
(1,2), renal epithelial cells (3) and chondrocytes (4–6) has
been reported, and it has been recognized that cell proliferation
and differentiation are largely influenced by culture conditions,
such as cell density and autocrine and/or paracrine regulators. In
a study investigating in vitro embryonic development, the
volume of micro-drop culture medium and the density of embryos were
important factors (2). Another
study demonstrated that cell viability was influenced by the media
glucose content, and with increasing volumes of culture medium
there are increasing amounts of glucose (3,5).
Furthermore, the rates of lactate reuptake appear to be highly
dependent on the culture medium volume, indicating there may be a
volume-induced stimulation of oxidative lactate metabolism
(3). In addition, the
concentration of oxygen in the medium has been demonstrated to
decrease in line with increasing medium depth (6,7).
Collectively, this indicates that the medium volume may impact a
variety of cell culture factors.
Bone homeostasis is maintained via a balance between
osteoblastic bone formation and osteoclastic resorption. This
remodeling is controlled by a wide variety of systemic and local
factors including hormones, cytokines and mechanical stresses.
Osteoblasts undergo proliferation, matrix maturation and
extracellular matrix mineralization (8). During this process, osteoblasts
synthesize extracellular matrix components including type I
collagen. Notably, the activity of osteoblast alkaline phosphatase
(ALP) increases upon reaching confluence in culture (9,10).
ALP hydrolyzes substrates, and the extracellular matrix is
subsequently mineralized, which occurs by increasing the local
calcium phosphate concentration. ALP activity has therefore been
used as an important indicator of bone formation (11,12).
Osteoblastic MC3T3-E1 cells undergo a process of proliferation and
differentiation, and then produce mineralized nodules (13). Osteoclasts are derived from
hematopoietic cells and are regulated by various cytokines and
hormones including interleukin (IL)-1, IL-6 and parathyroid hormone
(14). It was previously reported
that macrophage colony-stimulating factor and receptor activator of
nuclear factor-κB (RANK) ligand (RANKL) are necessary and
sufficient for osteoclast differentiation (15,16).
Osteoprotegerin (OPG) is the decoy receptor for RANKL and inhibits
RANKL-RANK signaling (17).
Osteoblasts express RANKL and OPG, and thus coordinate osteoclast
differentiation and bone resorption (18,19).
In vitro, osteoclast formation may be induced using a
co-culture of bone marrow and stromal cells (20). Cellular contact is essential for
osteoclastogenesis (21).
Further clarification on the importance of medium
volume in osteoblastic and osteoclastic cell culture systems in
vitro is required. Therefore, the present in vitro study
examined the impact of medium volume on the mineral deposition of
osteoblasts and differentiation of osteoclastic precursor cells.
The results indicated that the suppression of mineralization in
osteoblastic cells and stimulation of the fusion process in
osteoclasts is influenced by the medium volume.
Materials and methods
Osteoblast cell culture
To investigate in vitro mineralization,
osteoblastic MC3T3-E1 cells were donated by Dr H. Kodama (Ohu
University, Kōriyama, Japan) and were cultured in fresh α-minimum
essential medium (α-MEM; Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 2 mM
L-alanyl-L-glutamine (Wako Pure Chemical Industries, Ltd.,), 284 µM
L-ascorbic acid 2-phosphate (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and 66.7 mg/ml kanamycin sulfate (Meiji Seika Kaisha,
Ltd., Tokyo, Japan) in 24- and 12-well plates. Cells were cultured
in 0.4, 0.6, 0.8, 1.0, 1.5 or 2.0 ml medium, and the culture medium
was changed every other day. All cultures were maintained at 37°C
in a humidified atmosphere with 5% CO2. After culture
for 30 days, the cells were fixed with 10% neutral formalin (Wako
Pure Chemical Industries, Ltd.) and subjected to von Kossa staining
as previously described (13).
Briefly, a solution of 1% aqueous silver nitrate was added to each
dish under direct light for 15 min.
Measurement of ALP activity
After culture for 30 days, ALP activity was assayed
using the method described by Suzuki et al (22), which is based on the method
developed by Bessey et al (23). Briefly, MC3T3-E1 cell homogenates
were prepared by ultrasonication and preincubated at 37°C in a
reaction mixture (0.5 ml) containing 50 mM sodium carbonate (pH
9.7), 25 mM sucrose and 1 mM MgCl2 with or without 5 mM
levamisole. Assays were initiated by adding
p-nitrophenylphosphate (pNPP) substrate (0.1 ml) to
the reaction mixture to a final concentration of 20 mM. After 15
min at 37°C, 0.5 ml reaction mixture was added to 1.5 ml 0.6 M NaOH
solution for color development. Hydrolysis of pNPP was
measured colorimetrically at 420 nm using a microplate reader. The
protein concentration of MC3T3-E1 cell homogenates was determined
using a DC Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Osteoclast cell culture
A total of 20 C57BL/6J Jms Slc male mice were
obtained from Sankyo Labo Service Corporation, Inc., (Tokyo,
Japan). All animal experiments were performed under an
institutionally approved protocol for use of animal research at
Hokkaido University (no. 10-0070). Mice were kept in the animal
facility at a temperature of 23°C and controlled humidity, under a
12 h light/dark cycle, and had free access to food and water. To
investigate in vitro osteoclastogenesis, bone marrow cells
were collected by flushing femoral shafts of 4-week-old mice were
sacrificed with CO2, using a 26-gauge sterile needle.
Bone marrow cells (1×105 cells/well) were co-cultured
with proliferating MC3T3-E1 cells, using fresh α-MEM supplemented
with 10% FBS, 2 mM L-alanyl-L-glutamine, 284 µM L-ascorbic acid
2-phosphate, 100 nM dexamethasone, 10 nM 1-alpha,
25-dihydroxyvitamin D3 (Sigma-Aldrich; Merck KGaA) and
66.7 µg/ml kanamycin sulfate in a 24-well plate. Cells were
cultured in 0.4, 0.6, 0.8, 1.0, 1.5 or 2.0 ml medium. Following
co-culture for 6 days, cells were fixed and stained for
tartrate-resistant acid phosphatase (TRAP) activity as described
previously (24). Briefly, TRAP
staining solution contained acetate buffer (pH 5.0), naphthol AS-MX
phosphate and red violet LB (all from Sigma-Aldrich; Merck KGaA),
in the presence of 50 mM sodium tartrate (Wako Pure Chemical
Industries, Ltd.). TRAP-positive multinuclear cells were considered
to be osteoclasts. The numbers of TRAP-positive multinuclear cells
were expressed as the mean ± standard deviation (SD) of triplicate
cultures.
Semi-quantitative measurement of RANKL
mRNA
MC3T3-E1 cells were co-cultured as aforementioned
for 6 days. Total RNA was isolated using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
First strand cDNA was synthesized using a Revatra Ace FSK-101 kit
(Toyobo Co., Ltd., Osaka, Japan). The reaction buffer contained the
following components: 50 mM Tri-HCl (pH 7.5), 100 mM NaCl, 0.1 mM
EDTA, 10 mM dithioerythritol, 0.01% Nonidet P-40 and 50% glycerol.
The reaction conditions for the reverse transcription (RT)
procedure were as follows: Annealing at 30°C for 10 min, extension
at 42°C for 20 min, denaturation at 99°C for 5 min followed by
cooling at 4°C. KOD-Dash DNA polymerase reaction mixture (Toyobo
Co., Ltd.) was used for amplification. Polymerase chain reaction
(PCR) amplification was performed with a GeneAmp PCR system 9700
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
following thermocycling conditions: 23 cycles for GAPDH and 30
cycles for RANKL of 94°C for 30 sec, annealing at 60°C for 2 sec
and extension at 72°C for 30 sec. The first cycle was conducted at
94°C for 10 min and the final extension cycle at 72°C for 10 min.
The following primers were used: RANKL, forward
5′-TATGATGGAAGGCTCATGGT-3′ and reverse 5′-TGTCCTGAACTTTGAAAGCC-3′;
GAPDH, forward 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. Amplification products were
separated on a 1% agarose gel and stained with ethidium bromide.
Densitometry was performed using Image J software version 1.48a
(National Institutes of Health, Bethesda, MD, USA). GAPDH was used
as the housekeeping gene for normalizing expression values
(25).
Measurement of OPG content
OPG content recovered from the osteoclast co-culture
system conditioned medium was measured using a mouse OPG Analyza
ELISA kit (catalog no. 10047; Bio-Techne Co, Minneapolis, MN, USA),
according to the manufacturer's protocol. In brief, standard
solutions and conditioned medium were plated in a 96-well
microplate pre-coated with monoclonal antibody specific to mouse
OPG and incubated for 2 h at room temperature. After washing, a
peroxidase-labeled polyclonal antibody specific to OPG was added to
each well and incubated for 2 h. Subsequently, unbound
antibody-enzyme was removed by washing and the substrate was added
to the wells. Following incubation for 30 min at room temperature,
the absorbance was measured at a wavelength of 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
Values were expressed as the mean ± SD. Overall
comparisons were performed using a two-way analysis of variance
followed by the Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
The present study examined the impact of culture
medium volume on the in vitro differentiation of
osteoblastic MC3T3-E1 cells, using 0.4, 0.6, 0.8 1.0, 1.5 or 2.0
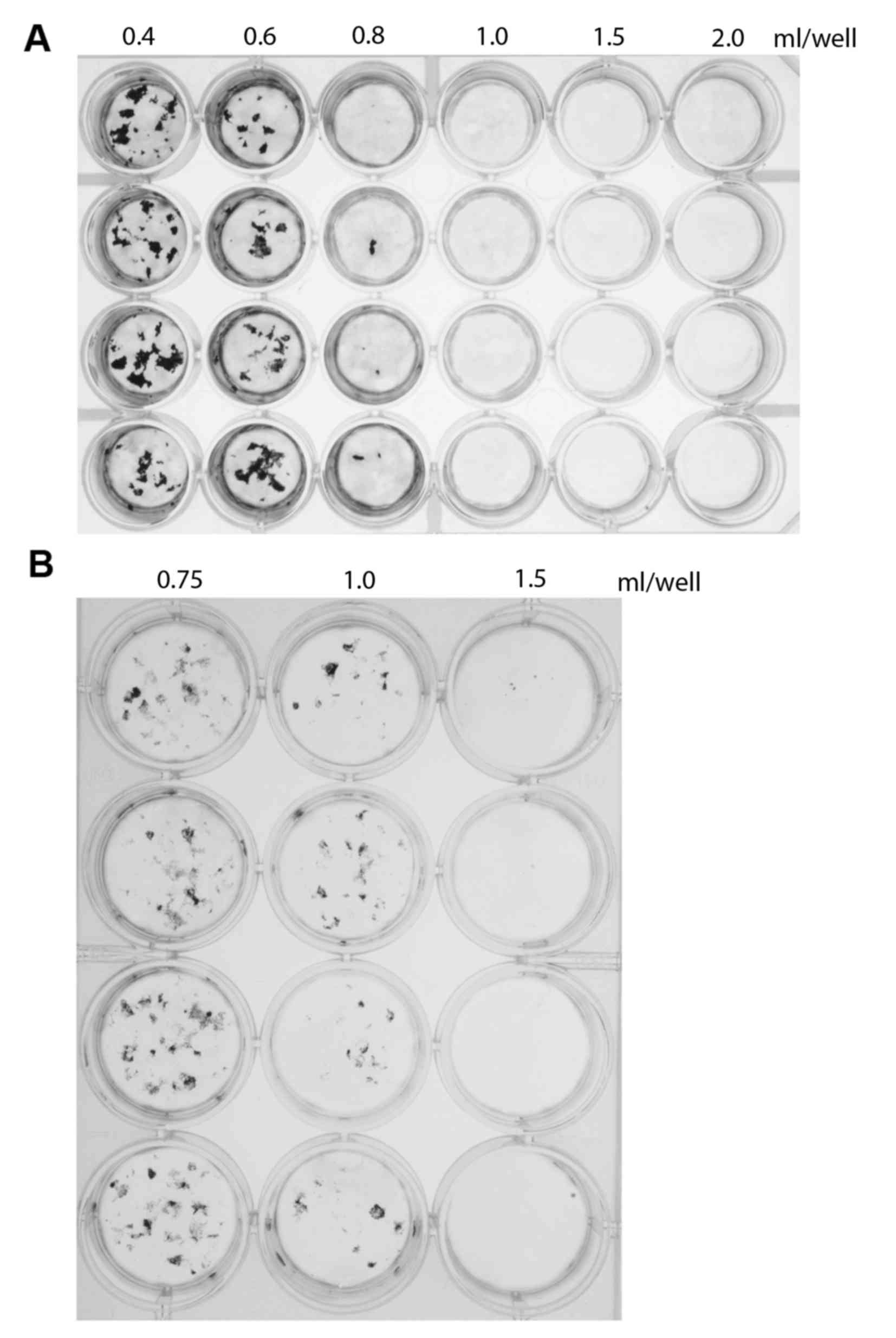
ml/well culture medium, in a 24-well plate. Mineral deposition was
measured after 30 days of culture. The results indicated that the
area of mineral deposition was inversely proportional to the volume
of medium (Fig. 1A). To verify
these results, mineral deposition was investigated using a 12-well
plate with 0.75, 1.0 or 1.5 ml/well. Once again, the area of
mineral deposition area was inversely proportional to the medium
volume (Fig. 1B), confirming the
results obtained in the initial experiment. Notably, there was no
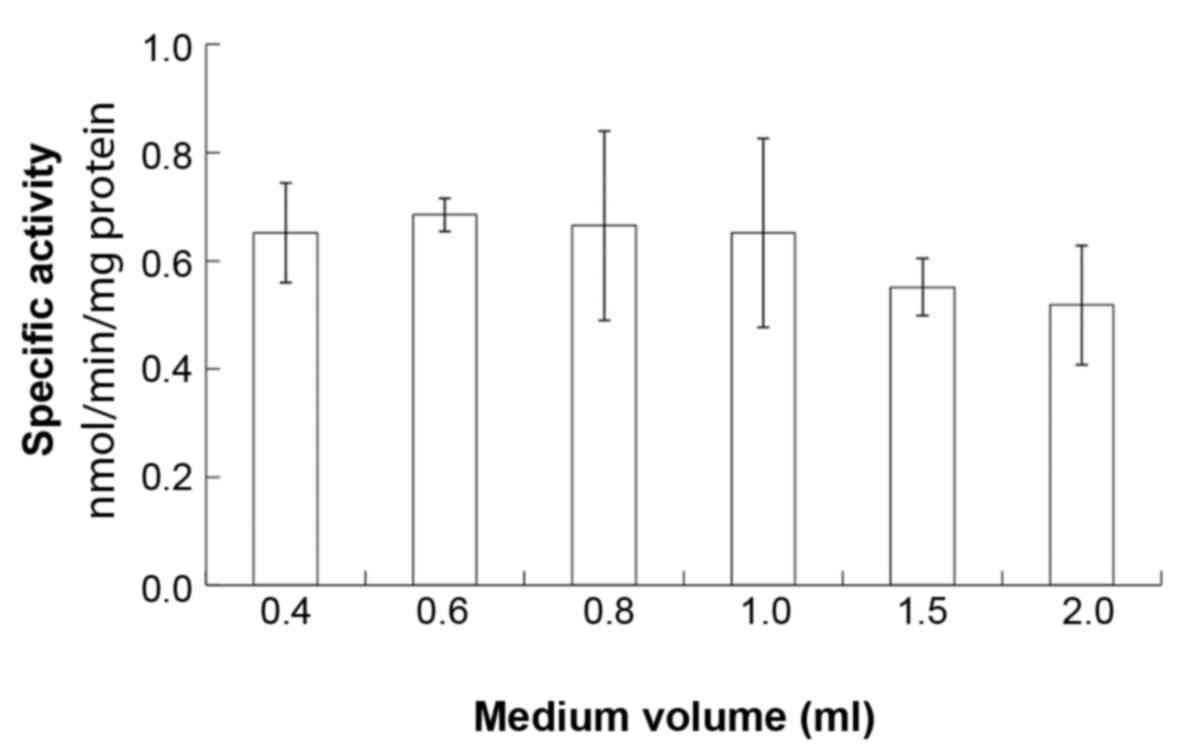
significant difference in the osteoblastic ALP activity amongst the
groups (P>0.05; Fig. 2).
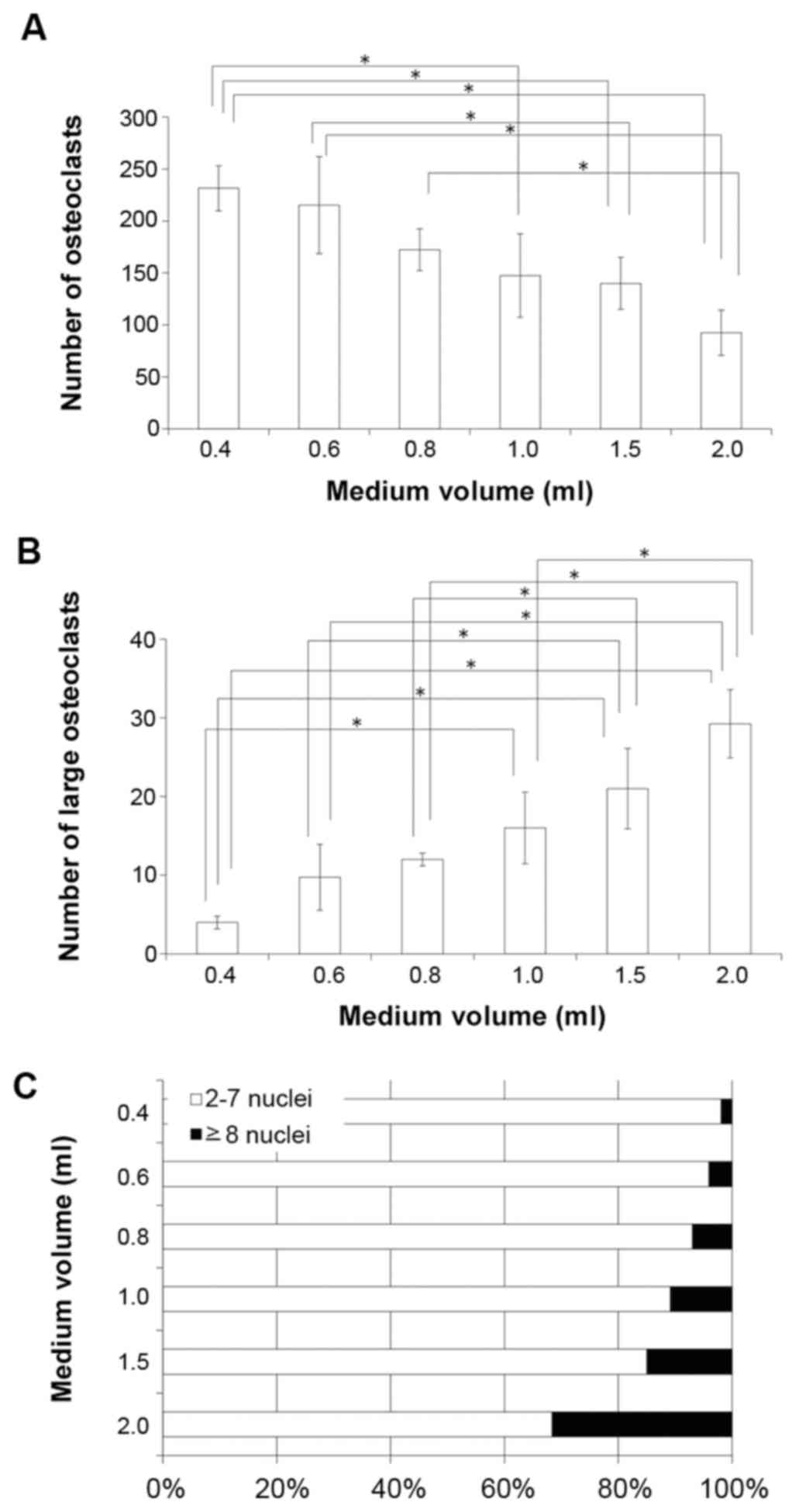
Co-culture of osteoblastic MC3T3-E1 cells with
C57BL/6 mouse-derived bone marrow cells was performed, and the
number of TRAP-positive cells was quantified. The total number of
TRAP-positive multinuclear cells was reduced in a medium
volume-dependent manner (Fig. 3A),
whereas the formation of large osteoclastic TRAP-positive
multinuclear cells (≥8 nuclei) was increased in a medium
volume-dependent manner (Fig. 3B).
Furthermore, the ratio of osteoclasts with 2–7 nuclei to
osteoclasts with ≥8 nuclei decreased with increasing media volume
(Fig. 3C).
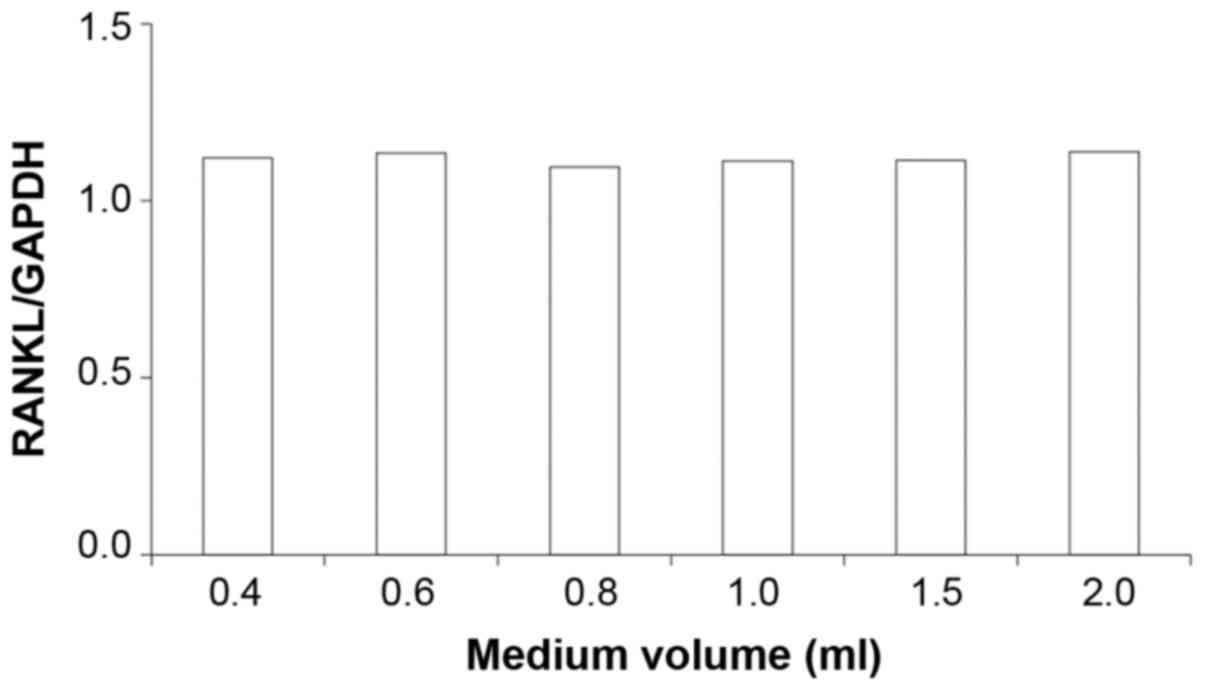
To investigate the impact of media volume on RANKL
mRNA levels, the expression of RANKL mRNA was semi-quantified using
RT-PCR. The expression of RANKL mRNA did not appear to differ
between groups (Fig. 4). ELISA was
used to investigate the effects of medium volume on OPG content.
The concentration of OPG was inversely proportional to the volume
of media (P<0.05; Fig. 5A),
however the total content of OPG did not differ between groups
(P>0.05; Fig. 5B).
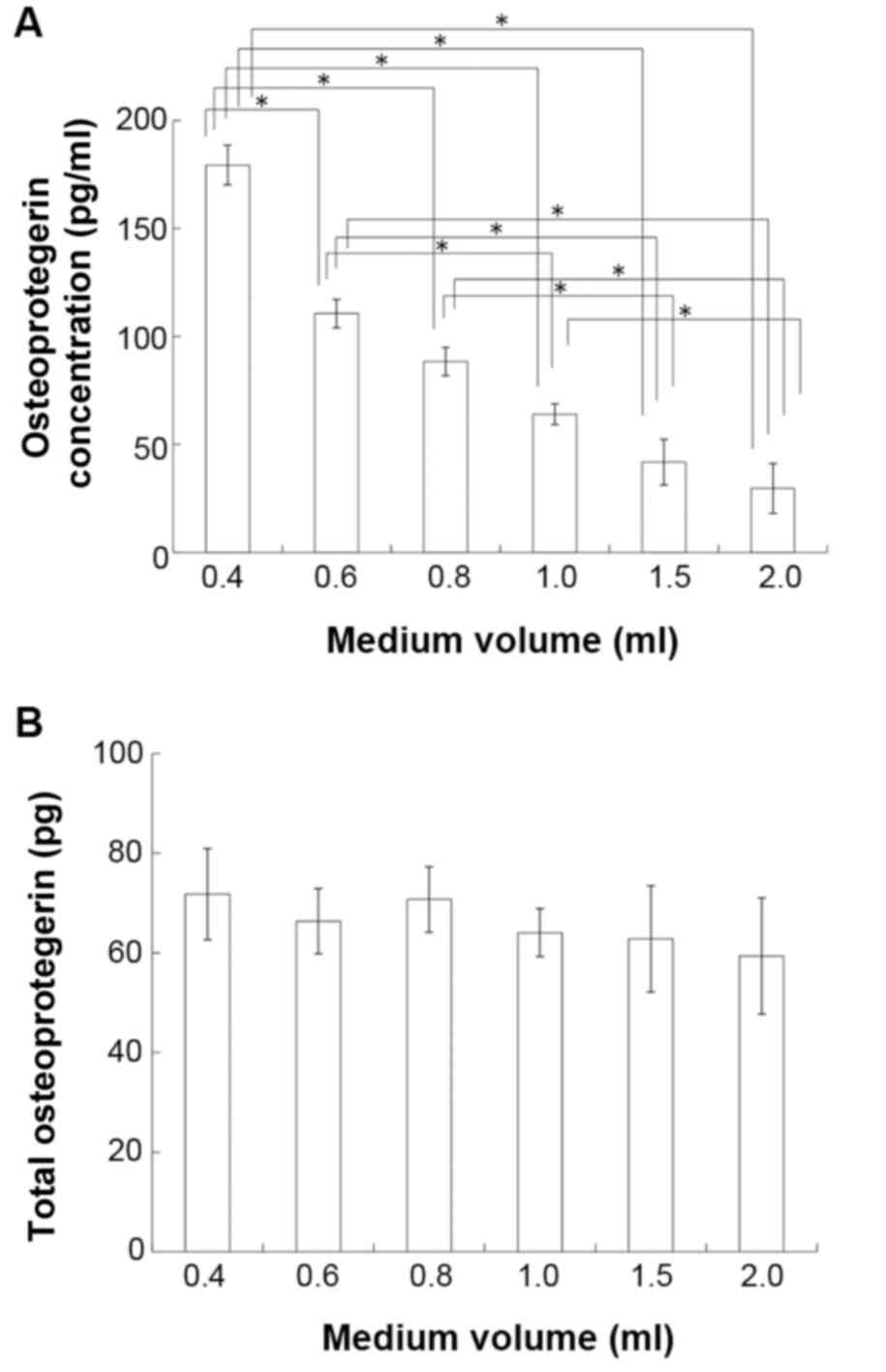 | Figure 5.Effects of medium volume on OPG
production in MC3T3-E1 cells. The OPG content of conditioned media
was measured by ELISA. (A) OPG concentration in 0.4, 0.6, 0.8, 1.0,
1.5 and 2.0 ml/well. (B) Total OPG content in 0.4, 0.6, 0.8, 1.0,
1.5 and 2.0 ml/well. Data are presented as the mean ± standard
deviation (n=6). *P<0.05, with comparisons indicated by
brackets. OPG, osteoprotegerin |
Discussion
The results indicated that there was a negative
association between the volume of media and the occurrence of
mineralization in cultured osteoblasts (Fig. 1). Previous research has indicated
that cell viability is influenced by glucose availability, and
increasing volumes of culture medium therefore contain increasing
amounts of glucose (3,5). However, the area of mineral
deposition was inversely proportional to medium volume. Although
the total content of cytokines would most likely be the same within
this culture system, the different media volumes result in
concentration or dilution of the total cytokine content, and the
cytokine concentrations were therefore inversely proportional to
the medium volume. This result indicates that osteoblast
mineralization may be promoted in an autocrine manner, regardless
of the glucose content. Previous studies have also demonstrated
that oxygen concentrations in the culture media decrease in line
with increasing medium depth (6,7). The
media volumes of 0.4, 0.6, 0.8, 1.0, 1.5 and 2.0 ml in a 24-well
plate (2 cm2/well) correspond to media depths of 2, 3,
4, 5, 7.5 and 10 mm, respectively. The concentration of oxygen in
the medium affects matrix synthesis and differentiation in murine
chondrogenic cell culture (6), and
therefore the altered osteoblastic mineralization may be related to
oxygen concentration. ALP activity has been used as an important
indicator of bone formation; however, in the present study, there
were no significant differences in the ALP activity of MC3T3-E1
cells among the cultures. Therefore, these results suggested that
ALP activity is not influenced by these conditions.
RANKL is an essential factor in osteoclast
differentiation and activation (17). Osteoblasts express RANKL and OPG,
and thus coordinate osteoclast differentiation and bone resorption
(15,26). Osteoclastogenesis is dependent on
RANKL concentration (27);
therefore, the present study employed a co-culture system with
osteoblastic cells and bone marrow cells without RANKL. In this
co-culture system, the total number of TRAP-positive multinuclear
osteoclasts was reduced in a medium volume-dependent manner,
although the formation of large TRAP-positive multinuclear
osteoclasts (≥8 nuclei) was increased, indicating that
osteoclastogenesis was increased under these conditions. To
investigate whether the inductive effect of medium volume on
osteoclasto genesis is dependent on the amount of RANKL and/or OPG
produced by osteoblasts, the amount of RANKL mRNA and the secretion
of OPG into the conditioned medium were measured. OPG is the decoy
receptor for RANKL, and it inhibits RANKL-RANK signaling (5). No significant impact of medium volume
on RANKL mRNA expression was observed. Furthermore, there were no
significant differences in the total amount of OPG amongst the
various culture conditions; however, the concentration of OPG was
inversely proportional to medium volume. This indicated that a
balance between RANKL and OPG concentrations influences osteoclast
fusion.
Increasing the volume of medium exerts mechanical
stress, such as hydraulic pressure, on cells. Human periodontal
ligament cells under hydraulic pressure display reduced OPG mRNA
expression in a medium volume-dependent manner (28); although these results appear to
conflict with the present study, this discrepancy may be
attributable to inherent differences between human periodontal
ligament cells and murine osteoblasts. A recent study demonstrated
that osteoclastogenesis was accelerated by an optimal compressive
force (24), where a compressive
force was directly applied to the osteoclast-precursor cells,
without the use of a co-culture system. Furthermore,
osteoclastogenesis appears to be accelerated by increasing volumes
of culture medium and a concomitant increase in nutrients,
including glucose (3,5). Glucose was the principal energy
source required for bone degradation (29), and hypoxia-inducible factor 1 alpha
was stabilized in osteoclasts, leading to osteoclast activation
(30). This suggests that the
differentiation of large multinuclear osteoclasts may be influenced
by the glucose content, and also by low concentrations of oxygen in
the medium (6,7). Notably, the total number of large
osteoclasts (≥8 nuclei) was observed to increase in a medium
volume-dependent manner. These results indicated that, in an in
vitro system, the volume of medium may have a greater effect on
promoting the osteoclast fusion process, compared with the
differentiation of mononuclear osteoclasts and/or osteoclastic
precursors. By contrast, the formation of 2–7 nuclei osteoclasts
appeared to be reduced in a medium volume-dependent manner; this
may be due to the concentrations of oxygen or of osteoclast
inducible cytokines, such as IL-1 and tumor necrosis factor-α
(TNF-α), which are reportedly inversely proportional to medium
volume (31). This may be due to
IL-1 and TNF-α stimulating osteoclast differentiation from
mononuclear pre-osteoclasts to small osteoclasts via RANKL-RANK
signaling (31).
Thus, the nutrient availability of the culture
medium appears to be influenced by the medium volume, and this may
directly influence osteoblast mineralization and osteoclast
differentiation (3). The volume of
media used in cell culture is therefore an important consideration
in the culture of osteoblasts and osteoclasts, and culture systems
should be optimized to ensure an optimal in vitro
microenvironment is achieved.
References
|
1
|
Kito S, Iritani A and Bavister BD: Effects
of volume, culture media and type of culture dish on in vitro
development of hamster 1-cell embryos. Theriogenology. 47:541–548.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai SJ, Xu CL, Wang J, Sun YP and Chian
RC: Effect of culture medium volume and embryo density on early
mouse embryonic development: Tracking the development of the
individual embryo. J Assist Reprod Genet. 29:617–623. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gstraunthaler G, Seppi T and Pfaller W:
Impact of culture conditions, culture media volumes, and glucose
content on metabolic properties of renal epithelial cell cultures.
Are renal cells in tissue culture hypoxic? Cell Physiol Biochem.
9:150–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heywood HK, Sembi PK, Lee DA and Bader DL:
Cellular utilization determines viability and matrix distribution
profiles in chondrocyte-seeded alginate constructs. Tissue Eng.
10:1467–1479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heywood HK, Bader DL and Lee DA: Glucose
concentration and medium volume influence cell viability and
glycosaminoglycan synthesis in chondrocyte-seeded alginate
constructs. Tissue Eng. 12:3487–3496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oze H, Hirao M, Ebina K, Shi K, Kawato Y,
Kaneshiro S, Yoshikawa H and Hashimoto J: Impact of medium volume
and oxygen concentration in the incubator on pericellular oxygen
concentration and differentiation of murine chondrogenic cell
culture. In Vitro Cell Dev Biol Anim. 48:123–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pettersen EO, Larsen LH, Ramsing NB and
Ebbesen P: Pericellular oxygen depletion during ordinary tissue
culturing, measured with oxygen microsensors. Cell Prolif.
38:257–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinri MS, Kennedy MB, Pockwines S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationship in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: An in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deyama Y, Takeyama S, Koshikawa M, Shirai
Y, Yoshimura Y, Nishikata M, Suzuki K and Matsumoto A: Osteoblast
maturation suppressed osteoclastogenesis in coculture with bone
marrow cells. Biochem Biophys Res Commun. 274:249–254. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anderson HC: Molecular biology of matrix
vesicles. Clin Orthop Relat Res. 266–280. 1995.PubMed/NCBI
|
|
12
|
Fleish H and Neuman WF: Mechanism of
calcification. Role of collagen, polyphosphatases, and phosphate.
Am J Physiol. 200:1296–1300. 1961.PubMed/NCBI
|
|
13
|
Deyama A, Deyama Y, Matsumoto A, Yoshimura
Y, Nishikata M, Suzuki K and Totsuka Y: A low calcium environment
enhances AP-1 transcription factor-mediated gene expression in the
development of osteoblastic MC3T3-E1 Cells. Miner Electrolyte
Metab. 25:147–160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suda T, Takahashi N and Martin TJ:
Modulation of osteoclast differentiation. Endocr Rev. 13:66–80.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida H, Hayashi S, Kunisada T, Ogawa M,
Nishikawa S, Okamura H, Sudo T, Shultz LD and Nishikawa SI: The
murine mutation osteopetrosis is in the coding region of the
macrophage colony stimulating factor gene. Nature. 345:442–444.
1990. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Udagawa N, Takahashi N, Yasuda H, Mizuno
A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K
and Suda T: Osteoprotegerin produced by osteoblasts is an important
regulator in osteoclast development and function. Endocrinology.
141:3478–3484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mochizuki A, Takami M, Kawawa T, Suzumoto
R, Sasaki T, Shiba A, Tsukasaki H, Zhao B, Yasuhara R, Suzawa T, et
al: Identification and characterization of the precursors committed
to osteoclasts induced by TNF-related activation-induced
cytokine/receptor activator of NF-kappa B ligand. J Immunol.
177:4360–4368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi N, Akatsu T, Udagawa N, Sasaki
T, Yamaguchi A, Moseley JM, Martin TJ and Suda T: Osteoblastic
cells are involved in osteoclast formation. Endocrinology.
123:2600–2602. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suda T, Udagawa N, Nakamura I, Miyaura C
and Takahashi N: Modulation of osteoclast differentiation by local
factors. Bone. 17:(2 Suppl). 87S–91S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki K, Yoshimura Y, Hisada Y and
Matsumoto A: Sensitivity of intestinal alkaline phosphatase to
L-homoarginine and its regulation by subunit-subunit interaction.
Jpn J Pharmacol. 64:97–102. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bessey OA, Lowry OH and Brock MJ: A method
for the rapid determination of alkaline phosphatase with five cubic
millimeters of serum. J Biol Chem. 164:321–329. 1946.PubMed/NCBI
|
|
24
|
Hayakawa T, Yoshimura Y, Kikuiri T,
Matsuno M, Hasegawa T, Fukushima K, Shibata K, Deyama Y, Suzuki K
and Iida J: Optimal compressive force accelerates
osteoclastogenesis in RAW264.7 cells. Mol Med Rep. 12:5879–5885.
2015.PubMed/NCBI
|
|
25
|
Nakai T, Yoshimura Y, Deyama Y, Suzuki K
and Iida J: Mechanical stress up-regulates RANKL expression via the
VEGF autocrine pathway in osteoblastic Mc3T3-E1 cells. Mol Med Rep.
2:229–234. 2009.PubMed/NCBI
|
|
26
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki N, Yoshimura Y, Deyama Y, Suzuki K
and Kitagawa Y: Mechanical stress directly suppresses osteoclast
differentiation in RAW264.7 cells. Int J Mol Med. 21:291–296.
2008.PubMed/NCBI
|
|
28
|
Nakao K, Goto T, Gunjigake KK, Konoo T,
Kobayashi S and Yamaguchi K: Intermittent force induces high RANKL
expression in human periodontal ligament cells. J Dent Res.
86:623–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Williams JP, Blair HC, McDonald JM,
McKenna MA, Jordan SE, Williford J and Hardy RW: Regulation of
osteoclastic bone resorption by glucose. Biochem Biophys Res
Commun. 235:646–651. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyauchi Y, Sato Y, Kobayashi T, Yoshida
S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Miyamoto K, et
al: HIF1α is required for osteoclast activation by estrogen
deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci USA.
110:16568–16573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takayanagi H: Osteoimmunology: Shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007. View
Article : Google Scholar : PubMed/NCBI
|