Introduction
Dexamethasone is a glucocorticoid that has been
reported to act on normal mammary epithelial cells and breast
cancer cells (1,2); however, its inhibitory effects on
cancer cell growth remain controversial. Dexamethasone has been
reported to suppress estrogen-dependent breast cancer growth
(2) and induce breast cancer cell
apoptosis in vitro (3,4).
Contradictory reports have demonstrated that dexamethasone exerted
protective effects against cell death (5) and promoted the proliferation of MCF-7
breast cancer cells (6). Notably,
dexamethasone has also been reported to enhance the sensitivity of
cancer cells to the effects of chemotherapy in vitro
(7) and in vivo (8).
The effects of dexamethasone on the sensitivity of
cancer cells to anticancer drugs have been associated with
ATP-binding cassette (ABC) transporters, including ABC transporter
subfamily G member 2 (ABCG2; originally termed breast cancer
resistance protein) (9,10). Side population (SP) cells have been
identified in breast cancer and have been reported to efflux the
fluorescent dye Hoechst 33342, possibly via the ABCG2 transporter
(11). SP cells are rare
populations among breast cancer cells (12), and have also been detected among
embryonic (13) and adult stem
cells (14). SP cells have
demonstrated self-renewing and differentiating capabilities
(15), tumorigenic activity
(16) and have been reported to
give rise to heterogeneous cell populations during cancer
development (17). Therefore, it
may be hypothesized that SP cells act as cancer stem-like cells and
serve a critical role in the development of multidrug resistance
(18,19). ABC transporters, including ABCG2,
have also been implicated in the development of multidrug
resistance, due to their role as efflux pumps for chemotherapeutic
agents (11).
The effects of dexamethasone in breast cancer and
breast cancer stem-like cells have yet to be thoroughly
investigated. The present study aimed to assess the putative
inhibitory effects of dexamethasone on cancer cell growth and
investigate the molecular mechanisms underlying its actions. The
present study demonstrated that dexamethasone exerted dose- and
time-dependent effects on MCF-7 cancer cell proliferation. The
Hoechst-based flow cytometry profiles suggested that dexamethasone
may target breast cancer stem-like cells, identified as the SP, in
accordance with previous studies (11,20).
Materials and methods
Cell culture
Human MCF-7 breast adenocarcinoma cells (American
Type Culture Collection, Manassas, VA, USA) were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). For the
creation of an anchorage-dependent culture, MCF-7 cells
(5×105) were seeded in a Falcon™ Standard Tissue Culture
Dish (Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C
in a humidified 5% CO2 atmosphere.
MCF-7 cell viability
MCF-7 cells (5×105) were seeded in DMEM
supplemented with 10% FBS. Following 24 h of culture, cells were
washed twice with PBS, and fresh medium was added. Various
concentrations (1, 10, 100 nM and 1 µM) of dexamethasone
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and/or the
glucocorticoid inhibitor RU486 (1 µM; Sigma-Aldrich; Merck KGaA)
were added to the cells. The numbers of viable cells were estimated
at a number of time points (following 24, 48 and 72 h of culture)
using trypan blue staining.
Flow cytometry
Following incubation for 72 h, cells
(1×105) were fixed in 70% ethanol for 1 h at 4°C, washed
with PBS and treated with 100 µg/ml RNase A (Sigma-Aldrich; Merck
KGaA) for 1 h at 37°C. Cells were then stained with 25 µg/ml
propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for 15 min at
37°C. For Annexin V-fluorescein isothiocyanate (FITC) staining,
cells were washed with PBS, treated with diluted trypsin-EDTA
solution, centrifuged at 25°C for 3 min at 150 × g, washed twice
with cold PBS, and resuspended in binding buffer (10 mM HEPES pH
7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM
CaCl2). A 100 µl aliquot of the suspension
(~1×105 cells) was incubated with 5 µl Annexin V-FITC
and 5 µl PI for 15 min in the dark at room temperature. Binding
buffer (400 µl) was added to each mixture, and the samples were
analyzed by flow cytometry within 1 h. Flow cytometry was performed
using the FACSCalibur™ system (BD Biosciences, San Jose, CA, USA)
and data were analyzed using the BD FACStation™ software version
6.0 (BD Biosciences). Experiments were performed in triplicate.
SP analysis of MCF-7 cells
MCF-7 cells (5×105) were seeded in DMEM
supplemented with 10% FBS. Following 24 h incubation, cells were
washed twice with PBS, and fresh media were added. The cells were
then treated with ethanol (CTL), dexamethasone (100 nM), RU486 (1
µM), or RU486 (1 µM) and dexamethasone (100 nM) for 72 h. SP
analysis was performed as previously described (11). To detach cells, cultures were
trypsinized for 3 min and detachment was monitored under a
phase-contrast microscope. The number of viable cells was estimated
using trypan blue staining. Cells were centrifuged at 25°C for 3
min at 150 × g and resuspended in 5 ml PBS. To detect SP cells,
cells at a density of 1×106 cells/ml were incubated with
Hoechst 33342 dye (5 µg/ml) in DMEM supplemented with 10% FBS for
90 min at 37°C, with vortexing every 10 min. At the end of the
incubation, cells were centrifuged at 4°C for 3 min at 150 × g and
transferred to microcentrifuge tubes. PI (1 µg/ml) was added to the
tube for 15 min at 37°C prior to fluorescence-activated cell
sorting for the identification and exclusion of dead cells. Samples
were analyzed using the BD FACSAria™ system and the FACSDiva™
software version 6.1 (BD Biosciences). In order to confirm that
cells belonging to the SP were expressing the ABCG2 transporter,
Hoechst 33342 staining was also performed in cells additionally
treated for 90 min with the ABCG2 inhibitor verapamil (50 µM; Merck
KGaA).
Flow cytometric analysis of ABCG2
expression
Cells were detached using trypsin as aforementioned,
and 5 ml DMEM supplemented with 10% FBS were added to the culture
for trypsin inactivation. Cells were collected by centrifugation
for 3 min at 150 × g at 25°C, resuspended in 5 ml PBS and
centrifuged again. Cell pellets were resuspended in 500 µl total
volume of PBS containing FITC-conjugated anti-ABCG2 antibody (cat.
no. 332014; 1:50; BioLegend, Inc., San Diego, CA, USA). Following
incubation for 25 min at room temperature, cells were rinsed three
times with PBS and flow cytometric analyses were performed in
triplicate using the FACSCalibur™ system (BD Biosciences).
Statistical analysis
The statistical significance of the differences
between groups was assessed using Student's t-test using Microsoft
Excel software version 2016 (Microsoft Corporation, Redmond, WA,
USA). Data are expressed as the mean ± standard error of the mean
of at least three independent experiments. P<0.05 was considered
to indicate a statistically significant difference.
Results
Dexamethasone decreases MCF-7 cell
viability
The present study evaluated the effects of
dexamethasone on the viability of human MCF-7 breast adenocarcinoma
cells using trypan blue staining. Following treatment with various
concentrations of dexamethasone for 72 h, MCF-7 cell viability was
reduced to 92, 81, 54 and 45% of the control levels by 1, 10, 100
nM and 1 µM dexamethasone, respectively (Fig. 1A). Following 72 h of treatment, 100
nM dexamethasone significantly reduced the numbers of viable MCF-7
cells compared with control (Fig.
1B). Therefore, a dose of 100 nM dexamethasone was used in all
subsequent experiments. To confirm that the observed reduction in
MCF-7 cell viability was due to dexamethasone, the glucocorticoid
inhibitor RU486 was employed. The inhibitory effects of
dexamethasone on MCF-7 proliferation were abolished following
treatment with 1 µM RU486 (Fig.
1C).
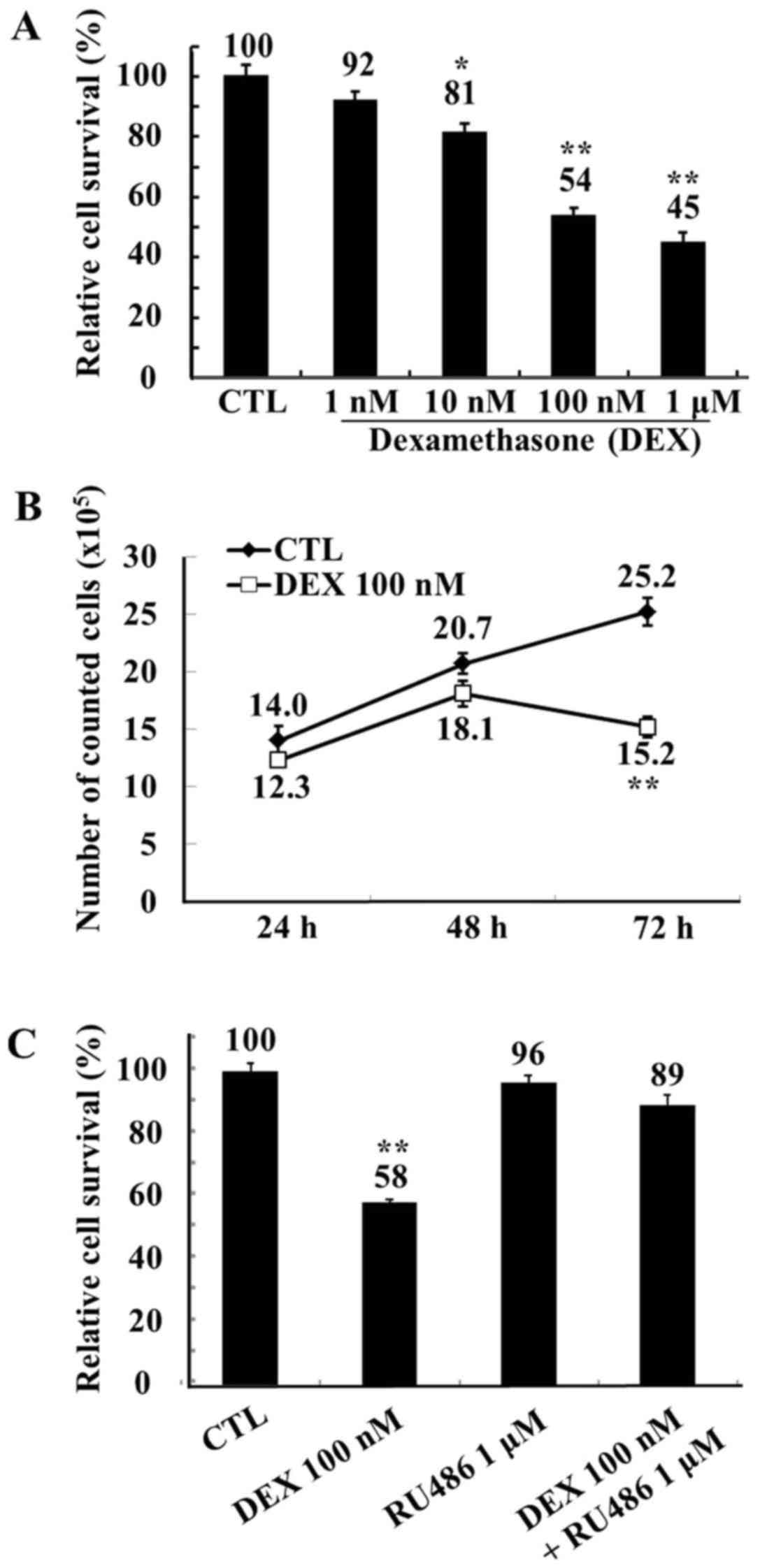 | Figure 1.Viability of MCF-7 cells following
treatment with DEX, assessed by trypan blue staining. (A) Human
MCF-7 breast adenocarcinoma cells were treated with ethanol (CTL)
or with DEX (1, 10, 100 nM and 1 µM) for 72 h. Relative cell
survival rate is presented as percentage survival vs. the survival
of control cells following treatment with DEX. (B) MCF-7 cells were
treated with ethanol (CTL) or with DEX (100 nM) for 24, 48, and 72
h. Cell survival is presented vs. the survival of control cells
following treatment with DEX. (C) MCF-7 cells were treated with
ethanol (CTL), the glucocorticoid inhibitor RU486 (1 µM), DEX (100
nM), or RU486 in combination with DEX for 72 h. Relative cell
survival rate is presented as percentage survival vs. the survival
of control cells following treatment with DEX. Data are expressed
as the mean ± standard error of the mean. *P<0.05 and
**P<0.01 vs. the CTL group. DEX, dexamethasone; CTL,
control. |
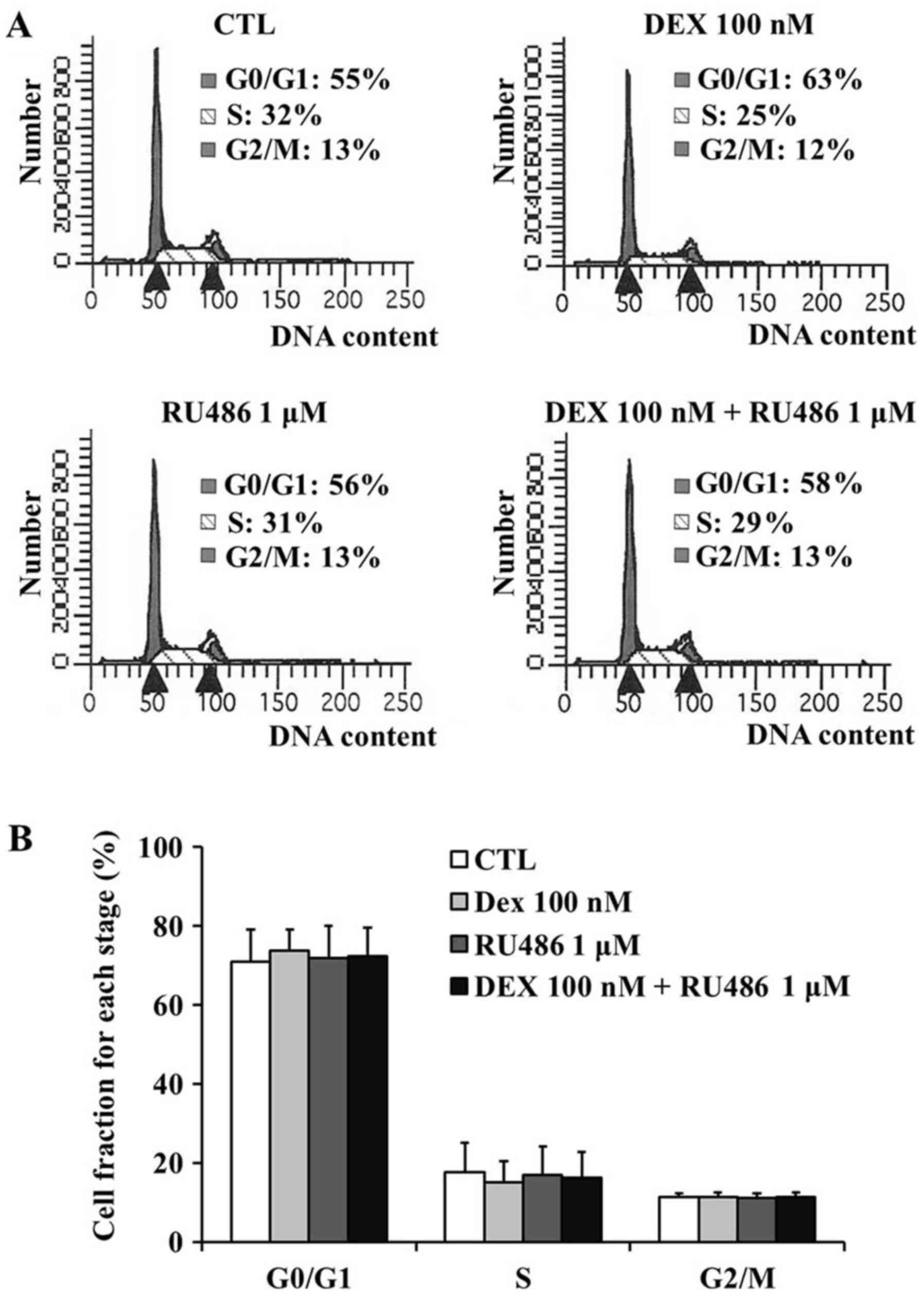
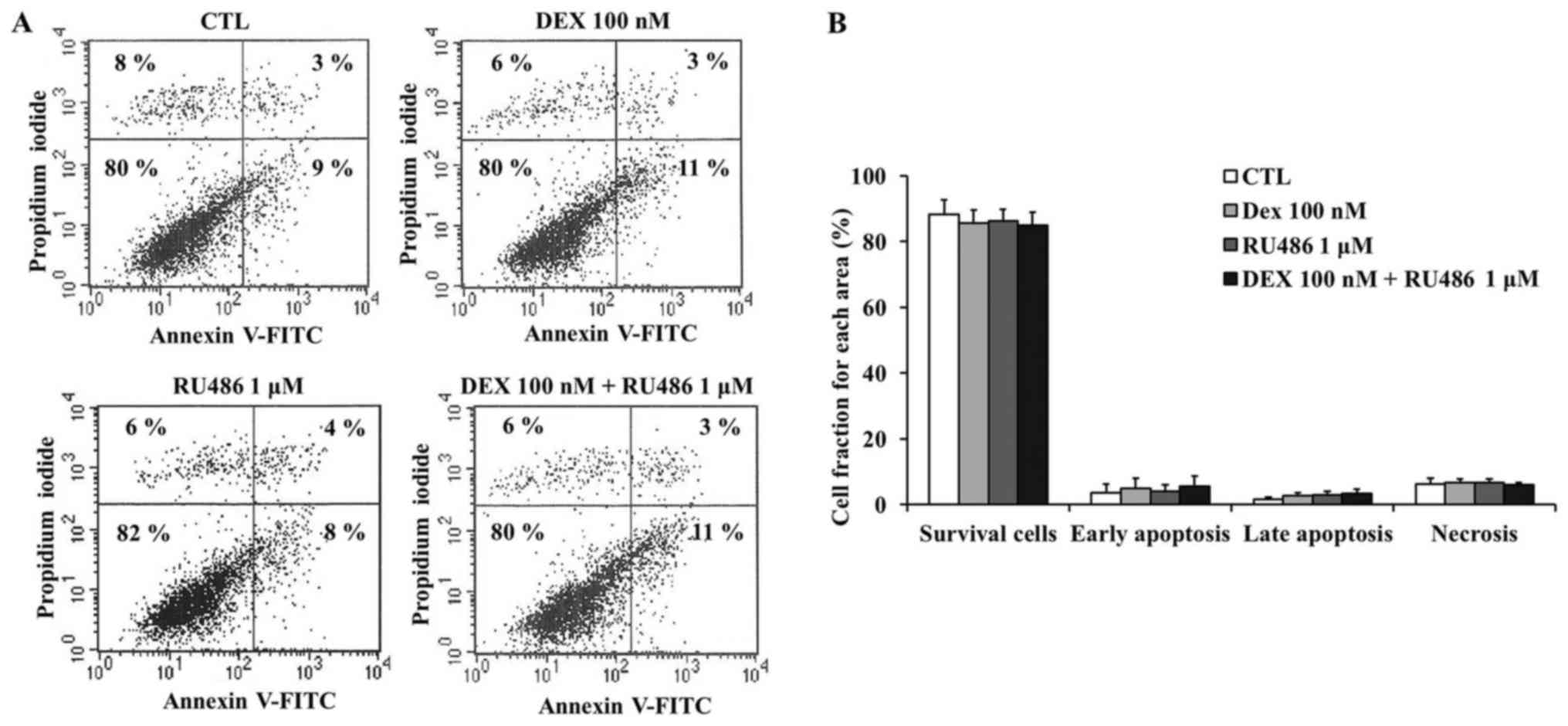
Dexamethasone does not affect cell
cycle distribution of MCF-7 cells
To determine whether the observed decrease in MCF-7
cell viability was the result of cell cycle arrest or apoptosis,
the DNA contents of the surviving cells were assessed using PI
staining followed by flow cytometry. The fraction of MCF-7 cells in
the G0/G1 phase demonstrated an upward trend
(~8% increase) following treatment with 100 nM dexamethasone
compared with untreated control cells; however, no statistical
significance was detected (Fig. 2A and
B). In detail, the G0/G1 fraction was
71.00±9.45% in untreated cells, 73.67±6.19% in cells treated with
100 nM dexamethasone, 72.00±9.33% in cells treated with 1 µM RU486
and 72.33±8.28% in cells treated with RU486 and dexamethasone. The
results of the apoptosis assay demonstrated that dexamethasone did
not produce any statistically significant effects on MCF-7 cell
apoptosis (Fig. 3A and B).
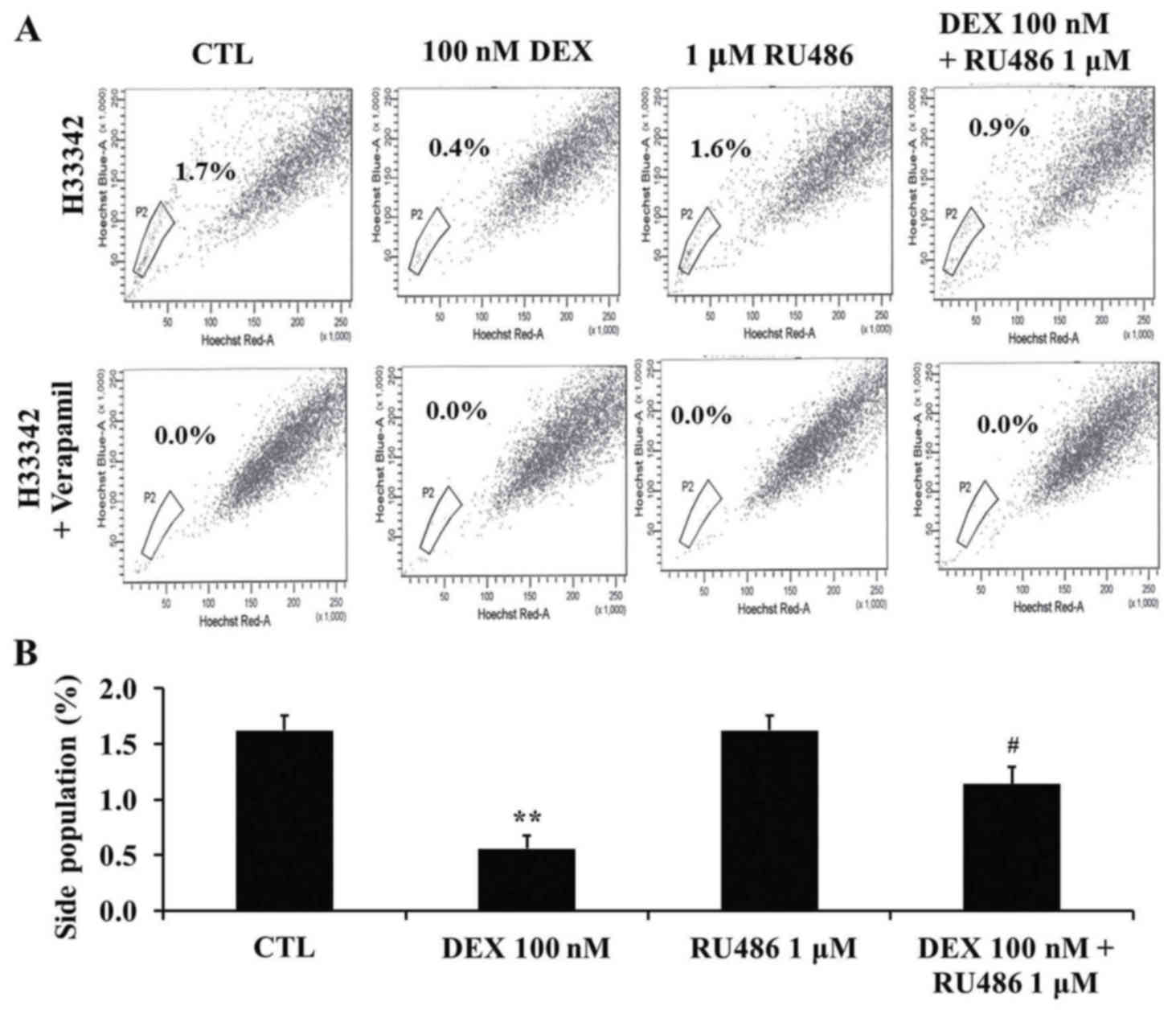
Dexamethasone decreases the SP
fraction of MCF-7 cells
To investigate the effects of dexamethasone
treatment on the SP fraction of MCF-7 cells, cells were treated
with 100 nM dexamethasone and 1 µM RU486 as aforementioned. SP
cells were detected using Hoechst 33342 staining followed by flow
cytometry. The size of the SP fraction was 1.6±0.1% in untreated
cells, 0.6±0.1% in cells treated with 100 nM dexamethasone,
1.6±0.1% in cells treated with 1 µM RU486 and 1.1±0.2% in cells
treated with RU486 and dexamethasone (Fig. 4A and B). The SP fraction was
significantly decreased following dexamethasone treatment
(P<0.01) compared with untreated cells, whereas
co-administration of RU486 attenuated the effects of dexamethasone
(P<0.05 compared with dexamethasone-treated cells). Following
treatment with verapamil, the SP fraction was not detected.
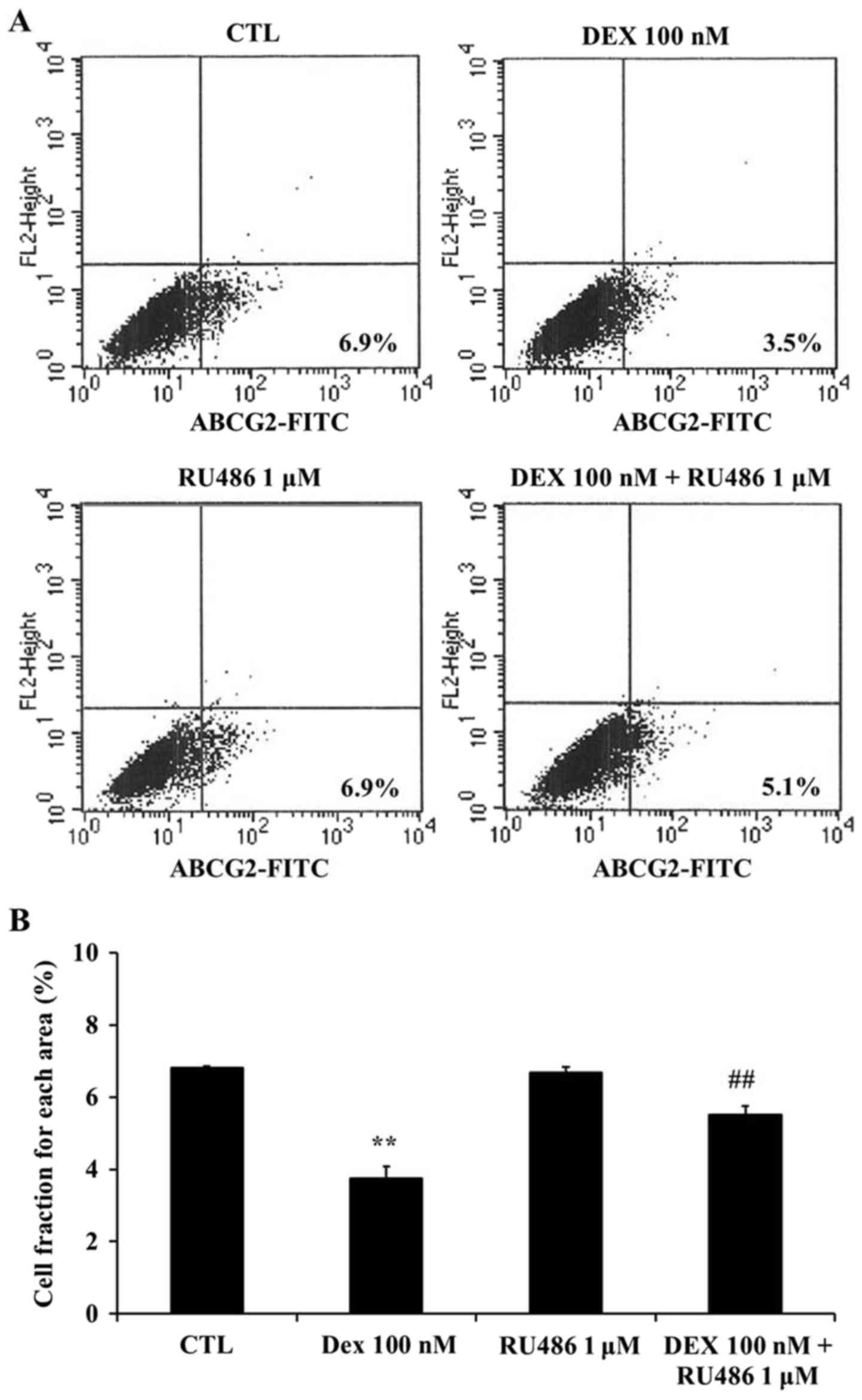
Dexamethasone downregulates ABCG2
expression in MCF-7 cells
To investigate whether the effects of dexamethasone
on the SP of MCF-7 cells may be associated with the ABCG2
transporter, the expression of ABCG2 was assessed using flow
cytometry. The present results demonstrated that ABCG2 expression
was significantly downregulated following treatment with
dexamethasone compared with untreated cells (P<0.01; Fig. 5). Furthermore, RU486
co-administration appeared to restore the dexamethasone-induced
ABCG2 downregulation in MCF-7 cells (P<0.01 compared with
dexamethasone-treated cells; Fig.
5). ABCG2-positive cells were detected as 6.8±0.06% in
untreated cells, 6.7±0.17% in the presence of 1 µM RU486, 3.7±0.32%
in the presence of 100 nM dexamethasone, and 5.5±0.24% in the
presence of both 1 µM RU486 and 100 nM dexamethasone.
Discussion
The inhibitory effects of dexamethasone on breast
cancer cell growth remain controversial, as dexamethasone has been
demonstrated to inhibit cell growth (2) and induce apoptosis (3,4),
whereas it has also been reported to prevent cell death (5) and promote cellular proliferation
(6). These contradictory results
may, in part, be attributed to alterations in protein expression
due to variations in the cell culture environment among the various
studies (21). Dexamethasone has
been demonstrated to enhance the efficacy of anticancer drugs on
breast cancer cells in vitro (4), whereas it has also been reported to
downregulate the expression of the ABCG2 transporter in breast
cancer cells (9,22–24).
Therefore, it may be hypothesized that dexamethasone inhibits
cancer cell growth via targeting cancer stem-like cells. The
results of the present study revealed that dexamethasone targeted
cells in the SP of MCF-7 breast cancer cells and downregulated the
expression of ABCG2, whereas its effects were abolished by the
glucocorticoid inhibitor RU486. Traditional chemotherapeutic
agents, including doxorubicin and docetaxel, have not been reported
to affect the SP fraction; however, the plant alkaloid berberine
has been demonstrated to decrease the SP fraction among breast
cancer cells, and this effect was associated with ABCG2
downregulation (11). These
results suggested that the molecular mechanisms underlying the
SP-suppressing actions of dexamethasone may involve the
downregulation of ABCG2 in cancer cells.
The pharmacological profile of dexamethasone is
diverse and has been reported to include antiemetic (25), anti-inflammatory (26) and pro-differentiating properties
(27,28). In addition, dexamethasone has been
reported to enhance the sensitivity of cancer cells to the effects
of chemotherapy in vitro (7) and in vivo (8). Furthermore, dexamethasone increased
the survival of patients with multiple myeloma in a phase II
clinical trial when used as an adjuvant treatment in combination
with anticancer drugs (29), and
enhanced the effects of prostate cancer chemotherapy in
vitro and in vivo (30). The present results demonstrated
that dexamethasone inhibited the growth and decreased the SP
fraction in human MCF-7 breast cancer cells, possibly through the
downregulation of ABCG2 expression.
In conclusion, the results of the present study
suggested that the inhibitory effects of dexamethasone on cancer
cell growth may be associated with a decrease in the SP fraction or
the number of cancer stem-like cells. Therefore, it may be
hypothesized that dexamethasone can target breast cancer cell SPs
and increase the sensitivity of tumor cells to chemotherapy, thus
holding potential as a chemosensitizer in the adjuvant treatment of
patients with breast cancer.
Acknowledgements
The present study was supported by a research grant
from Jeju National University (2015).
References
|
1
|
Boyd C and Náray-Fejes-Tóth A:
Steroid-mediated regulation of the epithelial sodium channel
subunits in mammary epithelial cells. Endocrinology. 148:3958–3967.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong H, Jarzynka MJ, Cole TJ, Le JH, Wada
T, Zhang B, Gao J, Song WC, DeFranco DB, Cheng SY and Xie W:
Glucocorticoids antagonize estrogens by glucocorticoid
receptor-mediated activation of estrogen sulfotransferase. Cancer
Res. 68:7386–7393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rachner TD, Benad P, Rauner M, Goettsch C,
Singh SK, Schoppet M and Hofbauer LC: Osteoprotegerin production by
breast cancer cells is suppressed by dexamethasone and confers
resistance against TRAIL-induced apoptosis. J Cell Biochem.
108:106–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buxant F, Kindt N, Laurent G, Noël JC and
Saussez S: Antiproliferative effect of dexamethasone in the MCF-7
breast cancer cell line. Mol Med Rep. 12:4051–4054. 2015.PubMed/NCBI
|
|
5
|
Machuca C, Mendoza-Milla C, Córdova E,
Mejía S, Covarrubias L, Ventura J and Zentella A: Dexamethasone
protection from TNF-alpha-induced cell death in MCF-7 cells
requires NF-kappaB and is independent from AKT. BMC Cell Biol.
7:92006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan S, Lopez-Dee Z, Kumar R and Ling J:
Activation of NFkB is a novel mechanism of pro-survival activity of
glucocorticoids in breast cancer cells. Cancer Lett. 337:90–95.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Wang Y, Rayburn ER, Hill DL,
Rinehar JJ and Zhang R: Dexamethasone as a chemosensitizer for
breast cancer chemotherapy: Potentiation of the antitumor activity
of adriamycin, modulation of cytokine expression, and
pharmacokinetics. Int J Oncol. 30:947–953. 2007.PubMed/NCBI
|
|
8
|
Pang D, Kocherginsky M, Krausz T, Kim SY
and Conzen SD: Dexamethasone decreases xenograft response to
Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol
Ther. 5:933–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honorat M, Mesnier A, Di Pietro A, Lin V,
Cohen P, Dumontet C and Payen L: Dexamethasone down-regulates ABCG2
expression levels in breast cancer cells. Biochem Biophys Res
Commun. 375:308–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JB, Ko E, Han W, Shin I, Park SY and
Noh DY: Berberine diminishes the side population and ABCG2
transporter expression in MCF-7 breast cancer cells. Planta Med.
74:1693–1700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, He Z, Zhu H, Chen X, Li J, Zhang H,
Pan X and Hu Y: Murine fertilized ovum, blastomere and morula cells
lacking SP phenotype. Sci China C Life Sci. 50:762–765. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scharenberg CW, Harkey MA and Torok-Storb
B: The ABCG2 transporter is an efficient Hoechst 33342 efflux pump
and is preferentially expressed by immature human hematopoietic
progenitors. Blood. 99:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dieterlen-Lièvre F: Lineage-switching by
pluripotent cells derived from adults. J Soc Biol. 195:39–46.
2001.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burkert J, Otto W and Wright N: Side
populations of gastrointestinal cancers are not enriched in stem
cells. J Pathol. 214:564–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Y, Kitisin K, Jogunoori W, Li C, Deng
CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS, et al:
Progenitor/stem cells give rise to liver cancer due to aberrant
TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 105:2445–2450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin L, Castagnino P and Assoian RK: ABCG2
expression and side population abundance regulated by a
transforming growth factor beta-directed epithelial-mesenchymal
transition. Cancer Res. 68:800–807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet J Jr, Badve S and
Nakshatri H: CD44+/CD24- breast cancer cells exhibit enhanced
invasive properties: An early step necessary for metastasis. Breast
Cancer Res. 8:R592006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pavek P, Merino G, Wagenaar E, Bolscher E,
Novotna M, Jonker JW and Schinkel AH: Human breast cancer
resistance protein: Interactions with steroid drugs, hormones, the
dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine,
and transport of cimetidine. J Pharmacol Exp Ther. 312:144–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elahian F, Kalalinia F and Behravan J:
Dexamethasone downregulates BCRP mRNA and protein expression in
breast cancer cell lines. Oncol Res. 18:9–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elahian F, Kalalinia F and Behravan J:
Evaluation of indomethacin and dexamethasone effects on
BCRP-mediated drug resistance in MCF-7 parental and resistant cell
lines. Drug Chem Toxicol. 33:113–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujii Y and Nakayama M: Reduction of
postoperative nausea and vomiting and analgesic requirement with
dexamethasone in women undergoing general anesthesia for
mastectomy. Breast J. 13:564–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Igarashi H, Medina KL, Yokota T, Rossi MI,
Sakaguchi N, Comp PC and Kincade PW: Early lymphoid progenitors in
mouse and man are highly sensitive to glucocorticoids. Int Immunol.
17:501–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Srivastava AS, Kaushal S, Mishra R, Lane
TA and Carrier E: Dexamethasone facilitates erythropoiesis in
murine embryonic stem cells differentiating into hematopoietic
cells in vitro. Biochem Biophys Res Commun. 346:508–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi YH, Ko SH, Kim SJ, Lee WY, Park JH
and Lee JM: Induction of cell death by photodynamic therapy with a
new synthetic photosensitizer DH-I-180-3 in undifferentiated and
differentiated 3T3-L1 cells. Biochem Biophys Res Commun.
337:1059–1064. 2004. View Article : Google Scholar
|
|
29
|
Hussein MA, Bolejack V, Zonder JA, Durie
BG, Jakubowiak AJ, Crowley JJ and Barlogie B: Phase II study of
thalidomide plus dexamethasone induction followed by tandem
melphalan-based autotransplantation and thalidomide-plus-prednisone
maintenance for untreated multiple myeloma: A southwest oncology
group trial (S0204). J Clin Oncol. 27:3510–3517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed S, Johnson CS, Reuger RM and Trump
DL: Calcitriol (1,25-dihydroxycholecalciferol) potentiates activity
of mitoxantrone/dexamethasone in an androgen independent prostate
cancer model. J Urol. 168:756–761. 2002. View Article : Google Scholar : PubMed/NCBI
|