Introduction
Pancreatic cancer, which is one of the most invasive
types of malignant tumor, is the sixth leading cause of mortality
in China, the fourth leading cause of cancer-associated mortality
in the United States, and has the worst prognosis among all solid
cancers (1–3). It was previously estimated that
53,070 new cases of pancreatic cancer and 41,780 cases of mortality
caused by pancreatic cancer would occur in the United States in
2016 (4). Several risk factors
contributing to pancreatic carcinogenesis have been identified,
including age, smoking, obesity and chronic pancreatitis (5–7). In
the past few decades, great progress in cancer research has led to
the discovery of novel diagnostic and surgical techniques. However,
although these have served to improve morbidity and postoperative
mortality, they have had no significant impact on survival
(8). The overall 5-year survival
rate for all stages of pancreatic cancer is only 5%, and the median
survival time is ~6 months (9).
The poor prognosis for patients with pancreatic cancer is mainly
attributed to non-specific symptoms, aggressive growth, early and
aggressive local invasion, metastatic potential, and resistance to
chemotherapy and radiotherapy (10–12).
Therefore, elucidation of the underlying mechanisms involved in
pancreatic cancer pathogenesis, as well as the development of novel
therapeutic treatments, is critical for improving the prognosis and
therapeutic efficacy in patients with pancreatic cancer.
Recently, microRNAs (miRNAs), a novel group of
endogenous, single-stranded, small, non-coding regulatory RNA
molecules with a length of 18–24 nucleotides, have garnered
attention (13). miRNAs decrease
gene expression through targeting partial complementary elements in
the 3′ untranslated regions (3′UTR) of their target genes, and
function via two mechanisms: Degrading the expression of target
genes and/or suppressing their translation (14). A single specific miRNA may regulate
a large number of target genes, often targeting numerous components
of complex intracellular networks (15). Key roles for miRNAs have been
established in various biological processes, including cell
proliferation, differentiation, metabolism and apoptosis, which
contribute to the pathogenesis of several diseases, including
cancer. Previous studies have demonstrated that the abnormal
expression of miRNAs and their target genes is correlated with
carcinogenesis and cancer progression (16–18).
Numerous miRNAs have been reported to be up- or downregulated in
pancreatic cancer, whereas the expression of specific miRNAs has
been correlated with poor disease prognosis, thus demonstrating the
potential of these molecules as novel therapeutic targets for
cancer therapy (19–21).
The present study aimed to investigate the pattern
of expression, biological role and mechanism of action of a
specific miRNA molecule, miR-296, in pancreatic cancer. In
addition, it was revealed that AKT2 is a direct target gene of
miR-296 in pancreatic cancer, indicating that miR-296 may hold
potential as a molecular target in the treatment of pancreatic
cancer.
Materials and methods
Clinical specimens
Human pancreatic cancer tissue samples from patients
with pancreatic cancer (7 male and 5 female; age range, 39–68
years), and matched control normal tissue (7 male and 5 female; age
range, 39–68 years) samples were obtained at Weifang People's
Hospital (Weifang, China). These specimens were immediately
snap-frozen and stored in liquid nitrogen until further processing.
The present study was approved by the Ethics Committee of Weifang
People's Hospital. Written informed consent was also obtained from
all patients participating in the present study.
Cell lines, culture conditions and
oligonucleotide transfection
Human pancreatic carcinoma cell lines PANC-1,
BxPC-3, Capan-2, SW-1990 and AsPC-1, and the human normal
pancreatic cell line HPDE6c7 were purchased from American Type
Culture Collection (Manassas, VA, USA). HEK293T cells were obtained
from the Shanghai Institute of Biochemistry and Cell Biology
(Shanghai, China). All cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), and maintained at 37°C in a 5% CO2
atmosphere.
miR-296 mimics and negative controls (NC) were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
AKT2 (5′-AGTGACCATGAATGACTTC-3′) and NC (5′-TCTACGAGTCGCGGCATTC-3′)
small interfering RNA (siRNA) were obtained from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). PANC-1 and Capan-2 cells were
transfected with these oligonucleotides using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as the
delivery agent, following to the manufacturer's protocol.
RNA isolation and reverse
transcription quantitative-polymerase chain reaction (RT-qPCR)
Total RNA was isolated from clinical specimens and
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using PrimeScript RT reagent kit
(Takara Biotechnology Co., Ltd, Dalian, China), following to the
manufacturer's protocol. qPCR analyses were performed on cDNA using
SYBR Green Premix Ex Taq (Takara Biotechnology Co., Ltd.) as
the fluorescent reporter probe. miR-296 expression was detected
with Hairpin-it miRNA qPCR Quantitation kit (Shanghai GenePharma
Co., Ltd.). The 20 µl reaction system for qPCR contained 10 µl SYBR
Green I mix, 2 µl forward primer, 2 µl reverse primer and 4 µl
double distilled water. The thermocycling conditions for qRCR were
as follows: 95°C for 30 sec; 40 cycles of 95°C for 5 sec; and 60°C
for 30 sec. The following primers were used: miR-296 forward,
5′-TGCCTAATTCAGAGGGTTGG-3′ and reverse, 5′-CTCCACTCCTGGCACACAG-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; AKT2 forward, 5′-TCCAGAACACCAGGCACCC-3′
and reverse, 5′-ATTGTCCTCCAGCACCTCA-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
RT-qPCR was performed in an ABI Prism 7700 Sequence Detector
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative
expression levels of miR-296 and AKT2 mRNA were calculated using
the the 2−ΔΔCq method (22) and the results were normalized to U6
expression for miR-296 and GAPDH expression for mRNA.
Cell Counting Kit 8 (CCK8) assay
Cell proliferation was evaluated with CCK8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Transfected cells
were harvested and re-suspended into a single cell suspension. A
total of 4×103 cells in 150 µl culture medium were
seeded per well in 96-well plates. At various time-points after
seeding (1, 2 and 3 days), cell proliferation was measured as
follows: 10 µl CCK8 assay solution was added to each well, and
incubated at 37°C for 4 h. The absorbance of the sample at 450 nm
was measured using a multiwell spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA).
Transwell migration and invasion
assays
The migratory and invasive capabilities of
pancreatic cancer cells were assessed using Costar Transwell
inserts (pore size, 8 µm; Corning Incorporated, Corning, NY, USA).
The membranes were pre-coated with 40 µg/well Matrigel (BD
Biosciences, San Jose, CA, USA) for the invasion assay. A total of
48 h post-transfection, the cells were harvested and re-suspended
into a single cell suspension in serum-free DMEM. Cells were seeded
into the upper chambers of the plate at a density of
5×104 cells in 250 µl serum-free medium; culture medium
supplemented with 20% FBS was added to the lower chambers. After
incubation for 48 h at 37°C, the cells on the top of the membrane
were removed carefully with cotton swabs. The cells on the lower
membrane were fixed with methanol, stained with crystal violet and
then counted under a light microscope (×200; Olympus Corporation,
Tokyo, Japan) to calculate their relative numbers.
Western blot analysis
A total of 72 h post-transfection, the cells were
collected and lysed in radioimmunoprecipitation assay lysis buffer
supplemented with protease inhibitors (Roche Diagnostics, Basel,
Switzerland) and phosphatase inhibitors (Merck KGaA, Darmstadt,
Germany). The concentration of total protein was determined using a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Equal
amounts of extracted protein samples (20 µg) were separated by 10%
SDS-PAGE and transferred onto a polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA). The membrane was blocked with
5% fat-free milk, followed by an overnight incubation at 4°C with
the following primary antibodies: Mouse anti-human monoclonal AKT2
antibody (1:1,000 dilution; cat. no. sc-5270; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-human monoclonal
GAPDH antibody (1:1,000 dilution; cat. no. sc-69778; Santa Cruz
Biotechnology, Inc.). After three washes with TBS containing 0.5%
Tween, the membranes were probed with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (1:5,000
dilution; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) for 2 h
at room temperature. The bands were visualized with enhanced
chemiluminescence Western Blotting Detection Reagent (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) according to the manufacturer's
protocol. GAPDH was used as a loading control for AKT2. Band
intensities were quantified using the FluorChem imaging system
(Alpha Innotec, GmbH, Kasendorf, Germany).
Bioinformatics analysis
In order to predict the putative targets of miR-296,
a bioinformatics analysis was performed using TargetScan
(http://www.targetscan.org/) and miRanda
(http://www.microrna.org/microrna/).
Luciferase reporter assay
The wild-type (Wt1 and Wt2) pMIR-AKT2-3′UTR and
mutant (Mut1 and Mut2) pMIR-AKT2-3′UTR were synthesized by Shanghai
GenePharma Co., Ltd. HEK293T cells were seeded in 24-well plates at
a density of 1.0×105 cells/well and transfected with
miR-296 mimics or NC, and pMIR-AKT2-3′UTR Wt (1,2) or
pMIR-AKT2-3′UTR Mut (1,2) using Lipofectamine 2000, according to
the manufacturer's protocol. A total of 48 h post-transfection, the
cells were collected and luciferase reporter assay was performed
using the Dual-Luciferase Reporter Assay system (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. Renilla luciferase activity was employed as an
endogenous control for firefly luciferase activity. The assay was
performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard deviation. Data were compared
using Student's t-test or analysis of variance followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-296 expression in pancreatic
cancer tissue and cell lines
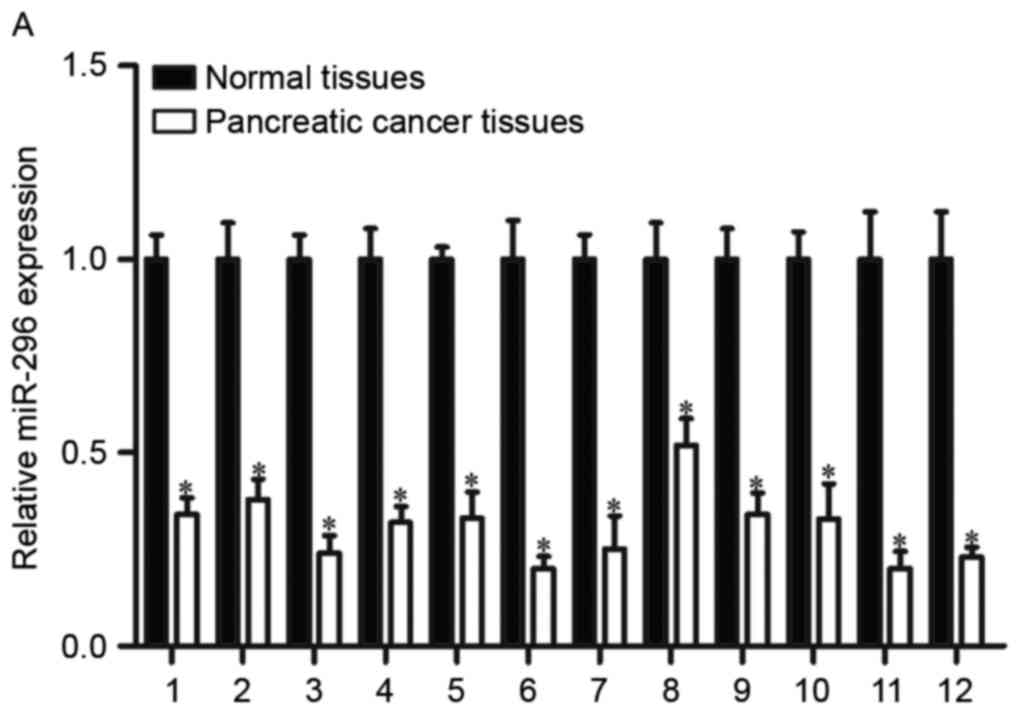
RT-qPCR was used to measure miR-296 expression in
human pancreatic cancer tissue samples and matched normal tissue
samples (Fig. 1A). miR-296
expression in pancreatic cancer tissue was reduced compared with
matched control tissue (P<0.05). The expression levels of
miR-296 were also assessed in human pancreatic carcinoma cell lines
and in the human normal pancreatic cell line HPDE6c7 (Fig. 1B). RT-qPCR revealed that miR-296
expression was significantly downregulated in all pancreatic cancer
cell lines compared with HPDE6c7 cells (P<0.05).
Overexpression of miR-296 inhibits
growth of PANC-1 and Capan-2 cells
In order to investigate the biological roles of
miR-296 in pancreatic cancer, miR-296 mimics were used to
upregulate miR-296 expression in pancreatic cancer cells. PANC-1
and Capan-2 cells, which exhibited the lowest miR-296 expression,
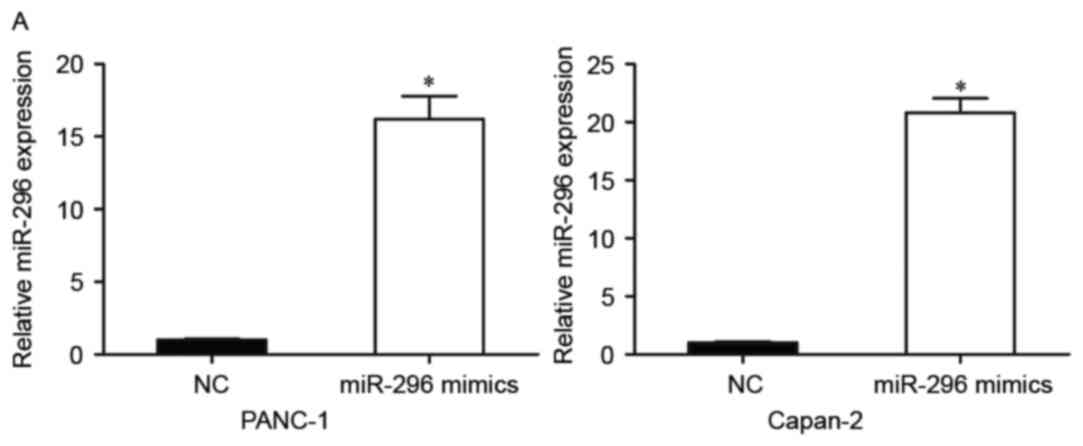
were transfected with miR-296 mimics or NC. A total of 48 h
post-transfection, the efficiency of transfection was confirmed via
RT-qPCR (Fig. 2A). The results
revealed that in PANC-1 and Capan-2 cell lines miR-296 levels were
significantly increased post-transfection (P<0.05). A CCK8 assay
was performed to investigate the effects of miR-296 on pancreatic
cancer cell proliferation (Fig.
2B). The assay revealed that cell growth was significantly
inhibited by miR-296 overexpression in PANC-1 and Capan-2 cells
(P<0.05).
Overexpression of miR-296 inhibits
migration and invasion of PANC-1 and Capan-2 cells
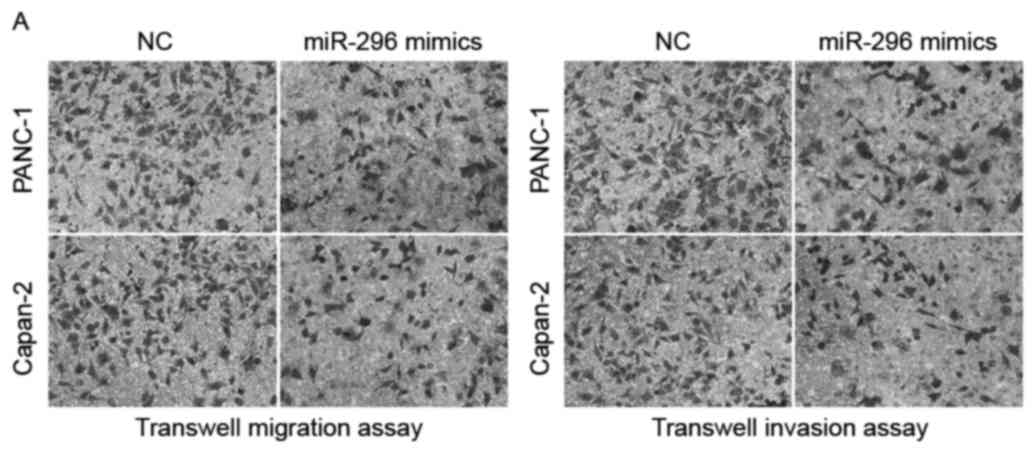
Transwell migration and invasion assays were
performed to explore the effects of miR-296 overexpression on the
migratory and invasive abilities of pancreatic cancer cells, which
are pivotal for malignant metastasis (Fig. 3A and B). PANC-1 and Capan-2 cells
transfected with miR-296 mimics exhibited significantly compromised
migratory and invasive capabilities compared with NC-transfected
cells (P<0.05). These findings suggested that miR-296 expression
is downregulated in pancreatic cancer, and its overexpression may
have tumor-suppressive properties through inhibiting the growth and
metastasis of pancreatic tumors.
miR-296 directly targets AKT2 in
pancreatic cancer
In order to explore the mechanism of action of
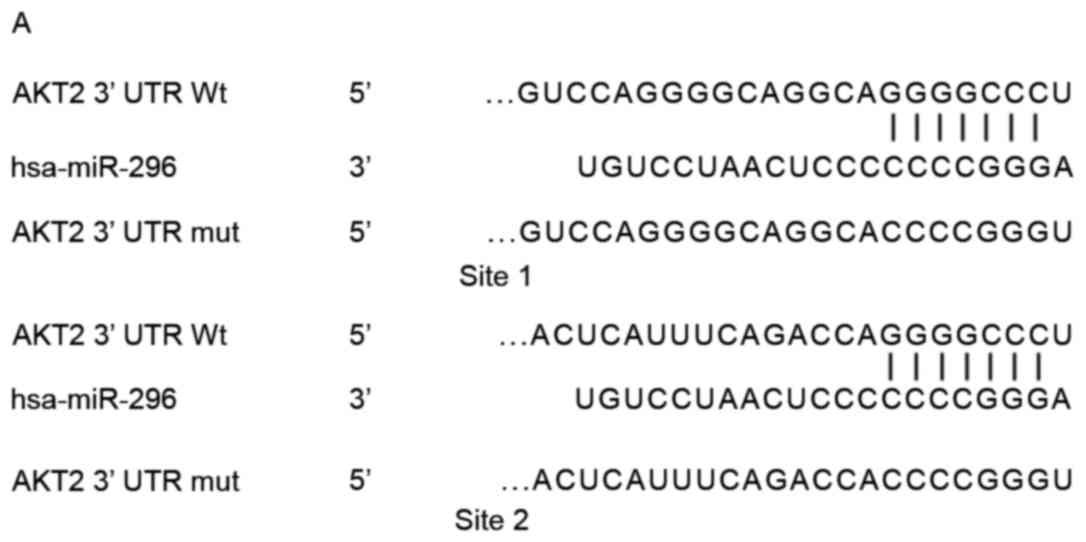
miR-296 in pancreatic cancer, bioinformatics analysis was employed
to identify potential target genes. Results suggested that the gene
coding for AKT2 contains two possible miR-296 binding sites in its
3′UTR (Fig. 4A). A luciferase
reporter assay was performed to investigate whether miR-296
directly targeted AKT2 (Fig. 4B).
The results revealed that miR-296 overexpression resulted in
significantly reduced luciferase activity in the AKT2-3′UTR Wt 1
and AKT2-3′UTR Wt 2 groups (P<0.05), whereas mutation of the
potential binding sites abolished the inhibitory effect of miR-296
overexpression. Furthermore, western blot analysis (Fig. 4C) demonstrated that miR-296
overexpression induced a significant decrease in AKT2 expression in
PANC-1 and Capan-2 cells (P<0.05). However, miR-296
overexpression had no effect on AKT2 mRNA expression in PANC-1 and
Capan-2 cells (Fig. 4D),
indicating that miR-296 inhibits AKT2 expression at the
post-transcriptional level. These results suggested that miR-296
may directly target AKT2 in pancreatic cancer cells.
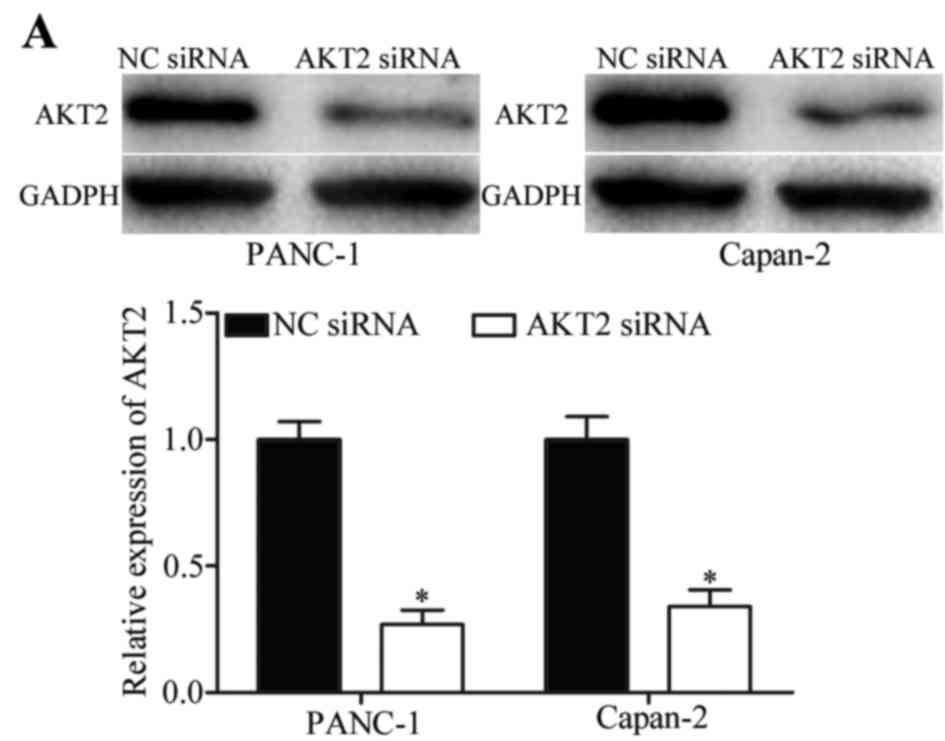
RNA interference was used to silence the expression
of AKT2 in PANC-1 and Capan-2 cells. The transfection efficiency
was assessed by western blot analysis (Fig. 5A), which revealed that in both cell
lines, transfection with AKT2 siRNA significantly decreased AKT2
expression levels (P<0.05). Post-transfection with AKT2 siRNA or
NC siRNA, CCK8 and transwell migration assays were performed to
investigate the effects of AKT2 on pancreatic cancer cell growth,
migration and invasion (Fig.
5B-D). The results indicated that silencing the expression of
AKT2 had similar inhibitory effects to miR-296 overexpression on
the growth, migration and invasion of PANC-1 and Capan-2 cells
(P<0.05). These results suggested that AKT2 may act as a direct
target of miR-296 in pancreatic tumor cells.
Discussion
Elucidating the molecular mechanisms underlying
pancreatic carcinogenesis and progression is crucial for developing
novel therapeutic strategies (23). Pancreatic cancer is an aggressive
cancer type, characterized by an accumulation of genetic mutations
in various genes, including oncogenes and tumor suppressor genes
(24,25). miRNAs can negatively modulate gene
expression via directly targeting specific mRNAs and inducing
suppression of translation or cleavage (14). They are implicated in the
development of human cancers, including pancreatic tumors (26,27).
Therefore, miRNAs hold great potential as a novel therapeutic
strategy for the treatment of pancreatic cancer.
The present study demonstrated that miR-296 is
downregulated in pancreatic cancer tissue and cell lines. In
addition, miR-296 overexpression can significantly inhibit the
proliferation, migration and invasion of pancreatic cancer cells.
Furthermore, AKT2 was revealed to be a direct target gene of
miR-296 in pancreatic cancer. To the best of our knowledge, this is
the first study to investigate the expression, biological role and
mechanism of action of miR-296 in pancreatic cancer.
Recent studies suggested that miR-296 acts as a
tumor suppressor in humans. miR-296 appeared to be downregulated in
non-small-cell lung cancer, whereas miR-296 overexpression reduced
cell viability by directly targeting Polo-like kinase 1 (28). Prostate cancer is characterized by
low intratumoral miR-296 levels, whereas restoration of miR-296
expression was demonstrated to inhibit growth and invasion of
prostate cancer cells through the downregulation of high mobility
group protein HMGA1 (29). In
addition, potentiated miR-296 expression decreased the
proliferation and anchorage-independent growth of prostate cancer
cell lines, through downregulation of Pin1, an isomerase whose
upregulation may be implicated in carcinogenesis (30). In human breast cancer tissue,
miR-296 levels appeared to be reduced, with low miR-296 expression
being associated with reduced disease-free survival. Additionally,
reduced miR-296 expression levels were significantly associated
with an earlier spread of cancer in the overall series and with
distant metastases. Furthermore, miR-296 has been demonstrated to
decrease tumor growth in vivo via blockade of SCRIB, a
protein possibly implicated in cancer progression (31). These findings suggested a
fundamental role for miR-296 in human carcinogenesis and the
progression of malignant tumors, and illustrate its potential as a
therapeutic target for various types of cancer.
Conflicting studies suggested that miR-296 may
function as an oncogene. In gastric cancer, miR-296 is upregulated
in tumor tissue, whereas ectopic miR-296 expression can enhance
cell growth by inhibiting cell cycle arrest and apoptosis, through
suppression of the intestine-specific transcription factor
Caudal-related homeobox 1 (32).
The expression of miR-296 also appeared to be upregulated in
esophageal squamous cell carcinoma, whereas miR-296 downregulation
suppressed the proliferation of esophageal squamous cell carcinoma
cells in vitro and in vivo by inhibiting the cell
cycle regulators cyclin D1 and p27 (33). These conflicting findings may arise
from fundamental differences between various cell types, and
demonstrate the complexity of the mechanisms underlying
tumorigenesis. The present study demonstrated that miR-296 was
downregulated in pancreatic cancer, and overexpression of miR-296
inhibited growth, migration and invasion of pancreatic cancer
cells.
In view of the key role miR-296 appears to serve in
carcinogenesis, the present study further investigated the gene
targets of miR-296 in an attempt to explore the mechanisms
underlying pancreatic carcinogenesis. Bioinformatics analysis was
employed to predict possible target genes for miR-296, and revealed
that AKT2 contains two possible miR-296 binding sites in its 3′UTR.
A luciferase reporter assay confirmed that AKT2 is a direct target
gene of miR-296 in pancreatic cancer cells, whereas RT-qPCR and
western blot analysis revealed that miR-296 negatively regulates
AKT2 expression at the post-transcriptional level. Finally, AKT2
silencing produced similar effects to miR-296 overexpression in
pancreatic tumor cells. These results indicated that miR-296 may
act as a tumor suppressor in pancreatic cancer through directly
targeting and inhibiting AKT2 expression.
AKT is a conserved Ser/Thr kinase that participates
in the phosphoinositide-3 kinase/AKT pathway, which regulates
various cellular processes, such as proliferation, migration,
invasion, metabolism and apoptosis (34,35).
Abnormalities in the AKT signaling pathway have been associated
with several types of human cancer, including breast, prostate,
lung and liver cancer, and glioblastoma (36,37).
AKT comprises three isoforms: AKT1, AKT2 and AKT3. In pancreatic
cancer, AKT2 has been reported to be upregulated at the mRNA and
protein level, whereas its catalytic activity is also potentiated
(38,39). These findings indicated a central
role for AKT2 in pancreatic cancer pathogenesis. The findings of
the present study identified AKT2 as a direct gene target of
miR-296, indicating that the miR-296/AKT2 pathway may be a
promising therapeutic target for the treatment of patients with
pancreatic cancer.
In conclusion, the present study reported that
miR-296 is downregulated in tissue from patients with pancreatic
cancer and pancreatic carcinoma cell lines. These findings
suggested that it may function as a tumor suppressor via inhibiting
the growth, migration and invasion of pancreatic cancer cells. In
addition, AKT2 was validated as a direct target of miR-296 in
pancreatic cancer cells, suggesting a role for miR-296 as a novel
therapeutic target for the treatment of pancreatic cancer.
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo X and Cui Z: Current diagnosis and
treatment of pancreatic cancer in China. Pancreas. 31:13–22. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hao J, Zhang S, Zhou Y, Hu X and Shao C:
MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in
pancreatic cancer. FEBS Lett. 585:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 66:7–30. 2017. View Article : Google Scholar
|
|
5
|
Fuchs CS, Colditz GA, Stampfer MJ,
Giovannucci EL, Hunter DJ, Rimm EB, Willett WC and Speizer FE: A
prospective study of cigarette smoking and the risk of pancreatic
cancer. Arch Intern Med. 156:2255–2260. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gapstur SM, Gann PH, Lowe W, Liu K,
Colangelo L and Dyer A: Abnormal glucose metabolism and pancreatic
cancer mortality. JAMA. 283:2552–2558. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michaud DS, Giovannucci E, Willett WC,
Colditz GA, Stampfer MJ and Fuchs CS: Physical activity, obesity,
height, and the risk of pancreatic cancer. JAMA. 286:921–929. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhayat SA, Abdeen B, Köhler G, Senninger
N, Haier J and Mardin WA: MicroRNA-100 and microRNA-21 as markers
of survival and chemotherapy response in pancreatic ductal
adenocarcinoma UICC stage II. Clin Epigenetics. 7:1322015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Couch FJ, Johnson MR, Rabe KG, Brune K, de
Andrade M, Goggins M, Rothenmund H, Gallinger S, Klein A, Petersen
GM and Hruban RH: The prevalence of BRCA2 mutations in familial
pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 16:342–346.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbruzzese JL: The challenge of pancreatic
cancer. Int J Gastrointest Cancer. 33:1–2. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hezel AF, Kimmelman AC, Stanger BZ,
Bardeesy N and Depinho RA: Genetics and biology of pancreatic
ductal adenocarcinoma. Genes Dev. 20:1218–1249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi C, Daniels JA and Hruban RH: Molecular
characterization of pancreatic neoplasms. Adv Anat Pathol.
15:185–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhardwaj A, Singh S and Singh AP:
MicroRNA-based cancer therapeutics: Big hope from small RNAs. Mol
Cell Pharmacol. 2:213–219. 2010.PubMed/NCBI
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boudreau RL, Jiang P, Gilmore BL, Spengler
RM, Tirabassi R, Nelson JA, Ross CA, Xing Y and Davidson BL:
Transcriptome-wide discovery of microRNA binding sites in human
brain. Neuron. 81:294–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gironella M, Seux M, Xie MJ, Cano C,
Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, et
al: Tumor protein 53-induced nuclear protein 1 expression is
repressed by miR-155 and its restoration inhibits pancreatic tumor
development. Proc Natl Acad Sci USA. 104:16170–16175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naderi E, Mostafaei M, Pourshams A and
Mohamadkhani A: Network of microRNAs-mRNAs interactions in
pancreatic cancer. Biomed Res Int. 2014:5348212014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin S, Zhu Y, Ai F, Li Y, Bai B, Yao W and
Dong L: MicroRNA-191 correlates with poor prognosis of colorectal
carcinoma and plays multiple roles by targeting tissue inhibitor of
metalloprotease 3. Neoplasma. 61:27–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu
J and Gao FH: MicroRNA-191 promotes pancreatic cancer progression
by targeting USP10. Tumour Biol. 35:12157–12163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He D, Miao H, Xu Y, Xiong L, Wang Y, Xiang
H, Zhang H and Zhang Z: MiR-371-5p facilitates pancreatic cancer
cell proliferation and decreases patient survival. PLoS One.
9:e1129302014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feldmann G, Beaty R, Hruban RH and Maitra
A: Molecular genetics of pancreatic intraepithelial neoplasia. J
Hepatobiliary Pancreat Surg. 14:224–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai Z, Sun J, Wang X, Wang H, Pei H and
Zhang Z: MicroRNA-153 is a prognostic marker and inhibits cell
migration and invasion by targeting SNAI1 in human pancreatic
ductal adenocarcinoma. Oncol Rep. 34:595–602. 2015.PubMed/NCBI
|
|
27
|
Subramani R, Gangwani L, Nandy SB,
Arumugam A, Chattopadhyay M and Lakshmanaswamy R: Emerging roles of
microRNAs in pancreatic cancer diagnosis, therapy and prognosis
(Review). Int J Oncol. 47:1203–1210. 2015.PubMed/NCBI
|
|
28
|
Xu C, Li S, Chen T, Hu H, Ding C, Xu Z,
Chen J, Liu Z, Lei Z, Zhang HT, et al: miR-296-5p suppresses cell
viability by directly targeting PLK1 in non-small cell lung cancer.
Oncol Rep. 35:497–503. 2016.PubMed/NCBI
|
|
29
|
Lee KH, Lin FC, Hsu TI, Lin JT, Guo JH,
Tsai CH, Lee YC, Lee YC, Chen CL, Hsiao M and Lu PJ:
MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in
prostate cancer by directly targeting Pin1. Biochim Biophys Acta.
1843:2055–2066. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Savi F, Forno I, Faversani A, Luciani A,
Caldiera S, Gatti S, Foa P, Ricca D, Bulfamante G, Vaira V and
Bosari S: miR-296/Scribble axis is deregulated in human breast
cancer and miR-296 restoration reduces tumour growth in vivo. Clin
Sci (Lond). 127:233–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei JJ, Wu X, Peng Y, Shi G, Basturk O,
Yang X, Daniels G, Osman I, Ouyang J, Hernando E, et al: Regulation
of HMGA1 expression by microRNA-296 affects prostate cancer growth
and invasion. Clin Cancer Res. 17:1297–1305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Lu YY, Zhao XD, Guo HQ, Liu CH, Li
H, Zhou L, Han YN, Wu KC, Nie YZ, et al: MicroRNA-296-5p increases
proliferation in gastric cancer through repression of
Caudal-related homeobox 1. Oncogene. 33:783–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong L, Han Y, Zhang H, Li M, Gong T, Sun
L, Wu K, Zhao Q and Fan D: The prognostic and chemotherapeutic
value of miR-296 in esophageal squamous cell carcinoma. Ann Surg.
251:1056–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agarwal E, Brattain MG and Chowdhury S:
Cell survival and metastasis regulation by Akt signaling in
colorectal cancer. Cell Signal. 25:1711–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang B, Hao C, Wang H, Zhang J, Xing R,
Shao J, Li W, Xu N, Lu Y and Liu S: Evaluation of
hepatic-metastasis risk of colorectal cancer upon the protein
signature of PI3K/AKT pathway. J Proteome Res. 7:3507–3515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buontempo F, Ersahin T, Missiroli S,
Senturk S, Etro D, Ozturk M, Capitani S, Cetin-Atalay R and Neri
ML: Inhibition of Akt signaling in hepatoma cells induces apoptotic
cell death independent of Akt activation status. Invest New Drugs.
29:1303–1313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu N, Lao Y, Zhang Y and Gillespie DA:
Akt: A double-edged sword in cell proliferation and genome
stability. J Oncol. 2012:9517242012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng JQ, Ruggeri B, Klein WM, Sonoda G,
Altomare DA, Watson DK and Testa JR: Amplification of AKT2 in human
pancreatic cells and inhibition of AKT2 expression and
tumorigenicity by antisense RNA. Proc Natl Acad Sci USA.
93:3636–3641. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Altomare DA, Tanno S, De Rienzo A,
Klein-Szanto AJ, Tanno S, Skele KL, Hoffman JP and Testa JR:
Frequent activation of AKT2 kinase in human pancreatic carcinomas.
J Cell Biochem. 87:470–476. 2002. View Article : Google Scholar : PubMed/NCBI
|