Introduction
Spinal cord injury (SCI) is a highly disabling
injury; with expensive treatments and other costs, loss of labor
and serious complications, SCI is a heavy burden for the patient's
family and society (1).
Epidemiological studies on traumatic SCI (TSCI) are regularly
conducted worldwide; however, these studies are less common in
China (2). The male to female
ratio ranges between 2.5:1 and 6:1, and as average life expectancy
increases the incidence increases every year in China (3). The mortality rate in patients with
TSCI is relatively high; in fact, the United States reported a
mortality rate of 25.5–30.0/106 with the cause of mortality most
commonly associated with diseases in the circulatory and
respiratory systems (3). In
addition, TSCI is associated with a number of severe complications,
including infection, bedsores and deep vein thrombosis (3). Owing to these severe complications,
it is important to investigate effective treatment methods to
constantly monitor TSCI progress (4). The underlying mechanisms of
TSCI-associated damage are beginning to be revealed; however,
identifying effective treatments for TSCI has remained challenging
(4). A number of different
drug-based treatments, surgical treatments and physical
rehabilitation methods have had some success; however, they do have
some limitations (4).
Schisandrin B and schizandrin are the main active
ingredients in the fruit of the Chinese magnolia vine
(Schisandra chinensis). Previous in vivo and in
vitro studies have demonstrated that schisandrin B scavenges
free radicals and inhibits lipid peroxidation (5). Schisandrin B has a high level of
activity in the central nervous system, the cardiovascular system
and the respiratory systems, and it has a regulatory role in the
stimulation of the cerebral cortex inhibition process (5,6). The
aim of present study was to evaluate the effects of schisandrin B
on TSCI, and to verify whether schisandrin B markedly inhibits the
expression of pro-inflammatory factors, oxidative stress and
apoptosis in TSCI rats, as well as the potential pathways
associated.
Materials and methods
Animals
Adult male Sprague-Dawley rats (6–10 weeks; weight,
230–300 g; n=40) were obtained from the Animal Center of the
General Hospital of Jinan Military Area Command (Jinan, China), and
were housed at 23–25°C under a 12 h light/dark cycle (relative
humidity 40–60%) and fed a standard laboratory diet and water ad
libitum. The present study was approved by the Scientific
Review Committee and the Institutional Review board of the General
Hospital of Jinan Military Area Command.
Drugs and chemicals
Methylprednisolone sodium succinate (MPSS) was
supplied by Nanfang Hospital of Guangzhou in China. Superoxide
dismutase (SOD; A001-3), malondialdehyde (MDA, A003-1), nuclear
factor (NF)-κB subunit p65 (H202), tumor necrosis factor-α (TNF-α,
R019) and caspase-3/9 ELISA kits (catalog nos. G015 and G018) were
obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
TSCI model rats
The SCI model was established as previously
described (7). Briefly, rats were
anaesthetized by intraperitoneal (i.p.) injection of pentobarbital
(30 mg/kg, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China).
Following anesthesia, the rat skin was shaved carefully, opened and
cleaned using betadine solution. In the thoracic region, a 20 mm
midline incision was made that exposed the vertebral column. At the
tenth thoracic vertebra (T10), alaminectomy was carried out, which
exposed the dorsal cord surface and left the dura intact. SCI was
induced by dropping a 10 g rod from a height of 5.0 cm onto the T10
level of the spinal cord.
Experimental design
Animals in each experiment were randomly divided
into 4groups (n=10): i) The control group, in which the surgical
area was exposed without SCI induction and the rats received
physiological saline (0.1 ml/100 g, i.p.); ii) the SCI group, in
which SCI was induced and the rats received physiological saline
(0.1 ml/100 g, i.p.); iii) the MPSS group, in which SCI was induced
and MPSS (100 mg/kg, i.p.) was administered; and iv) the
schisandrin B group, in which SCI was induced and the rats were
orally administered schisandrin B (50 mg/kg/day) for 5 days.
Behavioral examination
Following schisandrin B treatment for 5 days, the
rats (n=6) were executed to analyze motor behavior analysis, which
was performed using the Basso, Beattie and Bresnahan (BBB)
locomotor scale method. BBB scores range between 0 (no observable
hind-limb movements) and 21 (normal gait).
Inclined plate test
Following schisandrin B treatment for 5 days, rats
(n=6) were examined by the inclined plate test using a 6-mm-thick
rubber pad. Rats were placed with their body axis perpendicular
with orientation to the plate to evaluate the maximum vertical axis
of the inclined plate. The incline angle was gradually increased,
with the rats held on the inclined plate for 5 sec and the maximum
angle was recorded, with the procedure repeated three times.
Spinal cord water content
Following schisandrin B treatment for 5 days, rats
(n=6) were sacrificed and spinal cord samples were collected and
weighed (‘wet weight’) and dried at 120°C for 24–48 h. Dry spinal
cord samples were then weighed (‘dry weight’) and the results were
recorded. The spinal cord water content was assessed by the
following equation: [(wet weight -dry weight)/wet weight] ×100%
(8).
Histopathological study
Spinal cord samples were fixed in 10% neutral
buffered formalin for 72 h. The tissue samples were dehydrated with
gradient ethanol, soaked with xylene + ethanol for transparency and
embedded in paraffin, divided into 5-mm slices and stained using
hematoxylin-eosin sassy. SCI 0–4 scale: 0, none or minor; 1, modest
or limited; 2, intermediate; 3, widespread or prominent and 4,
widespread and prominent.
Measurement of inflammation factors
and oxidative stress
Following schisandrin B treatment for 5 days, rats
(n=6) were sacrificed and peripheral blood was collected. The blood
was centrifuged at 12,000 × g for 10 min at 4°C and the supernatant
was collected and stored at −80°C for further study. The expression
levels of SOD, MDA, NF-κB p65 and TNF-α were measured using the
corresponding ELISA kits (Nanjing Jiancheng Bioengineering
Institute) following the manufacturer's protocol.
Western blot analysis of caspase-3 and
phosphorylated (p)-p53 expression
Following schisandrin B treatment for 5 days, rats
(n=6) were sacrificed and spinal cord samples were collected. The
spinal cord was homogenized with liquid nitrogen and
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The homogenate was centrifuged at
12,000 × g for 10 min at 4°C and analyzed using a bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology). An equal
quantity (50 µg) of protein was separated by 8–10% SDS-PAGE, then
transferred onto nitrocellulose filter membranes. Membranes were
blocked with 5% skim milk powder in TBST for 1 h at 37°C and
detected using the following primary antibodies: Mouse
anti-caspase-3 (catalog no. sc-22139; dilution, 1:300; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); p-p53 expression (catalog
no. sc-12904-R; dilution, 1:1,000; Santa Cruz Biotechnology, Inc.);
or mouse anti-β-actin (catalog no. BB-2116-1;dilution, 1:5,000;
BestBio Inc., Shanghai, China) at 4°C overnight. Proteins of
interest were detected with horseradish peroxidase-conjugated goat
anti-mouse secondary antibody (catalog no. sc-2005; dilution,
1:5,000; Santa Cruz Biotechnology, Inc.) Proteins were observed
using an enhanced chemiluminescence detection ECL kit (BestBio
Inc.) and quantified using imaging software (BioSens Digital
Imaging 5; Shanghai Bio-Tech Inc., Shanghai, China).
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Statistical analysis was performed using two-way
analysis of variance followed by Dunnett's test and the SPSS 17.0
software package (SPSS, Inc., Chicago, IL, USA) was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
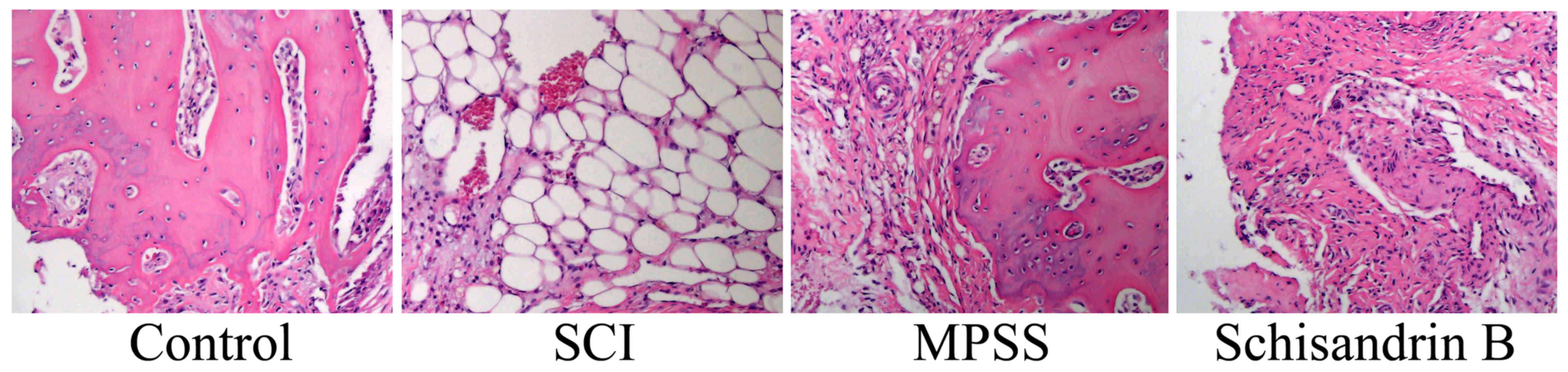
Effect of schisandrin B on histology
following SCI induction
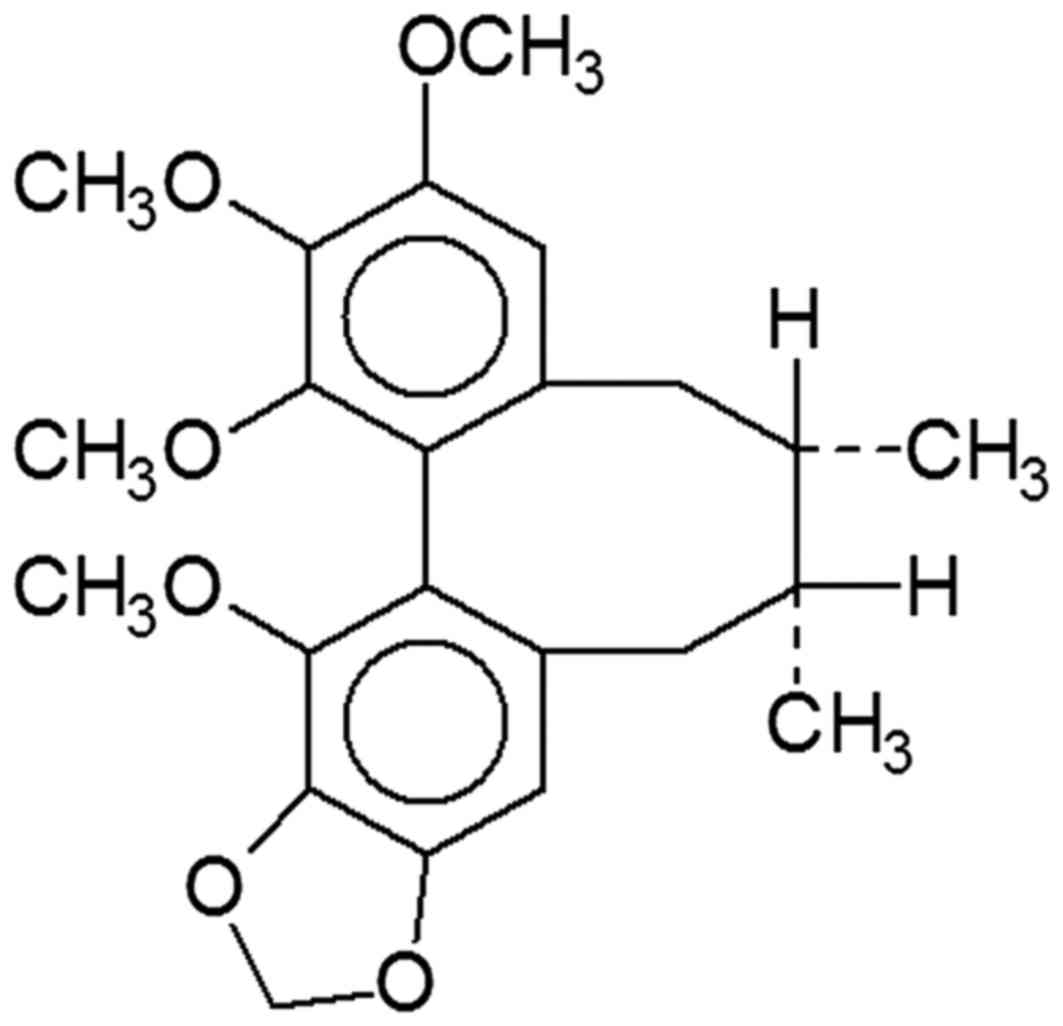
The chemical structure of schisandrin B is depicted
in Fig. 1. The histological SCI
scores were greater compared with control group (Fig. 2). Treatment with MPSS or
schisandrin B inhibited the generation of SCI histology in SCI rats
(Fig. 2).
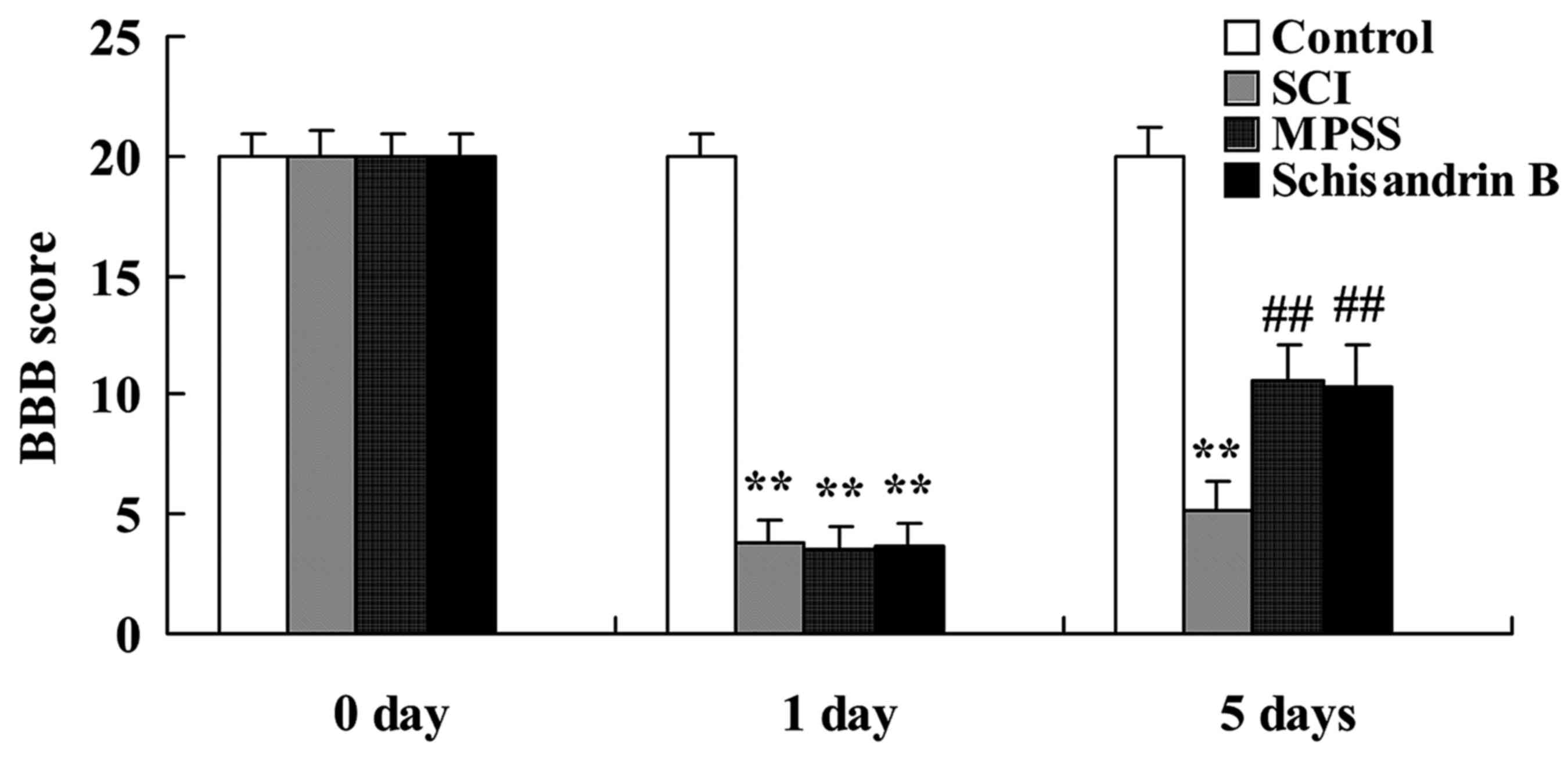
Effect of schisandrin B on behavioral
examination following SCI
Statistical analysis demonstrated a significant
decrease in the BBB score at day 1 in the SCI, MPSS and schisandrin
B groups compared with the control group (Fig. 3). At 5 days post SCI induction, the
BBB score was significantly lower in the SCI group than the control
group, and was significantly higher in the MPSS and schisandrin B
groups compared with the SCI group (Fig. 3). No significant inter-group
difference was identified between the schisandrin B and MPSS groups
for the BBB score in SCI rats (Fig.
3).
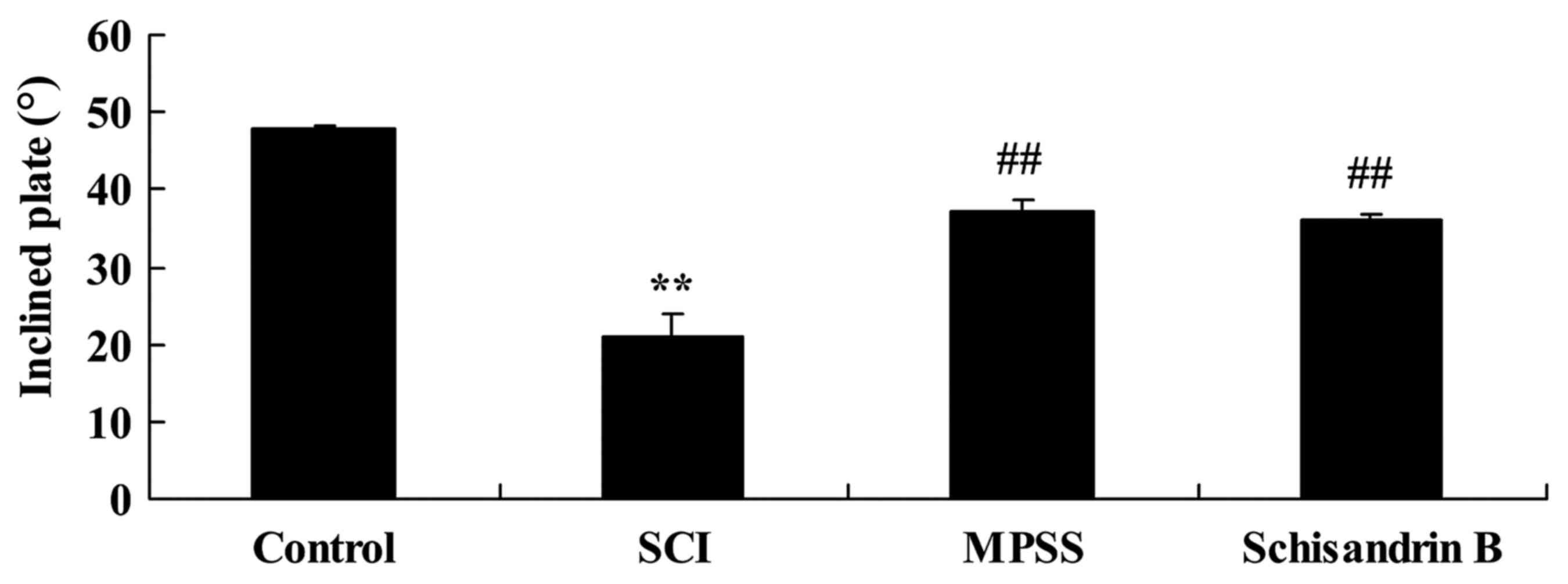
Effect of schisandrin B on the maximum
angle of inclined plate test following SCI
To evaluate the protective effect of schisandrin B
on SCI, the maximum angle of the inclined plate test was observed 5
days post-SCI induction. The maximum incline angle for SCI rats was
significantly lower than that of control group (Fig. 4). Schisandrin B treatment exhibited
a protective effect against SCI, as the maximum angle of the
inclined plate was significantly higher compared with the untreated
SCI group (Fig. 4). The effect of
schisandrin B on SCI was similar to that of MPSS-treated SCI rats,
which also exhibited significantly incline angles compared with the
SCI group (Fig. 4).
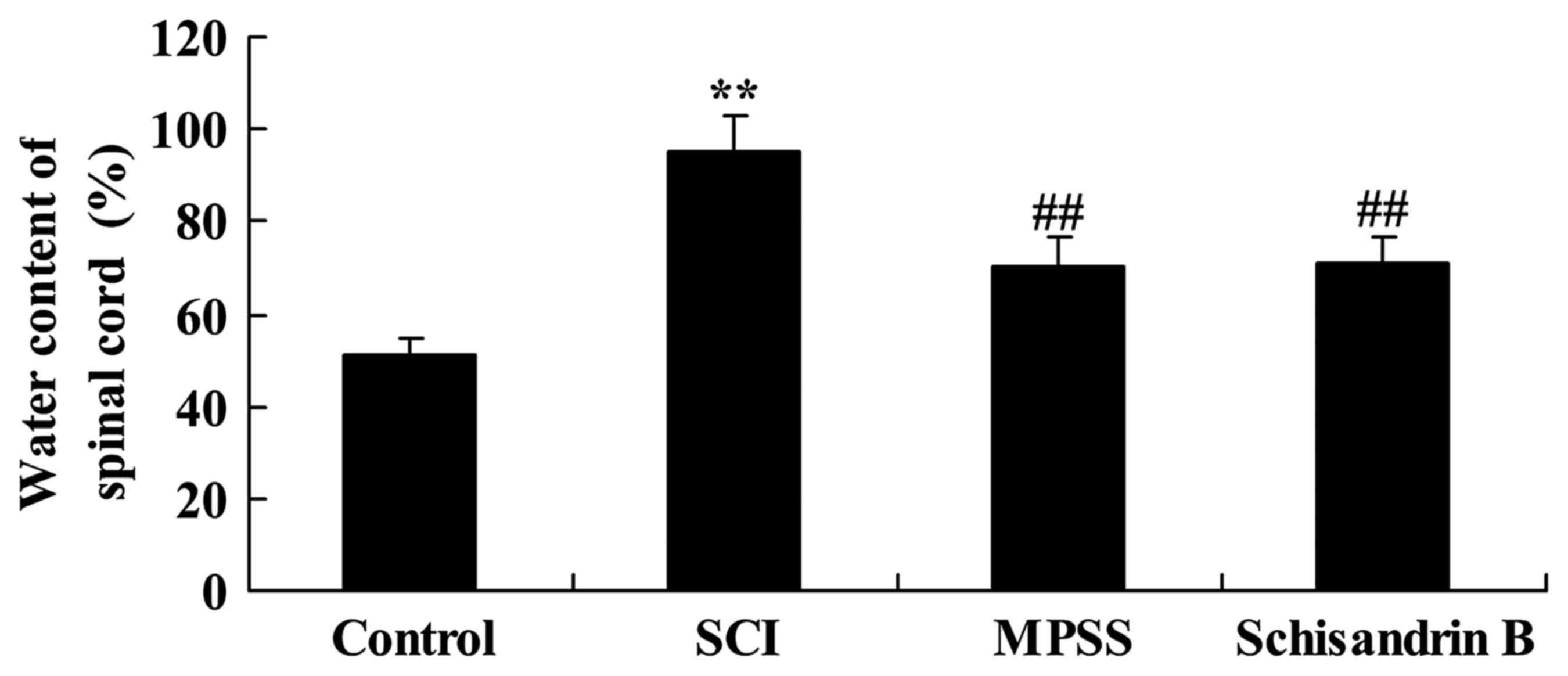
Effect of schisandrin B on spinal cord
water content following SCI
As shown in Fig. 5,
the spinal cord water content of SCI rats was significantly
increased compared with the control group. The results revealed no
significant difference in the post-SCI spinal cord water content
between the schisandrin B- and MPSS-treated groups; however,
schisandrin B and MPSS significantly lowered spinal cord water
content following SCI compared with the untreated SCI group
(Fig. 5).
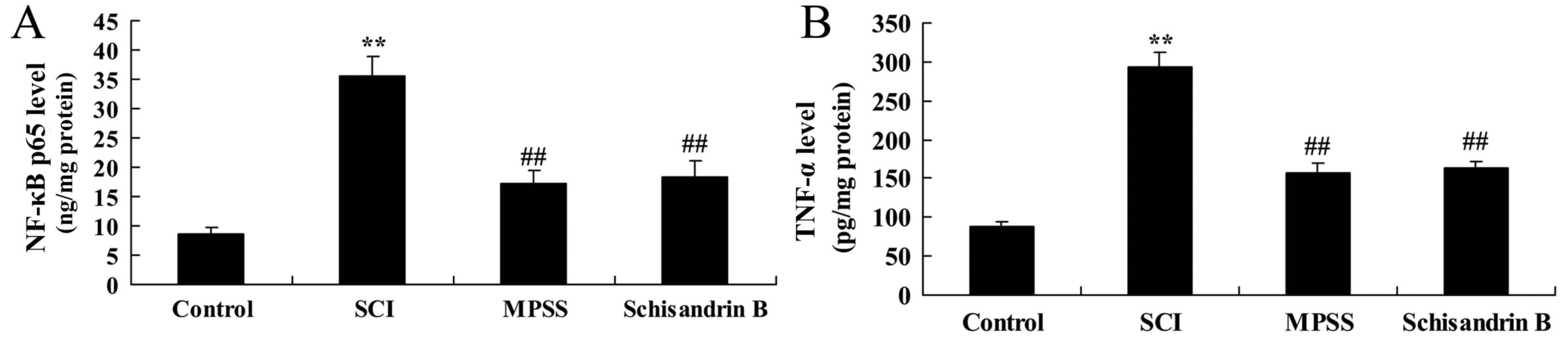
Effect of schisandrin B on NF-κB p65
and TNF-α expression following SCI
To evaluate the underlying mechanisms of schisandrin
B in SCI, the levels of NF-κB p65 and TNF-α expression were
measured 5 days following SCI induction. SCI induced significantly
higher NF-κB p65 and TNF-α expression compared with the control
group (Fig. 6). By contrast,
treatment with schisandrin B or MPSS significantly inhibited the
SCI-induced expression of NF-κB p65 and TNF-α in SCI rats (Fig. 6); no significant difference was
observed between the MPSS-treated and schisandrin B-treated groups
(Fig. 6).
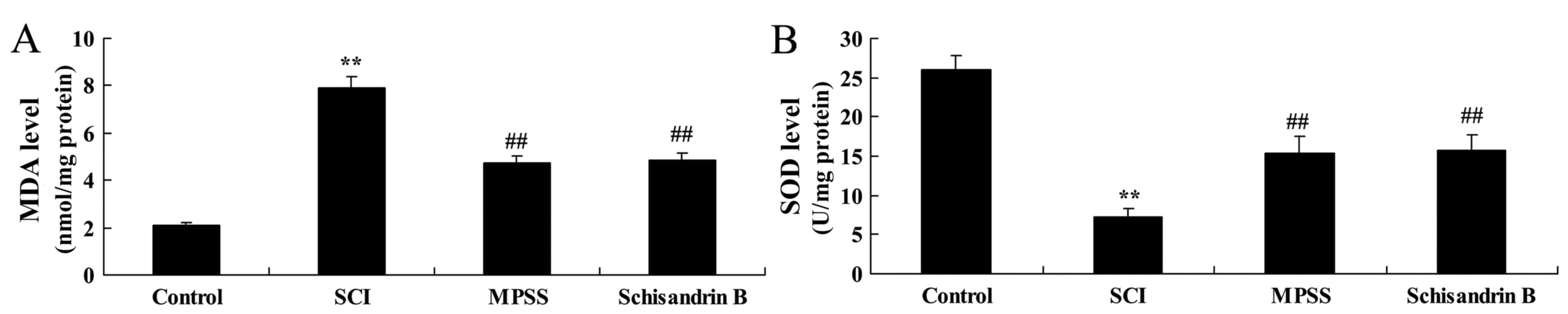
Effect of schisandrin B on oxidative
stress following SCI
MDA and SOD expression levels in spinal cord tissue
were also measured to fully investigate the mechanisms involved in
the effect of schisandrin B in SCI. As shown in Fig. 7, MDA expression was significantly
increased and SOD expression was significantly decreased in the SCI
group compared with the control group. However, treatment with
schisandrin B significantly lowered MDA expression levels and
significantly increased SOD expression compared with the untreated
SCI group (Fig. 7). The results
demonstrated that there were no significant differences in MDA and
SOD activity between the schisandrin B-and MPSS-treated groups at 5
days following the SCI surgery.
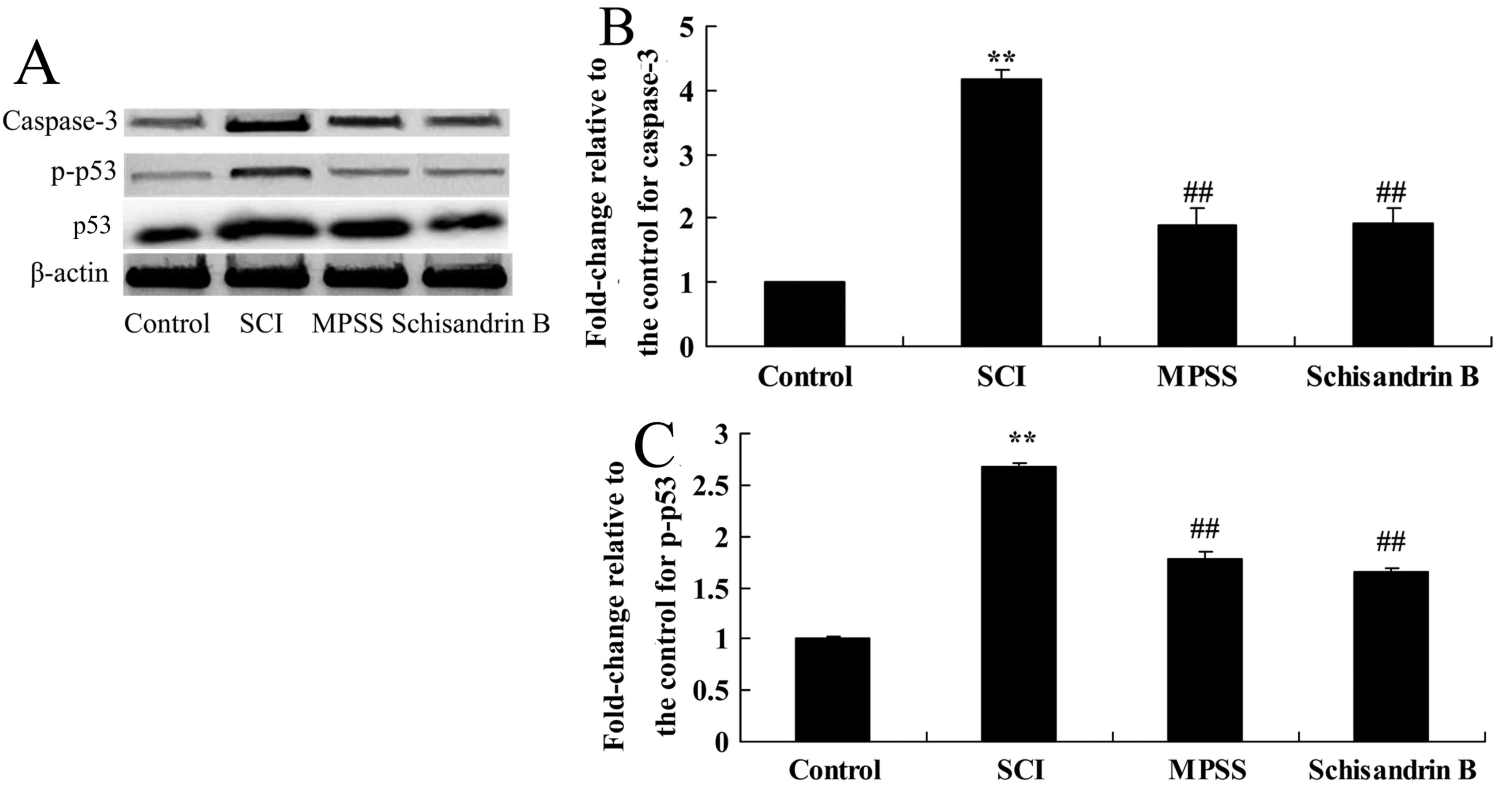
Effect of schisandrin B on apoptosis
following SCI
The effect of schisandrin B on apoptosis was
investigated by measuringcaspase-3 and p-p53 protein expression.
There was a significant increase in caspase-3 and p-p53 protein
expression following SCI compared with the control group (Fig. 8). Schisandrin B treatment
significantly alleviated this increase in caspase-3 and p-p53
protein expressions in SCI rats, compared with untreated SCI group
(Fig. 8). In addition, no
significant difference was identified in caspase-3 and p-p53
protein expression between the schisandrin B-and the MPSS-treated
groups (Fig. 8).
Discussion
TSCI is characterized by spinal cord damage caused
by a number of different factors, such as trauma and infection,
which can lead to symptoms of paralysis and serious debilitating
diseases. Rapid developments in transportation, engineering
construction and the sports entertainment industry have led to
arise in the incidence of TSCI (3). It has been reported that >3
million people are affected by TSCI worldwide. The pathological
process of acute secondary spinal cord damage is complex owing to
the release of a number of factors, including excitatory amino
acids, oxidizing metabolites and inflammatory cells, which lead to
irreversible damage as well as movement, sensory and autonomic
nerve dysfunction (4,9). In the present study, schisandrin B
treatment improved the BBB score and the maximum angle of the
inclined plate test, and reduced the spinal cord water content in
TSCI rats. These results indicate that schisandrin B possesses
beneficial properties against TSCI.
TSCI is one of the leading causes of disability, and
its pathological process includes primary and secondary injuries.
Secondary injury initially stimulates a large number of
inflammatory cells, which produce a strong inflammatory reaction
and eventually glial cell death (10). Inflammation factors is a major
neurotransmitter regulating immune response, it is the Bridges of
our immune cells and other cells (10). NF-κB p65 and TNF-α are secreted by
activated microglia and macrophages, neurons and glial cells during
the early stages of TSCI (11). As
neurotransmitters for intercellular signal transduction, NF-κB p65
and TNF-α regulate the central nervous system, inflammation and
autoimmune reactions (12). TNF-α
promotes polymorphonuclear leukocyte and macrophage infiltration
from the blood to the area of inflammation, and also promotes the
activation of astrocytes, resulting in the formation of a glial
scar that can induce a permanent loss of neurological function
(13). The present study
demonstrated that schisandrin B effectively inhibited the
SCI-induced activation of NF-κB p65 and TNF-α in TSCI rats.
Giridharan et al demonstrated that schisandrin B attenuates
cisplatin-induced oxidative stress and inflammation in mice
(14), and Thandavarayan et
al revealed that schisandrin B may be preventive against
doxorubicin-induced cardiac dysfunction through the inhibition of
oxidative stress and inflammation (6).
Oxidative stress is a basic protective mechanism to
regulate normal activities in the body, including cell signal
transduction, proliferation and apoptosis (15). Oxidative stress is characterized by
the increased production of reactive oxygen species (ROS) and
reactive nitrogen species (RNS). ROS and RNS can be generated in a
number of ways, including chemical and drug metabolism, cell
respiration (12). However, an
excessive level of free radicals inhibits the body's ability to
remove oxides, which can lead to a high level of oxidative damage
(16). The spinal cord contains a
large number of polyunsaturated fatty acids, has limited neuronal
regeneration ability and an active oxidative metabolism; however,
it has a low antioxidant capacity, therefore reactive oxygen
metabolites accumulate and antioxidants are excessively reduced in
this tissue (16). These
characteristics make neurons and glial cells particularly
susceptible to the influence of harmful stimulation and oxidative
stress (17). The present study
revealed that schisandrin B treatment effectively inhibited the
SCI-induced expression of MDA in TSCI rats. Schisandrin B treatment
also improved the SCI-induced reduction in SOC expression in TSCI
rats. Chen et al reported that schisandrin B protects
against cerebral ischemia/reperfusion injury through the
antioxidant status in rats (5).
Apoptosis is different from necrocytosis in that it
is a process of programmed cell death that is regulated by a
variety of signaling pathways and results in characteristic
morphological changes and DNA fragmentation (18). Caspases serve a key role in
apoptotic events, adjust cell death, and the appearance and
function of Caspase apoptotic features are closely associated
(19). They cut off the target
cells from the surrounding cells, reorganize the cytoskeleton,
lower the level of DNA replication and cell repair abilities,
damage the DNA and nuclear structure, attract phagocytes and
disintegration of the cell formation apoptotic body (20). Previous studies have confirmed that
promotion of caspase activity in TSCI model rats increases neural
cell apoptosis (19,20). The present study demonstrated that
schisandrin B treatment significantly reduced the SCI-induced
increase in caspase-3 protein expression in SCI rats. Wang et
al revealed that schisandrin B protects against amyloid β
(1–42)-induced neurotoxicity in cortical neurons by decreasing
caspase-9 and caspase-3 activities (21). Chiu et al demonstrated that
schisandrin B protects against hypoxia/reoxygenation-induced
apoptosis through the suppression of caspase-3 protein expression
in mouse AML12 hepatocytes (22).
p53 is important in TSCI and regulates spinal cord
apoptosis to accelerate the death of oligodendrocytes, microglia
and astrocytes (23). p53 may
additionally have a role in the inhibition of DNA repair (24). Future research may investigate how
to assess the development of this regulation, the damage to DNA
repair and how to improve the survival rate of neurons and glial
cells (24,25). In the present study, schisandrin B
significantly reduced the activation of p-p53 protein expression in
SCI rats.
The present study demonstrated that schisandrin B
attenuated the inflammatory response, oxidative stress and
apoptosis in TSCI model rats by inhibiting the p53 signaling
pathway. Further clinical studies are required to determine whether
schisandrin B would be effective in anti-inflammatory,
antioxidative and anti-apoptotic therapies for TSCI.
Acknowledgements
The present study was partly supported by The Youth
Innovation Fund of Inner Mongolia Medical University (grant no.
YKD2014QNCX021).
References
|
1
|
Cristante AF, Filho Barros TE, Marcon RM,
Letaif OB and Rocha ID: Therapeutic approaches for spinal cord
injury. Clinics (Sao Paulo). 67:1219–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paula AA, Nicolau RA, Mde Lima O, Salgado
MA and Cogo JC: Low-intensity laser therapy effect on the recovery
of traumatic spinal cord injury. Lasers Med Sci. 29:1849–1859.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chun S and Lee Y: ‘I am just thankful’:
The experience of gratitude following traumatic spinal cord injury.
Disabil Rehabil. 35:11–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahimi-Movaghar V, Niakan A, Haghnegahdar
A, Shahlaee A, Saadat S and Barzideh E: Early versus late surgical
decompression for traumatic thoracic/thoracolumbar (T1-L1) spinal
cord injured patients. Primary results of a randomized controlled
trial at one year follow-up. Neurosciences (Riyadh). 19:183–191.
2014.PubMed/NCBI
|
|
5
|
Chen N, Chiu PY and Ko KM: Schisandrin B
enhances cerebral mitochondrial antioxidant status and structural
integrity, and protects against cerebral ischemia/reperfusion
injury in rats. Biol Pharm Bull. 31:1387–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thandavarayan RA, Giridharan VV, Arumugam
S, Arumugam S, Suzuki K, Ko KM, Krishnamurthy P, Watanabe K and
Konishi T: Schisandrin B prevents doxorubicin induced cardiac
dysfunction by modulation of DNA damage, oxidative stress and
inflammation through inhibition of MAPK/p53 signaling. PLoS One.
10:e01192142015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gruner JA: A monitored contusion model of
spinal cord injury in the rat. J Neurotrauma. 9:123–128. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Li Y, He Z, Zhang B, Li Y, Hu J,
Han M, Xu Y, Li Y, Gu J, et al: Targeting endothelin receptors A
and B attenuates the inflammatory response and improves locomotor
function following spinal cord injury in mice. Int J Mol Med.
34:74–82. 2014.PubMed/NCBI
|
|
9
|
van Middendorp JJ, Barbagallo G, Schuetz M
and Hosman AJ: Design and rationale of a prospective, observational
European Multicenter Study on the efficacy of acute surgical
decompression after traumatic spinal cord injury: The SCI-POEM
study. Spinal Cord. 50:686–694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang D, Yue Y, Jiang S, Li A, Guo A, Wu
X, Xia X, Cheng H, Zhang J, Tao T and Gu X: GART expression in rat
spinal cord after injury and its role in inflammation. Brain Res.
1564:41–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang
J, Liang W and Ma Z: Curcumin modulates TLR4/NF-κB inflammatory
signaling pathway following traumatic spinal cord injury in rats. J
Spinal Cord Med. 38:199–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan J, Zhang F, Liang F, Wang Y, Li Z,
Yang J and Liu X: Protective effects of hyperbaric oxygen treatment
against spinal cord injury in rats via toll-like receptor 2/nuclear
factor-κB signaling. Int J Clin Exp Pathol. 7:1911–1919.
2014.PubMed/NCBI
|
|
13
|
Genovese T, Mazzon E, Crisafulli C, Di
Paola R, Muià C, Bramanti P and Cuzzocrea S: Immunomodulatory
effects of etanercept in an experimental model of spinal cord
injury. J Pharmacol Exp Ther. 316:1006–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giridharan VV, Thandavarayan RA, Bhilwade
HN, Ko KM, Watanabe K and Konishi T: Schisandrin B, attenuates
cisplatin-induced oxidative stress, genotoxicity and neurotoxicity
through modulating NF-κB pathway in mice. Free Radic Res. 46:50–60.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ordonez FJ, Rosety MA, Camacho A, Rosety
I, Diaz AJ, Fornieles G, Bernardi M and Rosety-Rodriguez M:
Arm-cranking exercise reduced oxidative damage in adults with
chronic spinal cord injury. Arch Phys Med Rehabil. 94:2336–2341.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurtoglu T, Basoglu H, Ozkisacik EA, Cetin
NK, Tataroglu C, Yenisey C and Discigil B: Effects of cilostazol on
oxidative stress, systemic cytokine release, and spinal cord injury
in a rat model of transient aortic occlusion. Ann Vasc Surg.
28:479–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Juang CL, Yang FS, Hsieh MS, Tseng HY,
Chen SC and Wen HC: Investigation of anti-oxidative stress in vitro
and water apparent diffusion coefficient in MRI on rat after spinal
cord injury in vivo with Tithonia diversifolia ethanolic extracts
treatment. BMC Complement Altern Med. 14:4472014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Wu F, Kong X, Yang J, Chen H,
Deng L, Cheng Y, Ye L, Zhu S, Zhang X, et al: Nerve growth factor
improves functional recovery by inhibiting endoplasmic reticulum
stress-induced neuronal apoptosis in rats with spinal cord injury.
J Transl Med. 12:1302014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ersahin M, Çevik Ö, Akakın D, Şener A,
Özbay L, Yegen BC and Şener G: Montelukast inhibits caspase-3
activity and ameliorates oxidative damage in the spinal cord and
urinary bladder of rats with spinal cord injury. Prostaglandins
Other Lipid Mediat. 99:131–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Springer JE, Azbill RD and Knapp PE:
Activation of the caspase-3 apoptotic cascade in traumatic spinal
cord injury. Nat Med. 5:943–946. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B and Wang XM: Schisandrin B protects
rat cortical neurons against Abeta1-42-induced neurotoxicity.
Pharmazie. 64:450–454. 2009.PubMed/NCBI
|
|
22
|
Chiu PY, Luk KF, Leung HY, Ng KM and Ko
KM: Schisandrin B stereoisomers protect against
hypoxia/reoxygenation-induced apoptosis and associated changes in
the Ca(2+)-induced mitochondrial permeability transition and
mitochondrial membrane potential in AML12 hepatocytes. Phytother
Res. 23:1592–1602. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Floriddia EM, Rathore KI, Tedeschi A,
Quadrato G, Wuttke A, Lueckmann JM, Kigerl KA, Popovich PG and Di
Giovanni S: p53 Regulates the neuronal intrinsic and extrinsic
responses affecting the recovery of motor function following spinal
cord injury. J Neurosci. 32:13956–13970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chopp M, Chan PH, Hsu CY, Cheung ME and
Jacobs TP: DNA damage and repair in central nervous system injury:
National Institute of Neurological Disorders and Stroke Workshop
Summary. Stroke. 27:363–369. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai Y, Li J, Yang S, Li P, Zhang X and Liu
H: CIBZ, a novel BTB domain-containing protein, is involved in
mouse spinal cord injury via mitochondrial pathway independent of
p53 gene. PLoS One. 7:e331562012. View Article : Google Scholar : PubMed/NCBI
|