Introduction
Carcinoembryonic antigen (CEA) is a glycoprotein,
which was first identified from human colon adenocarcinoma and the
fetal digestive system (1,2). CEA is overexpressed on the cell
surface of a variety of carcinomas (3). CEA overexpression is observed in
patients with a variety of carcinomas, including in the colon,
thyroid, lung, uterus, pancreas and ovary, and the serum levels of
CEA are increased in certain cancer types. CEA can be used as a
cancer marker in clinical testing; it can also be used as a
prognostic marker for cancer after radiotherapy and chemotherapy
(4,5), a predictive factor for cancer
treatment (6), and as a
therapeutic target (7,8). Radiolabeled monoclonal antibodies
(mAbs) have been developed for the treatment and diagnosis of
CEA-positive cancers (9).
Nevertheless, the pharmacokinetics of intact mAbs that exhibit slow
blood elimination and high liver uptake of are not ideal for their
use as cancer-targeted probes (10,11).
Smaller antibody fragments, such as antigen-binding fragment (Fab)
and single chain fragment variable, particularly nanobodies, have
better pharmacokinetics for use as cancer-targeted probes due to
their rapid metabolism and high uptake by tumors. The nanobodies
with a prolate shape of ~4.2 nm in length and 2.5 nm in diameter
(12) are the smallest intact
antigen-binding fragments (15 kDa) available, which were first
isolated from heavy-chain camelid antibodies, and have efficient
and specific cancer targeting ability (11,13).
Nanobodies have several advantages, including high stability, ease
of manufacturing with high yield, fast elimination and high
affinity to the target site; thus, they may be suitable as cancer
imaging probes and therapeutic vectors.
The incidence of cancer has sustained an increase in
the last decades, and lung cancer occupies a large proportion of
cancer case. It was estimated that lung and bronchus cancer
accounted for 13.3% of all new cancer cases in 2016, and the
percent survival in 2006–2012 was 17.7% in America (14). The major type of lung cancer is
non-small cell lung cancer (NSCLC), which accounts for up to 85% of
all types of lung cancer. Serum CEA level is useful as a prognostic
and predictive marker for overall survival, and risk of recurrence
and death in lung cancer, particularly in NSCLC, regardless of the
treatment received (15,16). Combined with other indicators for
lung cancer, CEA can be a diagnostic marker (17,18)
and useful target for NSCLC therapy (19).
The present study investigated the targeting of a
nanobody (code: 15.2m) to CEA, with fluorescein isothiocyanate
(FITC)-labeled immunofluorescent staining, radiotechnetium-labeled
cell binding and biodistribution assays on the H460 CEA-positive
human large cell lung cancer cell line. The results indicated that
the nanobody targets CEA-positive cells; therefore, it may serve as
a promising targeted probe for NSCLC.
Materials and methods
Chemicals
The nanobody (code, 15.2m; molecular weight, 15 kDa)
was obtained from a phage display library as described previously
(20,21). Fresh technetium-99 m
(99mTc)-pertechnetate eluant was purchased from the
China Institute of Atomic Energy (Beijing, China). Sodium
borohydride, HCl, sodium potassium tartrate tetrahydrate, sodium
carbonate, and fluorescent dyes (FITC and DAPI) were purchased from
Sigma-Aldrich (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Silica gel plates were obtained from Yantai Jiangyou Silica Gel
Development Co., Ltd. (Yantai, China). Carbon monoxide and carbon
dioxide were purchased from Tianjin KUNTENG Gas Marketing Co. Ltd.
(Tianjin, China). Ultracentrifugal filter units with a molecular
weight cut-off of 3,000 Da were purchased from EMD Millipore
(Billerica, MA, USA) and used as per the manufacturer's manual.
RPMI-1640 medium, penicillin, fetal bovine serum (FBS), and
streptomycin were purchased from Hyclone (GE Healthcare Life
Sciences, Logan, UT, USA). Human serum was obtained from the
Tianjin Blood Center (Tianjin, China). All procedures using human
sera were approved by and conducted in accordance with the
regulations of the Ethics Committee of the Institute of Radiation
Medicine, Chinese Academy of Medical Sciences (Tianjin, China).
Cell culture and animals
H460 cells were obtained from the Cell Resource
Center of the Institute of Basic Medical Sciences of the Chinese
Academy of Medical Sciences/Peking Union Medical College (Beijing,
China) and cultured in RPMI-1640 medium supplemented with 10% FBS,
100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C and 5%
CO2. Female BALB/c nude mice (age, 4–5 weeks; weight,
18–20 g) and female Wistar rats (age, 6–8 weeks; weight, 200–250 g)
were purchased from the Experimental Animal Center of Academy of
Military Medical Sciences (Beijing, China). All animals were kept
under controlled temperature (20–22°C) and 12 h light/dark cycles
with ad libitum access to food and water. All animal
procedures were approved by and conducted in accordance with the
regulations of the Ethics Committee of the Institute of Radiation
Medicine, Chinese Academy of Medical Sciences (Tianjin, China).
Immunofluorescent staining assay
The nanobody (1 mg) was diluted three times against
PBS; subsequently, 600 µl FITC (1 mg/ml in DMSO) was added, and the
mixture was gently stirred for 24 h at 4°C. The reaction mixture
was purified by ultrafiltration, and washed twice to remove the
unreacted dyes. The FITC-nanobody was obtained, and the absorbance
at 280 and 495 nm was measured to determine the concentration of
the nanobody and the ratio of FITC to the nanobody. Following
washing twice with PBS, the H460 cells seeded in 24-well plates at
a density of 4×104 cells/well were fixed with ethanol
for 10 min at room temperature; then 0.5 ml serum-free medium and
100 µl FITC-nanobody solution (concentration was adjusted according
to the absorbance) were added sequentially. Following incubation
for 80 min at 4°C, the fluorescent images were acquired using a
fluorescence microscope (DMI6000B; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA), and the nuclei were stained using
DAPI.
Radioactive technetium labeling
The nanobody was labeled with 99mTc at
its His6 tail, as described previously (22,23).
Briefly, 1 ml of the fresh 99mTc-pertechnetate eluant
(10 mCi) was added to a mixture of 4 mg sodium carbonate, 22 mg
sodium borohydride and 15 mg sodium potassium tartrate
tetrahydrate. The mixture was reacted in a boiling water bath for
20 min under atmospheric carbon monoxide to obtained the
[99mTc
(H2O)3(CO)3]+. After
adjusting to a neutral pH using 1 mol/l HCl, the
[99mTc(H2O)3(CO)3]+
was added to a nanobody solution (1 mg/ml) and incubated for 90 min
at 50°C.
Purification and radiochemical purity
test
The 99mTc-nanobody solution was purified
by ultrafiltration and washed twice using PBS to remove the
dissociative
[99mTc(H2O)3(CO)3]+.
Then the 99mTc-nanobody solution was passed through a
0.22-µm Millipore filter (EMD Millipore) to eliminate possible
aggregates. Thin layer chromatography (TLC) was then performed to
determine the labeling efficiency and radiochemical purity of the
99mTc-nanobody, directly after labeling and after
purification. The analytes were spotted on silica gel plates, which
were subsequently developed in acetone and detected using an
AR-2000 radio-TLC Imaging Scanner equipped with a 10% methane:argon
gas supply and the running analysis software Winscan version v.3
(Bioscan Inc., Washington, DC, USA). The analysis of crude mixtures
was used to calculate the yield, and the analysis of the purified
product was used to calculate the radiochemical purity.
In vitro stability
Two portions of 100 µl 99mTc-nanobody
were added to 500 µl normal saline at room temperature and 500 µl
human serum at 37°C, respectively. Radiochemical purities were
determined using thin layer chromatography as mentioned above at 1,
2, 6 and 24 h.
In vitro evaluation of the
99mTc-nanobody
H460 cells were seeded in 24-well plates, at a
density of 4×104 cells/well. Following overnight
incubation, the cells were washed twice using cold PBS then the
99mTc -nanobody was added at concentrations of 0.02 to
80 nM. The plates were incubated on ice for 1 h then washed using
cold PBS two times. Portions of 1 ml sodium hydroxide (1 mol/l)
were added and the plates were incubated at room temperature for 1
h. The lysates were collected and the radioactivity was measured by
a γ counter (2470 WIZARD2; PerkinElmer, Inc., Waltham, MA, USA).
The blocking experiment was performed by adding 50 µg cold nanobody
to the wells and incubating each for 30 min before adding the
99mTc-nanobody.
Blood clearance of the
99mTc-nanobody
Wistar rats (n=3) were injected with 10 µCi
99mTc-nanobody via the cauda vein. Blood samples were
collected using microcapillaries at 1, 5, 10, 20, 40, 60, 90, and
120 min after the injection, and the radioactivity was measured
using a γ counter to obtain a radioactivity-time curve. Data are
presented as the percentage injected activity per total blood
weight (% ID/g). Total blood weight was calculated as 7% of the
total body weight.
Biodistribution
The distribution of the 99mTc-nanobody
was investigated using nude mice bearing subcutaneously-implanted
human xenografts of H460 cells. The H460 cells (1×107)
in 200 µl PBS were subcutaneously injected into the left armpit of
female BALB/c nude mice (n=4) to establish the xenograft tumors.
After the tumor volume reached approximately 200 mm3,
the mice were injected with 100 µCi 99mTc-nanobody via
the tail vein. At 1, 2, and 6 h post-injection, four mice were
anesthetized, exsanguinated and dissected. Blood, tumor and normal
tissues were weighed, and radioactivity was measured using a γ
counter. Radioactivity uptake was calculated as % ID/g.
Statistical analysis
The statistical significance of the differences
between groups was assessed using two-tailed Student's t-test. Data
are expressed as the mean ± standard deviation of three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
the GraphPad Prism software version 5 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
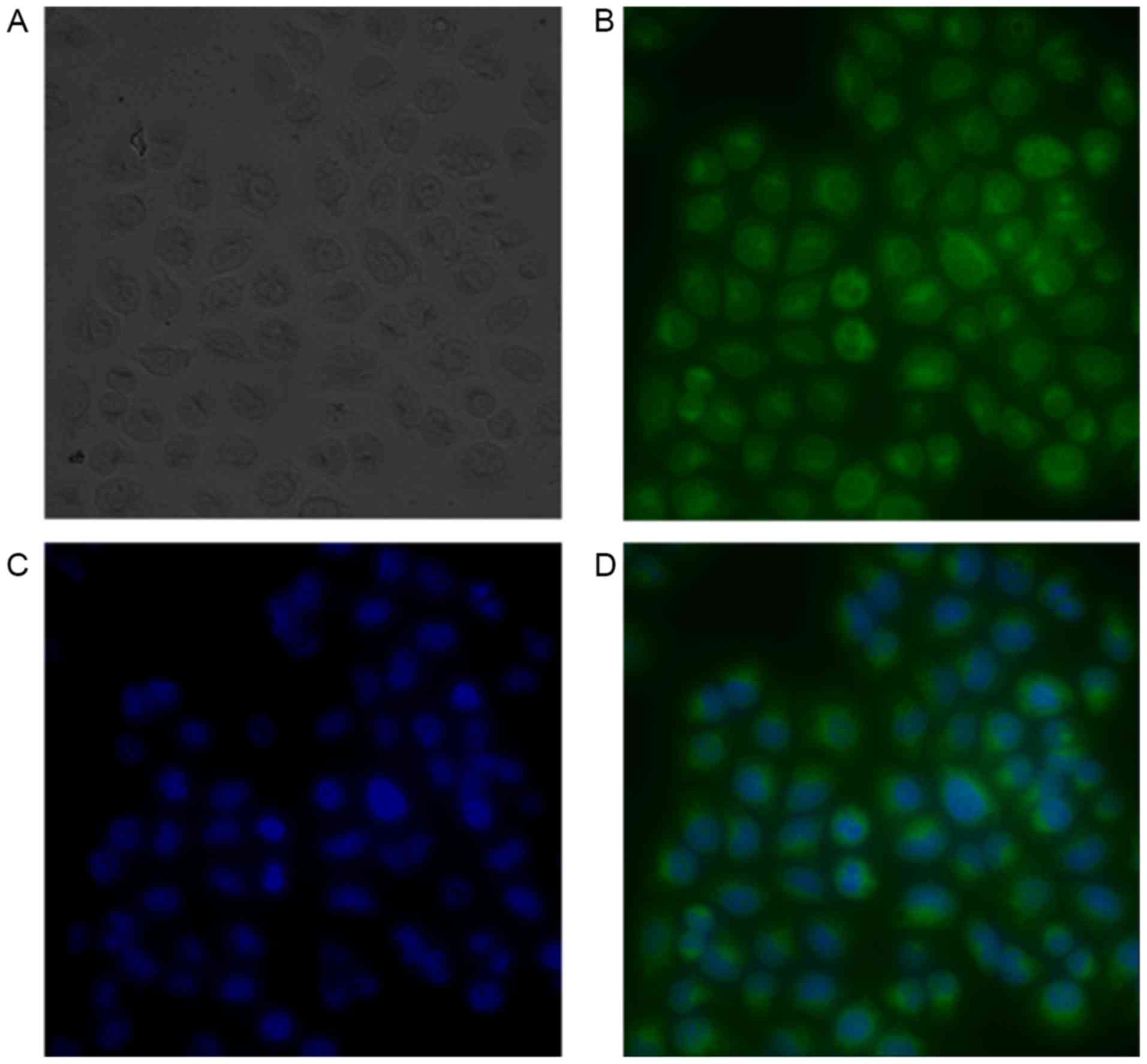
Immunofluorescent staining
The concentration of nanobody and the ratio of FITC
to nanobody were determined by [(A280-0.31xA495)/1.4] and
[3.1xA495/(A280-0.31xA495)] respectively and the results were 0.254
mg/ml and 10.1; the FITC-nanobody was diluted twice using PBS for
staining test. The fluorescent images are presented in Fig. 1.
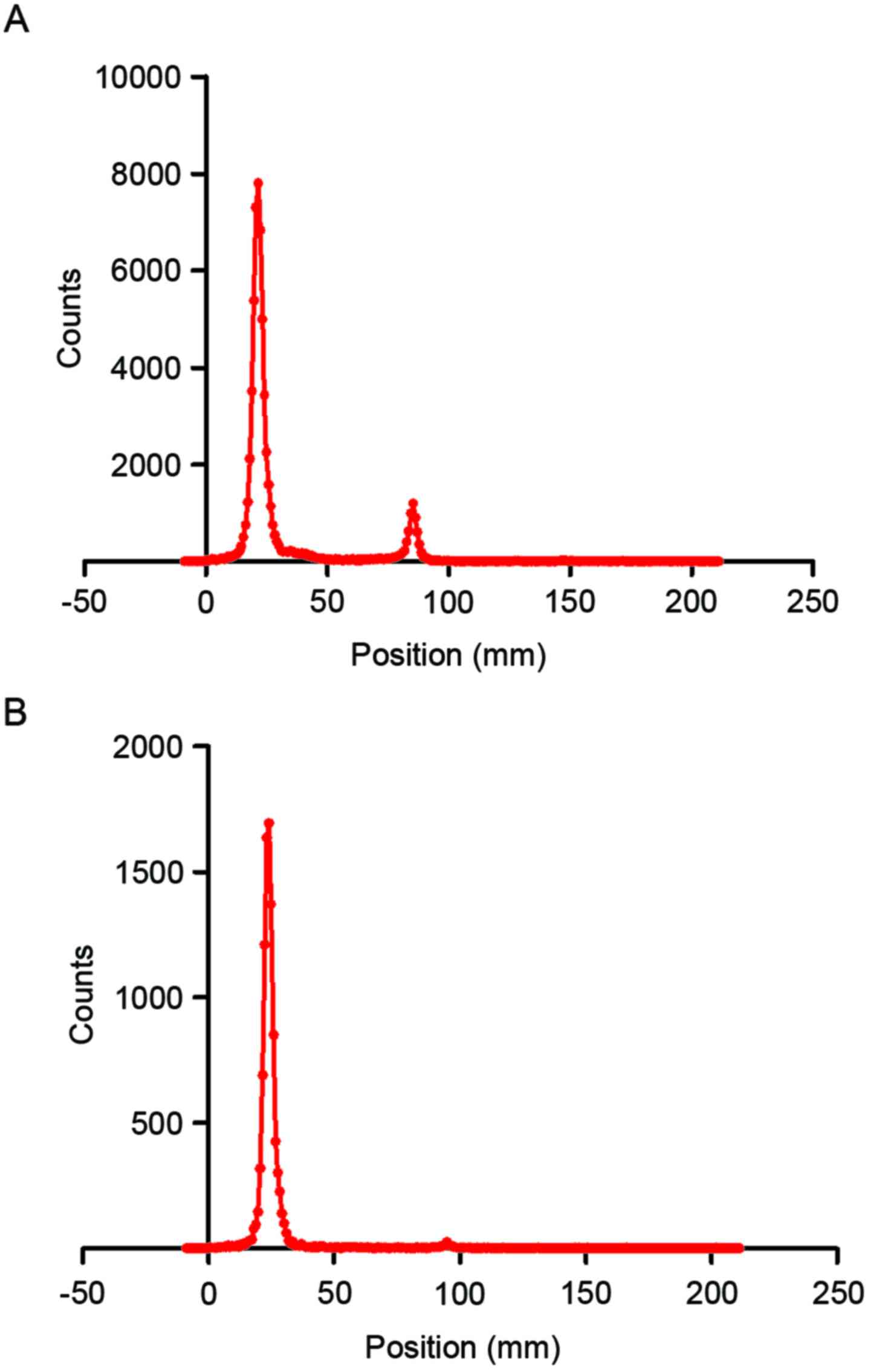
Purification and radiochemical
purity
The 99mTc-nanobody and the dissociative
[99mTc (H2O)3(CO)3]+ were separated well on silica gel
plates using acetone as the developing solvent. The radiolabeling
efficiency, which was determined using thin layer chromatography,
was 87.0% (Fig. 2A). The crude
product was then purified by ultrafiltration to obtain the
99mTc-nanobody, of which radiochemical purity was 97.1%
(Fig. 2B).
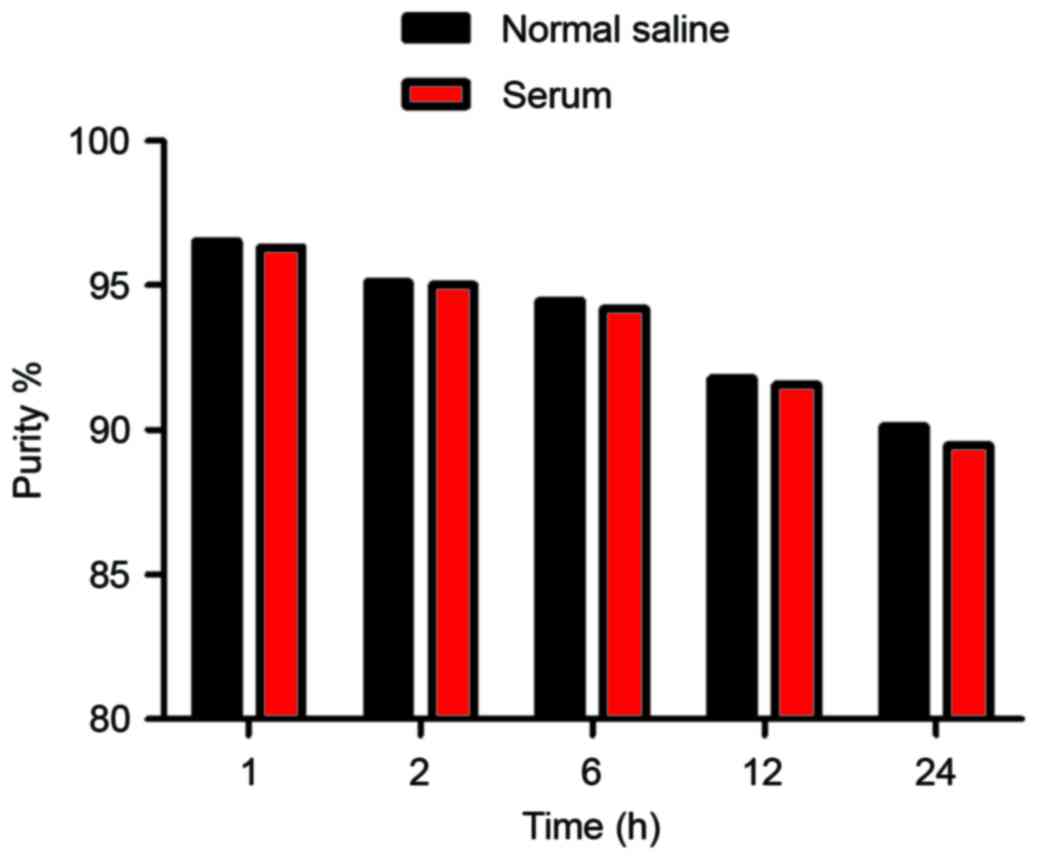
In vitro stability
The radiochemical purity of the
99mTc-nanobody was assessed in normal saline at 25°C and
in human serum at 37°C. Under both conditions, the
99mTc-nanobody exhibited good stability (Fig. 3).
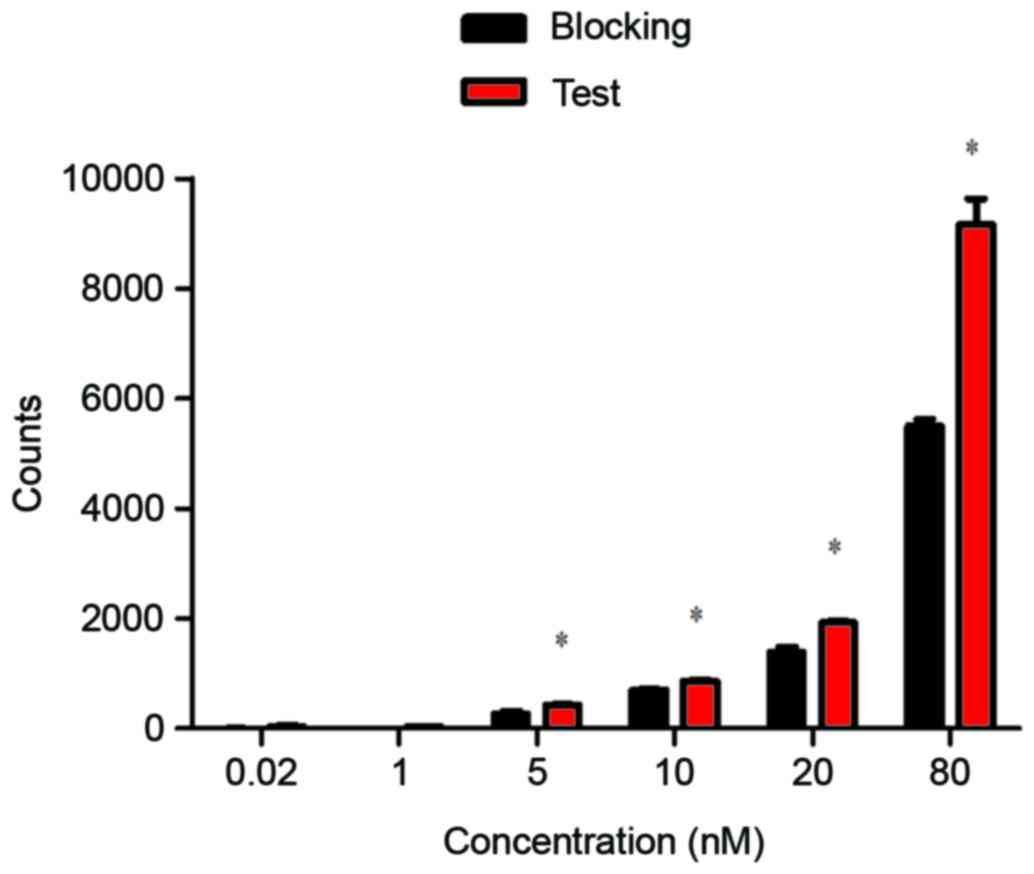
In vitro evaluation of the
99mTc-nanobody
An in vitro binding assay was performed using
CEA-positive H460 cells, the 99mTc-nanobody exhibited a
normal binding manner that was effectively blocked by cold nanobody
(Fig. 4).
Blood clearance of the
99mTc-nanobody
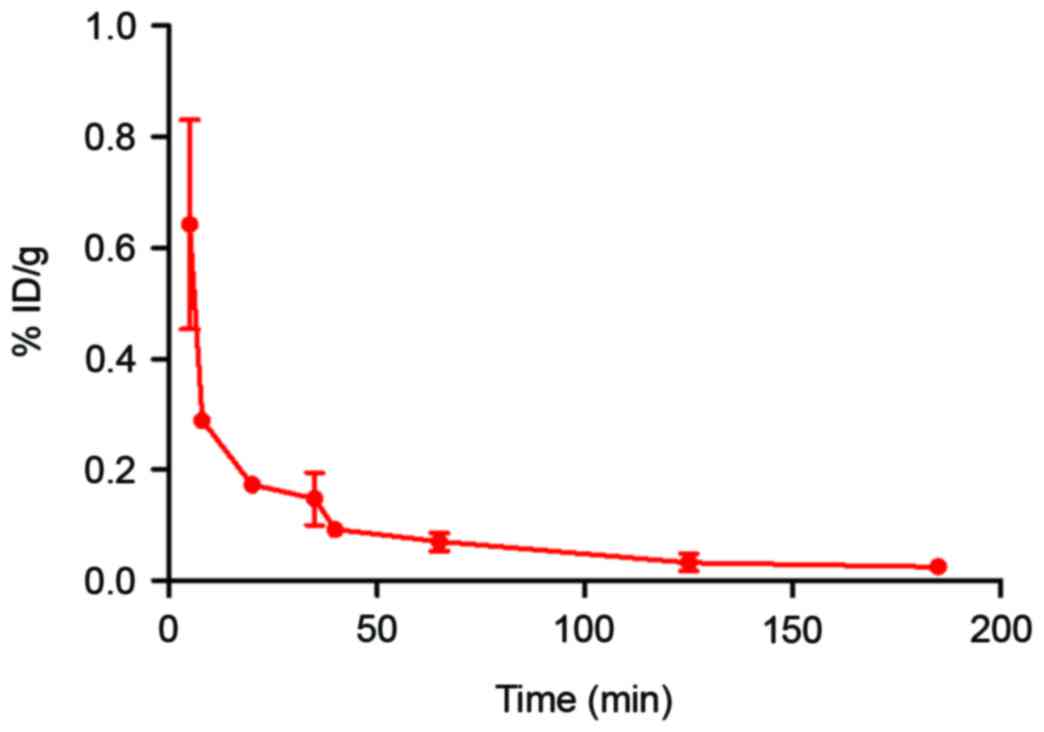
As per the radioactivity-time curve presented in
Fig. 5, the blood elimination was
fast during the initial 50 min and it then slowed. The half-lives
of distribution (T1/2α) and elimination
(T1/2β) were 20.2 and 143.5 min, respectively (Fig. 5).
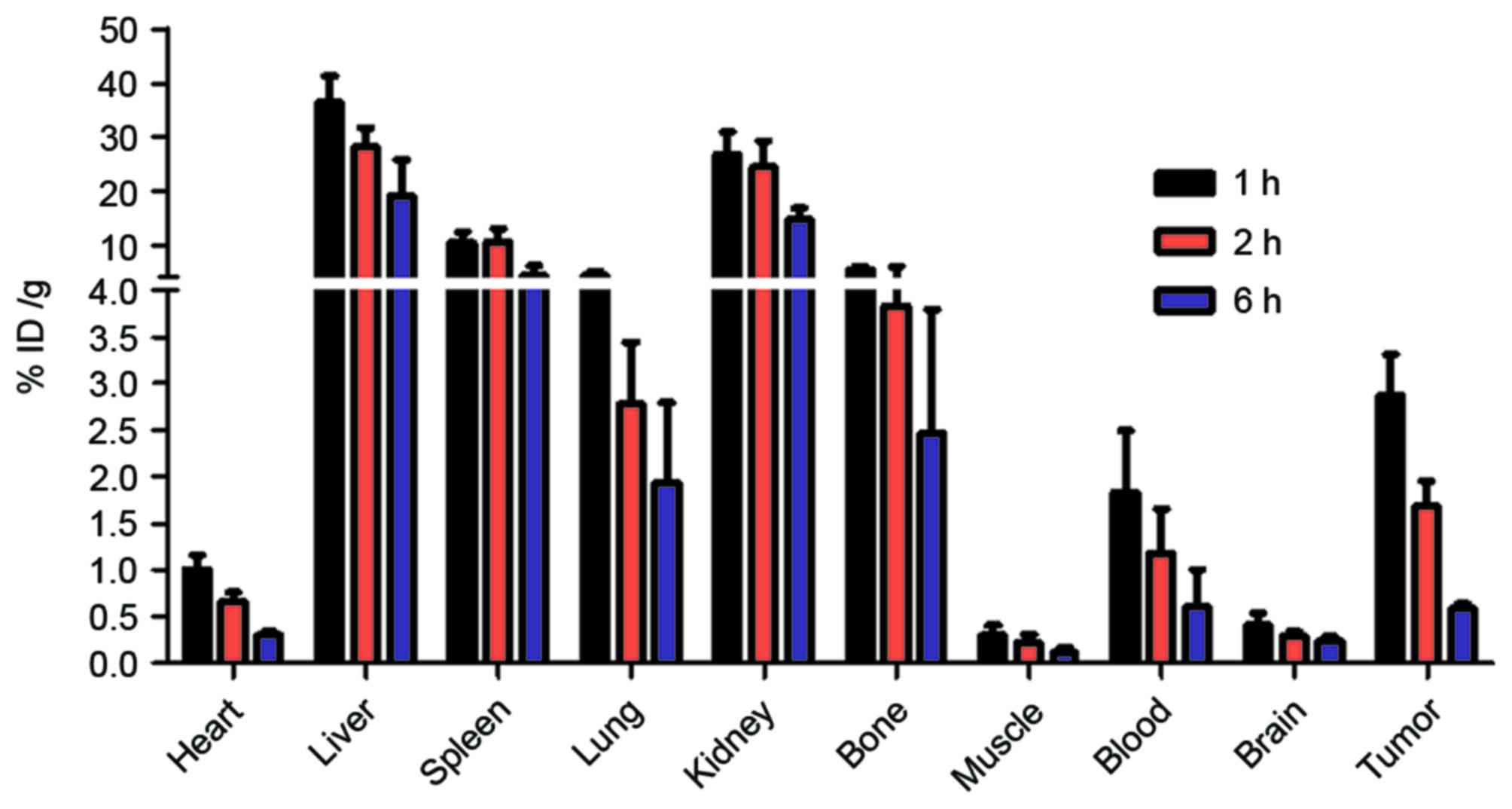
Biodistribution
Nude mice bearing H460 xenografts were injected with
the 99mTc-nanobody and sacrificed at 1, 2 and 6 h. The
radioactivity of different tissues was measured with a γ counter.
The result demonstrated that the radiolabeled nanobody was
predominantly excreted through the kidney, and partially
accumulated in the liver and spleen. A high uptake by tumor was
observed. The high uptake by bone may be caused by the dissociation
of the radionuclide from the 99mTc-nanobody in
vivo (Fig. 6).
Discussion
Molecular imaging has become an all-important tool
in cancer diagnosis and targeted therapy; it involves the use of
probes coupled to molecules with appropriate signal-emitting tags
(11,24,25).
Nanobodies are suitable for molecular imaging as they have several
advantages, including fast distribution and elimination, high
affinity and specificity, ease of manufacturing, and high
stability. This study evaluated the targeting profile of a nanobody
against CEA with the objective of developing a new probe for the
molecular imaging of NSCLC. On account of appropriate decay
characteristics and the ease of labeling with a His-tag that is far
from the activity site of the probe, 99mTc is the most
commonly used signal tag in nanobody imaging experiments (11). Therefore, 99mTc-labeling
was used to investigate the distribution and elimination of
CEA-targeting nanobody with the aim to develop a clinically
relevant single photon emission computed tomography probe.
In the present study, in vitro
immunofluorescent staining indicated efficient binding of the
nanobody to CEA-positive cells. The 99mTc-nanobody
exhibited good stability in normal saline and serum. The binding
and blocking experiment revealed that the 99mTc-nanobody
had normal and specific affinity for CEA-positive cells. The
T1/2α and T1/2β were 20.2 and 143.5 min,
respectively. The radioactivity-time curve revealed suitable
pharmacokinetics for its use in imaging. Biodistribution data in
nude mice with H460 xenografts revealed rapid tumor uptake and
specific tumor targeting by the 99mTc-nanobody. A high
tumor-to-background ratio further confirmed its use in CEA-positive
cancers. The radioactivity in tumor tissue was nine times higher
than the background of the muscle, indicating a favorable
distribution for imaging; a high uptake in the kidney indicated its
urinary excretion. All these results present the nanobody as a
potentially useful molecular probe for NSCLC.
In conclusion, a pilot study was conducted using a
CEA-targeted nanobody to investigate its NSCLC targeting effects.
In vitro binding, in vivo distribution and
pharmacokinetics assays were performed, and the results indicate
that the nanobody may be a promising molecular probe for
CEA-positive tumors, particularly in NSCLC cases.
Acknowledgements
The authors would like to thank Dr Jian-Feng Liu
(Institute of Radiation Medicine, Chinese Academy of Medical
Sciences and Peking Union Medical College, Tianjin, China) for his
suggestion in experiment design. Useful technical assistance was
provided by Professor Jian Tan (Tianjin Medical University General
Hospital, Tianjin, China). This study was funded by the National
Natural Science Foundation of China (grant nos. 1301983 and
81502759), The IRM-CAMS Research Fund (grant no. 1529) and Natural
Science Foundation of Tianjin (grant no. 15JCQNJC45800).
References
|
1
|
Gold P and Freedman SO: Demonstration of
tumor-specific antigens in human colonic carcinomata by
immunological tolerance and absorption techniques. J Exp Med.
121:439–462. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krupey J, Gold P and Freedman SO:
Purification and characterization of carcinoembryonic antigens of
the human digestive system. Nature. 215:67–68. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wahl RL, Philpott G and Parker CW:
Monoclonal antibody radioimmunodetection of human-derived colon
cancer. Invest Radiol. 18:58–62. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeon BG, Shin R, Chung JK, Jung IM and Heo
SC: Individualized cutoff value of the preoperative
carcinoembryonic antigen level is necessary for optimal use as a
prognostic marker. Ann Coloproctol. 29:106–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang KL, Yang SH, Liang WY, Kuo YJ, Lin
JK, Lin TC, Chen WS, Jiang JK, Wang HS, Chang SC, et al:
Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment
outcome of rectal cancer patients receiving pre-operative
chemoradiation and surgery. Radiat Oncol. 8:432013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eftekhar E and Naghibalhossaini F:
Carcinoembryonic antigen expression level as a predictive factor
for response to 5-fluorouracil in colorectal cancer. Mol Biol Rep.
41:459–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das A, Barik S, Banerjee S, Bose A, Sarkar
K, Biswas J, Baral R and Pal S: A monoclonal antibody against neem
leaf glycoprotein recognizes carcinoembryonic antigen (CEA) and
restricts CEA expressing tumor growth. J Immunother. 37:394–406.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conaghan P, Ashraf S, Tytherleigh M,
Wilding J, Tchilian E, Bicknell D, Mortensen NJ and Bodmer W:
Targeted killing of colorectal cancer cell lines by a humanised
IgG1 monoclonal antibody that binds to membrane-bound
carcinoembryonic antigen. Br J Cancer. 98:1217–1225. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koppe MJ, Bleichrodt RP, Soede AC,
Verhofstad AA, Goldenberg DM, Oyen WJ and Boerman OC:
Biodistribution and therapeutic efficacy of (125/131)I-, (186)Re-,
(88/90)Y-, or (177)Lu-labeled monoclonal antibody MN-14 to
carcinoembryonic antigen in mice with small peritoneal metastases
of colorectal origin. J Nucl Med. 45:1224–1232. 2004.PubMed/NCBI
|
|
10
|
Olafsen T and Wu AM: Antibody vectors for
imaging. Semin Nucl Med. 40:167–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chakravarty R, Goel S and Cai W: Nanobody:
The ‘magic bullet’ for molecular imaging? Theranostics. 4:386–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siontorou CG: Nanobodies as novel agents
for disease diagnosis and therapy. Int J Nanomedicine. 8:4215–4227.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Desmyter A, Decanniere K, Muyldermans S
and Wyns L: Antigen specificity and high affinity binding provided
by one single loop of a camel single-domain antibody. J Biol Chem.
276:26285–26290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cancer of the Lung and Bronchus-SEER Stat
Fact Sheets 2016. 2016.
|
|
15
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomita M, Shimizu T, Ayabe T, Yonei A and
Onitsuka T: Prognostic significance of tumour marker index based on
preoperative CEA and CYFRA 21-1 in non-small cell lung cancer.
Anticancer Res. 30:3099–3102. 2010.PubMed/NCBI
|
|
17
|
Ghosh I, Bhattacharjee D, Das AK,
Chakrabarti G, Dasgupta A and Dey SK: Diagnostic role of tumour
markers CEA, CA15-3, CA19-9 and CA125 in lung cancer. Indian J Clin
Biochem. 28:24–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai H, Liu J, Liang L, Ban C, Jiang J, Liu
Y, Ye Q and Wang C: Increased lung cancer risk in patients with
interstitial lung disease and elevated CEA and CA125 serum tumour
markers. Respirology. 19:707–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu GL, Liu X, Lv XB, Wang XP, Fang XS and
Sang Y: miR-148b functions as a tumor suppressor in non-small cell
lung cancer by targeting carcinoembryonic antigen (CEA). Int J Clin
Exp Med. 7:1990–1999. 2014.PubMed/NCBI
|
|
20
|
Bell A, Wang ZJ, Arbabi-Ghahroudi M, Chang
TA, Durocher Y, Trojahn U, Baardsnes J, Jaramillo ML, Li S, Baral
TN, et al: Differential tumor-targeting abilities of three
single-domain antibody formats. Cancer Lett. 289:81–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Li S, Zhang W, Sun J, Ren G and Dong
Q: A novel VHH antibody targeting the B cell-activating factor for
B-cell lymphoma. Int J Mol Sci. 15:9481–9496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xavier C, Devoogdt N, Hernot S, Vaneycken
I, D'Huyvetter M, De Vos J, Massa S, Lahoutte T and Caveliers V:
Site-specific labeling of his-tagged Nanobodies with
99mTc: A practical guide. Methods Mol Biol. 911:485–490.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gainkam LO, Huang L, Caveliers V, Keyaerts
M, Hernot S, Vaneycken I, Vanhove C, Revets H, De Baetselier P and
Lahoutte T: Comparison of the biodistribution and tumor targeting
of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole
SPECT/micro-CT. J Nucl Med. 49:788–795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aerts A, Impens NR, Gijs M, D'Huyvetter M,
Vanmarcke H, Ponsard B, Lahoutte T, Luxen A and Baatout S:
Biological carrier molecules of radiopharmaceuticals for molecular
cancer imaging and targeted cancer therapy. Curr Pharm Des.
20:5218–5244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teng FF, Meng X, Sun XD and Yu JM: New
strategy for monitoring targeted therapy: Molecular imaging. Int J
Nanomedicine. 8:3703–3713. 2013.PubMed/NCBI
|