Introduction
Hematopoietic stem cells (HSCs) have the ability of
self-renewal and multilineage differentiation potential. They are
capable of regenerating hematopoietic and immunological systems
following injury of bone marrow and thus have potential as
therapies for bone marrow failure, hematological malignancies and
immunodeficiencies (1).
Hematopoietic stem cell transplantation (HSCT) has been developed
rapidly in recent years. However, the limited number of HSCs and
homing failure hinder the further development of transplantation.
Promoting donor HSC homing and proliferation is one of the key
measures to prevent implantation dysfunction (2,3).
The chemokine stromal cell-derived factor 1α
(SDF-1α) is secreted by bone marrow mesenchymal stem cells (BMMSCs)
and only binds to C-X-C chemokine receptor 4 (CXCR4), which is
expressed on cluster of differentiation (CD) 34+ cells.
The CXCR4/SDF-1a (4–6) signaling axis is considered to serve a
pivotal role in homing. Peled et al (4) demonstrated that exposing human cord
blood-derived CD34+ cells to anti-CXCR4 antibody reduces
homing to bone marrow (BM) in non-obese diabetic/severe-combined
immunodeficient mice and indicated that CXCR4 is important for HSC
homing. Improving CXCR4 or SDF-1a expression may promote
CD34+ cell homing.
Prostaglandin E2 (PGE2) is a metabolic product of
arachidonic acid (AA). Cyclooxygenase (Cox)-1 or −2 converts AA
into prostaglandin H2 (PGH2) and PGE synthase subsequently converts
PGH2 into PGE2. PGE2 is involved in numerous physiological and
pathological systems (7). PGE2
inhibits T cell receptor signaling and may induce inflammation
(8), and also stimulates bone
resorption of osteoclasts (9). In
addition to this, PGE2 serves an important role in stem cells. A
previous study demonstrated that PGE2 promotes progenitor
proliferation in in vitro cell culture and in colony forming
unit-spleen assays following transplantation (10). North et al (11), using a Zebrafish embryo model,
confirmed that embryos exposed to exogenous 16,16-dimethyl-PGE2
(dmPGE2) exhibit a notable increase in HSC numbers. The increase in
PGE2 synthesis improved HSC numbers, while the inhibition of PGE2
synthesis decreased the numbers of HSCs. Current studies suggested
that PGE2 promotes human CD34+ cells homing in
vitro (12,13). However, PGE2 has four specific G
protein coupled E prostanoid (EP) receptors, PGE2 receptor EP1-4
subtypes (EP1-4). Sugimoto et al (14), using a knock-out mice model for
each EP subtype receptor, identified that PGE2 is mediated by each
EP sub-type receptor, which generates differences in signal
transduction and physiological effects. EP1, through the activation
of phospholipase C, regulates intracellular Ca2+ levels,
and EP2 and 4 increase the levels of cyclic (c) AMP by binding with
stimulatory G proteins. In contrast with EP2 and 4, EP3 inhibits
cAMP production via inhibitory G proteins. Applying PGE2 directly
to patients may produce a variety of serious adverse events. In
previous years, studies on EP agonists (EPAs) and EP antagonists
(EPAAs) have increased exponentially. EP1AA induces apoptosis in
breast cancer cells, inhibits the development of breast cancer
(15) and suppresses colon cancer
development in rats (16). EP2AA
possesses the potential for treatment of glaucoma (17). PGE2, via EP4, stimulates
anti-inflammation in the lung and provides a novel clinical
perspective for chronic airway inflammatory conditions (18). However, studies on the effects of
EPAs and EPAAs on HSCs have not been reported. Therefore, the
present study aimed to investigate the specific subtype receptor
mediating PGE2 promotion of human CD34+ cell homing.
More importantly, the results of the present study provide evidence
to develop a novel targeted treatment to prevent human HSC
implantation dysfunction in the future.
In the present study, it was demonstrated that EP2A
and EP4A upregulated CXCR4 and SDF-1a expression, and increased the
migratory ability of CD34+ cells towards SDF-1a and
BMMSCs.
Materials and methods
Reagents
Healthy donors were selected from the Department of
hematology, the First Affiliated Hospital of Sun Yat-sen University
(Guangzhou, China). The specimen handling was performed in
accordance with the requirements of the hospital ethics committee.
The present study was approved by the ethics committee of The First
Affiliated Hospital of Sun Yat-sen University and written informed
consent was obtained from all patients. Bone marrow was obtained
from healthy donors directly. Peripheral blood was collected after
the healthy donors received granulocyte colony stimulating factor
(G-CSF) mobilization. dmPGE2 was bought from the Cayman Chemical
Company (Ann Arbor, MI, USA). The EP2AA AH-6809 was bought from the
Cayman Chemical Company. The EP2A ONO-AE1-259, EP4A ONO-AE1-329 and
EP4AA ONO-AE3-208 were provided by Japanese ONO Pharmaceutical Co.,
Ltd. (Osaka, Japan).
Human CD34+ cell magnetic
sorting
A total of 3–5 ml peripheral blood was extracted
from allogeneic hematopoietic stem cell transplantation healthy
donor following G-CSF mobilization. Following 3–5-fold dilution
with PBS, the blood was treated with Ficoll-Paque fluid (1.077
g/ml; MP Biomedicals, Santa Ana, CA, USA) and centrifuged at 400 ×
g at 20°C for 30 min to separate mononuclear cells. Human
CD34+ cells were sorted using CD34+
immunomagnetic bead kit (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany), according to the manufacturer's protocol. Part of the
CD34+ cells were collected for purity testing by flow
cytometry (FCM; Beckman Coulter, Fulterton, CA, USA) and the other
part were used for the rest experiments.
BMMSC culture and identification
A total of 10 ml bone marrow fluid was collected and
bone marrow mononuclear cells isolated using Ficoll-Paque liquid.
The cells were subsequently resuspended in low glucose Dulbecco's
modified Eagle's media (L-DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and cultured at 37°C
containing 5% CO2 of the atmosphere in a 10-cm dish.
When adherent cells were >80% confluent, they were detached with
0.25% trypsin and 0.01% EDTA (Gibco; Thermo Fisher Scientific,
Inc.) and subsequently replated at a dilution of 1:3. Passage 3
cells were used for immunophenotypic analysis, and osteogenic and
adipogenetic induction. FCM was applied to evaluate the expression
of BMMSC surface antigens CD73, CD90, CD105, CD14, CD34, and
CD45.
BMMSC suspension (1×105; 100 µl) was
added to each of 6 1.5 ml Eppendorf tubes. Each tube then received
10 µl phycoerythrin (PE) -conjugated anti-human CD73 antibody
(PE-CD73; 550257; BD Biosciences, San Diego, CA, USA), 10 µl
phycoerythrincyanin 5 (PC5) -conjugated anti-human CD90 antibody
(PC5-CD90; 561972; BD Biosciences), 10 µl PE-conjugated anti-human
CD105 antibody (PE-CD105; 560839; BD Biosciences), 10 µl
PE-conjugated anti-human CD34 antibody (PE-CD34; 550761; BD
Biosciences), 10 µl floresceinisothiocyanate (FITC)-conjugated
anti-human CD14 antibody (FITC-CD14; 555397; BD Biosciences) and 10
µl PC5-conjugated anti-human CD45 antibody (PC5-CD45; 555484; BD
Biosciences) respectively. The tubes were incubated in the dark and
at room temperature for 30 min. The stained cells were washed twice
with PBS and analyzed by FCM (CellQuest Pro; Beckman Coulter,
Fulterton, CA, USA) at 488 nm. Formulated osteogenic and
adipogenetic induction medium (Cyagen, Santa Clara, CA, USA) was
used, according to the manufacturer's protocol. The detached third
passage cells were washed twice with PBS, and resuspended with
osteogenic and adipogenetic induction medium. The medium was
changed every 3 days. After 21 days, the supernatant was discarded,
and cells were stained for 30 min at room temperature with alizarin
red for osteogenic evaluation and oil red O for adipogenetic
evaluation, and observed under an inverted microscope
(magnifications, ×4 and ×10).
Human CD34+ cell
treatment
The sorted human CD34+ cells were
resuspended in RPMI 1640 (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) medium containing 10% FBS at a density of
5×105/ml and divided into 6 groups, including the
control [dimethyl sulfoxide (DMSO); 0.1%], the PGE2 group (1
µmol/l), the EP2A group (100 nmol/l), the EP2A (100 nmol/l) + EP2AA
group (100 nmol/l), the EP4A group (100 nmol/l), and the EP4A (100
nmol/l) + EP4AA group (100 nmol/l). All the groups received 100
ng/ml recombinant human stem cell factor (rhSCF; PeproTech, Inc.,
Rocky Hill, NJ, USA). The cells were incubated on ice for 2 h, and
then cultured at 37°C in an atmosphere containing 5% CO2
for 22 h.
Western blotting of CXCR4
Human CD34+ cells were lysed on ice in
radioimmunoprecipitation assay buffer [RIPA Lysis Buffer (Medium);
Beyotime Institute of Biotechnology, Shanghai, China] following the
above treatment. The protein concentration was determined using the
Bradford method. Equal amounts (30 µg) of protein were subjected to
SDS-PAGE on a 10% gel (the molecular weight CXCR4 is 40–47 kD so a
10% gel was selected) and transferred to a nitrocellulose membrane
(EMD Millipore, Billerica, MA, USA). Membranes were incubated
overnight at 4°C with primary antibody against CXCR4 (1:1,000;
SC-9046; Santa Cruz Biotechnology, Inc.) and GAPDH (1:2,000;
SC-47724; Santa Cruz Biotechnology, Inc.). Membranes were incubated
at room temperature for 1 h with the horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (1:5,000;
A32723; Pierce; Thermo Fisher Scientific, Inc.). Proteins were
visualized using the Super Signal West Pico chemiluminescence kit
(Pierce; Thermo Fisher Scientific, Inc.).
ELISA detection of SDF-1a levels
secreted by BMMSCs
The 3rd generation BMMSCs were resuspended in L-DMEM
containing 10% FBS at a density of 1×106 cells/ml and
divided into six groups: Control group (DMSO; 0.1%); PGE2 group (1
µmol/l); EP2A group (100 nmol/l); EP2A (100 nmol/l) + EP2AA group
(100 nmol/l); EP4A group (100 nmol/l); and EP4A (100 nmol/l) +
EP4AA group (100 nmol/l). All the above groups received rhSCF (100
ng/ml) and were cultured at 37°C in an atmosphere containing 5%
CO2 for 24 h. The supernatant was collected at 24 h, and
the concentration of SDF-1a was detected using an ELISA kit
(rhSDF-1a ELISA kit; DSA00; R&D Systems, Inc., Minneapolis, MN,
USA), according to manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) detection of CXCR4 and SDF-1a
expression
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse
transcribed to cDNA using the Superscript® III Reverse
Transcriptase (Thermo Fisher Scientific, Inc.) and oligo-dT primer.
qPCR was performed on a Bio-Rad fluorescent quantitative PCR
instrument using the Platinum® SYBR® Green
qPCR SuperMix (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min;
45 cycles of denaturation at 95°C for 15 sec, annealing at 62°C for
30 sec and extension at 65°C for 5 sec. Fluorescence intensity in
each cycle was monitored in real time. GAPDH was used as the
endogenous control. The results were analyzed using the
2-ΔΔCq method (19).
The primer sequences are listed in Table I.
 | Table I.Primer sequences used in the reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences used in the reverse
transcription-polymerase chain reaction.
|
| Primer
sequence |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| CXCR4 |
5′-CCTATGCAAGGCAGTCCATGT-3′ |
5′-GGTAGCGGTCCAGACTGATGA-3′ |
| SDF-1a |
5′-ACTGTGCCCTTCAGATTG-3′ |
5′-TTGGCTGTTGTGCTTACT-3′ |
| GAPDH |
5′-AAGGTGAAGGTCGGAGTCAAC-3′ |
5′-GGGGTCATTGATGGCAACAATA-3′ |
Chemotactic effect of rhSDF-1α on
human CD34+ cells
The transwell assay was performed using transwell
plates (3422; Corning Incorporated, Corning, NY, USA) to evaluate
human CD34+ cell migration. A total of 400 µl FBS-free
medium containing 100 ng/ml rhSDF-1a (PeproTech) was added to the
lower chamber. A total of 100 µl medium containing 2×105
CD34+ cells/ml were added to the upper chamber following
drugs treatment and twice washing in serum free medium. Plates were
incubated for 4 h. The cells in the lower chamber were collected
and fixed at room temperature with 4% paraformaldehyde for 1 h. FCM
(CellQuest Pro software; Beckman Coulter, Fulterton, CA, USA) was
used to count the CD34+ cells and thus calculate the
migratory rate.
Chemotactic effect of BMMSCs on human
CD34+ cells
1×105 BMMSCs were seeded into the lower
chamber, and treated with DMSO (control; concentration, 0.1%), PGE2
(1 µmol/l), EP2A (100 nmol/l), EP2A (100 nmol/l) + EP2AA (100
nmol/l), EP4A (100 nmol/l) and EP4A (100 nmol/l) + EP4AA (100
nmol/l) for 24 h. Equal amounts (1×105) of
CD34+ cells from the six treatment groups were added to
the upper chamber, receiving the same treatment as the BMMSCs in
lower chamber. Following a 6 h incubation, the cells in the lower
chamber were collected and fixed with 4% paraformaldehyde for 1 h.
FCM was used to count the CD34+ cells to calculate
migratory rate.
Statistical analysis
All statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). Values are
presented as the mean ± standard deviation. The Paired t-test was
used for data comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
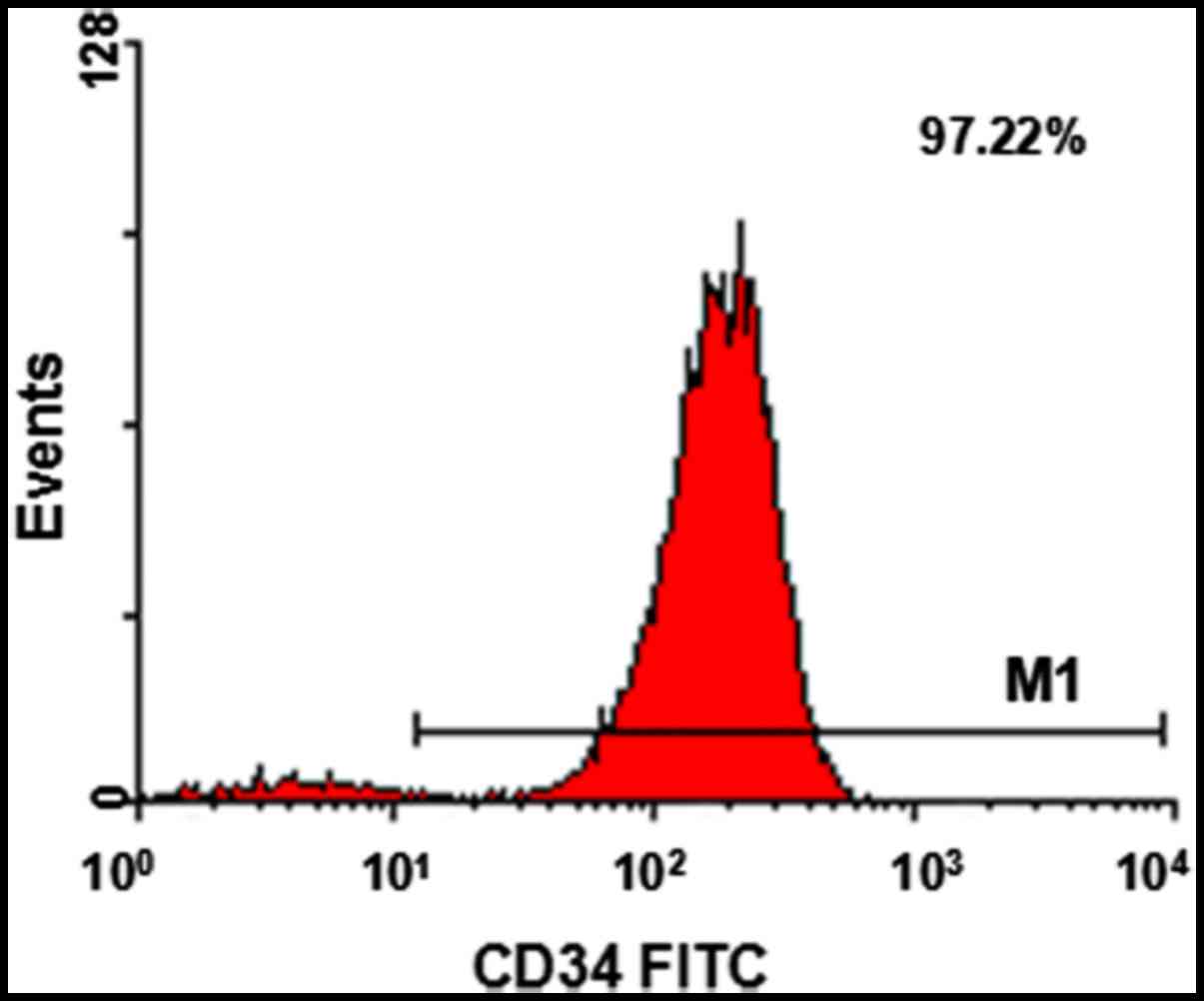
Human CD34+ cell sorting
and identification
Human CD34+ cells were isolated by
magnetic beads and identified by FCM for purity, their purity was
>90% (Fig. 1).
BMMSCs cultivation and
identification
BMMSCs were isolated from healthy volunteers for
cultivation. BMMSCs presented as fusiformis, spindle-shape, or
polygon after 1 week (Fig. 2A).
Subsequently, the cells were passaged for further purified
cultivation. Osteogenetic differentiation ability following
treatment with osteogenesis-induction medium for 3 weeks was
identified by staining with alizarin red. Red calcium nodules
observed under the inverted microscope (Fig. 2B). These results suggested that the
BMMSCs exhibited osteogenetic-differentiation capabilities. The
cells in the 3rd generation were treated with adipogenic-induction
medium for 3 weeks and stained with oil red O. Red lipid droplets
in grape-like clusters were observed under the inverted microscope
(Fig. 2C and D). These results
indicated that those cells exhibited the ability of adipogenic
differentiation. The 3rd generation cells were collected for FCM.
Those cells highly expressed CD73, CD90 and CD105, while negative
expressing CD14, CD34 and CD45 (Fig.
2E). These results revealed that BMMSCs present stem cell
surface antigens and not hematopoietic cell surface antigen.
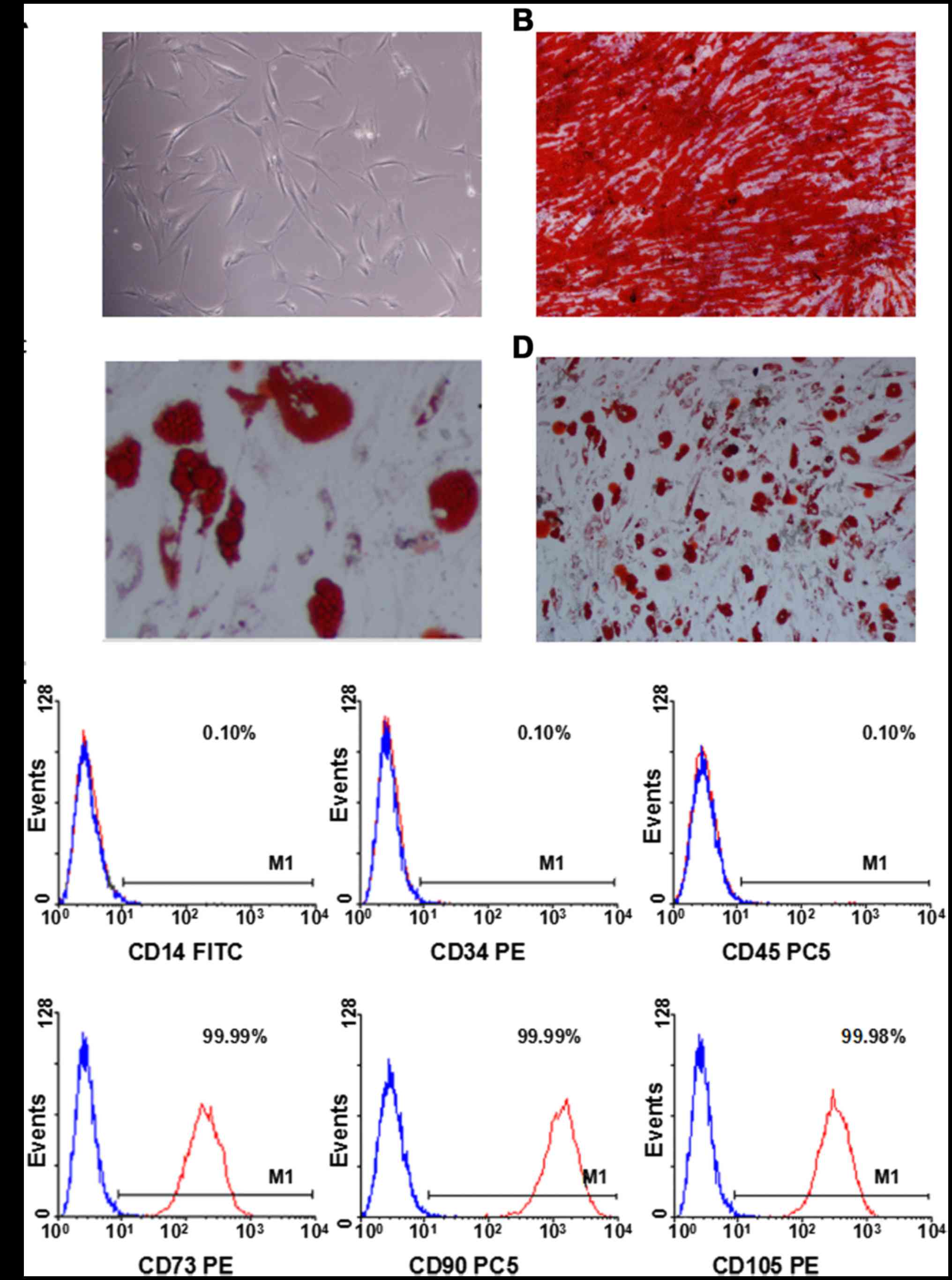 | Figure 2.In vitro morphology, osteogenic and
adipogenic differentiation, and immunophenotypic characterization
of BMMSCs. (A) The morphology of BMMSCs (magnification, ×10). (B)
Osteogenic differentiation of BMMSCs (magnification, ×4). (C)
Adipogenic differentiation of BMMSCs (magnification, ×10). (D)
Adipogenic differentiation of BMMSCs (magnification, ×4). (E)
Immunophenotypic characterization of BMMSCs using flow cytometry.
The expression of CD73, CD90 and CD105 is high in BMMSCs, while
expression of CD14, CD34 and CD45 is absent. BMMSC, bone marrow
mesenchymal stem cell; CD, cluster of differentiation; FITC,
fluorescein isothiocyanate; PE, phycoerythrin; PC5,
phycoerythrincyanin 5. |
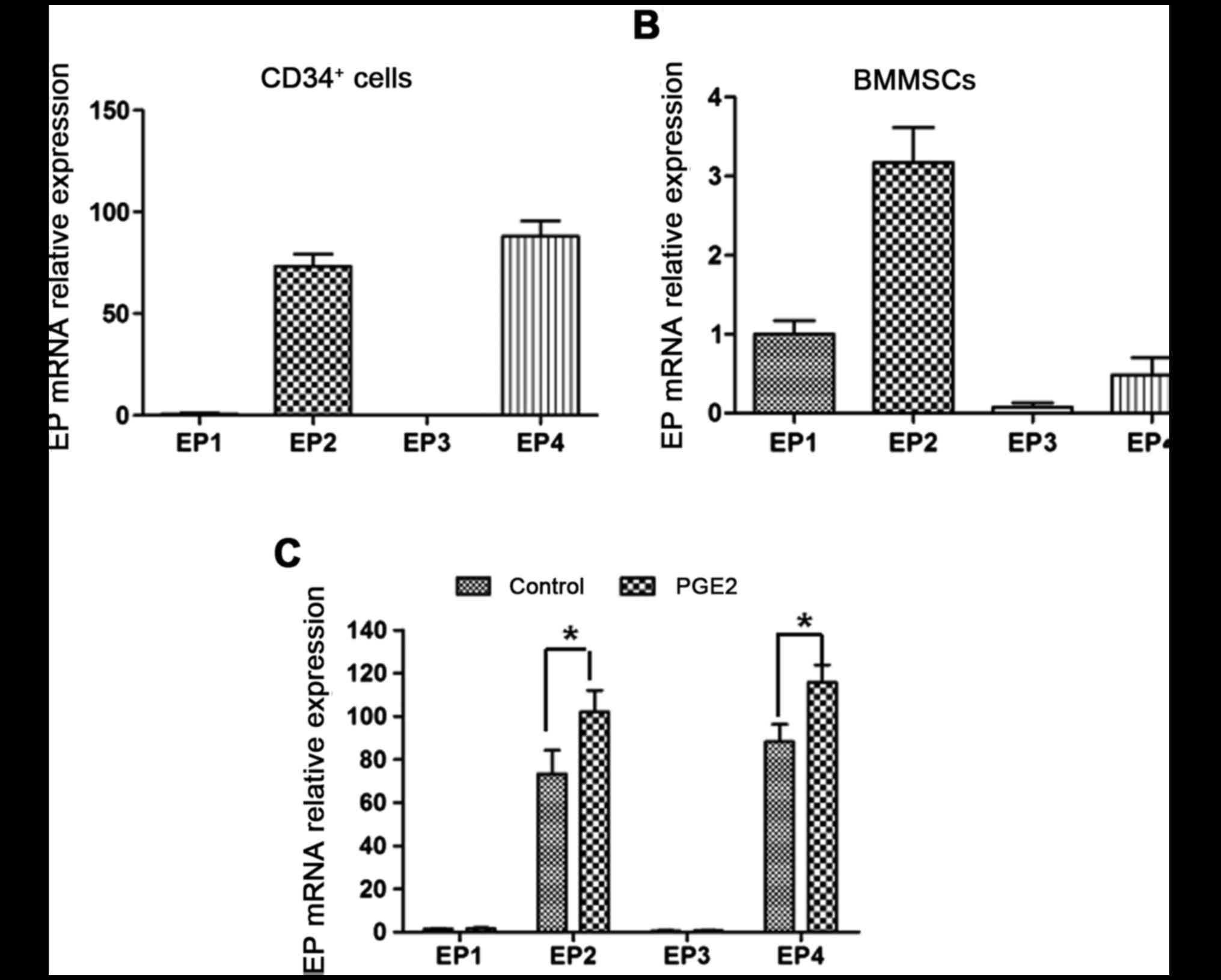
PGE2 receptors EP1-4 are expressed in
human CD34+ cells and BMMSCs
RT-qPCR was applied to detect the four PGE2 receptor
EP1-4 mRNA expression in human CD34+ cells and BMMSCs.
The results demonstrated that human CD34+ cells and
BMMSCs expressed mRNA of the four PGE2 receptors. CD34+
cells particularly expressed EP2 and 4 (Fig. 3A), while BMMSCs primarily expressed
EP1, 2 and 4 (Fig. 3B).
PGE2 elevates EP2 and 4 mRNA
expression in human CD34+ cells
Following treatment with PGE2, the level of EP2 and
4 mRNA in CD34+ cells was increased 1.44- (P=0.035) and
1.3-fold (P=0.029) compared with the control, respectively. EP1 and
3 mRNA levels did not exhibit a significant alteration (Fig. 3C). Therefore, PGE2 may serve a
critical role in human CD34+ cells through EP2 and 4.
Subsequently, EP2 and 4 were selected as the targets to investigate
the effect of EPAs on human CD34+ cells.
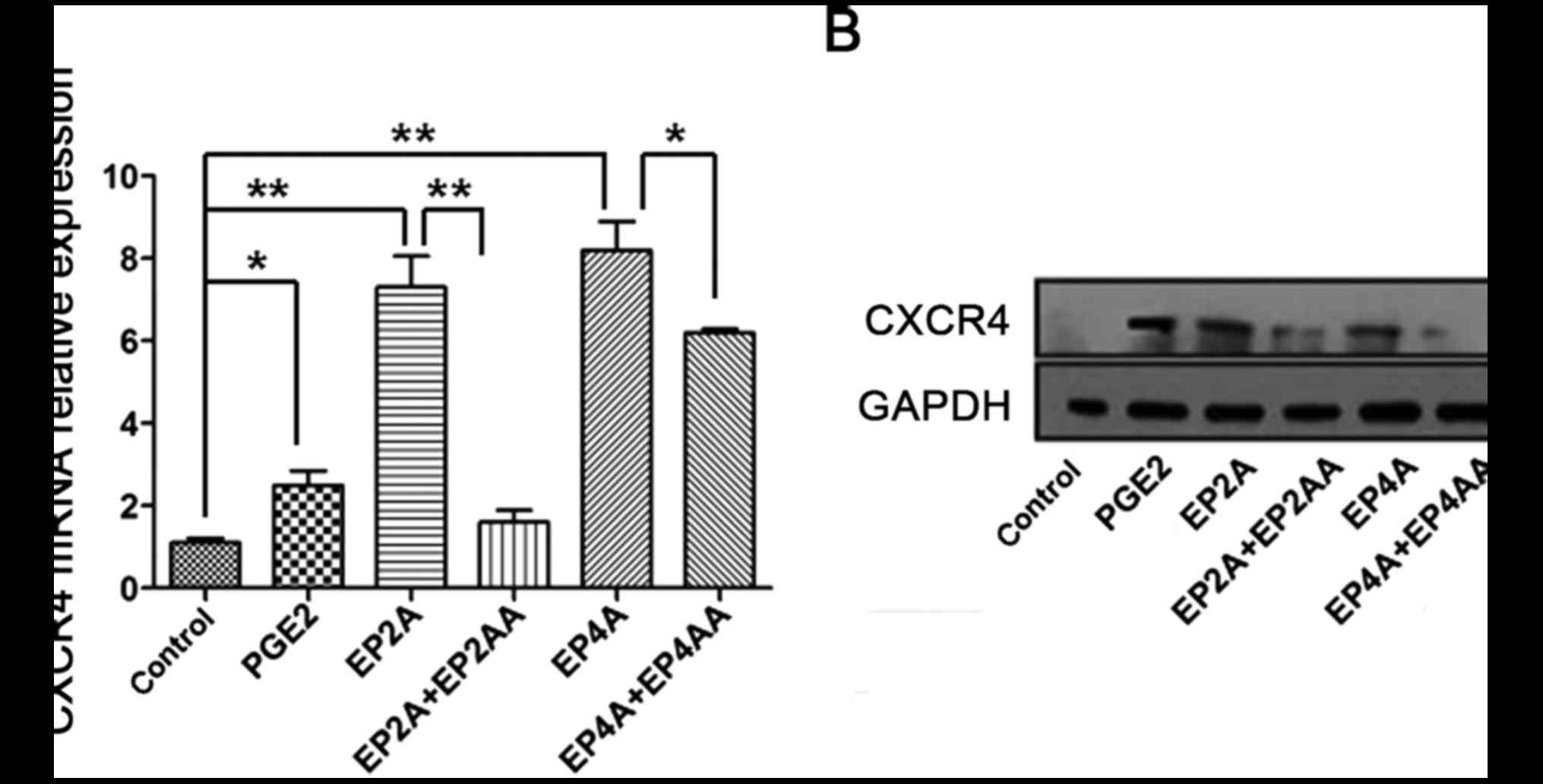
PGE2 promotes CXCR4 mRNA expression in
human CD34+ cells through EP2 and 4
RT-qPCR was performed to evaluate CXCR4 mRNA
expression in human CD34+ cells. It was observed that
compared with the control, CXCR4 mRNA expression levels in the
PGE2, EP2A and EP4A groups, were elevated 2.51- (P=0.016), 7.3-
(P=0.0012) and 8.17-fold (P=0.0005), respectively. Following
treatment with EP2AA (P=0.0022) or EP4AA (P=0.04), CXCR4 mRNA
levels decreased significantly (Fig.
4A).
PGE2 promotes CXCR4 protein expression
in human CD34+ cells through EP2 and 4
Western blotting was performed to test CXCR4 protein
expression in human CD34+ cells. It was revealed that
treatment of human CD34+ cells with PGE2, EP2A and EP4A
increased the expression of CXCR4 protein. Following treatment with
EP2AA or EP4AA, CXCR4 protein levels reduced markedly (Fig. 4B).
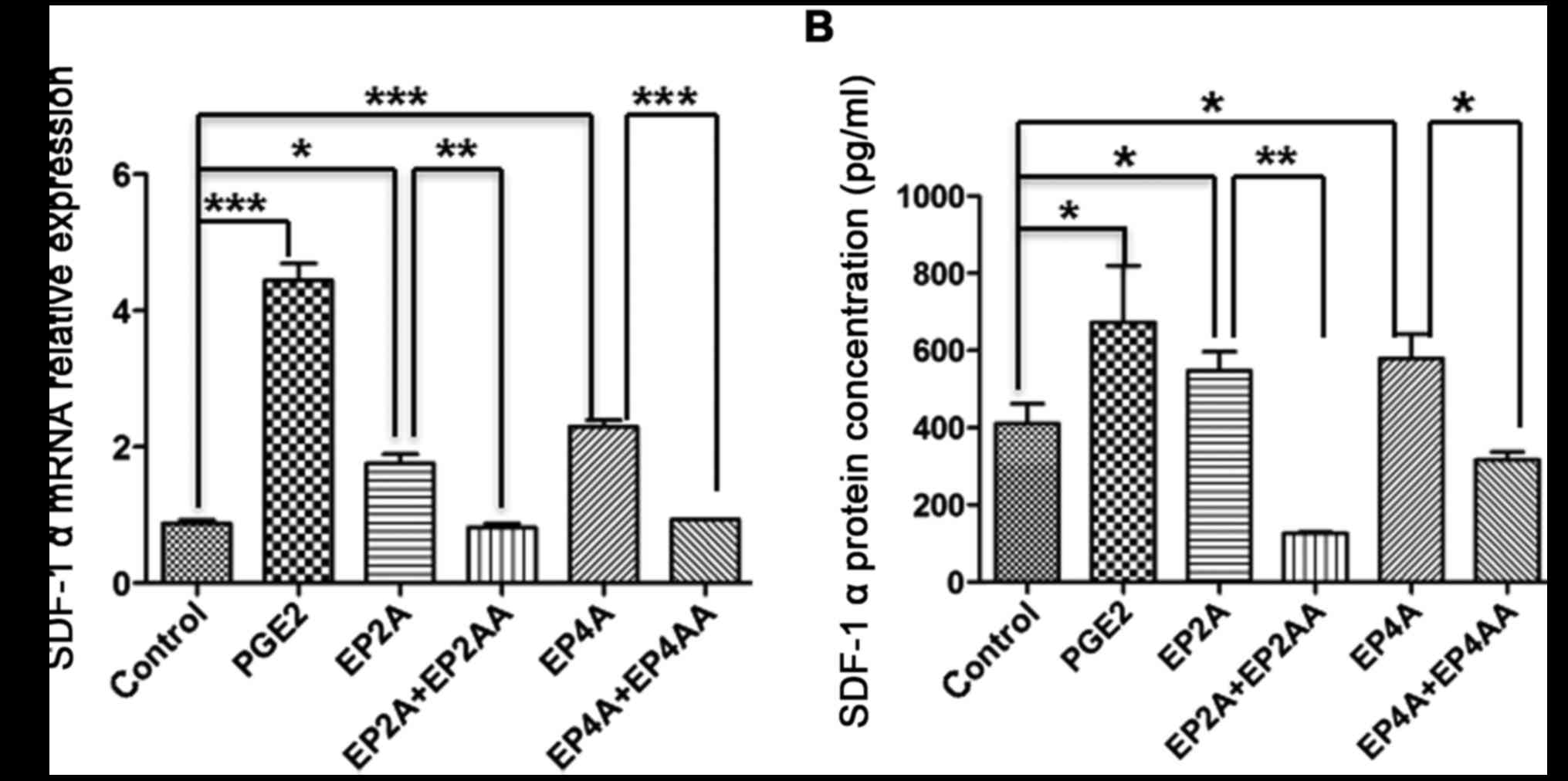
PGE2 promotes SDF-1a mRNA expression
in BMMSCs through EP2 and 4
RT-qPCR was performed to test SDF-1a mRNA expression
in human CD34+ cells. Compared with the control, the
SDF-1a mRNA expression levels in the PGE2, EP2A and EP4A groups
were increased 4.4- (P=0.0003), 1.72- (P=0.0158), and 2.32-fold
(P=0.0003), respectively. Following treatment with EP2AA (P=0.0073)
or EP4AA (P=0.0002), SDF-1a mRNA levels declined significantly
(Fig. 5A).
PGE2 promotes the secretion of SDF-1a
by BMMSCs through EP2 and 4
ELISA was applied to detect SDF-1a secretion in
BMMSCs. The results demonstrated that treatment with PGE2, EP2A and
EP4A increased SDF-1a protein secretion by 1.72- (P=0.021), 1.32-
(P=0.03) and 1.43-fold (P=0.018), respectively. Conversely, EP2AA
(P=0.009) and EP4AA (P=0.01) led to significantly decreased SDF-1a
secretion (Fig. 5B).
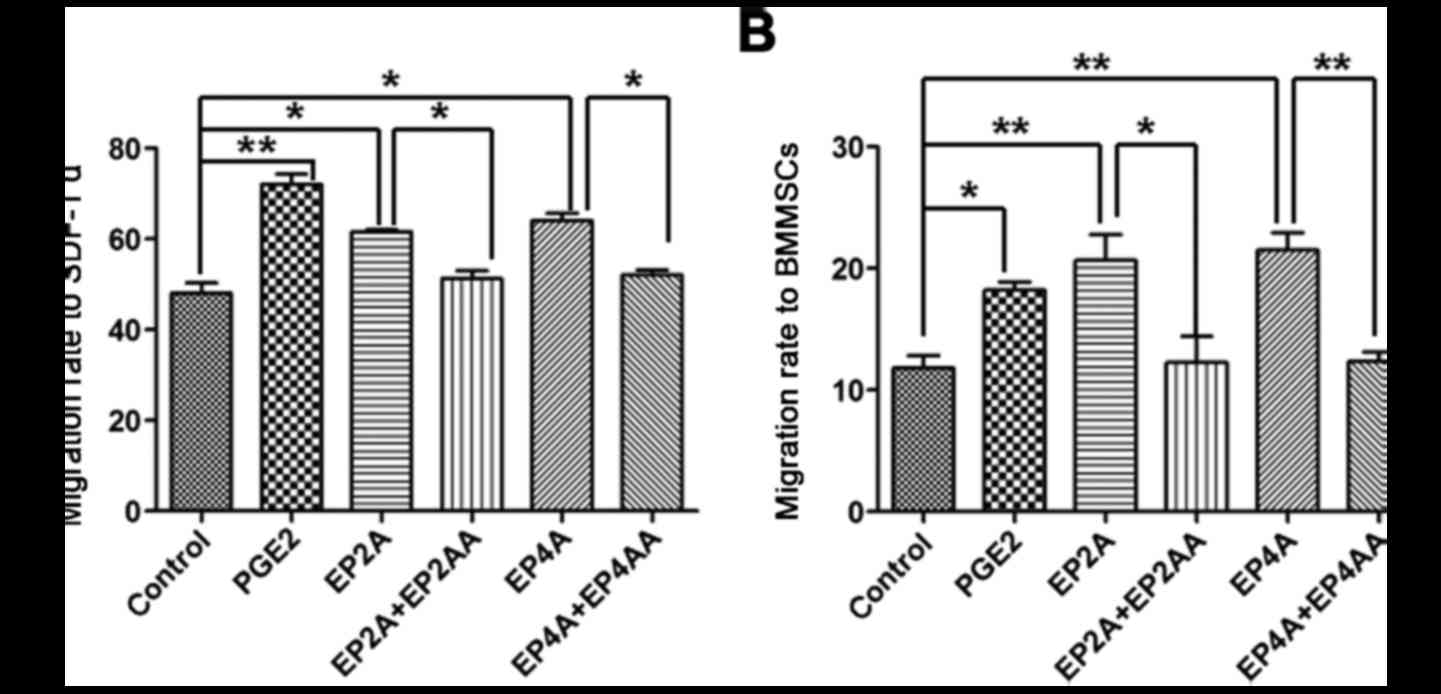
PGE2 promotes human CD34+
cell migration towards high SDF-1a concentrations through EP2 and
4
Transwell assays demonstrated that treatment with
PGE2, EP2A and EP4A enhanced human CD34+ cell migratory
ability towards high SDF-1a concentrations. The migratory rate
reached 71.9±3.52 (P=0.009), 62±0.58 (P=0.0178) and 64.2±2.77%
(P=0.0234) in the PGE2, EP2A and EP4A groups, respectively, which
was significantly increased compared with the control (48.4±3.45%).
Following treatment with EP2AA or EP4AA, migratory ability
decreased significantly to 51±2.94% (P=0.0228) and 52±2.02%
(P=0.0236), respectively (Fig.
6A).
PGE2 promotes human CD34+
cell migration towards BMMSCs through EP2 and 4
Transwell assays demonstrated that treatment with
PGE2, EP2A and EP4A increased human CD34+ cell migration
towards BMMSCs. The migration rate reached 17.9±0.64, 20.3±2.367,
and 21.2±1.27% in the PGE2 (P=0.0054), EP2A (P=0.041) and EP4A
(P=0.0038) groups, respectively, which was significantly increased
compared with the control. Following treatment with EP2AA or EP4AA,
the migratory ability decreased significantly to 12.3±2.13
(P=0.0361) and 12.4±0.81% (P=0.0043), respectively (Fig. 6B).
Discussion
PGE2, a subset of the eicosanoid family, is involved
in numerous physiological process, including stem cell development,
inflammation and cancer (20).
PGE2 exhibits a stable and direct hematopoietic regulatory ability
(1,11,21–25).
PGE2 promotes HSC homing following transplantation and prevents its
dysfunction with clinical application prospects. PGE2 serves a role
through four G-protein coupled receptors (EP1-4) on the cell
surface (26,27). Different receptors mediated
different physiological effects, and their expression levels varied
between different tissues and organs. Aside from promoting human
CD34+ cell homing, PGE2 may also induce other biological
effects through other sub-receptors (28–34).
Thus, the promoting effect of PGE2 on human CD34+ cells
is nonspecific, and its direct application to patients may result
in serious side effects. Therefore, it is necessary to investigate
the sub-type of receptor that mediates the promoting effect of PGE2
on CD34+ cell homing.
PGE2 regulates rat hematopoietic stem/progenitor
cells directly through the EP4 receptor and indirectly through
stromal progenitor cells (35).
PGE2 stimulates bone formation and prevents bone loss mediated by
EP4 (36). Dendritic cells develop
from hematopoietic progenitor cells through EP1 and 3 (37). However, few studies have
investigated the sub-receptor which mediates PGE2-induced human HSC
homing. In the present study, it was discovered by RT-qPCR that
human peripheral blood CD34+ cells expressed the four
receptors of PGE2, of which EP2 and 4 mRNA expression was
significantly increased compared with that of EP1 and 3. Following
treatment with PGE2 for 24 h, EP2 and 4 mRNA expression levels
increased, while EP1 and EP3 mRNA exhibited no significant
alteration. These results suggested that PGE2 may mediate its
effect on human HSC through EP2 and 4. Specific EP2 and 4 agonists
(EP2A and EP4A) and specific antagonists (EP2AA and EP4AA), were
used to investigate the roles of EP2 and human CD34+
cell homing.
The CXCR4/SDF-1a signaling axis serves a critical
role in the process of HSC homing (38–40).
CXCR4 is located on the surface of HSCs. Hoggatt and Pelus
(21) reported that PGE2 markedly
increased CXCR4 expression on the surface of mouse and human HSCs.
The results of the present study also confirmed that PGE2 promotes
CXCR4 mRNA and protein overexpression. It was observed that
treatment with EP2A and EP4A upregulated CXCR4 expression in human
CD34+ cells, while treatment with EP2AA and EP4AA
reduced CXCR4 expression. SDF-1a is primarily synthesized and
secreted by BMMSCs. It serves an important role in HSC
proliferation, mobilization and hematopoietic reconstruction
following HSCT (41–43), and provides directional power for
HSCs migration. The present study revealed that, similar to
treatment with PGE2, EP2A and EP4A also enhanced the chemotactic
ability of human CD34+ cells towards SDF-1a. PGE2, EP2A
and EP4A enhanced human CD34+ cell migration towards
BMMSCs, while EP2AA and EP4AA reduced human CD34+ cell
migration towards BMMSCs. Further investigation demonstrated that
PGE2, EP2A and EP4A promoted BMMSC secretion of SDF-1a, whereas
EP2AA and EP4AA treatment reduced SDF-1a secretion.
In conclusion, PGE2 promoted human CD34+
cell homing through EP2 and 4, which was primarily associated with
the effect of EP2 and 4 on CXCR4 expression on the surface of human
CD34+ cells and on the ability of BMMSCs to secrete
SDF-1a. The results of the present study highlighted the importance
of EP2 and 4 in HSC homing, and may provide novel evidence for the
clinical application of HSCT.
Acknowledgements
The authors of the present study would like to thank
the members of the Quentin Qiang Liu Laboratory (Guangzhou, China)
for their technical support and critical comments. The authors of
the present study would also like to thank Japanese ONO
Pharmaceutical Co., Ltd. (Osaka, Japan) for providing the
ONO-AE1-259, ONO-AE1-329 and ONO-AE3-208. The present study was
supported by the National Natural Science Foundation of China
(grant no. 81370663; to D. X.), and Science and Technology Planning
Project of Guangdong Province, China (grant no. 2013B021800239; to
D. X.).
Glossary
Abbreviations
Abbreviations:
|
PGE2
|
prostaglandin E2
|
|
EP1
|
PGE2 receptor EP2 subtype
|
|
EP4
|
prostaglandin E2 receptor EP4
subtype
|
|
EP2A
|
EP2 agonist
|
|
EP2AA
|
EP2 antagonist
|
|
EP4A
|
EP4 agonist
|
|
EP4AA
|
EP4 antagonist
|
|
BMMSC
|
bone marrow mesenchymal stem cell
|
|
HSC
|
hematopoietic stem cell
|
|
CXCR4
|
C-X-C chemokine receptor type 4
|
|
SDF-1α
|
stromal cell-derived factor 1α
|
References
|
1
|
Lord AM, North TE and Zon LI:
Prostaglandin E2: Making more of your marrow. Cell Cycle.
6:3054–3057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratajczak MZ and Suszynska M: Emerging
strategies to enhance homing and engraftment of hematopoietic stem
cells. Stem Cell Rev. 12:121–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma M, Afrin F, Tripathi RP and
Gangenahalli G: Transgene expression study of CXCR4 active mutants.
Potential prospects in up-modulation of homing and engraftment
efficiency of hematopoietic stem/progenitor cells. Cell Adh Migr.
8:384–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peled A, Petit I, Kollet O, Magid M,
Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al:
Dependence of human stem cell engraftment and repopulation of
NOD/SCID mice on CXCR4. Science. 283:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao L and Huang XJ: SDF-1/CXCR4 and
multiple myeloma osteolytic bone lesions-review. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 16:442–446. 2008.(In Chinese). PubMed/NCBI
|
|
6
|
Okada K, Kawao N, Yano M, Tamura Y,
Kurashimo S, Okumoto K, Kojima K and Kaji H: Stromal cell-derived
factor-1 mediates changes of bone marrow stem cells during bone
repair process. Am J Physiol Endocrinol Metab. 310:E15–E23.
2016.PubMed/NCBI
|
|
7
|
Miller SB: Prostaglandins in health and
disease: An overview. Semin Arthritis Rheum. 36:37–49. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiemer AJ, Hegde S, Gumperz JE and
Huttenlocher A: A live imaging cell motility screen identifies
prostaglandin E2 as a T cell stop signal antagonist. J Immunol.
187:3663–3670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsutsumi R, Xie C, Wei X, Zhang M, Zhang
X, Flick LM, Schwarz EM and O'Keefe RJ: PGE2 signaling through the
EP4 receptor on fibroblasts upregulates RANKL and stimulates
osteolysis. J Bone Miner Res. 24:1753–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fehér I and Gidáli J: Prostaglandin E2 as
stimulator of haemopoietic stem cell proliferation. Nature.
247:550–551. 1974. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
North TE, Goessling W, Walkley CR,
Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH,
Grosser T, et al: Prostaglandin E2 regulates vertebrate
haematopoietic stem cell homeostasis. Nature. 447:1007–1011. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pelus LM and Hoggatt J: Pleiotropic
effects of prostaglandin E2 in hematopoiesis; prostaglandin E2 and
other eicosanoids regulate hematopoietic stem and progenitor cell
function. Prostaglandins Other Lipid Mediat. 96:3–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoggatt J, Singh P, Sampath J and Pelus
LM: Prostaglandin E2 enhances hematopoietic stem cell homing,
survival, and proliferation. Blood. 113:5444–5455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugimoto Y and Narumiya S: Prostaglandin E
receptors. J Biol Chem. 282:11613–11617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamori T, Uchiya N, Nakatsugi S,
Watanabe K, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Sugimura T
and Wakabayashi K: Chemopreventive effects of ONO-8711, a selective
prostaglandin E receptor EP(1), on breast cancer development.
Carcinogenesis. 22:2001–2004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawamori T, Uchiya N, Kitamura T, Ohuchida
S, Yamamoto H, Maruyama T, Sugimura T and Wakabayashi K: Evaluation
of a selective prostaglandin E receptor EP1 antagonist for
potential properties in colon carcinogenesis. Anticancer Res.
21:3865–3869. 2001.PubMed/NCBI
|
|
17
|
Yanochko GM, Affolter T, Eighmy JJ, Evans
MG, Khoh-Reiter S, Lee D, Miller PE, Shiue MH, Trajkovic D and
Jessen BA: Investigation of ocular events associated with
taprenepag isopropyl, a topical EP2 agonist in development for
treatment of glaucoma. J Ocul Pharmacol Ther. 30:429–439. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Birrell MA, Maher SA, Dekkak B, Jones V,
Wong S, Brook P and Belvisi MG: Anti-inflammatory effects of PGE2
in the lung: Role of the EP4 receptor subtype. Thorax. 70:740–747.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Logan CM, Giordano A, Puca A and Cassone
M: Prostaglandin E2: At the crossroads between stem cell
development, inflammation and cancer. Cancer Biol Ther.
6:1517–1520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoggatt J and Pelus LM: Eicosanoid
regulation of hematopoiesis and hematopoietic stem and progenitor
trafficking. Leukemia. 24:1993–2002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gori JL, Chandrasekaran D, Kowalski JP,
Adair JE, Beard BC, D'Souza SL and Kiem HP: Efficient generation,
purification, and expansion of CD34(+) hematopoietic progenitor
cells from nonhuman primate-induced pluripotent stem cells. Blood.
120:e35–e44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goessling W, Allen RS, Guan X, Jin P,
Uchida N, Dovey M, Harris JM, Metzger ME, Bonifacino AC, Stroncek
D, et al: Prostaglandin E2 enhances human cord blood stem cell
xenotransplants and shows long-term safety in preclinical nonhuman
primate transplant models. Cell Stem Cell. 8:445–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durand EM and Zon LI: Newly emerging roles
for prostaglandin E2 regulation of hematopoiesis and hematopoietic
stem cell engraftment. Curr Opin Hematol. 17:308–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inman MD, Denburg JA, Ellis R, Dahlbäck M
and O'Byrne PM: The effect of treatment with budesonide or PGE2 in
vitro on allergen-induced increases in canine bone marrow
progenitors. Am J Respir Cell Mol Biol. 17:634–641. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruan D and So SP: Prostaglandin E2
produced by inducible COX-2 and mPGES-1 promoting cancer cell
proliferation in vitro and in vivo. Life Sci. 116:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawahara K, Hohjoh H, Inazumi T, Tsuchiya
S and Sugimoto Y: Prostaglandin E2-induced inflammation: Relevance
of prostaglandin E receptors. Biochim Biophys Acta. 1851:414–421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosuge Y, Miyagishi H, Shinomiya T,
Nishiyama K, Suzuki S, Osada N, Ishige K, Okubo M, Kawaguchi M and
Ito Y: Characterization of motor neuron prostaglandin E2 EP3
receptor isoform in a mouse model of amyotrophic lateral sclerosis.
Biol Pharm Bull. 38:1964–1968. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Callaghan G and Houston A: Prostaglandin
E2 and the EP receptors in malignancy: Possible therapeutic
targets? Br J Pharmacol. 172:5239–5250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nasrallah R, Hassouneh R, Zimpelmann J,
Karam AJ, Thibodeau JF, Burger D, Burns KD, Kennedy CR and Hébert
RL: Prostaglandin E2 increases proximal tubule fluid reabsorption,
and modulates cultured proximal tubule cell responses via EP1 and
EP4 receptors. Lab Invest. 95:1044–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Säfholm J, Manson ML, Bood J, Delin I,
Orre AC, Bergman P, Al-Ameri M, Dahlén SE and Adner M:
Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction
in human small airways through the E prostanoid subtype 2 receptor.
J Allergy Clin Immunol. 136:1232–1239.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mo C, Zhao R, Vallejo J, Igwe O, Bonewald
L, Wetmore L and Brotto M: Prostaglandin E2 promotes proliferation
of skeletal muscle myoblasts via EP4 receptor activation. Cell
Cycle. 14:1507–1516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
von der Emde L, Goltz D, Latz S, Müller
SC, Kristiansen G, Ellinger J and Syring I: Prostaglandin receptors
EP1-4 as a potential marker for clinical outcome in urothelial
bladder cancer. Am J Cancer Res. 4:952–962. 2014.PubMed/NCBI
|
|
34
|
Maślanka T, Spodniewska A, Barski D,
Jasiecka A, Zuśka-Prot M, Ziółkowski H, Markiewicz W and
Jaroszewski JJ: Prostaglandin E2 down-regulates the
expression of CD25 on bovine T cells, and this effect is mediated
through the EP4 receptor. Vet Immunol Immunopathol. 160:192–200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kushima YM, Arai F, Hosokawa K, Toyama H,
Takubo K, Furuyashiki T, Narumiya S and Suda T: Prostaglandin E(2)
regulates murine hematopoietic stem/progenitor cells directly via
EP4 receptor and indirectly through mesenchymal progenitor cells.
Blood. 121:1995–2007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshida K, Oida H, Kobayashi T, Maruyama
T, Tanaka M, Katayama T, Yamaguchi K, Segi E, Tsuboyama T,
Matsushita M, et al: Stimulation of bone formation and prevention
of bone loss by prostaglandin E EP4 receptor activation. Proc Natl
Acad Sci USA. 99:4580–4585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh P, Hoggatt J, Hu P, Speth JM, Fukuda
S, Breyer RM and Pelus LM: Blockade of prostaglandin E2 signaling
through EP1 and EP3 receptors attenuates Flt3L-dependent dendritic
cell development from hematopoietic progenitor cells. Blood.
119:1671–1682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lapidot T, Dar A and Kollet O: How do stem
cells find their way home? Blood. 106:1901–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marquez-Curtis LA, Turner AR, Larratt LM,
Letcher B, Lee SF and Janowska-Wieczorek A: CD34+ cell
responsiveness to stromal cell-derived factor-1alpha underlies rate
of engraftment after peripheral blood stem cell transplantation.
Transfusion. 49:161–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jung Y, Wang J, Schneider A, Sun YX,
Koh-Paige AJ, Osman NI, McCauley LK and Taichman RS: Regulation of
SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for
stem cell homing. Bone. 38:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goessling W, North TE, Loewer S, Lord AM,
Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT
and Zon LI: Genetic interaction of PGE2 and Wnt signaling regulates
developmental specification of stem cells and regeneration. Cell.
136:1136–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Regan JW: EP2 and EP4 prostanoid receptor
signaling. Life Sci. 74:143–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang D, Mann JR and DuBois RN: WNT and
cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle.
3:1512–1515. 2004. View Article : Google Scholar : PubMed/NCBI
|