Introduction
The prevalence of diabetes, especially type 2
diabetes mellitus (T2DM), is increasing in China and worldwide
(1–5). The International Diabetes Federation
(IDF) predicts that the global prevalence of diabetes will grow
from 382 million in 2013 to 592 million by 2035 (5). China is now home to a quarter of the
world's diabetes sufferers, which amounts to >100 million
people-nearly 12% of the population (3,6,7).
More than 600 million Chinese people suffer from prediabetes
(7). These are epidemic
proportions, and China may face difficulties controlling its crisis
compared with other more developed nations (8,9).
Therefore, urgent action is required (2,4,10).
Traditional Chinese medicine (TCM) has served an
important role in the healthcare system in the past (11,12).
At present, TCM is still well integrated in the Chinese health-care
system as one of the two mainstream medical practices due to
economic, cultural and historical evolutionary determinants
(13–17). Despite the fact that TCM may
possess synergistic anti-diabetic effects, a systematic
evidence-based strategy to improve T2DM treatment and/or reduce the
risk of complications is still not available. Nevertheless, several
preclinical and clinical trials were performed to collect evidence
on the benefits of TCM (17–21).
One TCM, Liu Wei Di Huang Wan, or Six Flavor Rehmanni, has been
demonstrated to possess a particular effect on diabetes or diabetic
complications (22). In the
clinical setting, TCMs are often used combined with Western
medicine, and a clinical study demonstrated that the combined
effect is an improvement on the use of Western medicine alone
(23). There are several
advantages in treating DM with TCM, including lower rates of
toxicity and/or side effects, holistic regulation of metabolic
problems, reversal of risk factors leading to T2DM and delaying
diabetic complications.
Tangningtongluo (TNTL), a TCM, has been widely used
for decades in southwest China. The traditional medical formula is
composed of Herba Plantagins, kewoluoqu, Flos
Lonicerae and Herba Agrimoniae. In 2014, the preparation
of TNTL was authorized by the local drug administration department
of Guizhou Provincial Food and Drug Administration (Guiyang, China;
authorization no. QYZZ-2014033). The aim of the present study was
to analyze the effectiveness of the TNTL treatment strategy and to
investigate the associated hypoglycemic mechanism.
Materials and methods
Preparation of the plant material
Plant material was collected from the Chinese Herbal
Medicine Planting Base of Guizhou Bailing Pharmaceutical Co., Ltd.
(Guizhou, China). This research was permitted by the director of
the planting base. Plant material was botanically authenticated and
a voucher specimen (no. BL-130012~130015) was deposited in the Miao
Medicine Herbarium of Guizhou Bailing Pharmaceutical Co., Ltd. The
company also provided an aqueous extract of the TNTL formula. To
obtain the aqueous extract, the herb formula [Herba
Plantagins (Cheqiancao), 1.16 kg; kewoluoqu, 1.6 kg; Flos
Lonicerae (Shanyinghua), 1.11 kg; and Herba Agrimoniae
(Xianhecao), 1.11 kg] was cleaned and extracted with hot water,
yielding a powdered extract (1.0 kg TNTL extract, 20.1% extract
percentage), which was used for bioassay. To reduce the variability
of TNTL among different batches, the species, origin, harvest time,
medicinal parts and concocted methods for each component were
strictly standardized according to good manufacturing practice.
Furthermore, for quality control, a fingerprint of TNTL was
established using the high-performance liquid chromatography
technique in our previous study (24). In 2014, the preparation of TNTL was
authorized by the local drug administration department
(authorization no. QYZZ-2014033).
Chemical reagents
A One-Touch Ultra Blood Glucose Meter and strips
(cat no. 3462320) were obtained from Johnson & Johnson Medical
Equipment Co., Ltd. (Shanghai, China). A carboxyl methyl cellulose
(0.5%) solution served as the vehicle. Metformin (Met; Squib
Pharmaceutical Co., Ltd., Shanghai, China), suspended in 0.5%
carboxyl methyl cellulose solution was used as the reference drug
for hypoglycemic activity measurement. Insulin receptor substrate-1
(ISR-1; ab52167), phosphorylated (p)-ISR-1 (phospho S522; ab65745)
and β-actin (ab8227) primary antibodies, goat anti-Rabbit IgG
H&L (HRP) (ab6721) and goat anti-mouse IgG H&L (HRP;
ab6789) second antibodies were purchased from Abcam (Cambridge, MA,
USA).
Experimental animals
To confirm the hypoglycemic activity and the effect
on pre-proliferative diabetic retinopathy, 6-week-old female
C57BL/KsJ-db/db mice (specific pathogen free; n=50; 45–50 g) were
purchased from SLAC Laboratories Animal Co., Ltd. (Shanghai, China)
for a T2DM model (25,26). C57BL female mouse (specific
pathogen free n=10, 5–6 weeks, 18–22 g) were purchased from SLAC
Laboratories Animal Co., Ltd. (Shanghai, China) as a normal control
group. The animals were housed under controlled conditions
(temperature, 23±2°C; relative humidity, 50±10%; 12-h light/dark
cycle) and allowed free access to a standard diet. All mice were
acclimated for 20 weeks for the emergence of pre-proliferative
diabetic retinopathy prior to the initiation of the drug
intervention and were maintained according to Beijing Laboratory
Animal Management Regulations. The experiment protocol was reviewed
and approved by the Animal Management Committee of the Animal
Resource Center, Institute of Medicinal Plant Development, Chinese
Academy of Medical Sciences (Beijing, China).
Experimental protocol
Fluorescein angiography (FA) was applied to detect
early diabetes-induced alterations in the microvasculature of the
retina with an Animal Fluorescein Imaging system (ISSLO/SOS2000;
Optoprobe Research Ltd., Burnaby, BC, Canada), including a 4D-isOCT
Retinal Microscope Imaging system (Optoprobe®; Optoprobe
Research Ltd.). After detection of the microvascular hemangioma of
diabetic retinopathy, 26-week-old db/db mice were randomly divided
into 5 groups, each containing 10 mice. The C57BL mouse group
(group 1, normal control group) and db/db diabetic controls (group
2, model control group, placebo) received an equal volume of
vehicle. Positive controls (group 3, model + Met group) received
the anti-diabetic agent, Met [140 mg/kg body weight (BW), an
equivalent dose]. TNTL-treated mice received 1.8 g, 0.9 g or 0.45
g/kg BW (groups 4, 5 and 6, respectively; model + TNTL groups). In
clinic practice, the recommend dose was 2.7~3.6 g per subject
daily, which is ~0.045 g/kg BW for an adult (average BW =60 kg).
The equivalent dose in mice was 0.45 g/kg. In the present study,
doses that were 2-fold (0.9 g/kg), 4-fold (1.8 g/kg) and an
equivalent dose (0.45 g/kg) were administered orally per day
continuously for 18 weeks in mice to evaluate the activity. All
mice received the assigned intervention daily and BW, food and
water intake were measured weekly.
Blood glucose determination
To determine fasting blood glucose levels, blood
samples were drawn from the tail vein of all mice every week. After
placing fresh blood (~50 µl) on duplicate test strips, a validated
One-Touch Basic Glucose Monitoring system was used to determine the
glucose content. After 18 weeks of intervention, all animals were
fasted for 5 h and anesthetized with diethyl ether (inhalation
dosage: 2.0% for induction, 0.2–0.3% for maintenance;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After injecting
fluorescein (20 µl) into the tail vein of the mice, FA was
performed to detect early diabetes-induced alterations in
microvasculature with an Animal Fluorescein Imaging system and a
4D-isOCT Retinal Microscope Imaging system. Subsequently, the blood
samples were collected for biochemical analysis.
Biochemical analysis
Blood samples were centrifuged at 3,000 × g for 15
min at 4°C and then removed and stored the plasma (−60°C) for
further analysis. The plasma insulin and C-peptide levels were
detected immediately using a radioimmunoassay. Commercial kits and
a standard assay method were used to estimate the plasma glycated
hemoglobin A1c (HbA1c; EXP210563) level and the plasma lipid
profile of triacylglycerol (TG; EXP210617), total cholesterol (TC;
EXP210602), high-density lipoprotein (HDL) and low-density
lipoprotein (LDL; EXP210603) (all from Beijing Expandbiotech Co.,
Ltd., Beijing, China).
The homeostatic model assessment of insulin
resistance (HOMA-IR) index was applied to estimate the alteration
in insulin resistance in TNTL-treated diabetic mice. The HOMA-IR
calculation was performed using the following formula: HOMA-IR =
fasting blood glucose (mmol/l) × insulin (µU/ml)/22.5 (27).
Protein extraction and western blot
analysis
To determine the protein expression level associated
with insulin resistance, the liver and gastrocnemius muscle tissue
were homogenized (1:10 w/v) on ice with radioimmunoprecipitation
assay buffer [50 mM Tris (pH 7.6), 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and 2 mM
EDTA; sterile solution] containing protease and phosphatase
inhibitors (26). Following this,
the supernatants were collected after centrifugation at 12,000 × g
for 25 min at 4°C. A bicinchoninic acid assay was used to determine
the protein concentration and the protein was stored at −80°C for
further western blot analysis. The protein fractions were separated
by electrophoresis on 10% SDS-PAGE gels and then transferred onto
nitrocellulose membranes in Tris-glycine buffer at 110 V for 1 h.
The membranes were blocked with 5% (w /v) skimmed milk
powder in Tris-buffered saline containing 0.1% (v/v) Tween-20
(TBST) and then incubated overnight with ISR-1, p-ISR-1 and β-actin
primary antibodies (1:1,000) at 4°C. Following this, the membranes
were washed twice with TBST and incubated with secondary antibodies
(goat anti-mouse IgG H&L; ab6789; 1:1,000; Abcam) for 2 h at
room temperature. The results were visualized by an Enhanced
Chemiluminescence kit (Thermo Scientific, Inc., Waltham, MA, USA).
The relative band intensity was determined using a computerized
densitometric analysis (Bio Image Intelligent Quantifier, version,
IQ-11; Bio Image Systems, Inc., Jackson, MI, USA).
Histopathological examination
Following the oral glucose tolerance test, all mice
were anesthetized with diethyl ether (10% inhalation dosage) and
sacrificed by cervical decapitation. The liver, kidney, pancreas
and gastrocnemius muscle tissue were quickly removed for
examination by light microscopy. The tissue sections were preserved
in 10% neutral phosphate-buffered formalin and processed by routine
paraffin sectioning (2 µm) and staining with H&E. Staining was
performed according to the manufacturer's protocol. Pathological
alterations were observed under an optical microscope using a Leica
Application Suite (Leica Microsystems, Inc., Buffalo Grove, IL,
USA).
Statistical analysis
Statistical analysis was performed with SPSS
software version 11.5 (SPSS Inc., Chicago, IL, USA). All data are
expressed as the mean ± standard deviation. Comparisons between
groups were analyzed by one-way analysis of variance and group
comparisons (the post hoc multiple comparison) were analyzed using
the Student-Newman-Keuls test, if equal variances assumed or
Thamhan's T2, if equal variances not assumed. The paired t-test was
used for continuous variables in the retrospective analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of TNTL on the metabolic
abnormalities in db/db mice
As presented in Fig.
1, the TNTL intervention resulted in a significant
time-dependent decrease in fasting blood glucose in db/db mice from
week 1 to 18. Compared with the placebo, TNTL (1.8, 0.9 and 0.45
g/kg BW) or Met (140 mg/kg BW) treatment did not significantly
affect the BW, food and water intake or feed efficiency ratio of
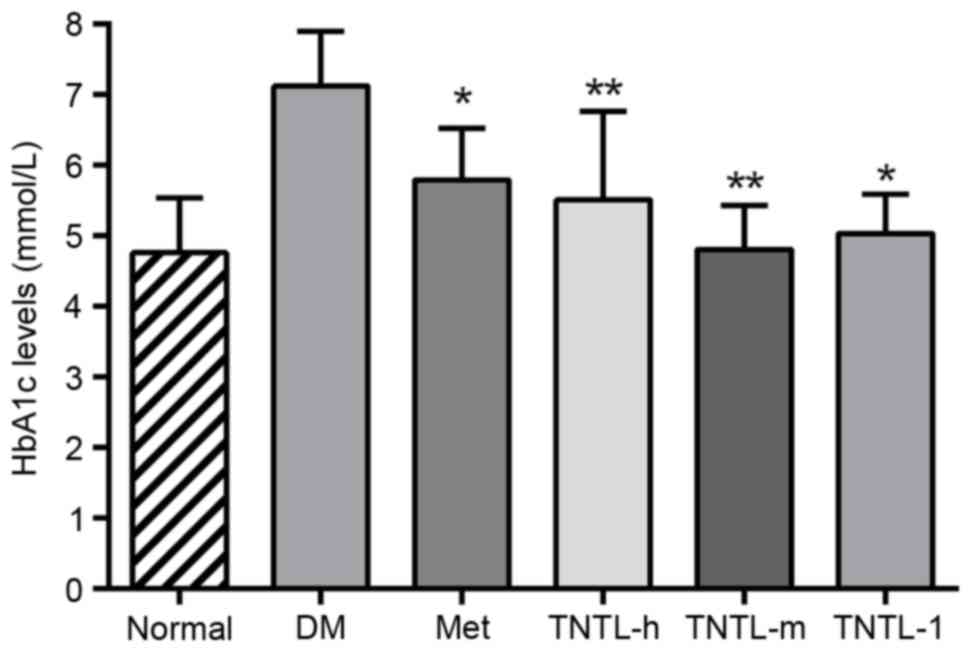
the db/db mice (data not shown). As presented in Fig. 2, the TNTL intervention resulted in
a significant dose-dependent decrease in HbA1c level (5.50±1.06,
5.02±0.56 and 4.80±0.63 vs. 7.12±0.78 for 0.45, 0.9 and 1.8 g/kg BW
doses of TNTL vs. placebo, respectively). As expected, Met
significantly lowered the levels of blood glucose (Fig. 1) and HbA1c (5.78±0.72 vs.
7.12±0.78; Fig. 2) in db/db mice.
Therefore, TNTL exhibits a time-dependent hypoglycemic effect with
sustained medication.
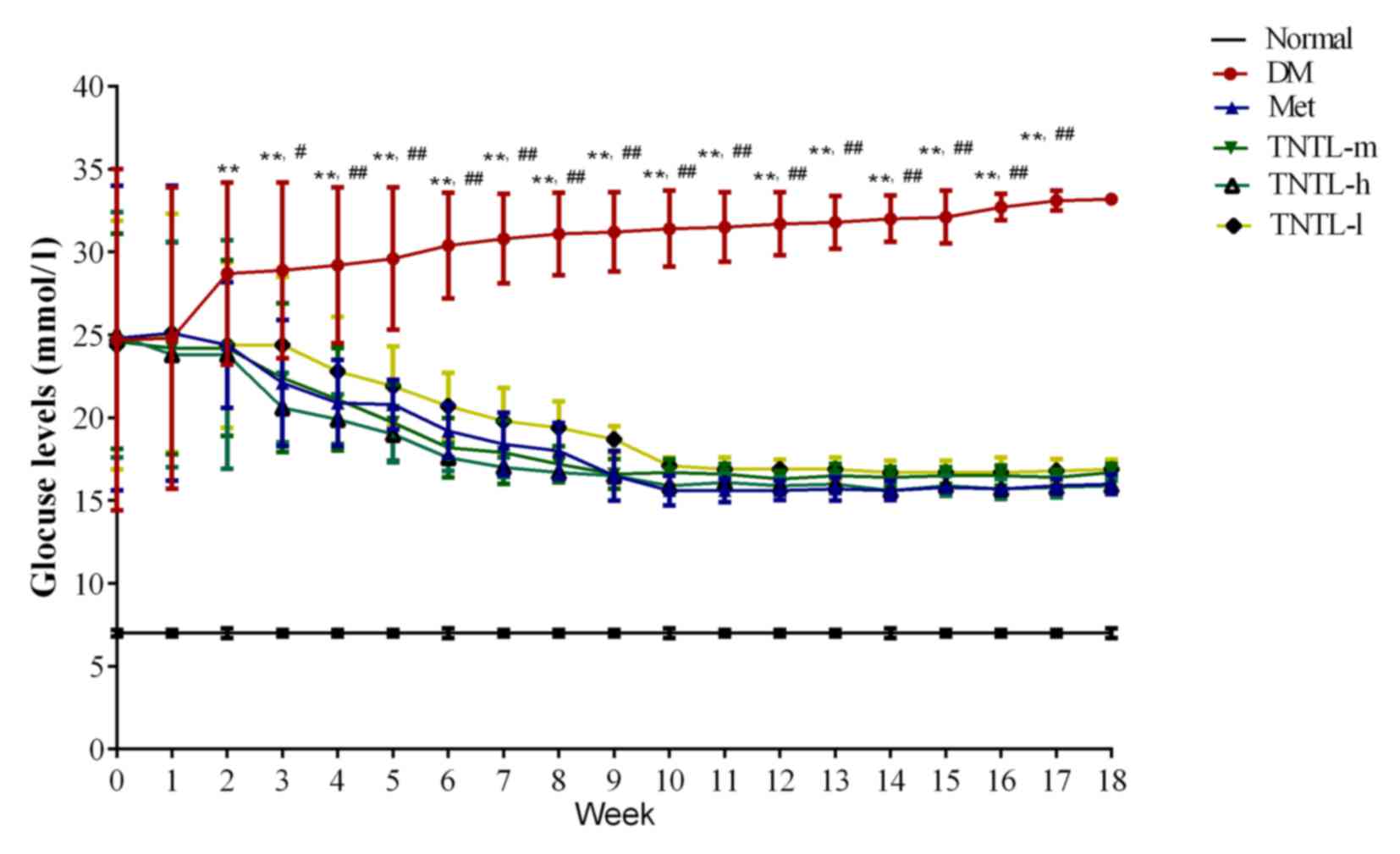 | Figure 1.Fasting blood glucose levels from
weeks 1 to 18. Sustained TNTL treatment exerts a time-dependent
hypoglycemic effect. Data are presented as the mean ± standard
deviation. **P<0.01 vs. Normal mice, #P<0.05 vs.
other 4 treatment groups (Met, TNTL-m, TNTL-h and TNTL-l),
##P<0.01 vs. other 4 treatment groups (Met, TNTL-m,
TNTL-h and TNTL-l). Normal, C57BL mice; DM, untreated C57BLdb/db
mice; Met, db/db mice treated with metformin; TNTL-h, TNTL-m and
TNTL-l, C57BLdb/db mice treated with 1.8, 0.9 and 0.45 g/kg body
weight Tangningtongluo, respectively. |
Serum lipid levels of TG, TC and HDL were increased
significantly in the model group compared with normal mice,
indicating dyslipidemia in db/db diabetic mice. Compared with the
diabetic control group, the TNTL treatment markedly reduced plasma
concentrations of TG, TC and LDL in a dose-dependent manner
(Table I).
 | Table I.Serum lipid levels in C57BL/KsJ-db/db
mice (mg/dl). |
Table I.
Serum lipid levels in C57BL/KsJ-db/db
mice (mg/dl).
| Group | Dose (g/kg) | TC | HDL | LDL | TG |
|---|
| Normal | – |
51.88±11.93b |
20.25±3.21b |
11.23±4.10b |
14.73±3.39b |
| Model | – | 89.33±9.73 | 10.01±4.33 | 17.09±2.38 | 25.09±4.47 |
| Model + Met | 0.14 | 83.01±7.91 |
17.40±1.52a | 14.88±3.25 | 22.59±1.49 |
| Model + TNTL | 1.8 |
69.67±5.51b |
19.86±4.02b |
12.35±1.82b |
17.17±3.40a |
| Model + TNTL | 0.9 |
75.83±9.91a |
18.67±2.33a |
14.67±3.11a | 19.92±3.01 |
| Model + TNTL | 0.45 | 82.20±10.33 | 17.83±3.49 | 15.00±1.82 | 21.97±2.01 |
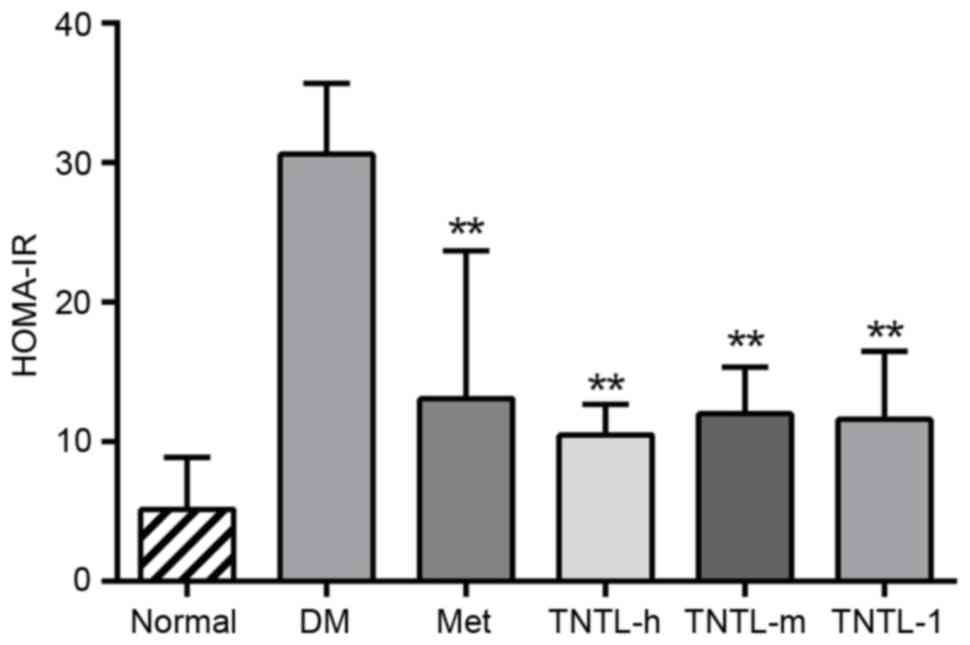
The plasma insulin and C-peptide levels, as assessed
by HOMA-IR index, increased significantly in db/db mice compared
with the normal control group. Treatment with Met resulted in a
significant decrease in the HOMA-IR index compared with the db/db
model (12.86±8.91 vs. 30.61±5.43. TNTL treatment (1.8, 0.9 and 0.45
g/kg BW) resulted in the same effect compared with the db/db model
group (10.55±1.55, 11.85±2.56 and 11.23±4.693 vs. 30.61±5.43,
respectively; Fig. 3).
Effect of TNTL on retinal degeneration
in db/db mice
Fundus examination results from FA prior to drug
intervention are presented in Fig.
4A and results from after 18 weeks of treatment are presented
in Fig. 4B. Compared with normal
control mice (Fig. 4Aa), db/db
mice exhibited deterioration of pathological neovascularization as
T2DM progressed (Fig. 4Ab).
However, 1.8 and 0.9 g/kg BW TNTL treatment significantly reduced
the density of vascular calibers in the fundus oculi (Fig. 4Ad and e and Bd and e).
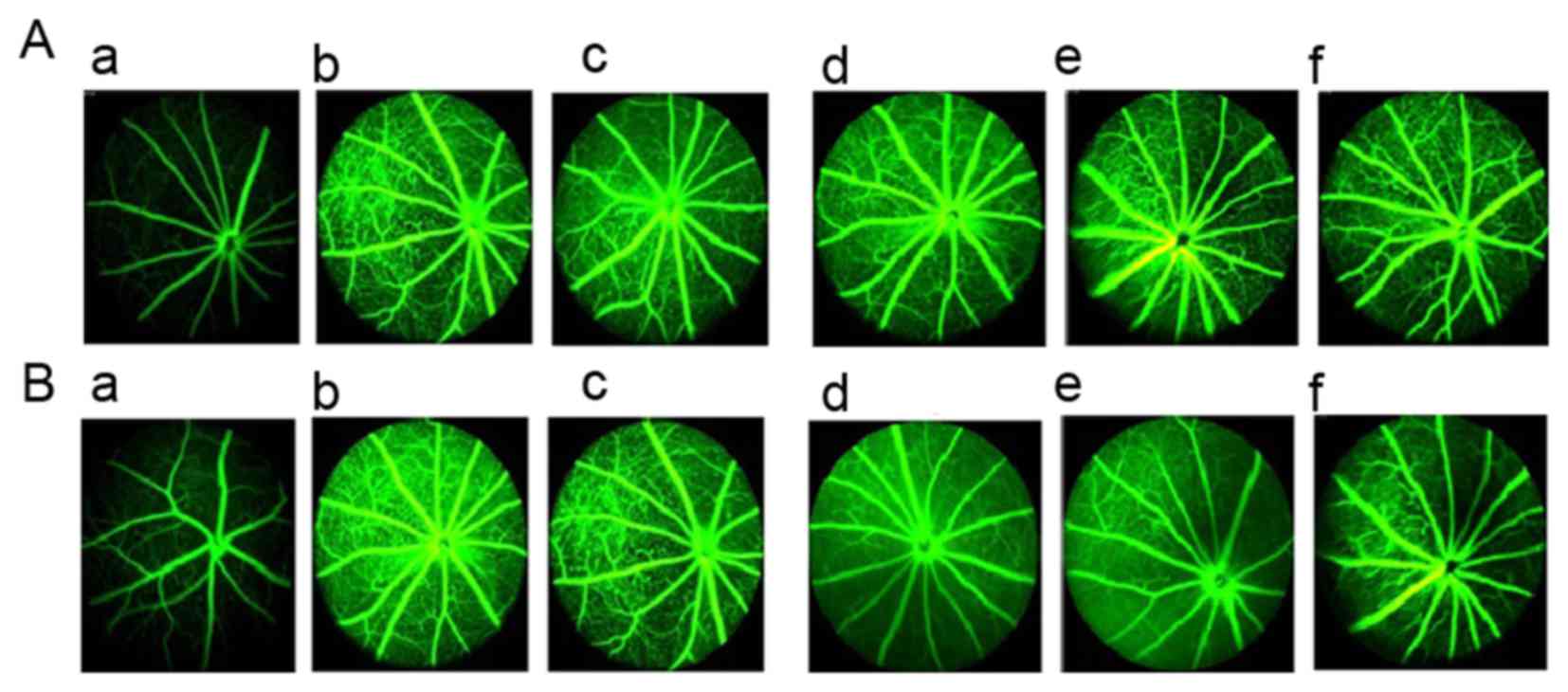 | Figure 4.Retinal imaging using FA in each
group. (A) Results of the fundus examination using FA prior to drug
intervention in the (a) normal, (b), DM, (c), Met, (d), TNTL-h, (e)
TNTL-m and (f) TNTL-1 groups. (B) Results of the fundus examination
using FA after the 18-week drug intervention in the (a) normal,
(b), DM, (c), Met, (d), TNTL-h, (e) TNTL-m and (f) TNTL-1 groups.
Magnification, ×40. Normal, C57BL mice; DM, untreated C57BLdb/db
mice; Met, C57BLdb/db mice treated with metformin; TNTL-h, TNTL-m
and TNTL-l, C57BLdb/db mice treated with 1.8, 0.9 and 0.45 g/kg
body weight Tangningtongluo, respectively; FA, fluorescein
angiography. |
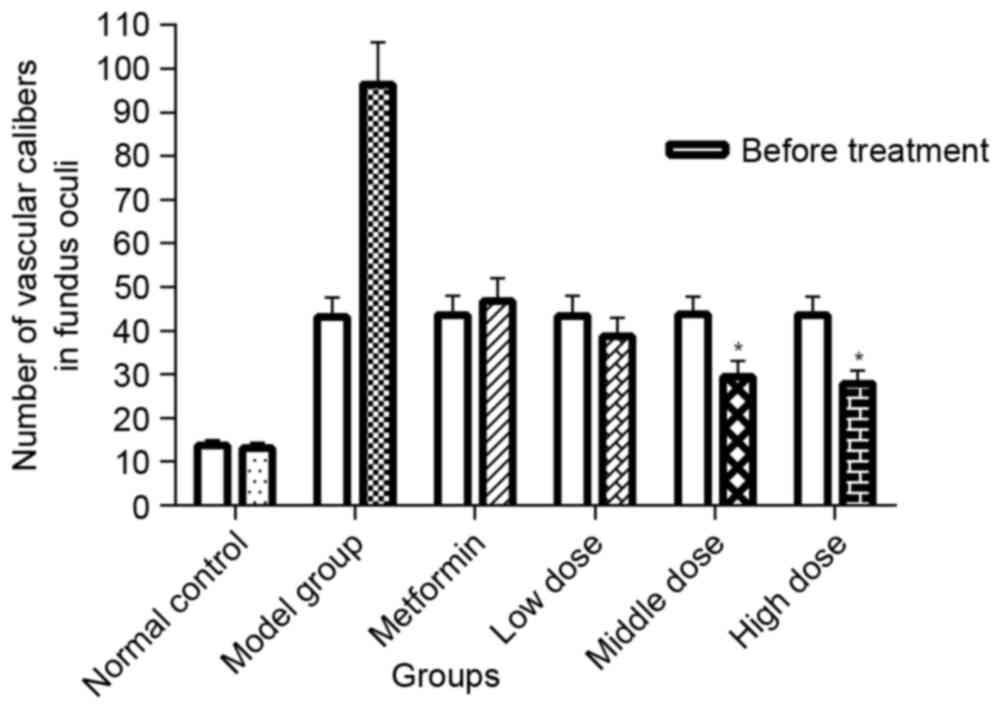
In addition, the number of vascular calibers (i.e.
pathological neovascularization) in the fundus of db/db mice was
counted to quantitatively analyze the effect of TNTL. In diabetic
mice, the number of vascular calibers increased in the model + Met
group along with the progression of T2DM. Quantitative analysis of
the density of vascular calibers in the fundus oculi showed that
TNTL significantly decreased the number of vascular calibers; in
contrast, the positive control group (Met) exhibited no significant
alleviation (Fig. 5).
Effect of TNTL on histopathological
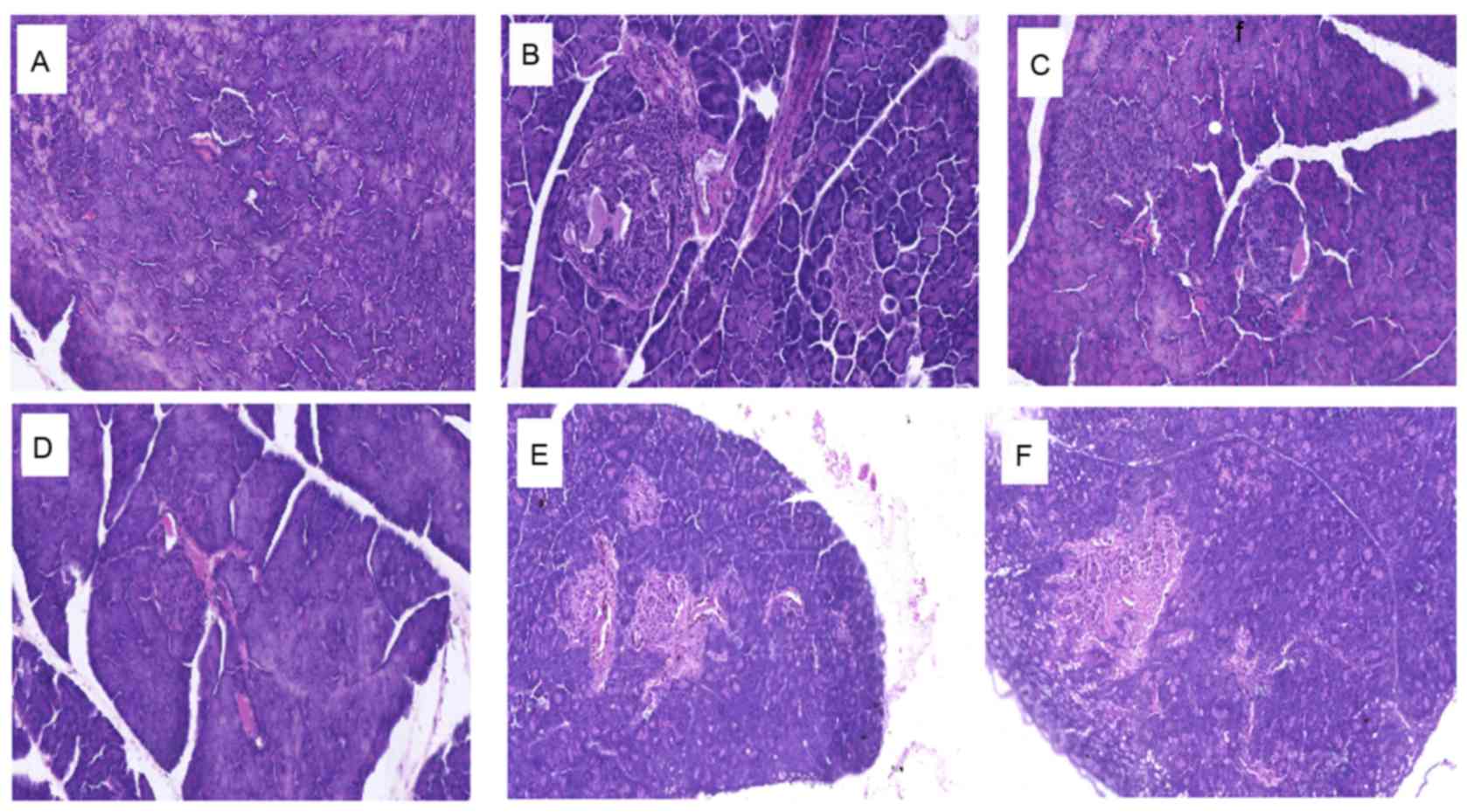
alterations in the pancreas of db/db mice
Histopathological analyses of mouse pancreas
sections are presented in Fig. 6.
Compared with the normal control group, which exhibited a typical
histological structure of islets in the pancreas (Fig. 6A), the diabetic control group
demonstrated evidence of severe damage characterized by a reduced
number and area of islets, transformation of the borders,
vacuolation and degranulation of cells (Fig. 6B). Administration of 1.8, 0.9 and
0.45 g/kg BW TNTL restored the damaged islets and healed the
structure (Fig. 6D-F,
respectively).
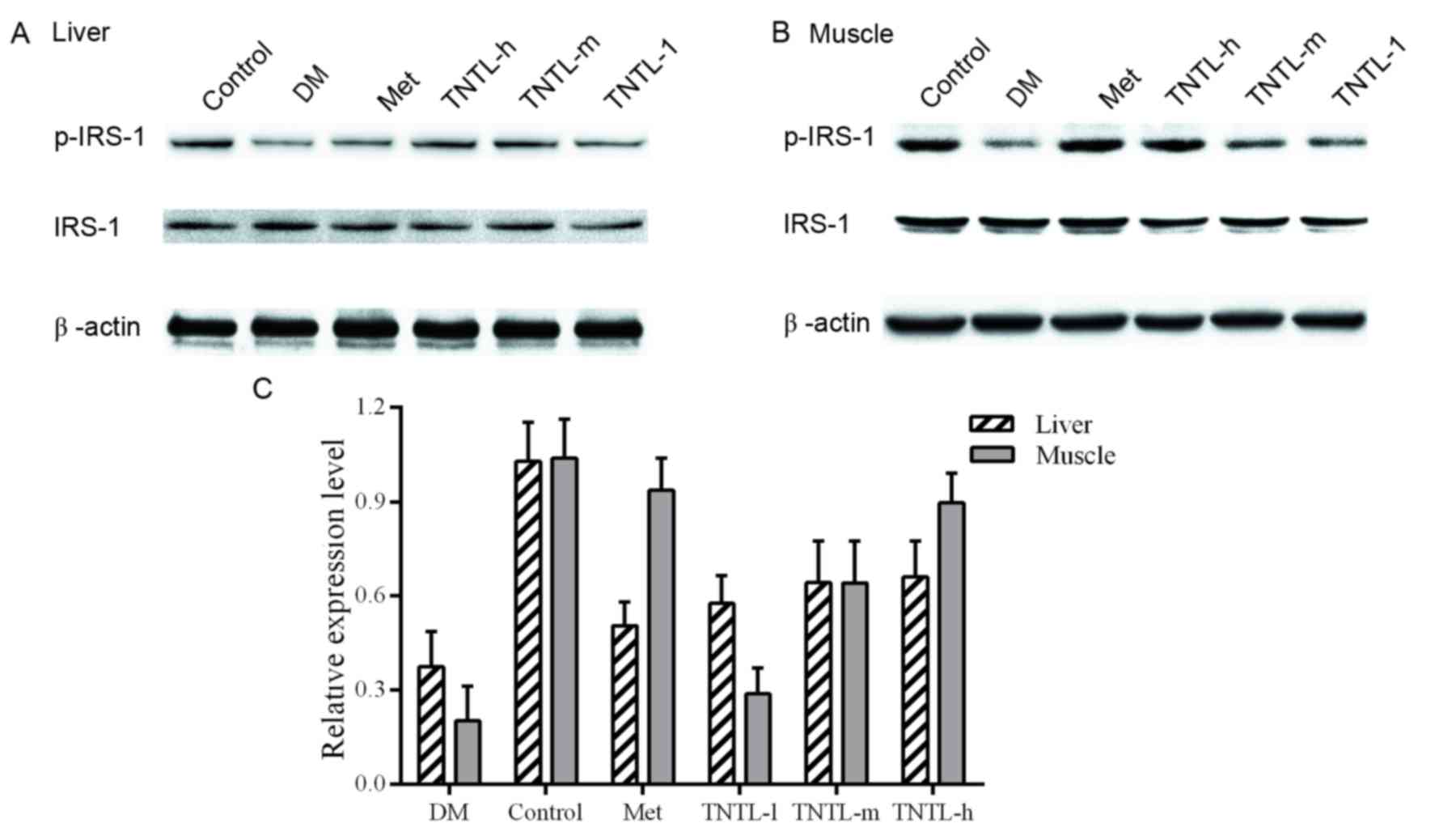
Effect of TNTL on ISR-1 and p-ISR-1
protein expression
Representative western blot images of protein
expression levels of ISR-1 are presented in Fig. 7A and B for liver and muscle tissue,
respectively. Phosphorylation levels of ISR-1 were reduced in the
db/db mice, compared with the normal control group (21.6±8.1% vs.
100.0±5.3% in muscle tissue, 39.8±8.9% vs. 100.0±6.4% in liver
tissue). Increased p-ISR-1expression was observed in the TNTL
group. The administration of TNTL led to higher expression compared
with the diabetic model group, and the difference was statistically
significant. As expected, the p-ISR-1 expression increased
significantly in the Met group.
Discussion
TCM is used widely to treat T2DM not only in China
but also worldwide (13,20,28).
However, the role of TCM and other herbal medicines in the
management of T2DM remains to be established. For most conditions,
there is a lack of scientific evidence on the underlying mechanisms
of TCM methods. Over 80% of the people in developing countries
depend on herbal medicine for their basic health care (19–21,29,30).
Therefore, it is urgent and necessary to confirm the efficacy of
TCM in the management of T2DM.
The present study aimed to evaluate the efficacy of
TNTL, a TCM, to investigate the underlying mechanism. It was
demonstrated that TNTL has an anti-diabetic effect in diabetic
animal models, as evidenced by its hypoglycemic activity.
FA was performed to detect the progressive neural
retinal pathology in db/db mice. The speed of retinal degeneration
was reduced significantly in the TNTL treatment groups, compared
with the placebo and Met-treated control groups. Therefore, TNTL
treatment may markedly reduce or reverse this pathological
neovascularization. The TNTL-induced reduction in the risk of
microvascular complications (diabetic retinopathy) demonstrated the
clinical advantage of comprehensive treatment effects of TCM.
Furthermore, the present study indicated that TNTL treatment
reduced insulin resistance, as evidenced by the decrease in
HOMA-IR. This finding may partially explain the mechanism of the
hypoglycemic effect. Collectively, these results demonstrated that
TNTL exerts a strong hypoglycemic effect and reverses retinal
degeneration. Therefore, TNTL may be beneficial in preventing the
progression of T2DM.
ISR-1, a docking protein that engages downstream
molecules in the insulin signaling network, is a key mediator of
insulin resistance (31–33). IRS-1 is downregulated in the liver
and skeletal muscle of diabetic animals (34). The protein expression levels of
ISR-1 and p-ISR (its active form) were investigated to elucidate
the molecular mechanism underlying the alleviation of insulin
resistance and the hyperglycemic effect. The present study
confirmed that TNTL administration elevated p-IRS-1 protein
expression levels in the liver and skeletal muscle of db/db mice
compared with non-diabetic mice, suggesting that TNTL may restore
the impaired insulin signaling pathway. The result indicated that
the phosphorylation of IRS-1 caused by TNTL administration was
strongly associated with the improvement in insulin resistance.
In conclusion, the results of the present study
demonstrated that TNTL exerts a strong hypoglycemic effect, reduces
insulin resistance and reverses retinal degeneration. In addition,
it was demonstrated that the anti-diabetic effects of TNTL are
strongly involved in the regulation of ISR-1 phosphorylation,
suggesting a potential mechanism. Therefore, TNTL may be an
anti-diabetic agent for the effective treatment of T2DM and an
alternative therapeutic to current drugs. Further investigations
are required to elucidate the precise molecular mechanism by which
TNTL exerts anti-diabetic effects and reverses retinal
degeneration.
Acknowledgements
The present study was supported by the Beijing
Science and Technology Projects (grant no. Z141100002114044), the
National Major Drug Discovery Projects (grant no. 2012ZX09501001
and 2012ZX09301002); The Open Project of Key Laboratory of Zunyi
Medical University (ZY-2015-01); and Guizhou Bailing Pharmaceutical
Co., Ltd. The authors thank scientific editors at American Journal
Experts for providing professional English language editing of this
paper.
Glossary
Abbreviations
Abbreviations:
|
TNTL
|
Tangningtongluo
|
|
TCM
|
traditional Chinese medicine
|
|
db/db
|
C57BL/KsJ-db/db
|
|
T2DM
|
type 2 diabetes mellitus
|
|
IDF
|
International Diabetes Federation
|
|
HbA1c
|
glycated hemoglobin A1c
|
|
Met
|
metformin
|
|
HOMA-IR
|
homeostatic model assessment of
insulin resistance
|
References
|
1
|
Chan JC, Malik V, Jia W, Kadowaki T,
Yajnik CS, Yoon KH and Hu FB: Diabetes in Asia: Epidemiology, risk
factors, and pathophysiology. Jama. 301:2129–2140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan JC, Zhang Y and Ning G: Diabetes in
China: A societal solution for a personal challenge. Lancet
Diabetes Endocrinol. 2:969–979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ning G and Bloomgarden Z: Diabetes in
China: Prevalence, diagnosis, and control. J Diabetes. 5:3722013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weng J and Bi Y: Diabetes in China: The
challenge now. J Diabetes Investig. 1:170–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
L'Heveder R and Nolan T: International
Diabetes Federation. Diabetes Res Clin Pract. 101:349–351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu S, Sun Z, Zheng L, Guo X, Yang H and
Sun Y: Prevalence of Diabetes and Impaired Fasting Glucose in
Hypertensive Adults in Rural China: Far from Leveling-Off. Int J
Environ Res Public Health. 12:14764–14779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu D, Sun L, Fu P, Xie J, Lu J, Zhou J, Yu
D, Whelton PK, He J and Gu D: Prevalence and risk factors for type
2 diabetes mellitus in the Chinese adult population: The InterASIA
Study. Diabetes Res Clin Pract. 84:288–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu D, Fu P, Xie J, Chen CS, Yu D, Whelton
PK, He J and Gu D: MS for the InterASIA Collaborative Group:
Increasing prevalence and low awareness, treatment and control of
diabetes mellitus among Chinese adults: The InterASIA study.
Diabetes Res Clin Pract. 81:250–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia W: Diabetes: A challenge for China in
the 21st century. Lancet Diabetes Endocrinol. 2:e6–e7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chi C, Lee JL, Lai JS, Chen SC, Chen CY
and Chang SK: Utilization of Chinese medicine in Taiwan. Altern
Ther Health Med. 3:40–53. 1997.PubMed/NCBI
|
|
12
|
Hesketh T and Zhu WX: Health in China.
Traditional Chinese medicine: One country, two systems. BMJ.
315:115–117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pang B, Zhou Q, Zhao TY, He LS, Guo J,
Chen HD, Zhao LH and Tong XL: Innovative thoughts on treating
diabetes from the perspective of traditional Chinese medicine. Evid
Based Complement Alternat Med. 2015:9054322015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang C, Ye Y, Feng Y and Quinn RJ: TCM,
brain function and drug space. Nat Prod Rep. 33:6–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong XL, Dong L, Chen L and Zhen Z:
Treatment of diabetes using traditional Chinese medicine: Past,
present and future. Am J Chin Med. 40:877–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Xu Q, Sun Q, Fan FF, Guo XR and
Guo F: Assessment of the reporting quality of randomized controlled
trials on the treatment of diabetes mellitus with traditional
chinese medicine: A systematic review. PLoS One. 8:e705862013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang TT and Jiang JG: Active ingredients
of traditional Chinese medicine in the treatment of diabetes and
diabetic complications. Expert Opin Investig Drugs. 21:1625–1642.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang HL, Niu JJ, Zhang WF, Huang WJ, Zhou
MY, Sha WJ, Li JY, Li FF, Zhu T, Xia X, et al: The role of central
nervous system on hypoglycemia and the feasibility of the brain
theory in traditional Chinese medicine on treatment of diabetes
mellitus. J Integr Med. 12:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lao L and Ning Z: Integrating traditional
Chinese medicine into mainstream healthcare system in Hong Kong,
China-A model of integrative medicine in the HKU-SZ Hospital. J
Integr Med. 13:353–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Wu T, Shang H and Yang K: all
subcentres attending the Chinese EBM Working Meeting in December
2008: Strategies for promoting the development of evidence-based
medicine in China. J Evid Based Med. 2:47–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao PY, Hsu PC, Chen JM, Chiang JY and Lo
LC: Diabetes with pyogenic liver abscess-A perspective on tongue
assessment in traditional Chinese medicine. Complement Ther Med.
22:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin YJ, Ho TJ, Yeh YC, Cheng CF, Shiao YT,
Wang CB, Chien WK, Chen JH, Liu X, Tsang H, et al: Chinese herbal
medicine treatment improves the overall survival rate of
individuals with hypertension among type 2 diabetes patients and
modulates in vitro smooth muscle cell contractility. PLoS One.
10:e01451092015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Q and Fan H: The research for the
clinical curative effect through combing traditional Chinese
medicine with insulin to cure diabetes. Pak J Pharm Sci. 27:(4
Suppl). S1057–S1061. 2014.
|
|
24
|
Cheng L, Meng XB, Lu S, Wang TT, Liu Y,
Sun GB and Sun XB: Evaluation of hypoglycemic efficacy of
tangningtongluo formula, a traditional Chinese Miao medicine, in
two rodent animal models. J Diabetes Res. 2014:7454192014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Charlat O, Tartaglia LA, Woolf EA,
Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et
al: Evidence that the diabetes gene encodes the leptin receptor:
Identification of a mutation in the leptin receptor gene in db/db
mice. Cell. 84:491–495. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kodama H, Fujita M and Yamaguchi I:
Development of hyperglycaemia and insulin resistance in conscious
genetically diabetic (C57BL/KsJ-db/db) mice. Diabetologia.
37:739–744. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wallace TM, Levy JC and Matthews DR: Use
and abuse of HOMA modeling. Diabetes Care. 27:1487–1495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poon TY, Ong KL and Cheung BM: Review of
the effects of the traditional Chinese medicine Rehmannia Six
Formula on diabetes mellitus and its complications. J Diabetes.
3:184–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji L, Tong X, Wang H, Tian H, Zhou H,
Zhang L, Li Q, Wang Y, Li H, Liu M, et al: Efficacy and safety of
traditional chinese medicine for diabetes: A double-blind,
randomised, controlled trial. PLoS One. 8:e567032013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei J, Wu R and Zhao D: Analysis on
traditional Chinese medicine syndrome elements and relevant factors
for senile diabetes. J Tradit Chin Med. 33:473–478. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Angiolillo DJ, Bernardo E, Zanoni M, Vivas
D, Capranzano P, Malerba G, Capodanno D, Prandini P, Pasquali A,
Trabetti E, et al: Impact of insulin receptor substrate-1 genotypes
on platelet reactivity and cardiovascular outcomes in patients with
type 2 diabetes mellitus and coronary artery disease. J Am Coll
Cardiol. 58:30–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suer Orkunoglu FE, Mergen H, Bolu E and
Ozata M: Molecular scanning for mutations in the insulin receptor
substrate-1 (IRS-1) gene in Turkish with type 2 diabetes mellitus.
Endocr J. 52:593–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Sun CM, Hu XQ and Zhao Y:
Relationship between melatonin receptor 1B and insulin receptor
substrate 1 polymorphisms with gestational diabetes mellitus: A
systematic review and meta-analysis. Sci Rep. 4:61132014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karolina DS, Armugam A, Tavintharan S,
Wong MT, Lim SC, Sum CF and Jeyaseelan K: MicroRNA 144 impairs
insulin signaling by inhibiting the expression of insulin receptor
substrate 1 in type 2 diabetes mellitus. PLoS One. 6:e228392011.
View Article : Google Scholar : PubMed/NCBI
|