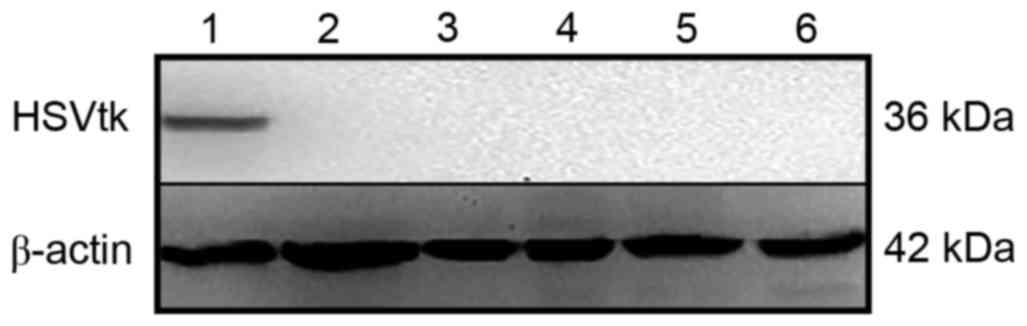
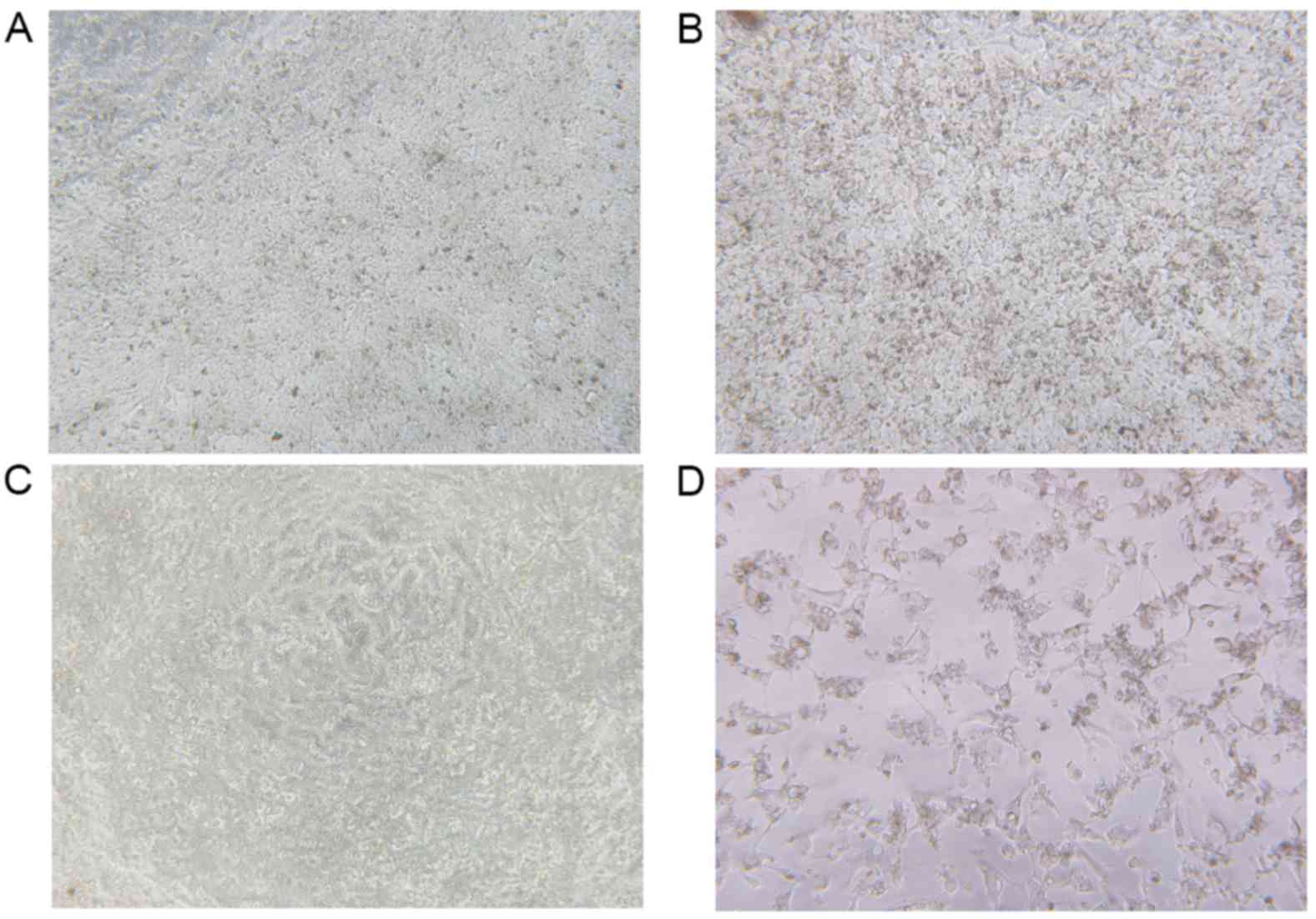
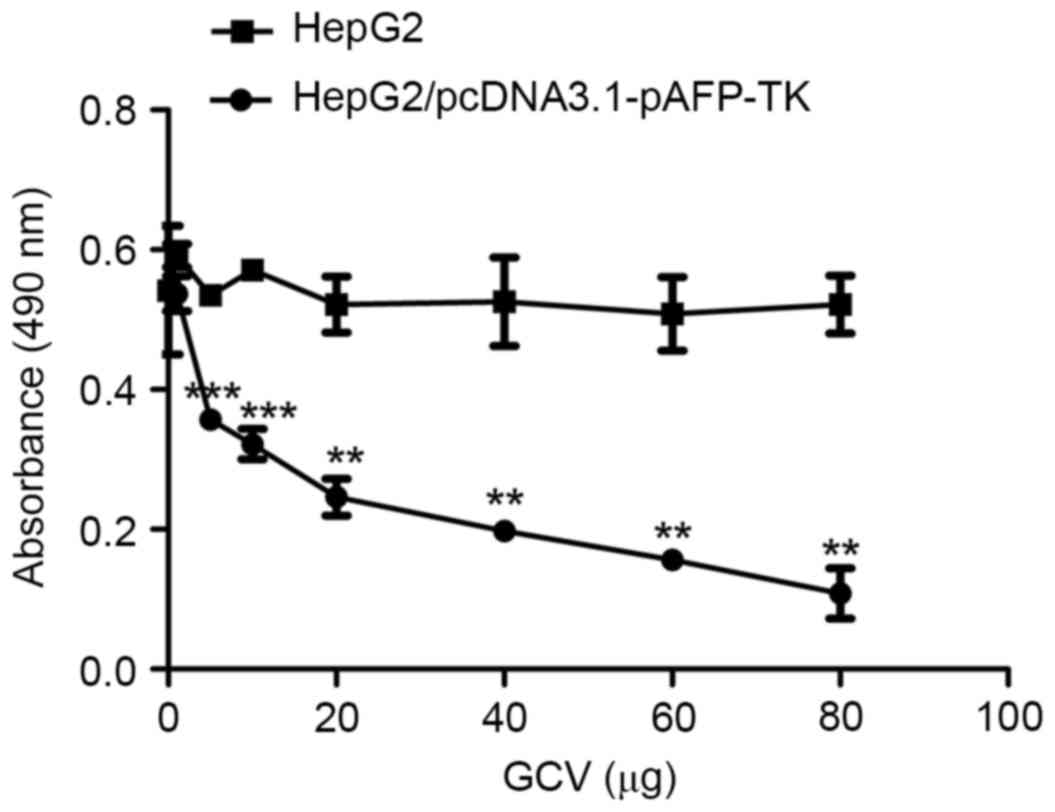
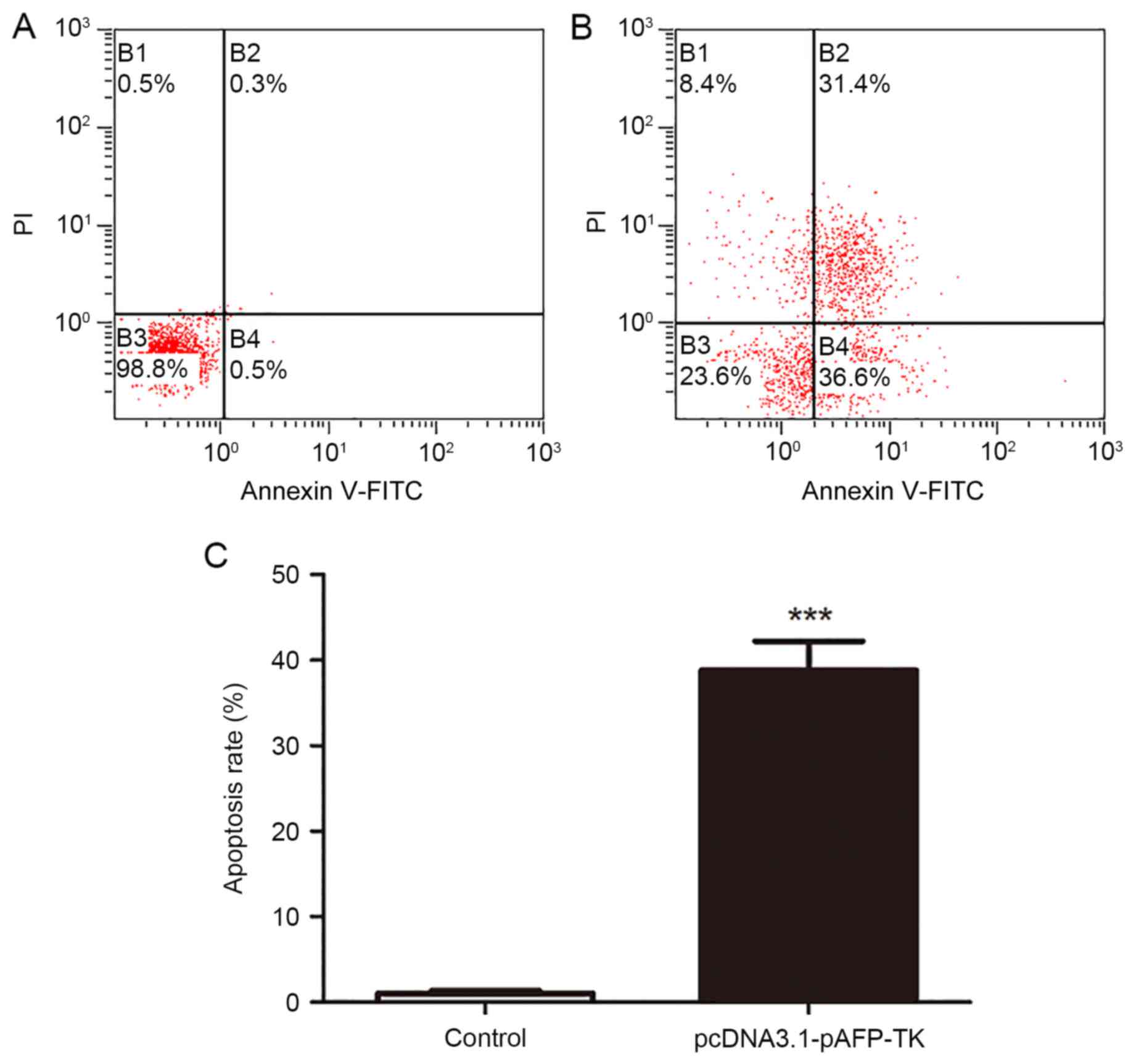
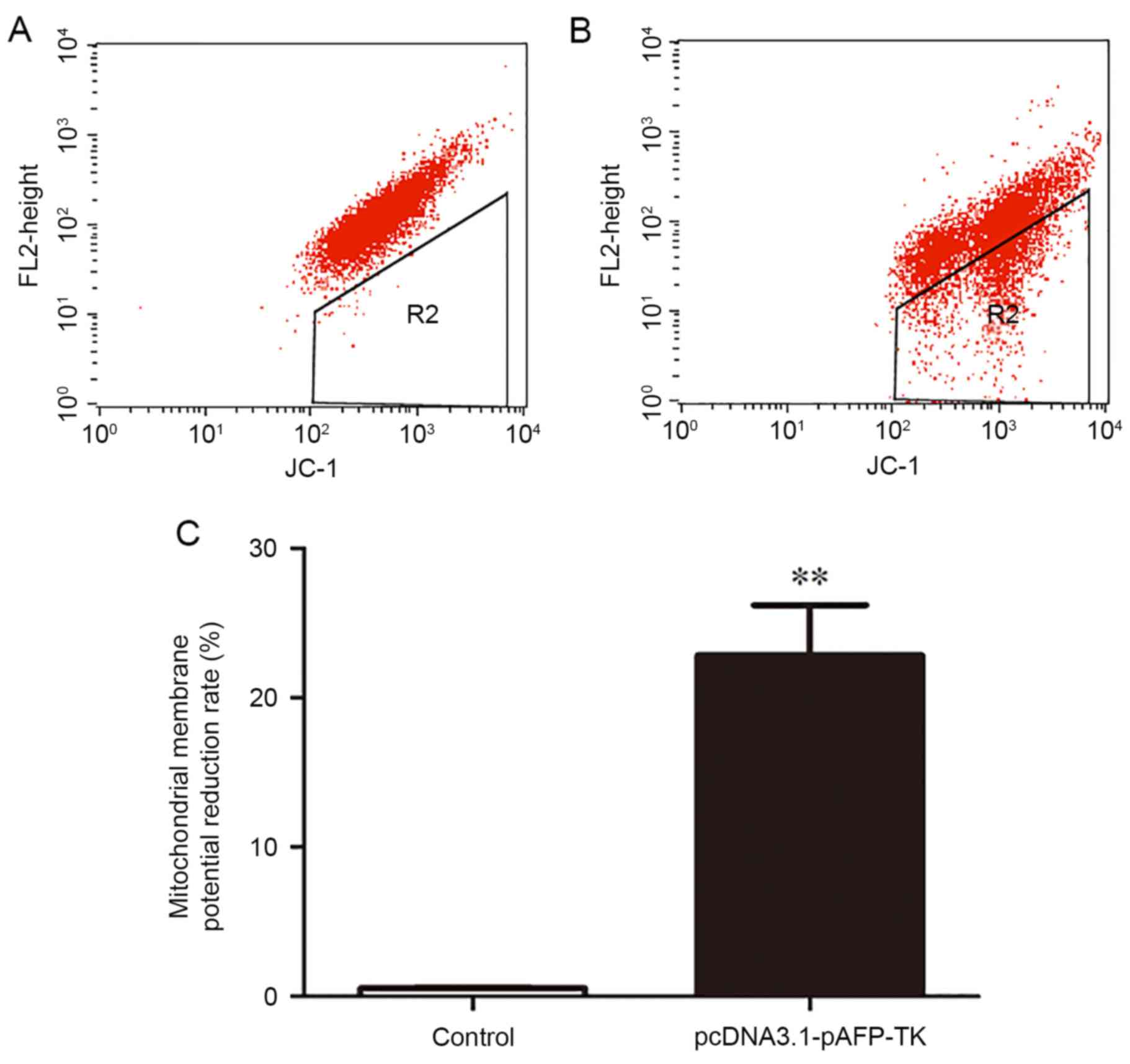
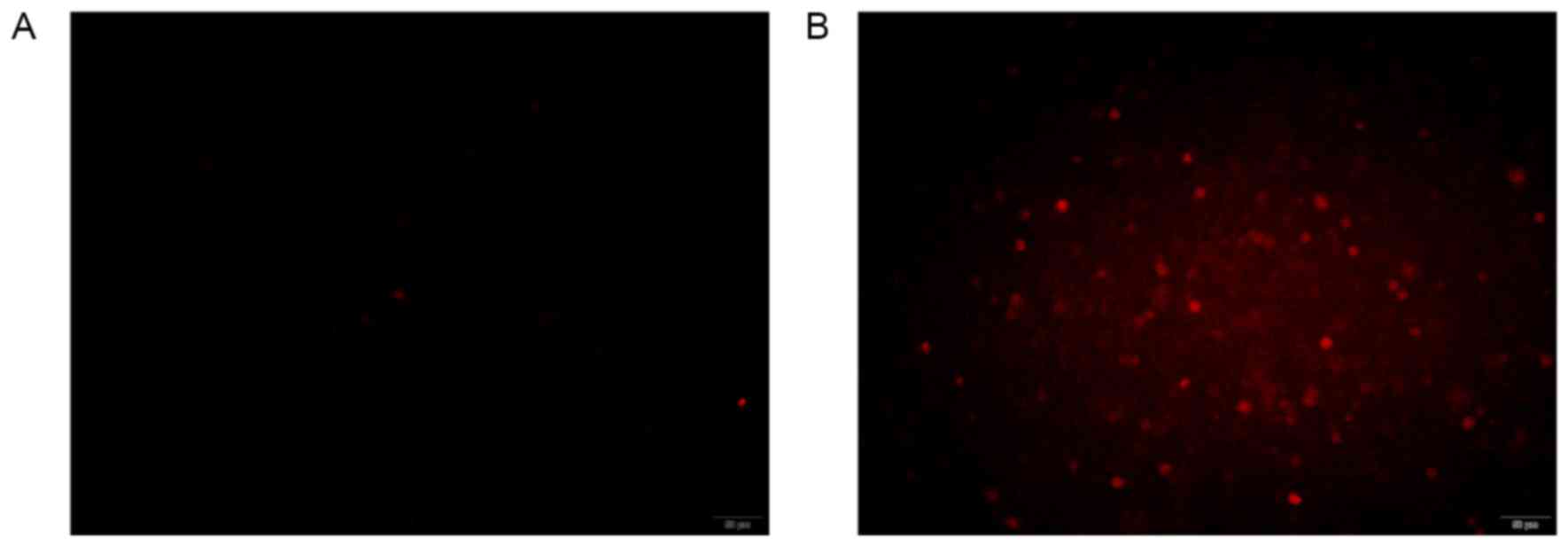
|
1
|
Bosch FX, Ribes J, Diaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127:(5 Suppl 1). S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levin B and Amos C: Therapy of
unresectable hepatocellular carcinoma. N Engl J Med. 332:1294–1296.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arii S, Yamaoka Y, Futagawa S, Inoue K,
Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K and Yamada
R: Results of surgical and nonsurgical treatment for small-sized
hepatocellular carcinomas: A retrospective and nationwide survey in
Japan. The liver cancer study group of Japan. Hepatology.
32:1224–1229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rongrui L, Na H, Zongfang L, Fanpu J and
Shiwen J: Epigenetic mechanism involved in the HBV/HCV-related
hepatocellular carcinoma tumorigenesis. Curr Pharm Des.
20:1715–1725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liver Cancer Study Group of Japan: Primary
liver cancer in Japan. Clinicopathologic features and results of
surgical treatment. Ann Surg. 211:277–287. 1990.PubMed/NCBI
|
|
8
|
Schmitz V, Qian C, Ruiz J, Sangro B,
Melero I, Mazzolini G, Narvaiza I and Prieto J: Gene therapy for
liver diseases: Recent strategies for treatment of viral hepatitis
and liver malignancies. Gut. 50:130–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dachs GU, Dougheay GJ, Stratford IJ and
Chaplin DJ: Targeting gene therapy to cancer: A review. Oncol Ras.
9:313–325. 1997.
|
|
10
|
Ferrara F, Staquicini DI, Driessen WH,
D'Angelo S, Dobroff AS, Barry M, Lomo LC, Staquicini FI, Cardó-Vila
M, Soghomonyan S, et al: Targeted molecular-genetic imaging and
ligand-directed therapy in aggressive variant prostate cancer. Proc
Natl Acad Sci USA pii: 201615400. 2016. View Article : Google Scholar
|
|
11
|
Anderson WF: Gene therapy for cancer. Hum
Gene Ther. 5:1–2. 2008. View Article : Google Scholar
|
|
12
|
Huber BE, Richards CA and Krenitsky TA:
Retroviral-mediated gene therapy for the treatment of
hepatocellular carcinoma: All innovative approach for therapy. Proc
Natl Acad Sci USA. 88:8039–8043. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yashiyasu K and Ayumi T: Gene therapy of
hepatoma: Bystander effects and non-apoptotic cell death induced by
thymidine kinase and ganciclovir. Cancer Lett. 96:105–110. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma WJ, Wang HY and Teng LS: Correlation
analysis of preoperative serum alpha-fetoprotein (AFP) level and
prognosis of hepatocellular carcinoma (HCC) after hepatectomy.
World J Surg Oncol. 11:2122013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su H, Chang JC, Xu SM and Kan YW:
Selective killing of AFP-positive hepatocellular carcinoma cells by
adeno-associated virus transfer of the herpes simplex virus
thymidine kinase gene. Hum Gene Ther. 7:463–470. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cahill BA and Braccia D: Current treatment
for hepatocellular carcinoma. Clin J Oncol Nurs. 8:393–399. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varela M, Sala M, Llovet JM and Bruix J:
Treatment of hepatocellular carcinoma: Is there an optimal
strategy. Cancer Treat Rev. 29:99–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huber BE, Austin EA, Richards CA, Davis ST
and Good SS: Metabolism of 5-fluorocytidineto5-fluorouracic in
human colorectal tumor cells transduced with the cytosine deaminase
gene: Significant antitumor effect when only a small percentage of
tumor cells express cytosine deaminase. Proc Natl Acad Sci USA.
91:8302–8306. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XP, Liu L, Wang P and Ma SL: Human
sulfatase-1 improves the effectiveness of cytosine deaminase
suicide gene therapy with 5-Fluorocytosine treatment on
hepatocellular carcinoma cell line HepG2 in vitro and in vivo. Chin
Med J (Engl). 128:1384–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuchiya K, Asahina Y, Matsuda S, Muraoka
M, Nakata T, Suzuki Y, Tamaki N, Yasui Y, Suzuki S, Hosokawa T, et
al: Changes in plasma vascular endothelial growth factor at 8 weeks
after sorafenib administration as predictors of survival for
advanced hepatocellular carcinoma. Cancer. 120:229–237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sadeghi M, Lahdou I, Oweira H, Daniel V,
Terness P, Schmidt J, Weiss KH, Longerich T, Schemmer P, Opelz G
and Mehrabi A: Serum levels of chemokines CCL4 and CCL5 in
cirrhotic patients indicate the presence of hepatocellular
carcinoma. Br J Cancer. 113:756–762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu L, Wang Y, Gong L, Zhu J, Gong R and Si
J: Suicide gene therapy for hepatocellular carcinoma cells by
surviving promoter-driven expression of the herpes simplex virus
thymidine kinase gene. Oncol Rep. 29:1435–1440. 2013.PubMed/NCBI
|
|
24
|
Yu BF, Wu J, Zhang Y, Sung HW, Xie J and
Li RK: Ultrasound-targeted HSVtk and Timp3 gene delivery for
synergistically enhanced antitumor effects in hepatoma. Cancer Gene
Ther. 20:290–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tilghman SM and Belayew A: Transcriptional
control of the murine albumin/alpha-fetoprotein locus during
development. Proc Natl Acad Sci USA. 79:5254–5257. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakai M, Morinaga T, Urano Y, Watanabe K,
Wegmann TG and Tamaoki T: The human alpha-fetoprotein gene.
Sequence organization and the 5′flanking region. J Biol Chem.
260:5055–5060. 1985.PubMed/NCBI
|
|
27
|
Kanai F, Shiratori Y, Yoshida Y, Wakimoto
H, Hamada H, Kanegae Y, Saito I, Nakabayashi H, Tamaoki T, Tanaka
T, et al: Gene therapy for alpha-fetoprotein-producing human
hepatoma cells by adenovirus-mediated transfer of the herpes
simplex virus thymidine kinase gene. Hepatology. 23:1359–1368.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gerolami R, Uch R, Faivre J, Garcia S,
Hardwigsen J, Cardoso J, Mathieu S, Bagnis C, Brechot C and Mannoni
P: Herpes simplex virus thymidine kinase-mediated suicide gene
therapy for hepatocellular carcinoma using HIV-1-derived lentiviral
vectors. J Hepatol. 40:291–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakai Y, Kaneko S, Nakamoto Y, Kagaya T,
Mukaida N and Kobayashi K: Enhanced anti-tumor effects of herpes
simplex virus thymidine kinase/ganciclovir system by codelivering
monocyte chemoattractant protein-1 in hepatocellular carcinoma.
Cancer Gene Ther. 8:695–704. 2001. View Article : Google Scholar : PubMed/NCBI
|