Introduction
Embryonic stem (ES) cells are derived from the inner
cell mass of a blastocyst of preimplantation embryos (1). They have pluripotency because they
are capable to differentiate into all of the three germ layers:
Ectoderm, endoderm, and mesoderm. Particular transcription factors
and molecular markers are known to regulate pluripotency of ES
cells and empower them ability to differentiate into cells of the
three germ layers (2,3). POU5F1, SOX2, and NANOG proteins may
modulate the pluripotency of ES cells by adjusting how ES cells
differentiate to any germ layer (4). Specifically, POU5F1 is known to
suppress neural ectodermal differentiation and promote mesodermal
differentiation. On the other hand, SOX2 inhibits mesodermal
differentiation and promotes neural ectodermal differentiation
(5). NANOG also helps the actions
of pluripotent factors such as POU5F1 and SOX2 to regulate the
target genes that play an important role in the maintenance of ES
cell pluripotency (6). Using these
established markers to regulate the pluripotency of stem cells may
enable to anticipate how stem cell-specific characteristics are
changed by various factors (7).
Estrogen and progesterone are among the primary
female sex hormones which are involved in the menstrual cycle,
pregnancy, and embryogenesis of humans and other species (8,9). The
placenta synthesizes increased levels of female hormones during
pregnancy (10,11). Extraordinarily, high levels of
these hormones enable the uterus and placenta to improve
vascularization and transfer of oxygen and nutrients, and support
the developing fetus via their receptors (12,13).
They also regulate differentiation in human embryonic stem cells
(hESCs). Estrogen compounds, estradiol (E2) and estriol (E3), have
effects on endodermal and mesodermal differentiation of human
embryoid bodies (14). In another
study, the effects of E2 on differentiation of neural cells were
investigated. E2 promoted differentiation of hESCs into dopamine
neurons via cross-talk between insulin-like growth factors-1 and
estrogen receptor β (15).
Moreover, pregnancy hormones, human chorionic gonadotropin and
progesterone, induced hESCs proliferation and differentiation into
neuroectodermal rosettes. hESCs expressed P4 receptor A, and
treatment of hESCs colonies with P4 induced neurulation, as
demonstrated by the expression of nestin and the formation of
columnar neuroectodermal cells that organize into neural tubelike
rosettes. Suppression of P4 signaling by treating with the
P4-receptor antagonist RU-486 inhibited the differentiation of hESC
colonies into EB's and rosettes (16). These results demonstrate that these
hormones influence on the differentiation of hESCs.
In addition, they play an important role in the
epithelial-mesenchymal transition (EMT) process (17). EMT is a multiple step of conserved
cellular programs that enable polarized immotile epithelial cells
to transform to motile mesenchymal cells. EMT and its reverse
process, MET (mesenchymal-epithelial transition), are critical for
embryonic development and the formation of various tissues or
organs (18). During development,
the EMT program has been observed in a variety of tissue remodeling
events, including mesoderm formation, neural crest development,
heart valve development, and secondary palate formation (19,20).
During EMT, epithelial cells lose apical-basal polarity and cell
junctions like tight junctions to acquire a mesenchymal phenotype
(21). Adherens and gap junctions
are also dissolved, and cell surface proteins such as E-cadherin
and epithelial-specific integrins that mediate epithelial
connections to neighboring cells and the basement membrane,
respectively, are replaced by N-cadherin and mesenchymal integrins.
Inside the cells, cytokeratins, the epithelial intermediate
filaments, are replaced by Vimentin (22,23).
Consequently, mesenchymal cells devoid of cell-cell contacts, which
are derived from epithelial cells via EMT, move into and invade
surrounding stroma. As a highly coordinated and specific series of
events, EMT is modulated by diverse regulators including SNAIL/SLUG
(SNAI1/SNAI2), Twist, and Six1 and signaling pathways (23).
In this study, we investigated whether female sex
steroid hormones influence EMT and maintenance of pluripotent
propensity of human ES cells using a new culture method including
conditioned medium (CM) that can maintain undifferentiated state of
ES cells without the mouse feeder cells. Generally, mouse feeder
cells have been used to help the growth and maintenance of human ES
cells, however, there may be a risk of contamination of culture
media by viruses or other macromolecules, which already exists in
the mouse cells and can transfer to the ES cells (24).
Our findings demonstrated that hormone treatment
leads to the process of EMT through the up- or down-regulation of
cellular markers and signaling proteins. In addition, its treatment
altered protein expression of transcriptional factors such as
POU5F1, SOX2, and NANOG, which are known to help maintain
pluripotent ability of ES cells. From this study, we confirmed that
E2 and P4 could affect the EMT in human ES cells and ES cell
differentiation due to the loss of pluripotency. It is hoped that
this study will provide new insights for the relationships between
female sex steroid hormones and embryo developmental process.
Materials and methods
Reagents and human ES cells
17β-Estradiol (E2), progesterone (P4), fulvestrant
(ICI 182,780), and mifepristone (RU486, a progesterone receptor
(PR) antagonist) were purchased from Sigma-Aldrich (St. Louis, MO,
USA) and diluted in dimethyl sulfoxide (DMSO; Junsei Chemical Co.,
Ltd., Tokyo, Japan). The prepared solution was stored at room
temperature. H9 human ES cells were obtained from Dr Jaejin Cho
(Department of Dental Regenerative Biotechnology, Seoul National
University, Seoul, Republic of Korea). The use of human ES cells in
this study was approved by IRB Committee of Chungbuk National
University (CBNU-201407-ETC-064-01).
Preparation of human ES cells
medium
A culture medium for human ES cells is composed of
Dulbecco's modified Eagle's medium/Ham's F-12 medium (DMEM/F12;
Gibco, Grand Island, New York, USA) supplemented with 20% knockout
serum replacement (KSR; Gibco), 0.25 M Peni/Strep (A&E
Scientific, Logan, UT, USA), 0.1 mM nonessential amino acids
(Gibco), and 0.1 mM 2-mercaptoethanol. For a prolonged culture, 4
ng/ml recombinant human fibroblast growth factor-basic (bFGF;
R&D Systems, Inc., Minneapolis, MN, USA) was required to be
added in the medium just before use.
Preparation of CM
Mouse embryonic fibroblast (MEF) feeder cells were
cultured in Dulbecco's modified Eagle's medium (DMEM/F12; Gibco)
supplemented with 10% heat inactivated fetal bovine serum (FBS;
Hyclone Laboratories, Inc., Logan, UT, USA), 1% Peni/Strep (A&E
Scientific), 2% Antibiotic-Antimycotic (GIBCO Invitrogen, Grand
Island, New York, USA), 1% glutamax (Gibco), 1% nonessential amino
acids (Gibco), and 0.1% 2-mercaptoethanol in a humidified
atmosphere of 5% CO2 containing air. When MEF feeder
cells reached approximately 80% of confluent growth, the MEF medium
was removed, and the cells were washed twice with 1X PBS. Fresh
medium for human ES cells was added to the MEF dishes, and CM was
collected every day. MEF cells can be used for up to 7 days to
produce CM. The CM collected from the MEF culture dishes after 24 h
was sterilized with a 0.2-µm filter and was stored at −20°C before
adding bFGF for later use.
Preparation of Matrigel-coated
plates
Matrigel (BD Biosciences, Bedford, MA, USA) was
slowly thawed on ice at 4°C for at least 1 h to prevent the
formation of gel clot. To prepare the working solution, Matrigel
(BD Biosciences) stock solution was diluted to 1:4 in cold CM
prepared beforehand. Each bottom of a 35 mm culture plate was
coated with 1 ml of Matrigel working solution. Next, the working
solution was removed, and the Matrigel-coated plates were incubated
for 1–2 h at room temperature, or overnight at 4°C until the plate
is completely dried.
Culture of human ES cells in a
feeder-free condition
A sharpened tip was made by heating a sterile
pasteur pipette with the alcohol lamp. The morphology of human ES
cells was checked under the microscope. Advised shape of ES cell
colonies is round, and the color is recommended to be dark. When
cells begin to differentiate, they look like dim because they grow
being overlapped so that the light of the microscope is difficult
to permeate the cells. It should be careful that the cell surface
is not clean or uneven. When the suitable number of colonies is
determined, the culture medium for human ES cells was completely
removed and washed with CM twice. The ES cell colonies were
separated into small numbers about 20–30 using a pasteur pipette
tip with a sharpened edge. After collecting the colonies using a
200 µl pipette, they were moved to the conical tube containing
approximately 1 ml of CM. After adding an appropriate amount of CM,
the cells were dissociated into small clusters (50–100 cells) by
gentle pipetting. Human ES cells were seeded into each well of
Matrigel-coated plate and were feed with 2–2.5 ml of CM
supplemented with 4 ng/ml bFGF per well every day.
RNA extraction and cDNA synthesis
mRNA expression of pluripotency-related markers was
identified in human ES cells cultured in a conventional medium as
well as a CM containing E2 and P4. Human ES cells were treated with
E2 (10−8 M) and P4 (10−6 M) for 24 h. RNA was
extracted with the Cell Lysis & RT Kit for qPCR (Toyobo Co.,
Ltd., Osaka, Japan) with a following protocol recommended by the
manufacturer. Briefly, 50 µl lysis solution with gDNA remover was
added to plates, and the solution was mixed gently using a 1 ml
pipette and incubated at room temperature for 5 min. Next, 10 µl
stop solution with RNase inhibitor was added to wells, and the
solution was mixed with tapping hands for 30 sec and incubated at
room temperature for 1.5 min. When the RNA extraction is completely
prepared, 8 µl 5X RT Master Mix was add to 24 µl nuclease-free
water in a tube. 32 µl reaction mixture was transferred into a PCR
plate, and 8 µl of the RNA lysate was added. The solution was mixed
gently and span down. And then, the mixture was incubated as
follows: for 15 min at 37°C, 5 min at 50°C, and 5 min at 98°C.
Polymerase chain reaction (PCR)
PCR amplification was performed for 30 cycles of
denaturation for 30 sec at 95°C, annealing for 30 sec at 58°C, and
extension for 30 sec at 72°C. The composition of the reaction
product is composed of cDNA template, Taq polymerase (Intron
Biotechnology, Seoul, Korea), dNTP, 10x PCR buffer (Intron
Biotechnology), and forward and reverse primers (Table I). The PCR products were loaded
onto a 1.5% agarose gel pretreated with EtBr, and the band sizes
were compared with 100-bp ladders. The bands were analyzed with Gel
Doc 2000 software (Bio-Rad Laboratories Inc, Hercules, CA, USA).
GAPDH was used as an endogenous control for normalization.
 | Table I.Oligonucleotide primer sequences of
genes for RT-PCR. |
Table I.
Oligonucleotide primer sequences of
genes for RT-PCR.
| Gene | Primer sequences
(5′→3′) | Expected size |
|---|
| GAPDH | F:
5′-ATGTTCGTCATGGGTGTGAACCA-3′ | 356 bp |
|
| R:
5′-TGGCAGGTTTTTCTAGACGGCAG-3′ |
|
| POU5F1 | F:
5′-CGTGAAGCTGGAGAAGGAGAAGCTG-3′ | 245 bp |
|
| R:
5′AAGGGCCGCAGCTTACACATGTTC-3′ |
|
| SOX2 | F:
5′-ACACCAATCCCATCCACACT-3′ | 224 bp |
|
| R:
5′-GCAAACTTCCTGCAAAGCTC-3′ |
|
| NANOG | F:
5′-TGCAAATGTCTTCTGCTGAGAT-3′ | 286 bp |
|
| R:
5′-GTTCAGGATGTTGGAGAGTTC-3′ |
|
Western blot assay
Human ES cells were treated with E2 (10−8
M), P4 (10−6 M), ICI 182,780 (10−6 M) or
RU486 (10−8 M) for 48 h. Cells were harvested with RIPA
buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl, 1% Triton X-100
(Sigma-Aldrich), 0.5% deoxycholic acid (Sigma-Aldrich), and 0.1%
SDS. Western blot analysis was performed as previously described
(25). Briefly, protein
concentration was determined by a bicinchoninic acid
(Sigma-Aldrich) assay. Total cell proteins (40 µg) were separated
on a 10% SDS-PAGE gel and then transferred to a polyvinylidene
fluoride (PVDF) membrane (BioRad, Laboratories, Inc., Berkeley, CA,
USA). The membrane was then incubated with E-cadherin, N-cadherin,
Snail, Slug, POU5F1, SOX2, and NANOG. Mouse monoclonal antibodies
against GAPDH (1A10), N-cadherin (5D5), Slug (1G7), POU5F1 (C-10),
SOX2 (E-4), NANOG (H-2) (1:12,000, 1:1,000, 1:1,000, 1:1,000,
1:1,000 and 1:1,000 dilution, respectively; Abcam, Cambridge, UK),
and rabbit polyclonal antibodies against E-cadherin (1:2,50
dilution; Abcam) and Snail (1:1,000; Abcam) were overnight at 4°C.
Horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin
(Ig) G or anti-rabbit IgG (1:3,000, 1:2,500 dilution, respectively;
Thermo Fisher Scientific, Inc., Rockford, IL, USA) was used as a
secondary antibody. Immunoreactive bands were detected using a West
Q chemiluminescent substrate Kit Plus (GenDEPOT, Barker, TX, USA).
Densities of target protein bands were quantified using Gel Doc
2000 (BioRad Laboratories, Inc.).
Statistics analysis
All experiments were repeated at least 3 times, and
data are presented as the mean ± SD. Statistical analysis was
conducted by using one-way ANOVA test, followed by Dunnett's
multiple comparison test or Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
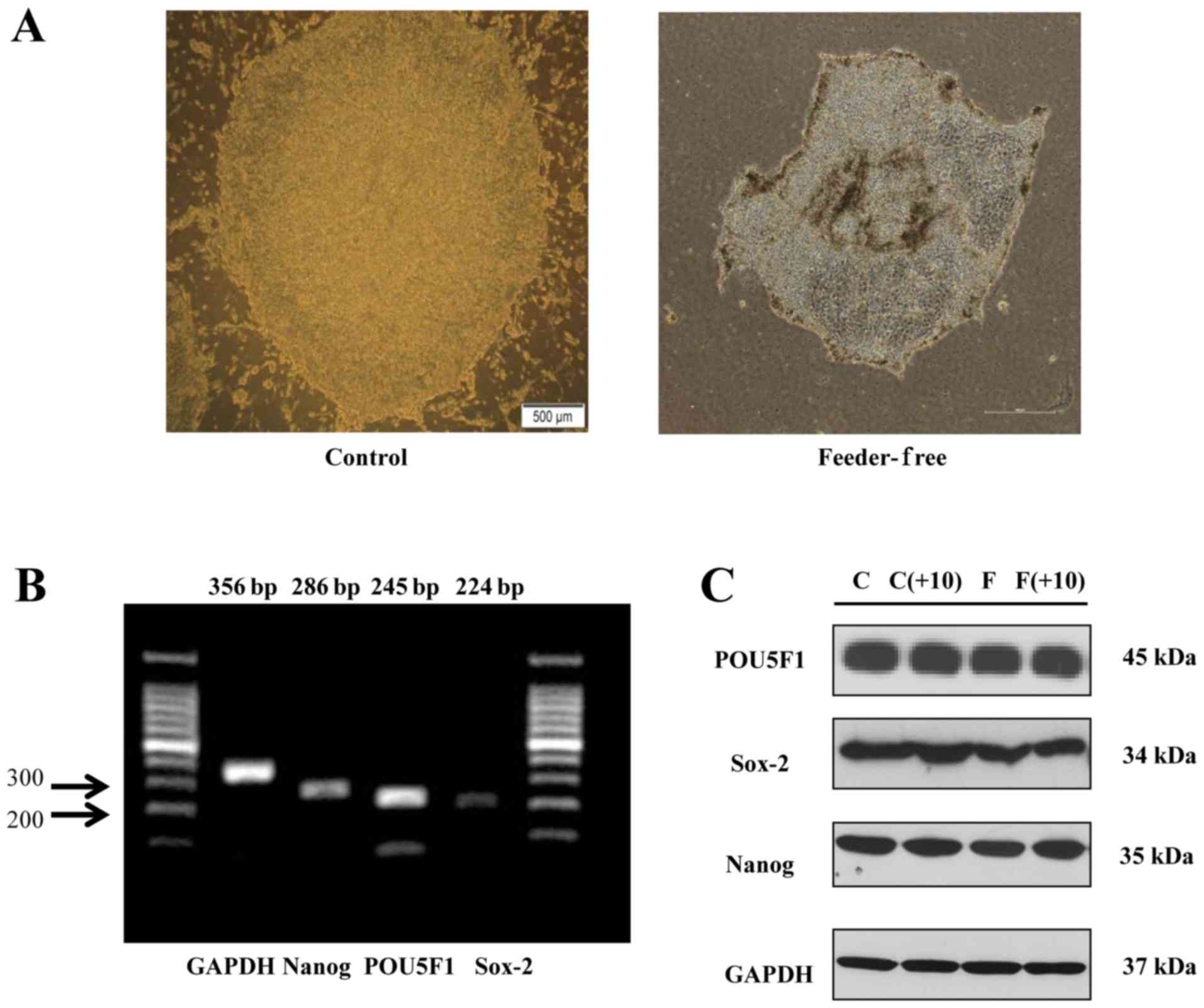
Maintenance of undifferentiation of ES
cells in a feeder-free condition
Undifferentiated human ES cells usually form round
colonies with clear margins. The morphology of ES cell colony from
conventional culture seen in Fig.
1A (Control) displayed the propensity of small, tightly packed
cells that grow in a monolayer. The colony looked clean, defined
edges, with little or no differentiation. Different cell features
were identified because they were tightly packed with each other
suggesting close cell membrane contact. Cells were grown to
overlapping, so it seems like dark. As we expected, however, the
colony of human ES cells cultured in a feeder-free condition showed
a round and well-defined edge (Fig.
1A, Feeder-Free), which is similar to that of ES cell colony
from conventional culture. The surface of the colony looks like
bumpy, because feeder-free medium did not contain MEF cells, which
support ES cells. In Fig. 1B,
RT-PCR data showed that human ES cells cultured in a conventional
method expressed mRNAs of undifferentiated ES cell-markers such as
POU5F1, SOX2, and NANOG genes. By western blotting,
protein expression levels of POU5F1, SOX2, and NANOG were similar
to mRNA expression, as shown in Fig.
1C. Specifically, we also observed that the protein expression
levels of POU5F1, SOX2, and NANOG were constantly maintained in a
conventional medium as well as a feeder-free CM after the 10th
passage of human ES cell culture as in the initial passage
(Fig. 1C).
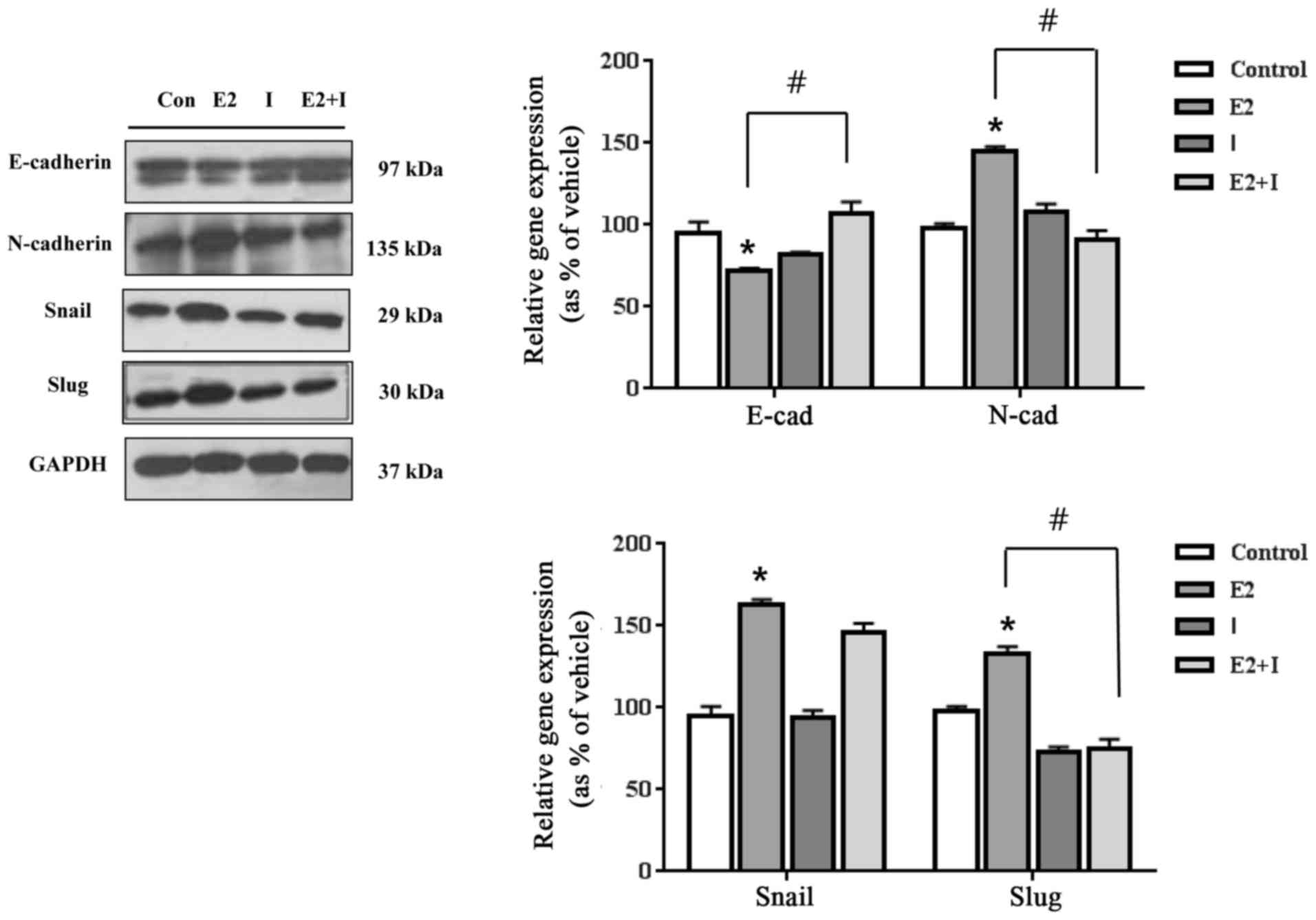
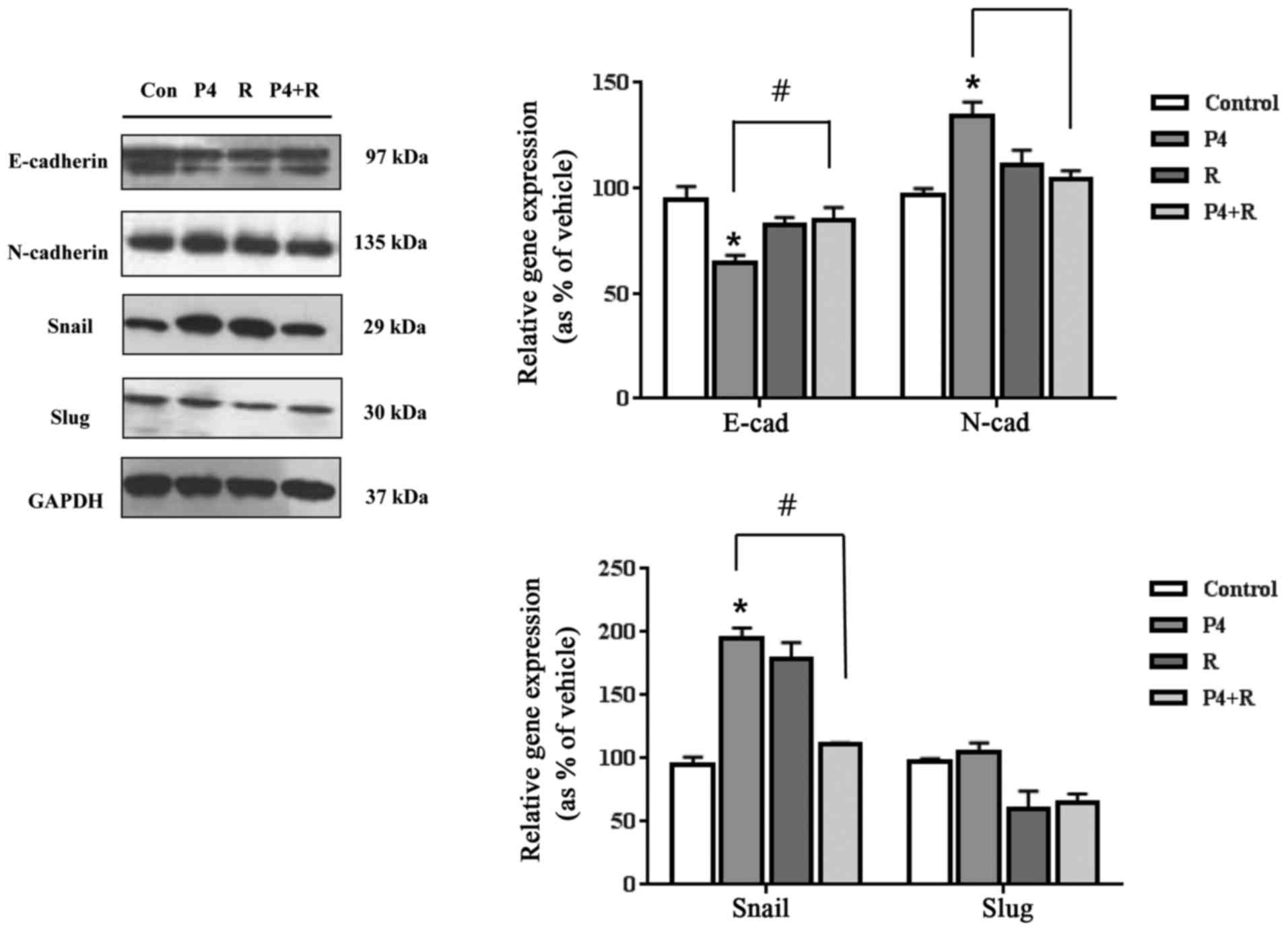
Sex steroid hormones affected EMT of
ES cells through their cognate receptors
To investigate whether sex hormones influence on EMT
of human ES cells, E2, P4, and inhibitors of their cognate
receptors (estrogen receptor inhibitor; ICI 182,780 and
progesterone receptor inhibitor; RU486) were treated to human ES
cells individually or by mixture. As shown in Figs. 2 and 3, E2 (10−8 M) and P4
(10−6 M) treatment induced the EMT process in human ES
cells. The protein expression of an epithelial cell marker,
E-cadherin, was reduced by E2 and P4. In contrast, the protein
expression of a mesenchymal cell marker, N-cadherin, was increased
by E2 and P4 as shown in Figs. 2
and 3. When ES cells were
co-treated with E2 in the presence of ICI 182,780, the protein
expression levels of E-cadherin and N-cadherin were distinctly
restored to the control levels as seen in Fig. 2. In addition, P4 distinctly
regulated these proteins, and the expression levels of E-cadherin
and N-cadherin were restored to the control levels in the presence
of RU486 (Fig. 3). These results
suggest that E2 and P4 may induce EMT by altering protein
expression of E-cadherin and N-cadherin via their receptors.
In addition to cell specific markers, the alteration
of transcription factors that control EMT was further investigated.
E2 increased protein expression of Snail, an E-cadherin repressor,
and Slug, an EMT regulator gene, while ICI 182 780 reversed
E2-induced increases via an ER-dependent manner (Fig. 2). It was of interest that P4
treatment increased Snail only in these cells as seen in Fig. 3. However, when ES cells were
co-treated with P4 and RU486, the altered expression levels of
Snail or Slug by P4 were restored to the control levels (Fig. 3). As a result, these results
indicate that E2 and P4 appear to induce the EMT process of ES
cells through their cognate receptors.
Effect of sex steroid hormones on mRNA
and protein expressions of cell pluripotency-related genes
POU5F1, SOX2 and NANOG are essential transcription
factors that maintain the self-renewal and pluripotency of ES cells
(5). Through RT-PCR and western
blot analyses, we investigated whether the female sex steroid
hormones could affect the expression of these pluripotency-related
genes in human ES cells. When ES cells were treated with E2
(10−8 M), mRNA expression of POU5F1 and
NANOG genes was decreased compared to the control (Fig. 1). When ES cells were treated with
P4 (10−6 M), mRNA expression of NANOG gene was
decreased compared to the control (Fig. 1). However, mRNA expression level of
SOX2 gene was not altered by E2 or P4 treatment (Fig. 4A). For the protein expression of
these genes, the expression levels of SOX2 and NANOG
genes were shown to be reduced by E2 and P4 compared to the control
level (Fig. 4B and C). When ES
cells were co-treated with E2 or P4 in combination with each
inhibitor, the altered expression levels of SOX2 or NANOG proteins
were recovered to the control levels (Fig. 4B and C). These results indicated
that E2 and P4 may reduce the pluripotency of human ES cells by
inhibiting the expression of pluripotency-associated markers such
as POU5F1, SOX2, and NANOG genes in transcriptional
or translational levels.
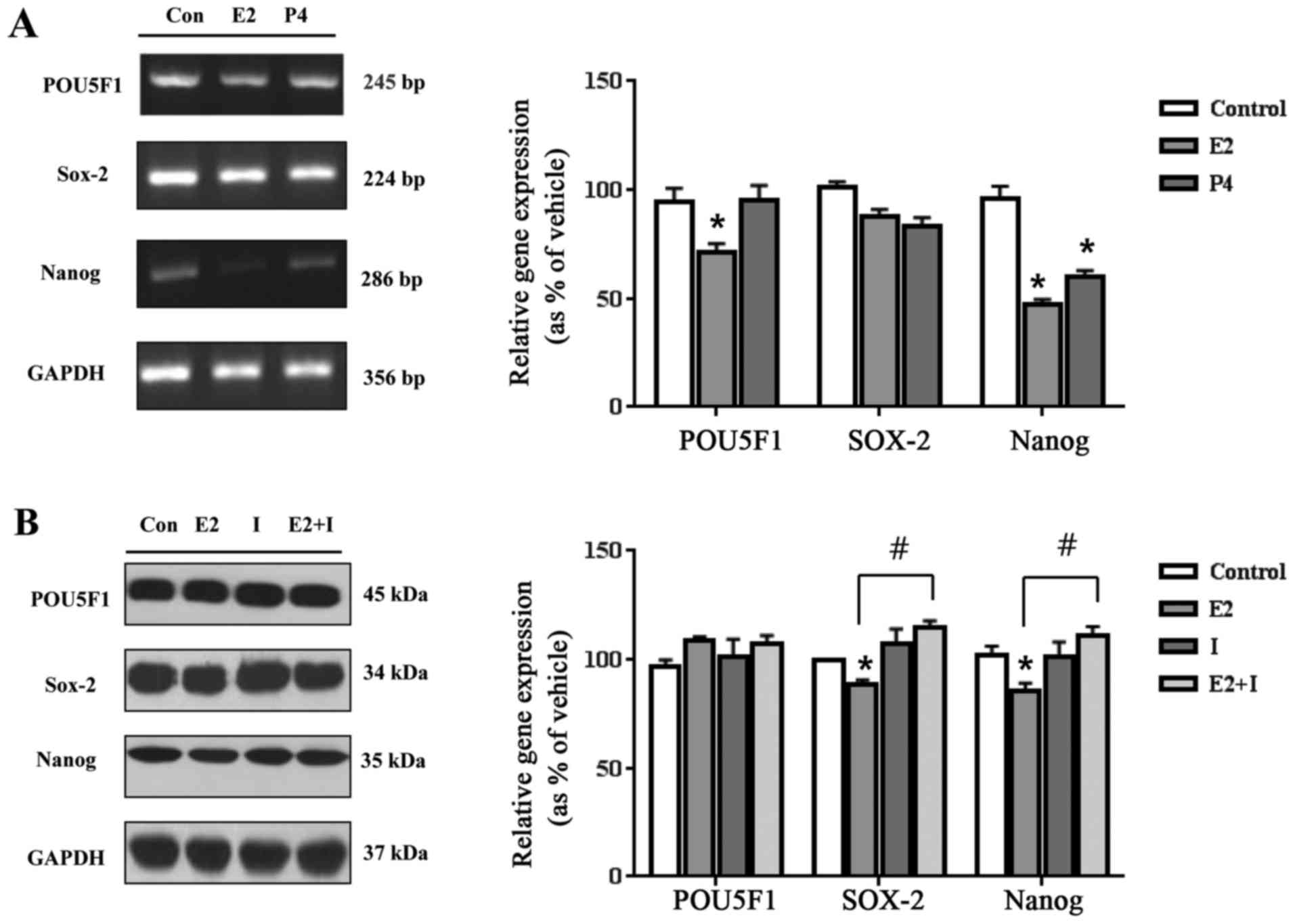 | Figure 4.Effects of E2 and P4 on mRNA and
protein expressions of pluripotency-related markers. (A) After the
treatment of E2 (10−8 M) and P4 (10−6 M) in
human ES cells for 24 h, total RNAs were extracted, and bands
corresponding to POU5F1, SOX2, and NANOG genes were detected by
RT-PCR and quantified using Gel Doc 2000. *Significant elevation or
reduction in gene expressions by each treatment comparing with
control (P<0.05 in Dunnett's multiple comparison test). (B)
After the treatment of E2 (10−8 M), ICI 182,780
(10−6 M), and E2 (10−8 M)+ICI 182,780
(10−6 M) in human ES cells for 48 h, total proteins were
extracted, and bands corresponding to POU5F1, SOX2, and NANOG were
detected by western blotting and quantified using Gel Doc 2000.
*Significant elevation or reduction in gene expressions by each
treatment comparing with control (P<0.05 in Dunnett's multiple
comparison test). #Significant elevation or reduction in
gene expressions by treatment of E2+ICI 182,780 (I) comparing with
treatment of E2 (P<0.05 in Student's t test). (C) After the
treatment of P4 (10−6 M), RU486 (10−8 M), and
P4 (10−6 M)+RU486 (10−8 M) in human ES cells
for 48 h, total proteins were extracted, and bands corresponding to
POU5F1, SOX2, and NANOG were detected by western blotting and
quantified using Gel Doc 2000. *Significant elevation or reduction
in gene expressions by each treatment comparing with control
(P<0.05 in Dunnett's multiple comparison test).
#Significant elevation or reduction in gene expressions
by treatment of P4+RU486 (R) comparing with treatment of P4
(P<0.05 in Student's t-test). |
Discussion
Pluripotency is the main propensity of ES cells, and
maintenance of pluripotency requires a balance between survival,
proliferation, and self-renewal mechanisms (26). In fact, the precise mechanism that
regulates pluripotency of stem cells remains largely unknown.
Recently, through the in vitro and in vivo studies,
several genetic regulators have been identified to play important
roles in regulating the pluripotency in human and mouse ES cells.
They are closely related with extracellular signaling factors,
transcription factors, cell-cycle regulators, microRNAs, and DNA
methylation (27,28). Investigations for these regulators
might be useful to understand basic cellular biology and to exploit
human ES cells as a promising source for cell therapy to treat
patients with degenerative diseases (29,30).
Mouse feeder cells have been used to help the growth
and maintenance of human ES cells, but they must be removed before
use of ES cells in certain applications (31,32).
Frequently, the remaining feeder cells cause a problem because they
generate confusion in measuring cellular responses of human ES
cells to a certain stimulus (33).
In the present study, to minimize the possibility of contamination
by feeder cells, a feeder-free culture protocol for human ES cells
was adopted to properly evaluate the effects of female sex steroid
hormones on specific propensities of ES cells such as EMT and
maintenance of pluripotency without a concern deriving from the
mixed culture of ES cells and feeder cells. The procedure requires
Matrigel-coated culture plates with CM from MEF feeder cell culture
and monolayer culture of human ES cells. As shown in Fig. 1, these techniques provided a robust
culture platform for human ES cells and an environment for the
proper expression of stem cell markers without direct intervention
of MEF feeder cells.
After establishing the single culture condition for
human ES cells, the effects of female sex hormones such as E2 and
P4 on specific characteristics of ES cells were investigated. Most
animal cells are present in two types. Epithelial cells are
composed of basement membrane and held together through several
types of interactions, polarity, and immobility (18), while the characteristics of
mesenchymal cells are opposite: They are loosely associated each
other with no polarity and high mobility (34). Two types of transition, EMT and
MET, have been observed during embryonic development, and
interconversion between EMT and MET is likely to occur (35,36).
During early embryonic development, the mesoderm generated by EMT
develops into multiple tissue types, and later in development,
mesodermal cells generate epithelial organs such as kidney and
ovary via MET (35,37).
In the present study, when E2 and P4 were treated in
human ES cells, the expression of epithelial cell marker,
E-cadherin, was reduced, but the expression of mesenchymal cell
marker, N-cadherin, and EMT regulator genes such as Snail
and Slug was increased, indicating that female sex steroid
hormones induce EMT through the regulation of expression of
EMT-associated genes through their cognate receptors. However, the
single treatment of RU486 also increased the Snail expression, but
it had no statistical significance. RU486 is not a specific
antagonist for PR. However, it surely has an antagonistic effect on
P4. Since the combined treatment of P4 and RU486 restored the Snail
expression, which was increased by P4, to the control level as
shown in Fig. 3, it can be
estimated that P4 induced Snail expression via its receptor,
PR.
Next, the effect of sex hormones on the pluripotency
of human ES cells was examined. As a result, E2 and P4 were
revealed to reduce the pluripotency of human ES cells by inhibiting
the expression of pluripotency-associated markers such as
POU5F1, SOX2, and NANOG genes in both transcriptional
and translational levels.
More specifically, previous studies have
demonstrated the influence of EMT on characteristics of ES cells.
Li et al considered EMT as an early and necessary step in
lineage specification by showing that stimuli for differentiation
induced EMT prior to the differentiation processes and that
inhibition of EMT suppressed differentiation of ES cells (38). Likewise, the combinatorial
suppression of EMT and apoptotic pathways through many pathways
regulated by microRNAs maintained the self-renewal and inhibited
the differentiation of ES cells (39). In terms of the interrelationship
between EMT process and pluripotency of ES cells, the cell-adhesion
molecule E-cadherin is also known to play an important role in
pluripotency and reprogramming of ES cells (37). For instance, interference with
E-cadherin caused the EMT process and ES cell differentiation, and
the expression of E-cadherin in undifferentiated ES cells was
decreased immediately after ES cells initiated differentiation
(40). In addition, E-cadherin
stabilized cortical actin cytoskeletal arrangement in mouse or
human ES cells, leading to the prevention of cell surface
localization of the promigratory 5T4 antigen, which is associated
with very early phase of differentiation and altered motility of ES
cells (41,42). Although the absolute requirement of
E-cadherin for pluripotency is still under debate, it seems obvious
at least that E-cadherin partially contributes to survival,
self-renewal, and pluripotency of ES cells (43). Actually, E-cadherin, together with
proteins like SSEA1, alkaline phosphatase, POU5F1, NANOG, and
others have been used to maintain undifferentiated state of ES
cells (44,45). Our results showing the reduced
expression of E-cadherin by E2 and P4 to induce EMT process may be
also interpreted that these sex hormones regulate the pluripotency
of human ES cells by reducing the expression of well-known
pluripotency markers such as POU5F1, SOX2, and NANOG
genes as well as E-cadherin. Therefore, these results may also
support that EMT process is related with stemness and
differentiation of ES cells and can be reflected in the field of
application for developing induced pluripotent stem cells (iPS
cells).
In summary, we investigated whether female sex
steroid hormones influence on EMT and maintenance of pluripotent
propensity of human ES cells using a new culture method that can
help maintain undifferentiated state of ES cells without the
intervention of MEF feeder cells. Collectively, female sex hormones
can lead to the induction of EMT through the up- and
down-regulation of cellular markers and signaling proteins. In
addition, they down-regulated the expressions of POU5F1,
SOX2, and NANOG, which are known to maintain the
pluripotency of ES cells. Taken together, E2 and P4 can affect
differentiation of human ES cells by inducing EMT and the loss of
pluripotency of human ES cells as demonstrated in Fig. 5. It is expected that this study may
provide new insights for understanding the roles of female sex
hormones in the EMT process and differentiation of human ES cells
for embryo developmental processes and for developing more
efficient avenues for human ES cell programming.
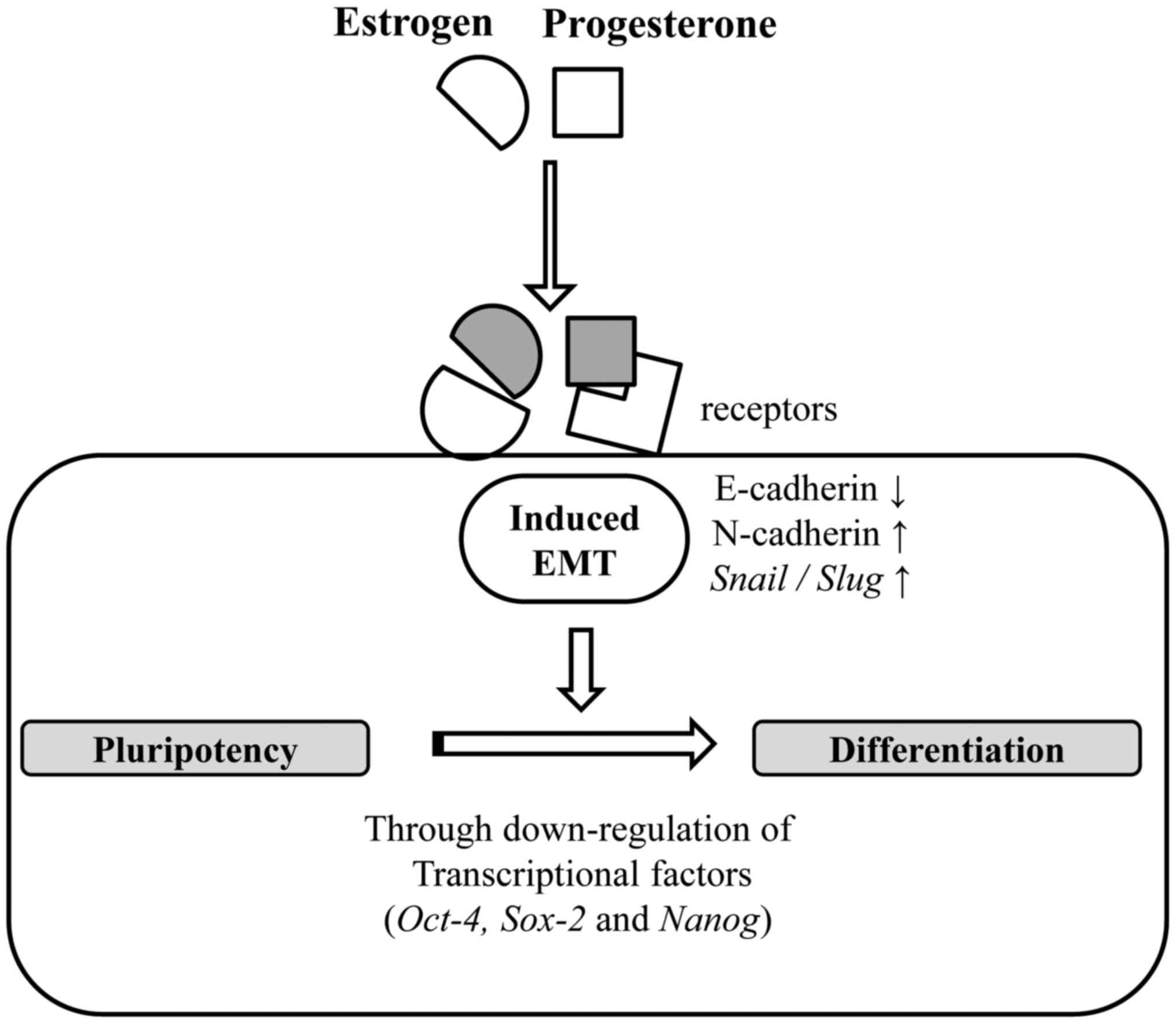 | Figure 5.Tentative mechanism of E2 and P4 in
regulating the EMT process and pluripotency of human ES cells. In
the present study, E2 and P4, female sex hormones, were revealed to
lead to the induction of EMT through the up and downregulation of
cellular markers and signaling proteins through their cognate
receptors. In addition, they down-regulated the expressions of
POU5F1, SOX2, and NANOG, which are known to maintain the
pluripotency of ES cells. As E-cadherin, POU5F1, NANOG, and others
have been known to maintain undifferentiated state of ES cells, E2
and P4 can induce the differentiation process such as embryo
development by promote the EMT process and the loss of pluripotency
of human ES cells. EMT, epithelial-mesenchymal transition; E2,
17β-estradiol; P4, progesterone; ES, embryonic stem. |
Acknowledgements
This study was supported by a grant from the
Next-Generation BioGreen 21 Program (no. PJ011355-2015), Rural
Development Administration, Republic of Korea.
Glossary
Abbreviations
Abbreviations:
|
ES
|
embryonic stem
|
|
E2
|
17β-estradiol
|
|
EMT
|
epithelial-mesenchymal transition
|
|
P4
|
progesterone
|
|
PR
|
progesterone receptor
|
|
MEF
|
mouse embryonic fibroblast
|
|
ECM
|
extracellular matrix
|
|
CM
|
conditioned medium
|
References
|
1
|
Das S, Bonaguidi M, Muro K and Kessler JA:
Generation of embryonic stem cells: Limitations of and alternatives
to inner cell mass harvest. Neurosurg Focus. 24:E42008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato N, Meijer L, Skaltsounis L, Greengard
P and Brivanlou AH: Maintenance of pluripotency in human and mouse
embryonic stem cells through activation of Wnt signaling by a
pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi N and Chen SY: From nerve to blood
vessel: A new role of Olfm2 in smooth muscle differentiation from
human embryonic stem cell-derived mesenchymal cells. J Biomed Res.
29:261–263. 2015.PubMed/NCBI
|
|
4
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W,
Chen X, Bourque G, George J, Leong B, Liu J, et al: The Oct4 and
Nanog transcription network regulates pluripotency in mouse
embryonic stem cells. Nat Genet. 38:431–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan G and Thomson JA: Nanog and
transcriptional networks in embryonic stem cell pluripotency. Cell
Res. 17:42–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi BR, Kim SU and Choi KC: Development and
application of neural stem cells for treating various human
neurological diseases in animal models. Lab Anim Res. 29:131–137.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bouman A, Heineman MJ and Faas MM: Sex
hormones and the immune response in humans. Hum Reprod Update.
11:411–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong SH, Lee JE, Kim HS, Jung YJ, Hwang D,
Lee JH, Yang SY, Kim SC, Cho SK and An BS: Effect of vitamin D3 on
production of progesterone in porcine granulosa cells by regulation
of steroidogenic enzymes. J Biomed Res. 30:203–208. 2016.PubMed/NCBI
|
|
10
|
Fowden AL, Forhead AJ, Sferruzzi-Perri AN,
Burton GJ and Vaughan OR: Review: Endocrine regulation of placental
phenotype. Placenta. 36:(Suppl 1). S50–S59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halasz M and Szekeres-Bartho J: The role
of progesterone in implantation and trophoblast invasion. J Reprod
Immunol. 97:43–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maliqueo M, Echiburú B and Crisosto N: Sex
steroids modulate uterine-placental vasculature: Implications for
obstetrics and neonatal outcomes. Front Physiol. 7:1522016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HR, Kim TH and Choi KC: Functions and
physiological roles of two types of estrogen receptors, ERα and
ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res.
28:71–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim H, Kim YY, Ku SY, Kim SH, Choi YM and
Moon SY: The effect of estrogen compounds on human embryoid bodies.
Reprod Sci. 20:661–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Ding C, Ding ZL, Ling M, Wang T,
Wang W and Huang B: 17β-Oestradiol promotes differentiation of
human embryonic stem cells into dopamine neurons via cross-talk
between insulin-like growth factors-1 and oestrogen receptor β. J
Cell Mol Med. Feb 28–2017.(Epub ahead of print).
|
|
16
|
Gallego MJ, Porayette P, Kaltcheva MM,
Bowen RL, Meethal Vadakkadath S and Atwood CS: The pregnancy
hormones human chorionic gonadotropin and progesterone induce human
embryonic stem cell proliferation and differentiation into
neuroectodermal rosettes. Stem Cell Res Ther. 1:282010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SH, Cheung LW, Wong AS and Leung PC:
Estrogen regulates Snail and Slug in the down-regulation of
E-cadherin and induces metastatic potential of ovarian cancer cells
through estrogen receptor alpha. Mol Endocrinol. 22:2085–2098.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beaman EM and Brooks SA: The extended
ppGalNAc-T family and their functional involvement in the
metastatic cascade. Histol Histopathol. 29:293–304. 2014.PubMed/NCBI
|
|
22
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klimanskaya I, Chung Y, Meisner L, Johnson
J, West MD and Lanza R: Human embryonic stem cells derived without
feeder cells. Lancet. 365:1636–1641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim CW, Go RE and Choi KC: Treatment of
BG-1 ovarian cancer cells expressing estrogen receptors with
lambda-cyhalothrin and cypermethrin caused a partial estrogenicity
via an estrogen receptor-dependent pathway. Toxicol Res.
31:331–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Babaie Y, Herwig R, Greber B, Brink TC,
Wruck W, Groth D, Lehrach H, Burdon T and Adjaye J: Analysis of
Oct4-dependent transcriptional networks regulating self-renewal and
pluripotency in human embryonic stem cells. Stem Cells. 25:500–510.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kashyap V, Rezende NC, Scotland KB,
Shaffer SM, Persson JL, Gudas LJ and Mongan NP: Regulation of stem
cell pluripotency and differentiation involves a mutual regulatory
circuit of the NANOG, OCT4, and SOX2 pluripotency transcription
factors with polycomb repressive complexes and stem cell microRNAs.
Stem Cells Dev. 18:1093–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burdon T, Smith A and Savatier P:
Signalling, cell cycle and pluripotency in embryonic stem cells.
Trends Cell Biol. 12:432–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schuldiner M, Yanuka O, Itskovitz-Eldor J,
Melton DA and Benvenisty N: Effects of eight growth factors on the
differentiation of cells derived from human embryonic stem cells.
Proc Natl Acad Sci USA. 97:11307–11312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishikawa S, Goldstein RA and Nierras CR:
The promise of human induced pluripotent stem cells for research
and therapy. Nat Rev Mol Cell Biol. 9:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludwig TE, Levenstein ME, Jones JM,
Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard
KR, Piekarczyk MS, et al: Derivation of human embryonic stem cells
in defined conditions. Nat Biotechnol. 24:185–187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiao E, Kmet M, Behr B and Baker J:
Derivation of human embryonic stem cells in standard and chemically
defined conditions. Methods Cell Biol. 86:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amit M, Margulets V, Segev H, Shariki K,
Laevsky I, Coleman R and Itskovitz-Eldor J: Human feeder layers for
human embryonic stem cells. Biol Reprod. 68:2150–2156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radisky DC: Epithelial-mesenchymal
transition. J Cell Sci. 118:4325–4326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeon SY, Hwang KA and Choi KC: Effect of
steroid hormones, estrogen and progesterone, on epithelial
mesenchymal transition in ovarian cancer development. J Steroid
Biochem Mol Biol. 158:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shook D and Keller R: Mechanisms,
mechanics and function of epithelial-mesenchymal transitions in
early development. Mech Dev. 120:1351–1383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Zhu L, Yang A, Lin J, Tang F, Jin S,
Wei Z, Li J and Jin Y: Calcineurin-NFAT signaling critically
regulates early lineage specification in mouse embryonic stem cells
and embryos. Cell Stem Cell. 8:46–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo WT, Wang XW, Yan YL, Li YP, Yin X,
Zhang Q, Melton C, Shenoy A, Reyes NA, Oakes SA, et al: Suppression
of epithelial-mesenchymal transition and apoptotic pathways by
miR-294/302 family synergistically blocks let-7-induced silencing
of self-renewal in embryonic stem cells. Cell Death Differ.
22:1158–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishii T, Fukumitsu K, Yasuchika K, Adachi
K, Kawase E, Suemori H, Nakatsuji N, Ikai I and Uemoto S: Effects
of extracellular matrixes and growth factors on the hepatic
differentiation of human embryonic stem cells. Am J Physiol
Gastrointest Liver Physiol. 295:G313–G321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Spencer HL, Eastham AM, Merry CL,
Southgate TD, Perez-Campo F, Soncin F, Ritson S, Kemler R, Stern PL
and Ward CM: E-cadherin inhibits cell surface localization of the
pro-migratory 5T4 oncofetal antigen in mouse embryonic stem cells.
Mol Biol Cell. 18:2838–2851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eastham AM, Spencer H, Soncin F, Ritson S,
Merry CL, Stern PL and Ward CM: Epithelial-mesenchymal transition
events during human embryonic stem cell differentiation. Cancer
Res. 67:11254–11262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen T, Yuan D, Wei B, Jiang J, Kang J,
Ling K, Gu Y, Li J, Xiao L and Pei G: E-cadherin-mediated cell-cell
contact is critical for induced pluripotent stem cell generation.
Stem Cells. 28:1315–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Redmer T, Diecke S, Grigoryan T,
Quiroga-Negreira A, Birchmeier W and Besser D: E-cadherin is
crucial for embryonic stem cell pluripotency and can replace OCT4
during somatic cell reprogramming. EMBO Rep. 12:720–726. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boiani M and Schöler HR: Regulatory
networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell
Biol. 6:872–884. 2005. View Article : Google Scholar : PubMed/NCBI
|