Introduction
The occurrence of tumors poses a serious threat to
life. The third leading cause of mortality is reported to be
hepatocellular carcinoma (HCC), particularly in China (1). Patients with chronic hepatitis are
more at risk for the onset of HCC, and this may have resulted in
the significant increase of HCC in previous years (2). Due to substantial deficiencies in
healthcare and physiological conditions, only a fraction of
patients are eligible for treatments, including liver
transplantation and resection. Therefore, the overall survival rate
for patients with HCC is relatively low (3). The majority of patients with HCC are
diagnosed during the late stages and, therefore, their prognosis is
poor, primarily due to the lack of early biomarkers. As a result,
identifying efficient and sensitive markers for HCC is urgently
required.
The heterogeneous nature of HCC also complicates
current understanding and effective treatment. Previous evidence
suggested that the variations in HCC may depend on stochastic
alterations during the period of carcinogenesis (4). The abnormal activation of several
pathways, including insulin growth factor and Akt signaling, are
also major causes of tumorigenesis (5,6).
In-depth knowledge of the determinant molecular mechanisms in
different HCC backgrounds presents a challenge in clinical and
experimental investigations. An effective therapeutic option cannot
be developed unless the genomic background of individuals has been
clearly characterized.
MicroRNAs are short-length, noncoding RNAs, which
can suppress gene expression though base-pairing to the
3′-untranslated region (3′-UTR) of targets (7,8).
Increasing evidence has shown that microRNAs may be pivotal in the
development of HCC (9–13). For example, microRNA (miR)-148b can
regulate cancer stem cell properties in HCC by directly targeting
Neuropilin-1, which is a transmembrane receptor critically
implicated in initiating angiogenesis and metastasis (13). miRNA-26a can suppress the
progression of HCC primarily by targeting the c-Met pathway
(14). The microRNA-based
classification of HCC has also been demonstrated and three clusters
have been identified (2). miR-517a
and miR-520c have been shown to be significantly positively
correlated with the malignant characteristics of HCC (2). Other microRNAs, including the let-7
family, miR-29, miR-183 and miR-122, showed reduced expression in
tumor tissues suggesting tumor suppressive roles for specific
microRNAs (15–18). Therefore, the role of microRNAs in
tumor development is complex and warrants further
investigation.
In the present study, the novel function of a less
well-known microRNA, miR-526a, in the tumorigenesis of HCC was
investigated. It was found that miR-526a was predominantly
downregulated in HCC cell lines and human specimens. The ectopic
expression of miR-526a significantly attenuated the proliferation,
migration and invasion of HCC cell lines in vitro.
Furthermore, miR-526a transfection decreased tumor volume in an
implantation assay in vivo. In addition, p21-activated
kinase 7 (PAK7) was identified as a potential miR-526a target in
HCC. The inverse correlation between PAK7 and miR-526a was
significant in tumor specimens. These results are the first, to the
best of our knowledge, to shown that miR-526a can serve as a
candidate tumor suppressor by targeting PAK7 in HCC, providing
crucial insight into effective therapeutic treatment, possibly by
targeting microRNAs.
Materials and methods
Cell culture and human specimens
All HCC cell lines (Huh7, SK-Hep1, SMMC-7721,
Bel7402, HepG2, Hep3B and MHCC97-H), together with the control cell
line (L02), were purchased from American Type Culture Collection
(Rockville, MD, USA). The culture media for these cell lines
comprised RPMI-1640 medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The medium was further supplemented with 5%
fetal bovine serum (FBS) and penicillin (100 U/ml) (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere of 5%
CO2 at 37°C. The HCC specimens included were all
surgical archives from patients at The Affiliated Hospital of
Guizhou Medical University (Guiyang, China) between September 2013
and July 2015. All specimens were maintained in liquid nitrogen at
−80°C following resection prior to experiments. All patients signed
informed consent forms. The protocols of experimental procedures
involving the use of human samples were formally approved by the
Human Research Ethics Committee of The Affiliated Hospital of
Guizhou Medical University (no. 2013-CF-0010).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The gene expression levels of miR-526a and PAK7 in
HCC were measured using RT-qPCR analysis. In brief, RNA was
extracted from the HCC cells and HCC human specimens using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and corresponding cDNA was obtained with a SYBR Premix Ex Taq™
kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. Briefly, 5 µm sections were cut from tissue archives and
placed in a micro-centrifuge tube. A total of 1 ml AutoDewaxer™ was
added to the sample and the tube was incubated for 15 min at 95°C.
The sample was then centrifuged for 1 min at room temperature at
10,000 × g. The incubatory and centrifugation steps were then
repeated three times. A total of 300 ml lysis buffer was added to
the deparaffinized tissue and it was vortexed for 1 min to obtain a
homogeneous solution. A TaqMan miRNA RT-qPCR kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used and
GAPDH was used as an internal control. For PAK7
detection, the SYBR-Green PCR Master Mix kit was used (Applied
Biosystems; Thermo Fisher Scientific, Inc.). All kinetic reactions
for RT-qPCR were performed using the ABI PRISM® 7000
sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). A total of 2 mg total RNA template was annealed
with 1 ml (500 ng) random primer in a sterile RNase-free
micro-centrifuge tube and heated at 70°C for 5 min. The following
reagents were then added: First-strand 5xbuffer (4 ml), 0.1 M DTT
(2 ml), 10 mM dNTP mix (2.5 mM each; 2 ml), RNasin™ (1 ml) and
SuperScript™ II (2 ml). PCR amplification was then carried out for
35 cycles in an ABI 9700 machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Reactions were carried out at 50°C for 2 min and
95°C for 10 min followed by 35 cycles of 95°C for 15 sec and 60°C
for 1 min. Gene expression was quantified as fold-change. A total
of 1 µl of the RNA was added to the Nanodrop™ to automatically
measure RNA at wavelengths of 260 and 280 nm (19). The primer sequences were as
follows: PAK7, forward 5′-ACGTACCGACAGTGCTGG-3′ and reverse
5′-TACGCCAATCGATGCAGGAGAA-3′; miR-526a, forward
5′-ACACTCGAGCTGGGGCTTGTTCGCTTGCCTT-3′ and reverse
5′-TGATGCTTCTGAGTCG-3′; GAPDH, forward
5′-CTCTAGGTCTATCGGT-3′ and reverse 5′-ATGTTATGCCGTAAGCAGT-3′.
Generation of stably transfected cell
lines
A lentiviral system was used to ectopically
overexpress miR-526a in the Huh7 and HepG2 cells in the present
study. The lentiviruses containing miR-526a mimics (miR-526a) and
negative controls were synthesized and purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). The
Lipofectamine® 2000 system (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for viral transfection. Cells
(1×105 cells/ml) were seeded into 12-well plates in 100
µl growth medium (RPMI-1640, Thermo Fisher Scientific, Inc.) at the
time of transfection. Then, 50 µl transfection lipoplexes were
added to each well. The cells were incubated in RPMI-1640 under 5%
CO2 at 37°C in a humidified incubator for 5 h. The cells
were then washed with PBS, medium replaced and cells were cultured
for another 48 h. At 24 h post-transfection, the culture medium was
replaced with fresh medium. All plasmids were experimentally
verified using RT-qPCR analysis.
Dual-luciferase reporter assay
The PAK7 gene was obtained from a cDNA
library (LIBEST_017384; www.ncbi.nlm.nih.gov/nucest/DR000640.1) and verified
using DNA sequencing. The 3′-untranslated region (UTR) of
PAK7 with the predicted binding site with hsa-miR-526a was
cloned into a Renilla luciferase reporter plasmid (phRL-TK;
Sigma-Aldrich; Merck Millipore) leading to the wild-type PAK7
luciferase reporter plasmid (PAK7 3′-UTR WT). The has-miR-526a
binding site for the 3′-UTR region of PAK7 was further mutated
using a Quick-Change™ Site-Directed Mutagenesis kit (Stratagene;
Agilent Technologies, Inc., Santa Clara, CA, USA). The mutated PAK7
3′-UTR was then inserted into the phRL-TK plasmid to produce the
mutated luciferase reporter plasmid for PAK7 (PAK7 3′-UTR MUT).
Co-transfection was performed for 24 h. The relative luciferase
units were determined using a dual-luciferase reporter assay
(Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol.
Invasion assay
The chemotaxis 96-well Transwell assay (Chemicon,
Temecula, CA, USA) was used to quantify the invasion of cells. The
upper chamber of the Transwell was covered with Matrigel
(Sigma-Aldrich; Merck Millipore) overnight. The lentivirus-treated
Huh7 and HepG2 cells were seeded within the upper chambers
(105 cells per well) in RPMI-1640 medium without serum
(Gibco; Thermo Fisher Scientific, Inc.). The lower chambers were
then filled with RPMI-1640 medium containing 2% FBS. After 24 h,
the migrated cells, which had migrated into the lower chambers were
fixed with 5% PFA (Sigma-Aldrich; Merck Millipore) and stained with
crystal violet (Sigma-Aldrich; Merck Millipore) at 20°C. Images
were captured through a Leica inverted fluorescent microscope
(DM-IRB; Leica Microsystems GmbH, Wetzlar, Germany). The invasion
was determined as fold-change relative to the control.
Prediction of miR-526a target
The present study used algorithms for target gene
prediction: TargetScan (www.targetscan.org), PicTar (pictar.mdc-berlin.de) and
miRDB (www.mirdb.org) as previously described
(20,21). The predicted targets were ranked by
Z scores. Top ranking and overlapping targets were selected
for further verification.
Proliferation assay
The Huh7 and HepG2 cells were seeded in 96-well
plates (104 cells per well) for 5 days. A Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was used. At each 24 h interval, MTT solution was added into
culture medium at a final concentration of 5 mg/ml. After 4 h, the
medium was removed and the crystalline formazan was dissolved in
100 µl SDS (15%; Sigma-Aldrich; Merck Millipore) solution for 24 h.
The plate was shaken for 5 min leading to complete solubilization.
Finally, the optical density at 490 nm was evaluated using a
Spectramax M5 microplate reader (Molecular Devices LLC, Sunnyvale,
CA, USA) according to the manufacturer's protocols.
Migration assay
Boyden Chambers (BD Biosciences, San Jose, CA, USA)
were used in a 12-well plate. At 24 h post-transfection,
105 cells from the RPMI-1640 medium were seeded in the
upper chamber. RPMI-1640 with 15% FBS was used as an attractant.
After 24 h, the non-migrated cells were removed, loaded into 5% PFA
and stained using crystal violet. The image was visualized by Leica
DM-IRB inverted microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
In vivo implantation assay and
immunohistochemical staining
BALB/c nude mice (6-week-old, average weight, 15.2
g; 5 male and 5 female) were purchased from the Model Animal
Research Center (Nanjing, China) and were maintained in SPF
conditions for an additional week. Mice were housed at 20°C, with
55–60% humidity and a light-dark cycle of 10–12 h. Ad
libitum access to food and water was provided. Animal
manipulation and experiments were performed in accordance with the
General Guide for the Use of Laboratory Animals and approved by the
Animal Experimental Ethics Committee of The Affiliated Hospital of
Guizhou Medical University (no. 2013-AF-0035). The HepG2 cells
transduced with lentivirus for 12 h were continuously cultured for
a further 24 h. The cells were then resuspended in serum-free
medium and 106 cells were implanted subcutaneously into
the null mice. Tumor volume in vivo was recorded each week.
After 30 days, all mice were sacrificed by an overdose of sodium
pentobarbital (5%, 250 mg/kg with intraperitoneal injection;
catalog no. 1507002; Sigma-Aldrich; Merck KGaA) and implants were
immunostained with Ki-67 (Sigma-Aldrich; Merck Millipore) for 30
min at 37°C. A rabbit monoclonal anti-mouse/human Ki-67 antibody
was used (catalog no. P6834; 1:1,000; Sigma-Aldrich; Merck
Millipore) for 15 min at 20°C. The immunohistochemistry was
performed with an enhanced biotin-free polymer one-step staining
technique with goat anti-rabbit secondary antibody (catalog no.
K5007; 1:1,000; Dako, Glostrup, Denmark) for 1 h at 37°C, according
to the manufacturer's protocols.
Western blot analysis
The Huh7 and HepG2 cells were resuspended and
harvested with cell lysis buffer containing 10% glycerol and 2%
NP-40 (Sigma-Aldrich; Merck Millipore). The protein extracts (150
µg) were then resolved on a 7% SDS-PAGE gel and transferred onto a
nitrocellulose membrane (Sigma-Aldrich; Merck Millipore). The
protein concentration was measured by Bradford protein assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primary
antibody against human PAK7 (catalog no. K3265; 1:1,000;
Sigma-Aldrich, Merck KGaA) was used at 4°C overnight, and
HRP-conjugated antibodies (catalog no. A9044; 1:1,000;
Sigma-Aldrich, Merck KGaA) at 20°C for 4 h. The blots were
monitored using a chemiluminescence kit (Sino-American
Biotechnology Co., Ltd., Shanghai, China).
Statistical analysis
All experiments were performed with three
replicates. The results are presented as the mean ± standard error
of the mean. Statistical differences were determined using
Student's t-test (SPSS 16.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-526a is frequently
downregulated in HCC
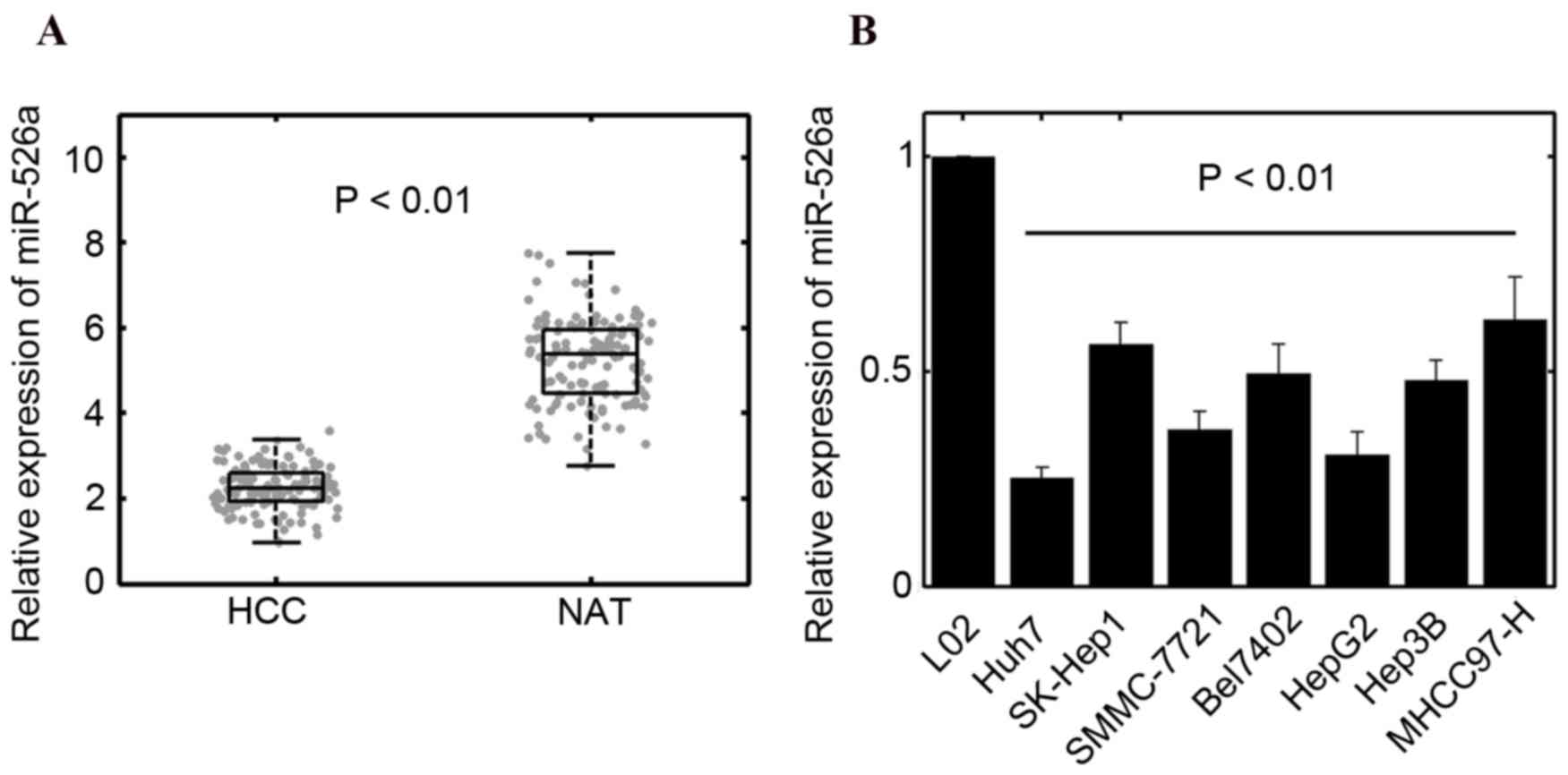
To determine the expression of miR-526a in HCC
tissues, RT-qPCR analysis was performed and the results showed that
the levels of miR-526a were significantly downregulated in HCC
specimens, compared with normal adjacent tissues (n=135; P<0.01;
Fig. 1A). Low expression of
miR-526a was associated with histological grade (P=0.01), vascular
invasion (P=0.002) and metastasis (P<0.001), as shown in
Table I. However, no significant
correlations were found between the expression of miR-526a and age
or gender (Table I). The
expression of miR-526a was further quantified in seven HCC cell
lines. The results of the RT-qPCR analysis also suggested that
miR-526a was reduced in tumor cells (Fig. 1B). Taken together, these results
suggested that miR-526a may be involved in regulating the
progression of HCC. It was found that the levels of miR-526a in
Huh7 and HepG2 cells were relatively low, compared with those in
other cell lines. Therefore, Huh7 and HepG2 cells were selected for
subsequent analyses.
 | Table I.Correlation between miR-526a and
clinicopathological features in hepatocellular carcinoma. |
Table I.
Correlation between miR-526a and
clinicopathological features in hepatocellular carcinoma.
|
|
| Expression of
miR-526a |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | n | High, n (%) | Low, n (%) | P-value |
|---|
| Age (years) |
|
<60 | 67 | 36 (53.7) | 31 (46.3) | 0.493 |
|
≥60 | 68 | 32 (47.1) | 36 (52.9) |
|
| Gender |
|
Male | 74 | 40 (54.1) | 34 (45.9) | 0.389 |
|
Female | 61 | 28 (45.9) | 33 (54.1) |
|
| Histological
grade |
|
Well/moderate | 72 | 44 (61.1) | 28 (38.9) | 0.01 |
|
Poor | 63 | 24 (38.1) | 39 (61.9) |
|
| Lymph node
metastasis |
|
Positive | 38 | 9 (23.7) | 29 (76.3) | <0.001 |
|
Negative | 97 | 59 (60.8) | 38 (39.2) |
|
| Vascular
invasion |
|
Positive | 70 | 27 (38.6) | 43 (61.4) | 0.002 |
|
Negative | 65 | 41 (63.1) | 24 (36.9) |
|
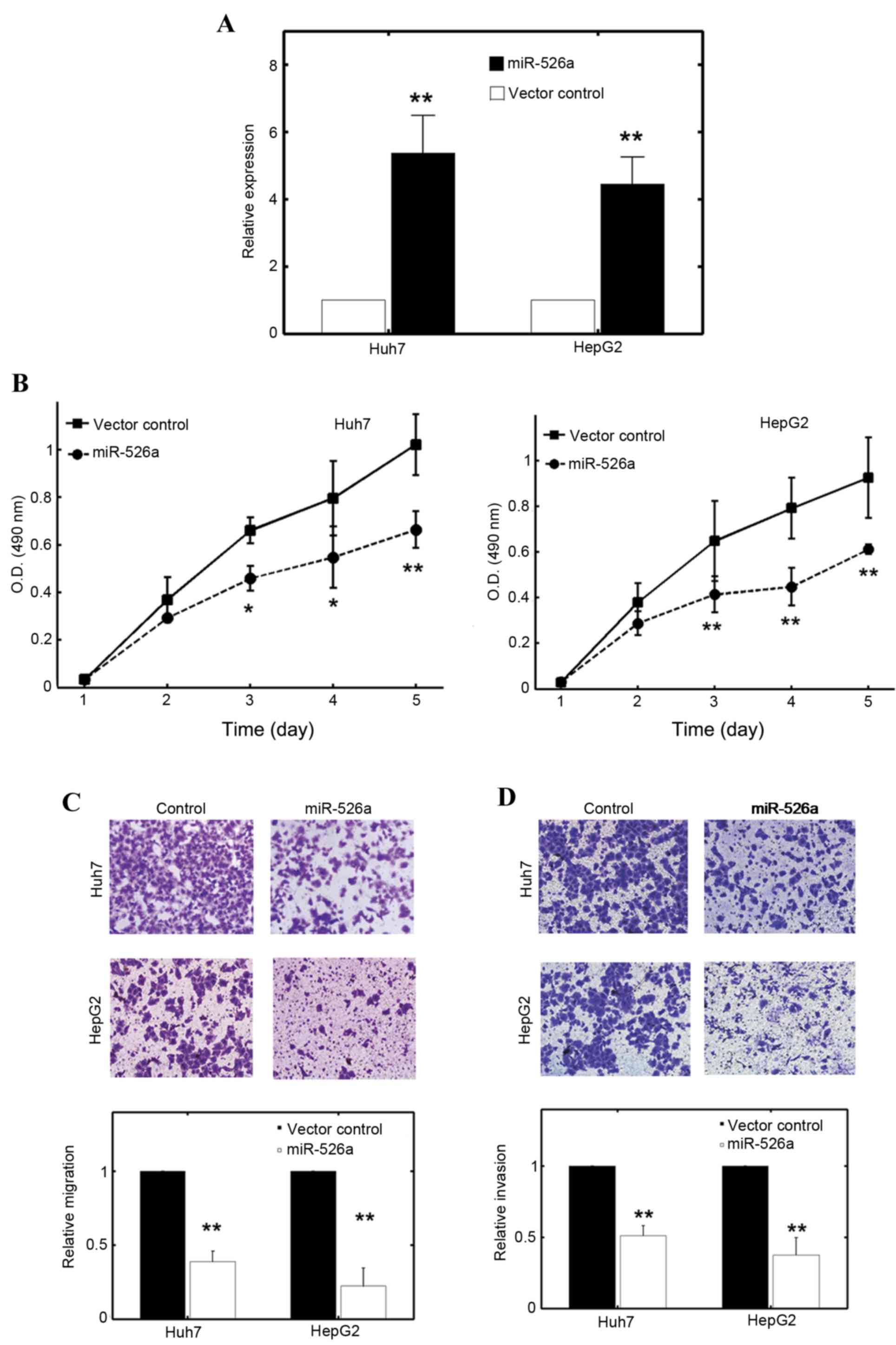
miR-526a inhibits the proliferation,
migration and invasion of HCC cell lines
As miR-526a was implicated in the regulation of HCC
tumorigenesis, the present study investigated the role of miR-526a
in the malignant characteristics of HCC cells. The Huh7 and HepG2
cells were stably transfected with miR-526a precursor or a control
vector, and the transfection efficiency was determined using
RT-qPCR. The results showed that miR-526a transfection
substantially elevated the intrinsic expression of miR-526a in Huh7
and HepG2 cells with an increase of ~4-5 fold (Fig. 2A; P<0.01). Following miR-526a
transfection, the proliferation rates of the Huh7 and HepG2 cells
were significantly inhibited (Fig.
2B). The migration assays also confirmed that miR-526a had a
suppressive role (Fig. 2C). The
effect was more evident in the HepG2 cells (Fig. 2C). The invasive capacities of Huh7
and HepG2 cells were also consistently attenuated with the
overexpression of miR-526a (Fig.
2D). These results suggested that miR-526a had a tumor
suppressive role in Huh7 and HepG2 cell lines.
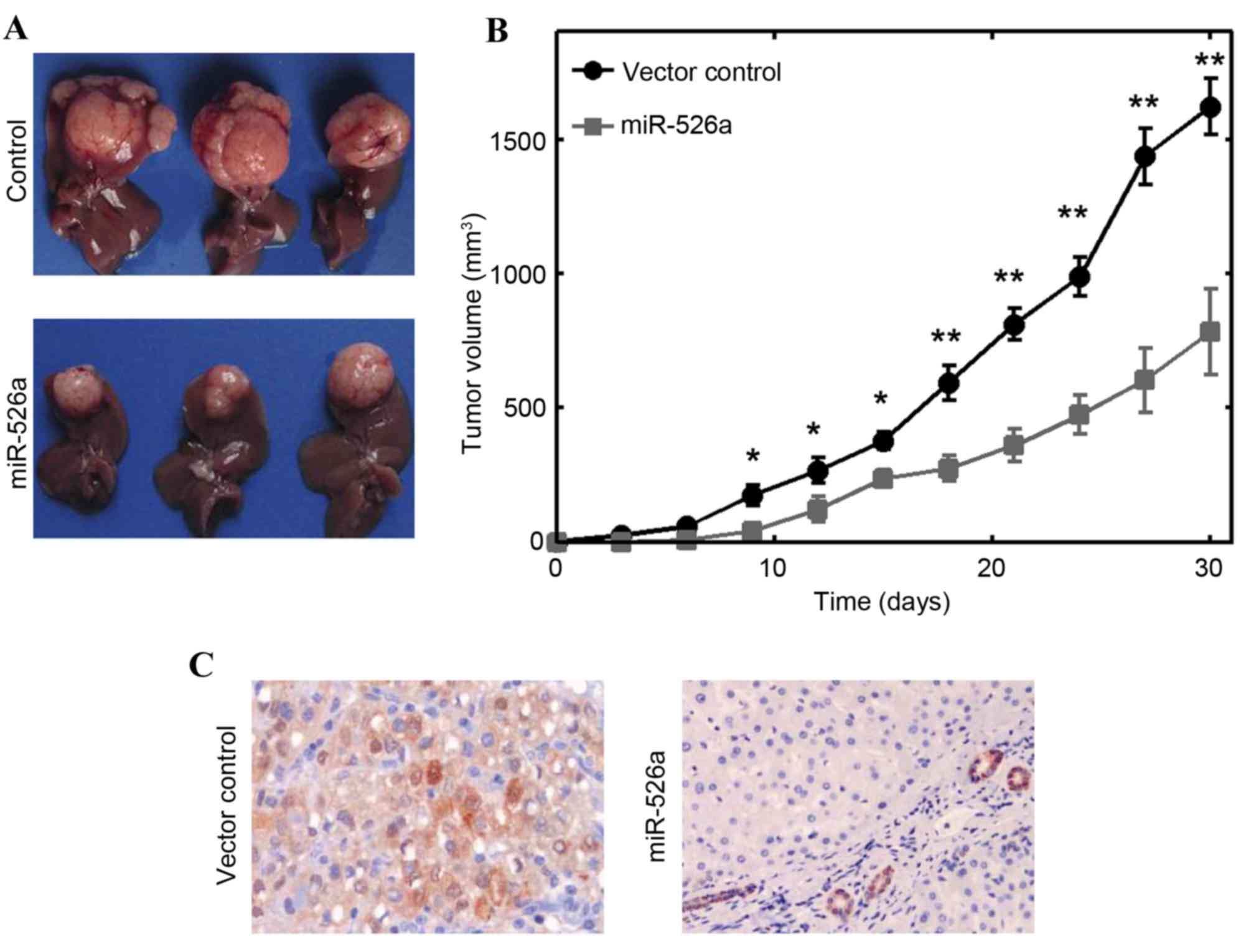
miR-526a can inhibit HCC tumor growth
in vivo
To further consolidate the role of miR-526a in
vivo, xenograft experiments were performed. A total of
106 HepG2 cells stably transfected with miR-526a or
vector controls were subcutaneously injected into nude mice, and
the growth of solid tumors was evaluated at intervals of 3 days. It
was found that the size of solid tumors were significantly
decreased, compared with those in the control vector transfection
group (P<0.01; Fig. 3A).
miR-526a transfection significantly decreased tumor volume as early
as 9 days post-injection (Fig.
3B). The Ki-67 immunostaining results showed that the growth of
HCC solid tumors was markedly inhibited by inducing the
overexpression of miR-526a (Fig.
3C). Collectively, these results showed that miR-526a also
inhibited tumor development in vivo.
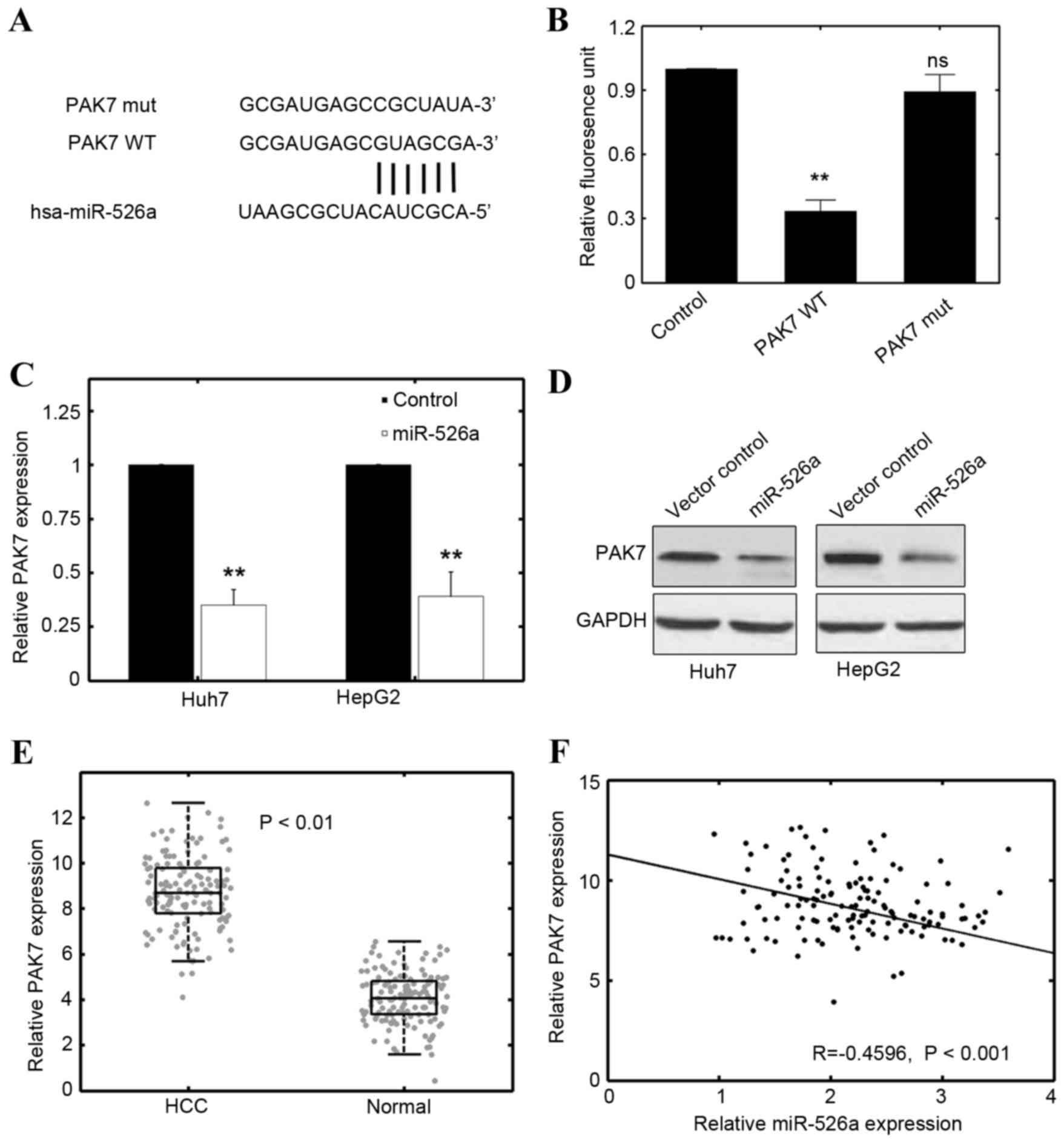
miR-526a suppresses the progression of
HCC by targeting PAK7
To determine the potential target of miR-526a,
several online databases were used including TargetScan (www.targetscan.org) PicTar (pictar.mdc-berlin.de) and
miRDB (www.mirdb.org). The cross verification
of several databases indicated that the oncogene, PAK7, may be a
promising candidate (Fig. 4A).
Using a luciferase reporter assay, it was further shown that
miR-526a transfection effectively decreased the luciferase
activities of the PAK7 group (Fig. 4B). However, if the base-paring
between PAK7 and miR-526a was mutated, the inhibitory effect of
miR-526a was absent (Fig. 4B).
Furthermore, the expression of PAK7 was markedly reduced at
the mRNA level by transfecting miR-526a precursor plasmids into the
Huh7 and HepG2 cells (Fig. 4C).
Consistently, the results of the western blot analysis showed that
the protein expression of PAK7 was inhibited by miR-526a
transfection (Fig. 4D). These
results suggested that miR-526a directly targeted PAK7 in the HCC
cell lines.
As the results showed that miR-526a was
significantly downregulated in HCC specimens and cell lines
(Fig. 1), the present study
determined whether the expression of PAK7 correlated with HCC
phenotype. The results of the RT-qPCR analysis revealed that the
levels of PAK7 were markedly increased in the HCC specimens,
compared with the normal adjacent tissues (n=135; Fig. 4E; P<0.01). A reverse correlation
was found between PAK7 and miR-526a in the HCC specimens
(Fig. 4F; R=−0.4596; P<0.01).
These results suggested that PAK7 may be a tumorigenic factor in
HCC and negatively correlate with the expression of miR-526a.
Discussion
HCC is a heterogeneous disease, in which multiple
factors collaborate with each other. Aberrant microRNA expression
has been reported to be involved in the tumorigenesis of several
types of cancer (22–24). Tipping the balance of microRNA
expression in tumor cells usually favors the uncontrolled
proliferation of tumor cells in addition to other malignant
characteristics (25). The fragile
nature of microRNA gene locus may increase the possibilities of
gene alterations and, therefore, increases the likelihood of
carcinogenesis (26). Therefore,
identifying the missing link between microRNA regulation and
tumorigenesis may provide critical insight into the pharmacological
intervention of cancer.
In the present study, it was found that miR-526a
functioned as a putative tumor suppressor in HCC, at least
partially by the fact that the expression of miR-526a was usually
downregulated in HCC specimens and cell lines (Fig. 1). Stable transfection with miR-526a
inhibited the malignant phenotypes of HCC, including proliferation,
migration and invasion (Figs. 2
and 3). PAK7 was predicted to be
the miR-526a target in HCC cell lines using overlapping
examinations from three independent databases. In addition, a
reverse correlation was found between PAK7 and miR-526a in HCC
specimens. p21-activated kinases (PAKs) are serine/threonine
kinases, which can be activated by GTPases (27). Two subgroups have been identified
for PAKs, termed group I PAKs (PAK1-3) and group II PAKs (PAK4-6).
PAK7 belongs to a different type of PAK, compared with group I and
group II PAKs (28). The
expression of PAK7 is usually elevated in several types of tumor,
including lung cancer, osteosarcoma, hepatocellular carcinoma,
colorectal cancer and pancreatic cancer (29–32).
Therefore, PAK7 may be implicated in the development of
various cancer types. A previous report showed that PAK7 promoted
cell mobility and proliferation by co-activating different survival
pathways (33). PAK7 can also
suppress apoptosis, possibly via altered subcellular localization
(34). The anti-apoptotic effect
may also be ascribed to post-translational modification of
anti-apoptotic proteins, including B cell lymphoma-2 family members
(33). Collectively, these
findings suggested that PAK7 may function as an oncogenic factor in
several tumor types. The post-transcriptional inhibition of the
expression of PAK7 by miR-526a may serve as an effective method of
releasing the intrinsic load of PAK7 in cancer cells, resulting in
tumor suppression.
Few reports have focused on miR-526a, particularly
in HCC. A previous study suggested that the decreased expression of
miR-526 was associated with increased viral infection and
attenuated apoptosis (35).
Consistently, another study argued that miR-526a may favor
RIG-I-dependent antiviral signaling (36). miR-526a may positively regulate the
production of type I interferon and suppress viral infection
(36). In addition, Fu et
al found that patients with decreased expression of miR-526a
correlated with active pulmonary tuberculosis (37). Therefore, miR-526a may also inhibit
infection and progression of tuberculosis (37). In a tumor-associated study, Zhang
et al (38) showed that
miR-526b can target Ku80 and suppress the incidence of non-small
cell lung cancer. The present study is the first, to the best of
our knowledge, to reveal a novel function of miR-526a in HCC. It
was found that miR-526a had a tumor suppressive role, possibly by
targeting PAK7, at least in the HCC cell lines assessed in the
present study. These preliminary findings may assist further
investigations on the role of miR-526a, particularly in
tumor-associated investigations.
In conclusion, the present study found a novel
function for miR-526a in HCC. The tumor suppressive effect of
miR-526a may function via targeting PAK7. As PAK7 has been
implicated in the progression of various tumor types, silencing the
expression of PAK7 via pairing with miR-526a may be an effective
strategy in tumor suppression. These results may improve current
understanding of microRNAs in modulating carcinogenesis and may
shed light on determining the heterogeneity in HCC.
Acknowledgements
This study was supported by A Brainstorm Project on
Social Development by the Department of Science and Technology of
Guizhou Province (grant no. SY[2015]3047), the National Natural
Science Foundation of China (grant nos. 81560477 and 81672906) and
the Youth Science and Technology Talent Development Project of
Guizhou Provincial Educational Department (grant no.
KY[2016]142).
References
|
1
|
Park JW, Chen M, Colombo M, Roberts LR,
Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS and
Sherman M: Global patterns of hepatocellular carcinoma management
from diagnosis to death: The BRIDGE Study. Liver Int. 35:2155–2166.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toffanin S, Hoshida Y, Lachenmayer A,
Villanueva A, Cabellos L, Minguez B, Savic R, Ward SC, Thung S,
Chiang DY, et al: MicroRNA-based classification of hepatocellular
carcinoma and oncogenic role of miR-517a. Gastroenterology.
140:1618–1628.e16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX,
Shen F, Zhou WP and Wu MC: Antiviral therapy improves postoperative
survival in patients with hepatocellular carcinoma: A randomized
controlled trial. Ann Surg. 261:56–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoshida Y, Toffanin S, Lachenmayer A,
Villanueva A, Minguez B and Llovet JM: Molecular classification and
novel targets in hepatocellular carcinoma: Recent advancements.
Semin Liver Dis. 30:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tovar V, Alsinet C, Villanueva A, Hoshida
Y, Chiang DY, Solé M, Thung S, Moyano S, Toffanin S, Mínguez B, et
al: IGF activation in a molecular subclass of hepatocellular
carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol.
52:550–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villanueva A, Chiang DY, Newell P, Peix J,
Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, et al:
Pivotal role of mTOR signaling in hepatocellular carcinoma.
Gastroenterology. 135:1972–1983, e1-e11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YQ, Ren YF, Song YJ, Xue YF, Zhang
XJ, Cao ST, Deng ZJ, Wu J, Chen L, Li G, et al: MicroRNA-581
promotes hepatitis B virus surface antigen expression by targeting
Dicer and EDEM1. Carcinogenesis. 35:2127–2133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su X, Wang H, Ge W, Yang M, Hou J, Chen T,
Li N and Cao X: An in vivo method to identify microRNA targets not
predicted by computation algorithms: p21 targeting by miR-92a in
cancer. Cancer Res. 75:2875–2885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shih YT, Wang MC, Zhou J, Peng HH, Lee DY
and Chiu JJ: Endothelial progenitors promote hepatocarcinoma
intrahepatic metastasis through monocyte chemotactic protein-1
induction of microRNA-21. Gut. 64:1132–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui W, Huang Z, He H, Gu N, Qin G, Lv J,
Zheng T, Sugimoto K and Wu Q: MiR-1188 at the imprinted Dlk1-Dio3
domain acts as a tumor suppressor in hepatoma cells. Mol Biol Cell.
26:1416–1427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Q, Xu Y, Wei S, Gao W, Chen L, Zhou T,
Wang Z, Ying M and Zheng Q: miRNA-148b suppresses hepatic cancer
stem cell by targeting neuropilin-1. Biosci Rep. 35:e002292015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X, Zhang XF, Lu X, Jia HL, Liang L,
Dong QZ, Ye QH and Qin LX: MicroRNA-26a suppresses angiogenesis in
human hepatocellular carcinoma by targeting hepatocyte growth
factor-cMet pathway. Hepatology. 59:1874–1885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J,
Qin Y, Sun Z and Zheng X: miR-183 inhibits TGF-beta1-induced
apoptosis by downregulation of PDCD4 expression in human
hepatocellular carcinoma cells. BMC Cancer. 10:3542010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenicity, and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
17
|
Coulouarn C, Factor VM, Andersen JB,
Durkin ME and Thorgeirsson SS: Loss of miR-122 expression in liver
cancer correlates with suppression of the hepatic phenotype and
gain of metastatic properties. Oncogene. 28:3526–3536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Viswanathan SR, Powers JT, Einhorn W,
Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA,
Lockhart VL, et al: Lin28 promotes transformation and is associated
with advanced human malignancies. Nat Genet. 41:843–848. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung JY and Hewitt SM: An optimized RNA
extraction method from archival formalin-fixed paraffin-embedded
tissue. Methods Mol Biol. 611:19–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:(Database Issue). D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim EL, Trinh DL, Scott DW, Chu A,
Krzywinski M, Zhao Y, Robertson AG, Mungall AJ, Schein J, Boyle M,
et al: Comprehensive miRNA sequence analysis reveals survival
differences in diffuse large B-cell lymphoma patients. Genome Biol.
16:182015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Xing Y, Liang C, Hu L, Xu F and
Chen Y: Crucial microRNAs and genes of human primary breast cancer
explored by microRNA-mRNA integrated analysis. Tumour Biol.
36:5571–5579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hata A and Kashima R: Dysregulation of
microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol.
51:121–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leonardo TR, Schultheisz HL, Loring JF and
Laurent LC: The functions of microRNAs in pluripotency and
reprogramming. Nat Cell Biol. 14:1114–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin H, Mali RS, Ma P, Chatterjee A,
Ramdas B, Sims E, Munugalavadla V, Ghosh J, Mattingly RR, Visconte
V, et al: Pak and Rac GTPases promote oncogenic KIT-induced
neoplasms. J Clin Invest. 123:4449–4463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Melzer J, Kraft KF, Urbach R and Raabe T:
The p21-activated kinase Mbt is a component of the apical protein
complex in central brain neuroblasts and controls cell
proliferation. Development. 140:1871–1881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu J, Li K, Li M, Wu X, Zhang L, Ding Q,
Wu W, Yang J, Mu J, Wen H, et al: A role for p21-activated kinase 7
in the development of gastric cancer. FEBS J. 280:46–55. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang HH, Zhang ZY, Che CL, Mei YF and Shi
YZ: Array analysis for potential biomarker of gemcitabine
identification in non-small cell lung cancer cell lines. Int J Clin
Exp Pathol. 6:1734–1746. 2013.PubMed/NCBI
|
|
31
|
Xie Q, Chen X, Lu F, Zhang T, Hao M, Wang
Y, Zhao J, McCrae MA and Zhuang H: Aberrant expression of microRNA
155 may accelerate cell proliferation by targeting sex-determining
region Y box 6 in hepatocellular carcinoma. Cancer. 118:2431–2442.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giroux V, Dagorn JC and Iovanna JL: A
review of kinases implicated in pancreatic cancer. Pancreatology.
9:738–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han K, Zhou Y, Gan ZH, Qi WX, Zhang JJ,
Fen T, Meng W, Jiang L, Shen Z and Min DL: p21-activated kinase 7
is an oncogene in human osteosarcoma. Cell Biol Int. 38:1394–1402.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wells CM and Jones GE: The emerging
importance of group II PAKs. Biochem J. 425:465–473. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang YW: Transcriptomic approach predicts
tempo of disease progression in HIV-1 infections. Clin Chem.
59:1143–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu C, He X, Zheng Z, Zhang Z, Wei C, Guan
K, Hou L, Zhang B, Zhu L, Cao Y, et al: Downregulation of microRNA
miR-526a by enterovirus inhibits RIG-I-dependent innate immune
response. J Virol. 88:11356–11368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu Y, Yi Z, Wu X, Li J and Xu F:
Circulating microRNAs in patients with active pulmonary
tuberculosis. J Clin Microbiol. 49:4246–4251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang ZY, Fu SL, Xu SQ, et al: By
downregulating Ku80, hsa-miR-526b suppresses non-small cell lung
cancer. Oncotarget. 6:1462–1477. 2015. View Article : Google Scholar : PubMed/NCBI
|