Introduction
Recurrent spontaneous abortion (RSA), also referred
to as recurrent miscarriage, habitual abortion or recurrent
pregnancy loss, is defined by more than three consecutive
miscarriages prior to 20 gestational weeks (1,2). RSA
occurs in 1–5% of women during pregnancy (3). The cause of RSA remains unknown;
thus, continuing clinical and laboratory investigations are
required (4,5). Previous studies have reported that
various etiologic factors are involved in certain RSA cases;
including chromosome abnormalities, endocrine diseases, uterine
abnormalities, placental anomalies, hormonal problems,
thrombophilia, infections, nutritional disorders, autoimmune
disease and anatomy (6–8). The etiology of RSA remains to be
fully elucidated despite numerous studies investigating the above
factors. Early prediction of the potential risk of RSA is required
to increase live birth rates in patients with RSA (9).
Biomarkers are currently widely used to refine
diagnoses, predict disease and monitor the effects of treatment
(10). It is established that the
human proteome regulates cellular function and determines the
phenotype; thus, the identification of relevant proteins is likely
to reveal reliable biomarkers for predicting disease (11). A range of potential biomarkers for
RSA have been previously reported. Stortoni et al (12) reported that expression levels of
thrombomodulin were reduced by 45% in patients with RSA compared
with healthy individuals, as determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). Bao
et al (13) determined by
RT-qPCR, western blot analysis and immunohistochemistry that serum
Dickkopf-related protein (Dkk) 1 levels were increased in RSA
patients compared with controls. Additional studies are required to
validate these potential biomarkers and their prognostic value.
Identifying novel RSA biomarkers may improve the diagnosis, safety
and efficacy of current therapies for RSA. As one of the most
intensely studied protein families in biomedical science, cytokines
have been widely investigated as potential disease biomarkers
(14). The introduction of
high-throughput and high-specificity detection of complex proteins
at picomolar and femtomolar quantities, and antibody arrays, are
now widely used for mining complex proteomes (15), facilitating simultaneous screening
of numerous secreted signal proteins in complex biological samples
(16). However, to the best of our
knowledge, no previous study has identified serum RSA biomarkers
using antibody array technology. Therefore, the present study used
a RayBio® Label-Based (L-Series) Human Antibody Array
1000 Membrane kit (RayBiotech, Inc., Norcross, GA, USA) to identify
reliable biomarkers for the prediction of RSA.
Patients and methods
Patients and controls
From January 2014 to March 2015, a total of 60
Chinese patients with a history of RSA were recruited as the
patient group from the Department of Traditional Chinese Medicine
at the Beijing Obstetrics and Gynecology Hospital. They had normal
endocrine levels, and their partners had normal spermatogenesis and
sperm function. ‘Blood stasis’ syndrome (BSS, also known as
Xueyu zheng in Chinese) is characterized in traditional
medicine as ‘pain that occurs in a fixed location, dark-purple face
or tongue, bleeding, blood spots under the skin, and an astringent
pulse’ among other features (17).
The concept of blood stasis has been interpreted, changed and
developed systematically since ancient times (18). All 60 RSA patients exhibited the
‘blood stasis’ features described above at the time of study.
Patient characteristics, including age at diagnosis, gravidities,
number of child births and timing of spontaneous abortion, are
summarized in Table I. For the
control group, 20 Chinese females who had experienced full-term
pregnancies were recruited from the Department of Traditional
Chinese Medicine at the Beijing Obstetrics and Gynecology
Hospital.
 | Table I.Characteristics of 60 patients with
recurrent spontaneous abortion. |
Table I.
Characteristics of 60 patients with
recurrent spontaneous abortion.
| Characteristic | Value |
|---|
| Age at
diagnosisa | 30±2.8 |
|
Graviditiesa | 3±0.5 |
| No. of
childbirths |
|
|
1b | 5 (8%) |
|
0b | 55 (92%) |
| Spontaneous
abortionsa | 3±0.5 |
|
2b | 1 (2%) |
|
1b | 12 (20%) |
|
0b | 47 (78%) |
Ethical approval and sample
collection
All participants signed informed consent forms prior
to participation. The present study was approved by the Ethics
Committee of the Beijing Obstetrics and Gynecology Hospital,
Capital Medical University (Beijing, China; approval no.
2014-KY-001). Whole blood samples were collected from each
participant. Serum was collected following blood centrifugation at
550 × g for 10 min at 4°C, and stored at −80°C. The sera of 23 RSA
patients and 10 healthy subjects were pooled into 6 samples
followed by standard processing (19). The samples included those that
could be classified as ‘blood stasis’ 1, 2 and 3, ‘non-blood
stasis’ 1, 2, and 3, and controls 1–6. The order of mixing is
presented in Table II. All
mixtures were obtained by mixing equal volumes of sera.
 | Table II.Pooling of serum samples. |
Table II.
Pooling of serum samples.
| Pooled serum
sample | Original
sample |
|---|
| Patients with
RSA |
|
| Blood
stasis group 1 (thrombus 1) | A1, A2, A3, A4 |
| Blood
stasis group 2 (thrombus 2) | A5, A6, A7, A8 |
| Blood
stasis group 3 (thrombus 3) | A9, A10, A11,
A12 |
|
Non-blood stasis group 1
(non-thrombus 1) | C1, C2, C3, C4 |
|
Non-blood stasis group 2
(non-thrombus 2) | C5, C6, C7, C8 |
|
Non-blood stasis group 3
(non-thrombus 3) | C9, C10, C11 |
| Control |
|
| 1 | E1, E2, E3 |
| 2 | E4, E5, E6 |
| 3 | E7 |
| 4 | E8 |
| 5 | E9 |
| 6 | E10 |
Antibody array assay
The 12 samples described above were assayed for the
relative expression of 1,000 human proteins. The target proteins
included cytokines, chemokines, adipokines, growth factors,
angiogenic factors, proteases, soluble receptors and soluble
adhesion molecules. A RayBio® Label-Based (L-Series)
Human Antibody Array 1,000 Membrane kit (consisting of a
combination of Human L-507 and L-493) was used for protein
detection in accordance with the manufacturer's protocol. The
signals were scanned at a wavelength of 532 nm using an InnoScan
300 Microarray Scanner (Innopsys, Carbonne, France; resolution, 10
µm) and analyzed using RayBio Analysis Tool software (AAH-BLG-1-SW
and AAH-BLG-2-SW; RayBiotech, Inc.).
Detection of protein levels by
ELISA
As determined by microarray analysis, serum markers
with significant differences in expression levels between patients
and healthy individuals were detected in 60 patients and 20
controls using ELISA kits (ELH-TRAPPIN2, ELH-IGFBPRP1, ELH-RAGE,
ELH-DKK3 and ELH-Angiopoietin-2; RayBiotech, Inc.) according to the
manufacturer's protocol. Trappin-2, insulin-like growth
factor-binding protein-related protein 1 (IGFBP-rp1)/IGFBP-7,
receptor for advanced glycation end products (RAGE), Dkk3, and
angiopoietin-2 levels were detected. Serum samples were incubated
at room temperature. Following washing with wash buffer, a prepared
biotinylated antibody was added into the microplate to capture the
target protein. Following this, horseradish peroxidase-conjugated
streptavidin was used to bind with biotin from the biotinylated
antibody. Finally, 1-Step 3,3′,5,5′-tetramethylbenzidine-ELISA
substrate solution was added followed by stop solution, and
absorbance was measured at a wavelength of 450 nm by absorbance
microplate reader ELx800 (BioTek Instruments, Inc., Winooski, VT,
USA).
Statistical analysis and
bioinformatics
All array data analyses were performed using RayBio
Analysis Tool software. Biostatistics and bioinformatics analysis
included discriminatory protein analysis and data mining cluster
analysis. Statistical differences between two groups were
determined by Student's t-test. Fold change values of proteins were
used as indicators of relative expression levels. Data mining
cluster analysis was used to identify potential biomarkers by
clustering all relevant proteins according to the similarity of
their expression profiles using Cluster software version 3.0
(http://cluster2.software.informer.com/3.0). ELISA data
was analyzed using SigmaPlot software version 12.0 (Systat
Software, Inc., San Jose, CA, USA). T- and F-tests were used to
analyze ELISA quantification. The receiver operating
characteristics curve (ROC) method was used to assess sensitivity
and specificity of potential biomarkers using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of antibody microarrays
A total of 1,000 proteins were measured in the serum
mixture using the microarray. The spectra of 1,000 proteins from
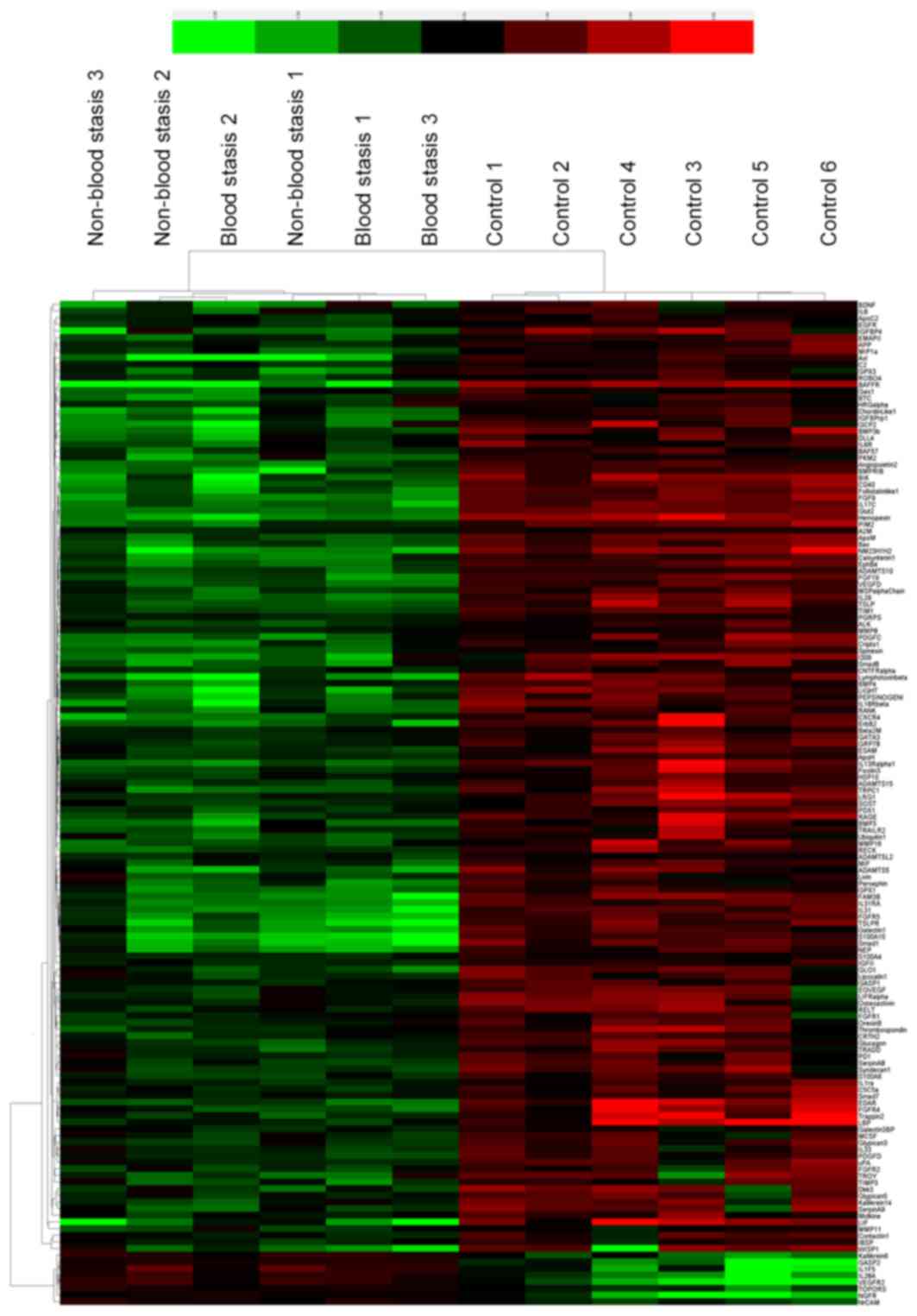
eight samples are presented in Fig.
1. The results demonstrated that 151 proteins had significantly
different expressions between the two groups. Of these differential
proteins, eight were significantly upregulated, and 143 proteins
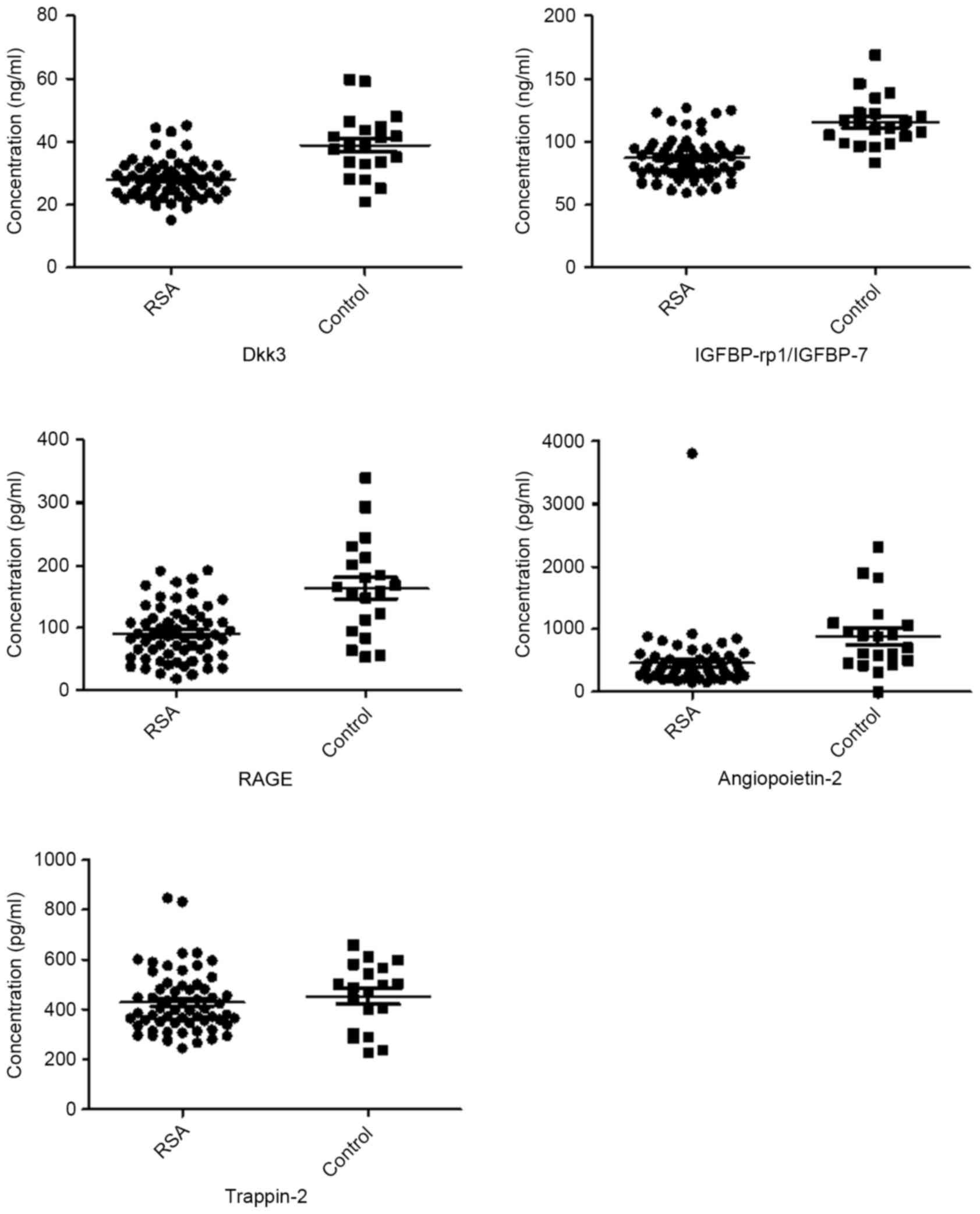
were downregulated in RSA patients compared with controls (Table III). Fig. 2 presents are boxplots of the
fluorescence signal values of eight differential proteins, selected
for signal strength, fold changes and clinical significance. Serum
mixture samples were arranged by similarities in the abundance of
these 151 markers in the sera clustering algorithm, which produced
two clusters that contained patients and healthy individuals
(Fig. 3).
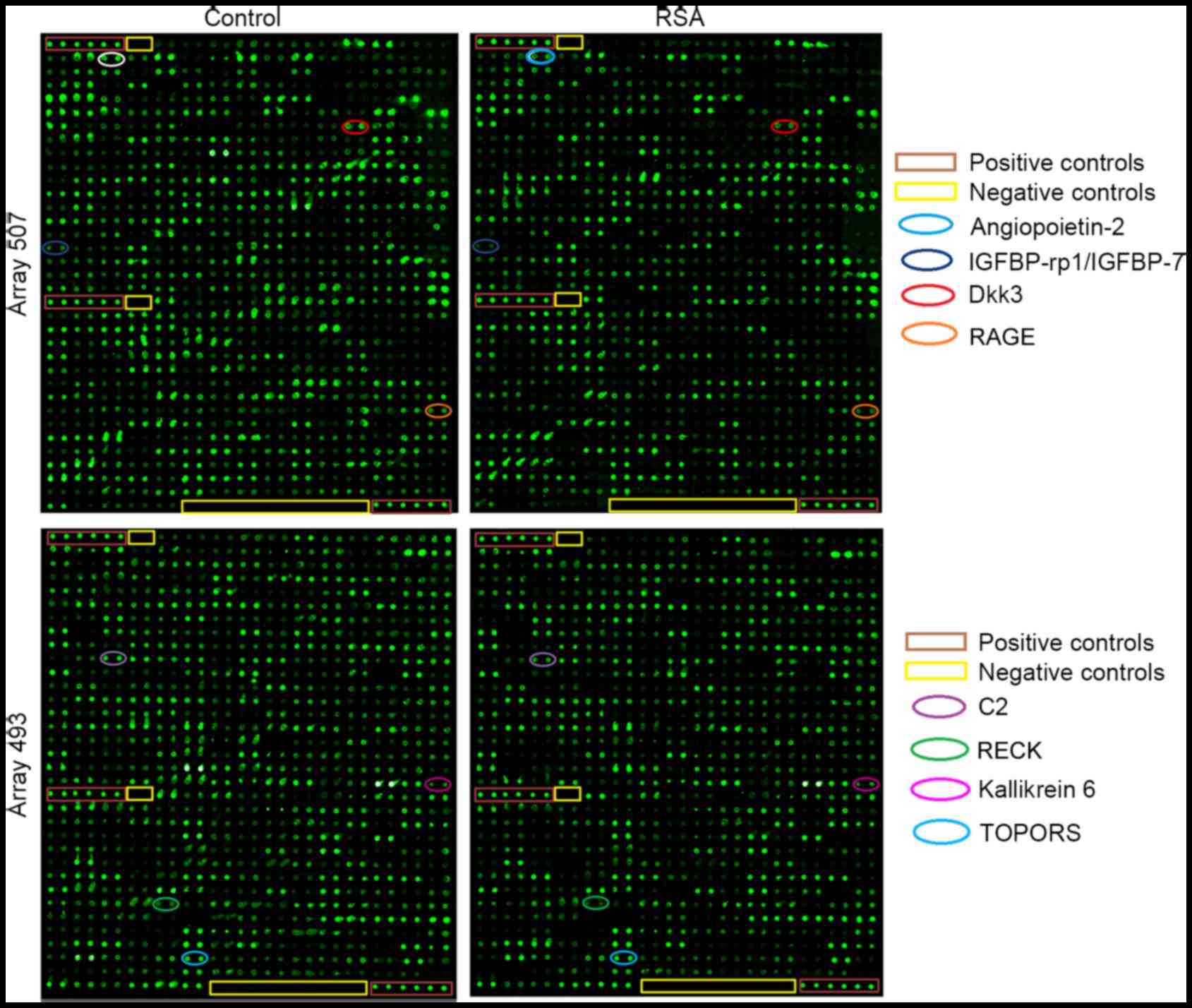 | Figure 1.Protein spectra from RayBio L-Series
Human 507 (507 proteins) and 493 (493 proteins) antibody arrays.
Representative images from human antibody arrays demonstrating the
reactivity of pooled serum samples to arrays L series (1,000
proteins) in healthy controls and RSA patients. Each protein was
measured in duplicate. A total of eight of significantly different
factors on the microarrays are marked in elliptical boxes.
IGFBP-rp1/IGFBP-7, insulin-like growth factor-binding
protein-related protein 1/insulin-like growth factor-binding
protein 7; Dkk3, Dickkopf-related protein 3; RAGE, receptor for
advanced glycation end products; RSA, recurrent spontaneous
abortion; TOPORS, topoisomerase I binding, arginine/serine-rich, E3
ubiquitin protein ligase; C2, complement C2; RECK,
reversion-inducing-cysteine rich protein with kazal motifs. |
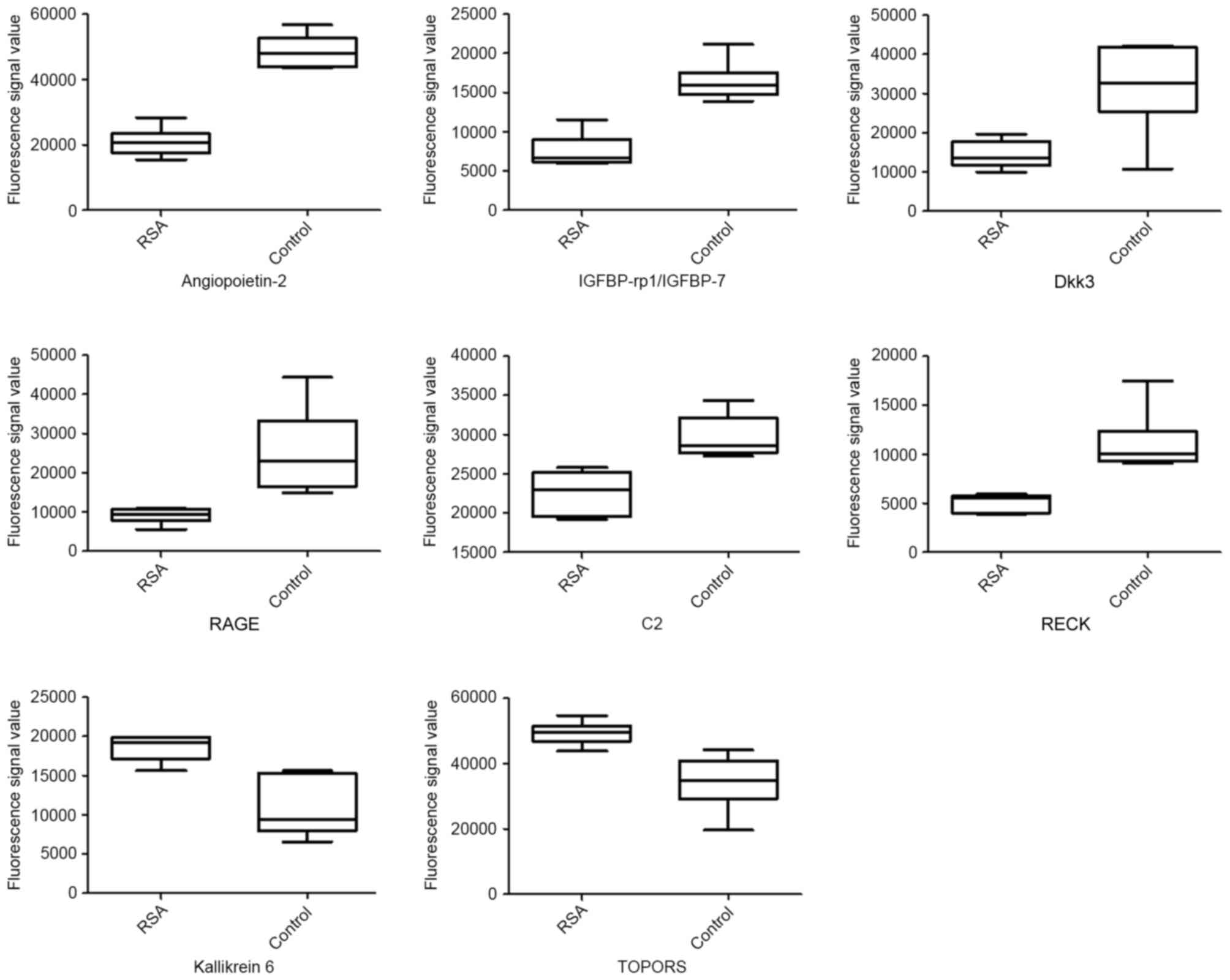 | Figure 2.Boxplots of differential serum
proteins between RSA patients and controls. P<0.05, RSA vs.
control for all presented proteins. ANG-2, angiopoietin 2;
IGFBP-rp1/IGFBP-7, insulin-like growth factor-binding
protein-related protein 1/insulin-like growth factor-binding
protein 7; Dkk3, Dickkopf-related protein 3; RAGE, receptor for
advanced glycation end products; RSA, recurrent spontaneous
abortion; TOPORS, topoisomerase I binding, arginine/serine-rich, E3
ubiquitin protein ligase; C2, complement C2; RECK,
reversion-inducing-cysteine rich protein with kazal motifs. |
 | Table III.A total of 151 proteins with
significantly different expression levels between patients with RSA
and controls. |
Table III.
A total of 151 proteins with
significantly different expression levels between patients with RSA
and controls.
|
| RSA | Control | RSA vs.
control |
|---|
|
|
|
|
|
|---|
| Target | Mean | SD | Mean | SD | F-test | t-test P-value | Fold-change |
|---|
| A2M | 4,650.558 | 373.053 | 6,374.645 | 269.687 | 0.494 | 0.000 | 0.730 |
| ADAMTS-10 | 7,862.456 | 1,306.692 | 13,925.383 | 550.378 | 0.081 | 0.000 | 0.565 |
| ADAMTS-15 | 2,999.115 | 479.112 | 5,705.655 | 1,924.875 | 0.008 | 0.017 | 0.526 |
| ADAMTS-5 | 558.209 | 250.695 | 1,466.103 | 483.403 | 0.176 | 0.002 | 0.381 |
| ADAMTS-L2 | 20,775.755 | 2,676.424 | 27,565.213 | 1,305.789 | 0.141 | 0.000 | 0.754 |
| ALK | 7,976.603 | 1,428.122 | 13,199.048 | 2,632.151 | 0.206 | 0.002 | 0.604 |
| Angiopoietin-2 | 20,789.029 | 4,292.976 | 48,570.398 | 4,977.529 | 0.753 | 0.000 | 0.428 |
| ApoC2 | 14,305.316 | 2,066.666 | 19,754.508 | 2,309.965 | 0.813 | 0.002 | 0.724 |
| ApoH | 4,898.886 | 462.884 | 7,760.884 | 1,065.437 | 0.091 | 0.000 | 0.631 |
| ApoM | 1,502.749 | 273.114 | 3,924.876 | 654.067 | 0.078 | 0.000 | 0.383 |
| APP | 12,633.899 | 1,318.521 | 19,428.245 | 5,101.616 | 0.010 | 0.021 | 0.650 |
| Axl | 1,510.055 | 1,117.320 | 4,936.198 | 495.598 | 0.099 | 0.000 | 0.306 |
| BAF57 | 1,181.342 | 265.140 | 1,906.967 | 361.301 | 0.513 | 0.003 | 0.619 |
| BAFF
R/TNFRSF13C | 130.169 | 152.078 | 1,362.334 | 162.415 | 0.889 | 0.000 | 0.096 |
| Bax | 15,217.862 | 3,242.826 | 38,315.362 | 7,712.389 | 0.080 | 0.000 | 0.397 |
| BDNF | 13,455.044 | 6,688.395 | 25,250.436 | 5,906.534 | 0.792 | 0.009 | 0.533 |
| β 2M | 1,320.861 | 133.263 | 1,910.310 | 294.761 | 0.106 | 0.001 | 0.691 |
| BIK | 311.407 | 134.485 | 1,179.513 | 269.458 | 0.153 | 0.000 | 0.264 |
| BMP-3 | 10,702.264 | 3,851.243 | 28,110.700 | 15,617.736 | 0.008 | 0.041 | 0.381 |
| BMP-3b/GDF-10 | 124.436 | 41.431 | 347.417 | 122.473 | 0.033 | 0.005 | 0.358 |
| BMP-4 | 901.991 | 2,56.578 | 1,767.609 | 343.897 | 0.536 | 0.001 | 0.510 |
| BMPR-IB/ALK-6 | 23,780.461 | 9648.386 | 62,589.402 | 6,422.893 | 0.393 | 0.000 | 0.380 |
| BTC | 11,944.615 | 4,921.916 | 21,760.107 | 3,620.813 | 0.517 | 0.003 | 0.549 |
| C2 | 22,604.021 | 2,719.955 | 29,647.219 | 2,700.060 | 0.988 | 0.001 | 0.762 |
| C5/C5a | 12,737.991 | 1,525.086 | 22,749.974 | 7,304.236 | 0.004 | 0.019 | 0.560 |
| Calsyntenin-1 | 1,911.570 | 607.215 | 4,505.218 | 691.477 | 0.782 | 0.000 | 0.424 |
| CD40/TNFRSF5 | 1,101.031 | 337.593 | 3,397.425 | 678.353 | 0.152 | 0.000 | 0.324 |
| Chordin-Like 1 | 1,391.942 | 585.338 | 3,128.589 | 566.356 | 0.944 | 0.000 | 0.445 |
| CNTF R α | 77,532.324 | 10,164.182 | 114,914.734 | 12,924.497 | 0.611 | 0.000 | 0.675 |
| Contactin-1 | 3,977.884 | 438.734 | 6,093.532 | 1,458.926 | 0.020 | 0.015 | 0.653 |
| Cripto-1 | 8,176.343 | 2,831.476 | 18,724.736 | 4,786.781 | 0.274 | 0.001 | 0.437 |
| CRTH-2 | 34,856.201 | 6,547.851 | 55,742.907 | 8,503.753 | 0.580 | 0.001 | 0.625 |
| CXCR4 (fusin) | 3,702.606 | 1,090.358 | 11,095.207 | 6,134.514 | 0.002 | 0.031 | 0.334 |
| Dkk-3 | 14,387.266 | 3,452.455 | 31,716.357 | 11,437.946 | 0.020 | 0.012 | 0.454 |
| DLL4 | 1,759.891 | 629.238 | 3,330.977 | 898.310 | 0.453 | 0.006 | 0.528 |
| EDAR | 1,240.627 | 223.958 | 4,773.756 | 1,971.378 | 0.000 | 0.007 | 0.260 |
| EGF R/ErbB1 | 11,026.167 | 1,733.072 | 15,758.956 | 2,371.519 | 0.508 | 0.003 | 0.700 |
| EG-VEGF/PK1 | 29,916.922 | 4,877.762 | 51,349.446 | 16,211.012 | 0.020 | 0.022 | 0.583 |
| EMAP-II | 10,666.342 | 2,600.714 | 19,708.795 | 3,896.837 | 0.395 | 0.001 | 0.541 |
| EphB4 | 1,962.256 | 406.898 | 5,161.814 | 945.891 | 0.088 | 0.000 | 0.380 |
| ErbB2 | 4,267.172 | 1,130.288 | 15,536.343 | 9,241.970 | 0.000 | 0.030 | 0.275 |
| ESAM | 2,854.186 | 495.592 | 5,990.106 | 2,581.532 | 0.002 | 0.030 | 0.476 |
| FAM3B | 1,270.946 | 447.324 | 4,455.137 | 885.200 | 0.160 | 0.000 | 0.285 |
| FGF R4 | 3,992.796 | 1,049.190 | 12,794.810 | 7,288.004 | 0.001 | 0.031 | 0.312 |
| FGF R5 | 2,826.176 | 1,013.809 | 7,878.562 | 1,003.601 | 0.983 | 0.000 | 0.359 |
| FGF-19 | 1,641.777 | 369.438 | 4,442.636 | 778.147 | 0.128 | 0.000 | 0.370 |
| FGF-9 | 6,413.361 | 1,443.488 | 22,180.535 | 5,185.520 | 0.014 | 0.000 | 0.289 |
| FGFR1 | 11,978.622 | 1,527.435 | 16,683.655 | 4,343.023 | 0.039 | 0.045 | 0.718 |
| FGFR2 | 13,703.293 | 2,914.828 | 23,307.209 | 7,653.038 | 0.054 | 0.017 | 0.588 |
| Ficolin-3 | 3,374.750 | 486.123 | 8,120.462 | 4,212.321 | 0.000 | 0.040 | 0.416 |
|
Follistatin-like1 | 2,481.289 | 721.297 | 7,152.976 | 1,161.224 | 0.319 | 0.000 | 0.347 |
| Galectin-1 | 1,435.877 | 634.069 | 3,694.218 | 803.285 | 0.616 | 0.000 | 0.389 |
| Galectin-3BP | 10,655.461 | 1,406.056 | 13,522.242 | 1,910.307 | 0.517 | 0.014 | 0.788 |
| Gas1 | 4,530.505 | 1,493.529 | 7,110.508 | 1,228.339 | 0.678 | 0.008 | 0.637 |
|
GASP-1/WFIKKNRP | 85,406.876 | 7,073.932 | 128,207.327 | 24,054.577 | 0.018 | 0.006 | 0.666 |
| GATA-3 | 7,625.978 | 591.277 | 13,276.727 | 2,655.282 | 0.005 | 0.003 | 0.574 |
| GCP-2/CXCL6 | 847.677 | 514.092 | 2,243.977 | 1,012.301 | 0.163 | 0.013 | 0.378 |
| GLO-1 | 997.491 | 290.387 | 2,106.667 | 501.997 | 0.255 | 0.001 | 0.473 |
| Glucagon | 61,025.896 | 9,853.803 | 128,506.268 | 35,031.652 | 0.015 | 0.004 | 0.475 |
| GluT2 | 11,546.660 | 704.259 | 37,514.070 | 2,261.457 | 0.023 | 0.000 | 0.308 |
| Glypican 3 | 1,389.140 | 249.379 | 2,554.322 | 649.947 | 0.056 | 0.002 | 0.544 |
| Glypican 5 | 15,529.644 | 2,266.574 | 25,519.026 | 7,257.053 | 0.023 | 0.018 | 0.609 |
| GPX1 | 2,008.854 | 557.930 | 4,383.148 | 782.893 | 0.475 | 0.000 | 0.458 |
| GPX3 | 3,009.541 | 1,283.488 | 5,083.531 | 927.399 | 0.493 | 0.009 | 0.592 |
| GRP78 | 2,266.940 | 302.999 | 4,387.312 | 1,150.085 | 0.011 | 0.005 | 0.517 |
| Hemopexin | 725.265 | 151.625 | 3,871.951 | 913.350 | 0.001 | 0.000 | 0.187 |
| HRG-α | 5,321.121 | 1,385.473 | 7,078.008 | 834.906 | 0.291 | 0.024 | 0.752 |
| HSP10 | 26,971.939 | 3,802.952 | 43,781.370 | 10,058.305 | 0.052 | 0.003 | 0.616 |
| I-309 | 4,283.951 | 2,116.728 | 13,856.485 | 3,913.389 | 0.204 | 0.000 | 0.309 |
| IBSP | 7,712.034 | 986.133 | 10,133.874 | 1,761.061 | 0.229 | 0.015 | 0.761 |
| IGFBP-4 | 1,453.473 | 684.730 | 4,146.757 | 1,511.595 | 0.107 | 0.003 | 0.351 |
|
IGFBP-rp1/IGFBP-7 | 7,523.924 | 2,135.058 | 16,380.247 | 2,502.068 | 0.736 | 0.000 | 0.459 |
| IGF-II | 65,869.138 | 5,912.640 | 94,321.230 | 10,392.485 | 0.241 | 0.000 | 0.698 |
| IL-1 ra | 361,872.941 | 34,398.268 | 563,878.996 | 45,414.321 | 0.011 | 0.011 | 0.642 |
| IL-13 R α1 | 6,360.695 | 1,012.833 | 24,083.997 | 13,484.474 | 0.000 | 0.023 | 0.264 |
| IL-17C | 2,345.533 | 541.033 | 7,048.395 | 748.914 | 0.493 | 0.000 | 0.333 |
| IL-18 R β/AcPL | 1,935.580 | 858.981 | 5,832.686 | 1,358.705 | 0.337 | 0.000 | 0.332 |
| IL-29 | 8,733.803 | 1,800.279 | 24,866.993 | 5,813.232 | 0.022 | 0.001 | 0.351 |
| IL-31 | 1,970.735 | 687.942 | 5,958.295 | 784.836 | 0.779 | 0.000 | 0.331 |
| IL-31 RA | 1,182.894 | 372.281 | 3,207.266 | 506.214 | 0.516 | 0.000 | 0.369 |
| IL-33 | 2,441.589 | 430.891 | 3,847.273 | 1,061.515 | 0.070 | 0.013 | 0.635 |
| IL-6 R | 3,540.308 | 909.773 | 6,566.421 | 1,881.755 | 0.137 | 0.005 | 0.539 |
| IL-8 | 25,002.761 | 6,204.334 | 34,829.214 | 6,706.528 | 0.869 | 0.025 | 0.718 |
| Kallikrein 14 | 2,979.489 | 615.746 | 5,825.747 | 1,585.646 | 0.058 | 0.002 | 0.511 |
| LBP | 3,409.164 | 463.893 | 13,342.910 | 8,052.946 | 0.000 | 0.029 | 0.256 |
| LIF R α | 13,797.957 | 2,293.325 | 27,604.197 | 8,923.296 | 0.010 | 0.012 | 0.500 |
| LIF | 332.827 | 242.463 | 1,628.345 | 875.661 | 0.014 | 0.014 | 0.204 |
| LIGHT/TNFSF14 | 2,092.869 | 917.268 | 6,360.354 | 1,227.569 | 0.538 | 0.000 | 0.329 |
| Lipocalin-1 | 20,832.310 | 4,151.253 | 34,570.502 | 9,172.736 | 0.107 | 0.007 | 0.603 |
| Livin | 2,707.067 | 795.904 | 4,194.195 | 745.424 | 0.889 | 0.007 | 0.645 |
| LRG1 | 11,113.403 | 863.592 | 28,581.729 | 14,531.460 | 0.000 | 0.032 | 0.389 |
| Lymphotoxin β
R/TNFRSF3 | 788.596 | 380.913 | 3,291.572 | 764.370 | 0.153 | 0.000 | 0.240 |
| M-CSF | 28,394.735 | 5,351.774 | 41,766.017 | 11,016.348 | 0.139 | 0.023 | 0.680 |
| Midkine | 3,267.246 | 433.600 | 5,032.121 | 1,617.032 | 0.012 | 0.044 | 0.649 |
| MIF | 3,951.056 | 885.814 | 6,986.334 | 1,120.792 | 0.618 | 0.000 | 0.566 |
| MIP-1α | 50,222.493 | 12,359.813 | 94,756.426 | 25,311.008 | 0.142 | 0.003 | 0.530 |
|
MMP-11/Stromelysin-3 | 54,153.970 | 12,843.620 | 69,764.345 | 7,386.564 | 0.250 | 0.027 | 0.776 |
| MMP-16/MT3-MMP | 11,330.829 | 2,524.054 | 26,764.023 | 11,916.806 | 0.004 | 0.024 | 0.423 |
| MMP-8 | 52,067.139 | 3,933.472 | 70,211.908 | 7,728.075 | 0.165 | 0.000 | 0.742 |
| MSP α Chain | 13,201.992 | 2,412.896 | 25,409.389 | 4,525.441 | 0.194 | 0.000 | 0.520 |
| NEP | 1,581.827 | 658.480 | 3,958.249 | 666.439 | 0.980 | 0.000 | 0.400 |
| NM23-H1/H2 | 550.675 | 189.223 | 2,651.125 | 1,022.967 | 0.002 | 0.004 | 0.208 |
| Orexin B | 43,718.747 | 7,996.118 | 74,138.593 | 18,347.514 | 0.092 | 0.004 | 0.590 |
|
Osteoactivin/GPNMB | 2,895.689 | 312.735 | 5,540.870 | 1,872.568 | 0.001 | 0.017 | 0.523 |
| PD-1 | 2,591.490 | 389.734 | 4,141.350 | 1,038.516 | 0.051 | 0.007 | 0.626 |
| PDGF-C | 9,264.920 | 2,881.398 | 24,377.899 | 9,157.178 | 0.024 | 0.008 | 0.380 |
| PDGF-D | 2,883.630 | 396.751 | 5,130.131 | 907.360 | 0.093 | 0.000 | 0.562 |
| PDX-1 | 2,270.140 | 363.742 | 3,981.057 | 824.869 | 0.097 | 0.001 | 0.570 |
| PEPSINOGEN I | 2,439.074 | 824.492 | 6,093.801 | 1,335.963 | 0.313 | 0.000 | 0.400 |
| Persephin | 2,515.448 | 1,011.303 | 4,523.807 | 838.775 | 0.691 | 0.004 | 0.556 |
| PGRP-S | 12,316.910 | 730.024 | 16,966.564 | 1,180.649 | 0.315 | 0.000 | 0.726 |
| PIM2 | 1,865.699 | 437.191 | 4,879.191 | 1,260.593 | 0.036 | 0.001 | 0.382 |
| PKM2 | 2,655.795 | 1,099.697 | 4,528.436 | 478.749 | 0.092 | 0.003 | 0.586 |
| RAGE | 9,020.357 | 1,947.254 | 25,325.305 | 10,739.884 | 0.002 | 0.013 | 0.356 |
| RANK/TNFRSF11A | 2,020.058 | 587.942 | 4,020.679 | 833.497 | 0.462 | 0.001 | 0.502 |
| RECK | 5,084.724 | 891.920 | 11,081.323 | 3,186.035 | 0.014 | 0.005 | 0.459 |
| RELT/TNFRSF19L | 31,871.125 | 4,886.989 | 61,866.224 | 17,568.797 | 0.014 | 0.007 | 0.515 |
| ROBO4 | 66,732.387 | 11,925.740 | 100,118.428 | 17,349.652 | 0.430 | 0.003 | 0.667 |
| S100A10 | 1,189.482 | 653.171 | 4,146.007 | 336.156 | 0.171 | 0.000 | 0.287 |
| S100A4 | 4,024.174 | 328.979 | 5,366.717 | 482.437 | 0.421 | 0.000 | 0.750 |
| S100A6 | 3,485.252 | 541.367 | 6,023.360 | 1,041.092 | 0.178 | 0.000 | 0.579 |
| Serpin A8 | 1,755.946 | 304.447 | 3,270.617 | 704.644 | 0.089 | 0.001 | 0.537 |
| Serpin A9 | 1,258.179 | 327.453 | 2,710.239 | 999.220 | 0.029 | 0.015 | 0.464 |
| Smad 1 | 1,028.273 | 540.825 | 3,720.431 | 748.020 | 0.494 | 0.000 | 0.276 |
| Smad 7 | 34,565.690 | 2,075.517 | 55,551.432 | 18,891.474 | 0.000 | 0.042 | 0.622 |
| Smad 8 | 6,886.663 | 2,599.832 | 15,373.512 | 4,688.229 | 0.221 | 0.003 | 0.448 |
| SOST | 3,117.271 | 461.906 | 6,218.660 | 1,701.605 | 0.012 | 0.006 | 0.501 |
| Spinesin | 20,626.782 | 4,848.625 | 38,828.941 | 4,444.642 | 0.853 | 0.000 | 0.531 |
| Syndecan-1 | 1,772.888 | 379.277 | 3,778.232 | 1,215.352 | 0.023 | 0.008 | 0.469 |
|
Thrombospondin-4 | 10,921.569 | 2,002.073 | 23,198.652 | 9,994.362 | 0.003 | 0.029 | 0.471 |
| TIM-1 | 2,132.294 | 259.727 | 4,438.018 | 697.608 | 0.049 | 0.000 | 0.480 |
| TIMP-3 | 4,008.775 | 1,259.727 | 6,988.724 | 1,853.036 | 0.417 | 0.009 | 0.574 |
| TRADD | 127,030.588 | 28,309.796 | 222,266.652 | 75,285.718 | 0.051 | 0.016 | 0.572 |
| TRAIL
R2/DR5/TNFRSF10B | 17,190.463 | 4,094.960 | 32,861.735 | 13,104.866 | 0.023 | 0.032 | 0.523 |
| Trappin-2 | 5,865.048 | 978.164 | 19,388.585 | 11,625.715 | 0.000 | 0.036 | 0.303 |
| TROY/TNFRSF19 | 2,324.349 | 786.973 | 5,012.391 | 1,945.216 | 0.069 | 0.011 | 0.464 |
| TRPC1 | 2,436.721 | 681.450 | 6,693.017 | 2,511.517 | 0.012 | 0.008 | 0.364 |
| TSLP | 1,853.969 | 303.884 | 5,110.938 | 2,119.578 | 0.001 | 0.013 | 0.363 |
| TSLP R | 1,905.470 | 965.346 | 6,488.242 | 1,625.517 | 0.277 | 0.000 | 0.294 |
| Ubiquitin+1 | 17,529.029 | 5,002.370 | 34,561.192 | 16,122.797 | 0.023 | 0.049 | 0.507 |
| uPA | 52,463.526 | 6,879.542 | 94,616.706 | 25,331.681 | 0.012 | 0.008 | 0.554 |
| VEGF-D | 24,467.055 | 5,668.534 | 52,501.169 | 6,836.652 | 0.691 | 0.000 | 0.466 |
| WISP-1/CCN4 | 2,330.112 | 1,166.842 | 5,516.779 | 2,727.490 | 0.086 | 0.025 | 0.422 |
| GASP-2/WFIKKN | 71,246.693 | 3,344.127 | 31,422.325 | 19,817.529 | 0.001 | 0.004 | 2.267 |
| IL-1 F5/FIL1δ | 30,158.276 | 6,083.967 | 13,083.494 | 8,056.469 | 0.553 | 0.002 | 2.305 |
| IL-28A | 71,058.941 | 7,420.008 | 30,883.742 | 18,108.416 | 0.072 | 0.001 | 2.301 |
| Kallikrein 6 | 18,588.432 | 1,695.500 | 10,786.937 | 3,766.885 | 0.104 | 0.001 | 1.723 |
| NGF R | 42,876.389 | 1,905.084 | 1,8740.985 | 13,128.177 | 0.001 | 0.006 | 2.288 |
| NrCAM | 73,805.167 | 6,435.403 | 5,1415.054 | 8,782.973 | 0.511 | 0.001 | 1.435 |
| TOPORS | 49,264.099 | 3,557.276 | 3,4240.228 | 8,383.960 | 0.083 | 0.002 | 1.439 |
| VEGF R2 (KDR) | 37,152.074 | 4,541.513 | 1,2904.085 | 9,743.845 | 0.119 | 0.000 | 2.879 |
Validation of microarray data by
ELISA
A total of five of the 151 proteins were selected
for validation assay in 60 RSA and 20 control samples. Serum levels
of trappin-2, IGFBP-rp1/IGFBP-7, RAGE, Dkk3 and angiopoietin-2 were
selected to be measured by ELISA based on the results from the
microarray experiments, previous reports on serum biomarkers in RSA
and the availability of commercial test kits. Levels of
IGFBP-rp1/IGFBP-7, Dkk3, RAGE and angiopoietin-2 were downregulated
in RSA patients compared with healthy controls, which was
consistent with the microarray results (P<0.05; Table IV and Fig. 4).
 | Table IV.ELISA analysis of cytokine levels in
the serum of patients with RSA and healthy controls. |
Table IV.
ELISA analysis of cytokine levels in
the serum of patients with RSA and healthy controls.
|
|
|
| Patients vs.
control |
|---|
|
|
|
|
|
|---|
| Cytokine | RSA | Control | F-test | t-test P-value | Fold-change |
|---|
| Trappin-2 | 429.17±125.17 | 453.26±132.34 | 0.718 | 0.473 | 0.947 |
|
IGFBP-rp1/IGFBP-7 |
86.94±16.49 | 115.63±20.12 | 0.246 | 0.000a | 0.752 |
| RAGE |
91.29±44.28 | 163.64±76.99 | 0.001 | 0.001a | 0.558 |
| Dkk3 |
28.16±6.22 |
38.96±10.05 | 0.005 | 0.000 | 0.723 |
| Angiopoietin-2 | 461.34±484.38 | 887.72±576.22 | 0.312 | 0.002a | 0.520 |
Analysis of sensitivity and
specificity of serum biomarkers for RSA
To validate whether IGFBP-rp1/IGFBP-7, Dkk3, RAGE
and angiopoietin-2 may be used as biomarkers for predicting RSA,
ROC curves were used to analyze sensitivity and specificity.
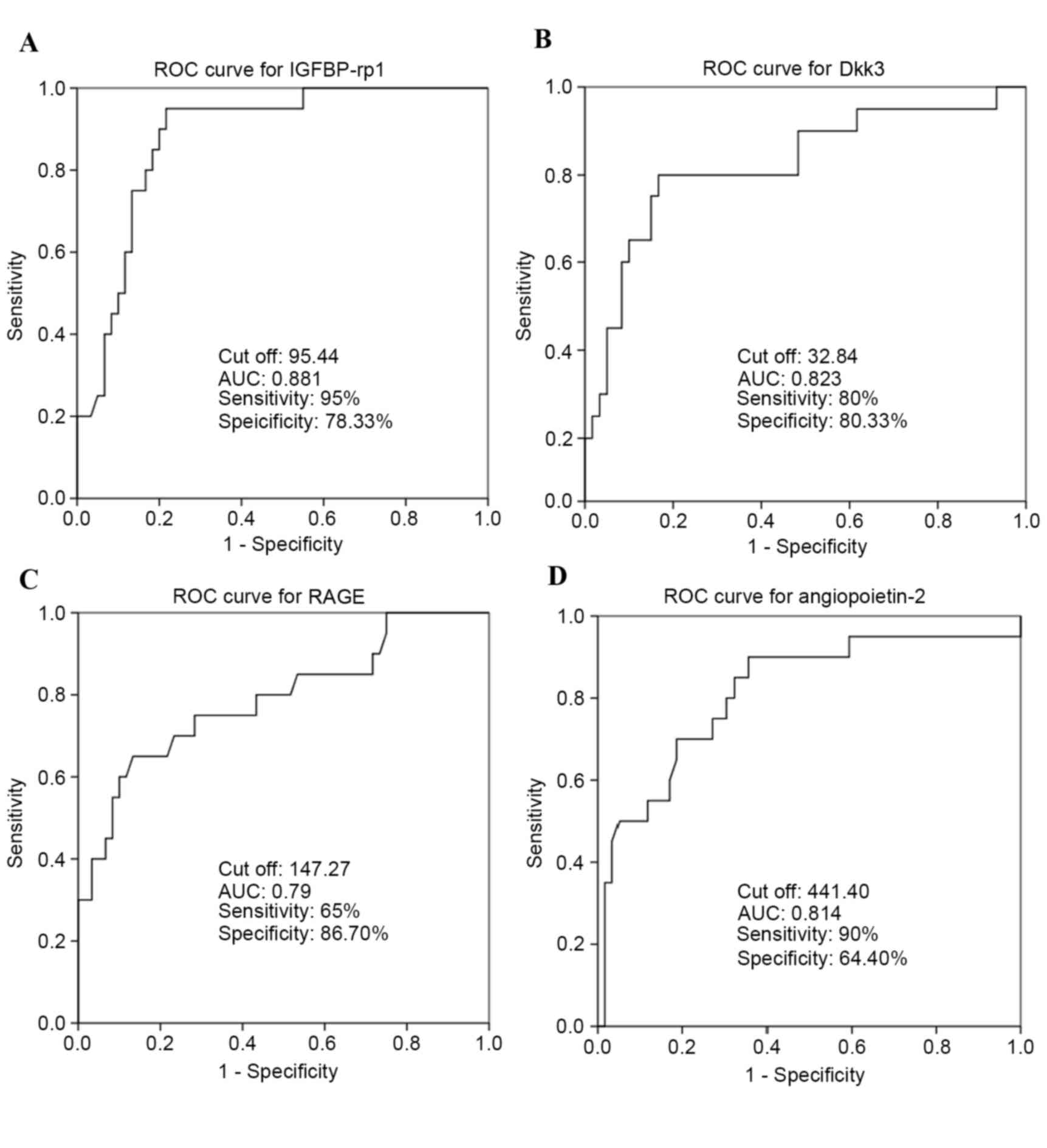
Area-under-ROC-curve values for IGFBP-rp1/IGFBP-7 (Fig. 5A), Dkk3 (Fig. 5B), RAGE (Fig. 5C) and angiopoietin-2 (Fig. 5D) cytokines were 0.881, 0.823, 0.79
and 0.814, respectively. IGFBP-rp1/IGFBP-7 had a sensitivity of 95%
and specificity of 78.33%. Dkk3 had a sensitivity of 80% and
specificity of 83.33%. RAGE had a sensitivity of 65% and
specificity of 86.70%. Angiopoietin-2 had a sensitivity of 90% and
specificity of 64.40%. All these were deemed suitable biomarkers
for the prediction of RSA.
Discussion
Potential biomarkers of RSA have previously been
reported. Khonina et al (20) investigated whether mixed lymphocyte
reaction blocking factor may be used as an indicator of the
efficacy for immunotherapy with paternal lymphocytes in females
with RSA. Metwally et al (21) performed a proteomic analysis of
obese and overweight women with RSA by 2-D gel electrophoresis,
principle component analysis and mass spectrometry, and
demonstrated that RSA patients exhibit a significant increase in
haptoglobin expression. Ibrahim et al (22) demonstrated that pentraxin-3
indicates the presence of abnormally exaggerated intrauterine
inflammation that may cause pregnancy failure in females with
unexplained RSA. Kim et al (23) identified RSA-associated factors in
human blood samples by 2-D gel electrophoresis, and analyzed spots
samples with matrix-assisted laser desorption/ionization-time of
flight/mass spectrometry, and reported that in RSA patients,
inter-α-trypsin inhibitor heavy chain family member 4 (ITI-H4)
expression was low and exhibited a molecular weight of 120 kDa in
controls; however, ITI-H4 was expressed at higher levels and at a
modified molecular weight of 36 kDa in the RSA patient group. This
indicated that ITI-H4 may be used as biomarker of RSA.
The present study used antibody array technology for
a primary screening of RSA biomarkers on pooled samples. The array
results revealed that the levels of eight cytokines were
significantly increased in the RSA patient group compared with
controls, and the levels of 143 of the tested 1,000 proteins were
significantly reduced in the RSA patient group compared with
controls. A total of 5 proteins, trappin-2, IGFBP-rp1/IGFBP-7,
Dkk3, RAGE and angiopoietin-2, were selected for ELISA validation
assay in a larger cohort of patient and control subjects. ELISA
results for these proteins were in accordance with the array
results. Sensitivity and specificity analysis by ROC revealed that
these four cytokines may be used as biomarkers of RSA.
To the best of our knowledge, the association
between IGFBP-rp1/IGFBP-7, Dkk3 and angiopoietin-2, and RSA has not
been reported. However, an isoform of the RAGE protein, sRAGE, has
been reported to be associated with RSA (24).
IGFBP-rp1/IGFBP-7
IGFs, which have characteristics of tissue growth
factors and circulating growth hormones, are potent mitogens and
anti-apoptotic agents (25). IGFs
include the hormones IGF-I and -II and their corresponding
receptors, and the IGFBPs (26).
The IGFBP superfamily includes six members (IGFBP-1-6) and 10
associated proteins (IGFBP-rp1-10) (27). IGFBP-7 has been demonstrated to be
a tumor suppressor in a variety of cancers. Benatar et al
(28) reported that treatment with
IGFBP-7 may have therapeutic potential for triple-negative breast
cancer. Liu et al (29)
demonstrated that IGFBP-7 was downregulated in gastric cancer, and
that it may be used as an indicator of poor prognosis in patients
with gastric cancer. IGFBP-7 has additionally been proposed as a
novel biomarker for assessing the risk of acute kidney injury
(30) and heart failure with
reduced ejection fraction, and has been demonstrated to have links
to the presence and severity of echocardiographic parameters of
abnormal diastolic function (31).
Dkk3
The Wnts are an evolutionarily conserved family of
secreted glycoproteins characterized by numerous conserved cysteine
residues (32). The Dkk proteins
are secreted Wnt inhibitors, inducing removal of the Wnt
co-receptor low-density lipoprotein receptor-related protein, and
consist of four primary members in vertebrates (Dkk1-4) (33,34).
Dkk-3 is downregulated in various types of cancer cells. Loss of
Dkk3 protein expression is associated with poor prognoses in
patients with gastric cancer, indicating that it may be a biomarker
for predicting lymph node involvement in these patients (35). Dkk3 has recently been implicated in
clear cell renal cell carcinoma, and may present a novel molecular
target for its diagnosis and treatment (36). Additionally, Dkk3 may represent a
therapeutic target for the treatment of heart failure following
myocardial infarction (37).
RAGE
RAGE is a cell-surface receptor that interacts with
AGEs, and is a member of the immunoglobulin superfamily (38). RAGE activation via its multiple
ligands, including S100 calcium-binding protein (S100A) 4 (39), high mobility group box 1 protein
(40) and amyloid-β protein
(41), serves important roles in
certain diseases. Dahlmann et al (42) demonstrated that the activity of
S100A4-RAGE induces RAGE-dependent increases in the migratory and
invasive capabilities of colorectal cancer cells. Guo et al
(43) identified RAGE as a
potential prognostic biomarker in renal cell carcinoma. RAGE/S100A7
signaling has been demonstrated to have a functional role in
linking inflammation to aggressive breast cancer development;
therefore, RAGE expression is currently regarded as a potential
biomarker for triple-negative breast cancer (44). Additionally, overexpression of RAGE
may be a useful marker to predict gastric cancer progression
(45).
Angiopoietin-2
As a member of the angiopoietin family,
angiopoietin-2 has complex and unique roles in regulating
angiogenesis, and has additional unconventional functions,
including stimulating tumor angiogenesis, invasion and metastasis
via Tie2-independent signaling pathways, involving
integrin-mediated signaling. Therefore, angiopoietin-2 may have
great potential as a therapeutic target, prognostic marker and
inhibitor of human cancer (46).
Angiopoietin-2 is expressed during vascular remodeling, thus
preventing vascular stability (47). A study by Morrissey et al
(48) demonstrated that
angiopoietin-2 inhibition impeded tumor growth of LuCaP 23.1
prostate cancer xenografts, and suggested that angiopoietin-2
inhibition in combination with other treatments is a potential
therapy for metastatic disease patients. Calfee et al
(49) reported that lowering
plasma angiopoietin-2 with fluid conservative therapy may be
beneficial, in part by decreasing endothelial inflammation. Goede
et al (50) demonstrated
that serum angiopoietin-2 represents a candidate biomarker for the
outcome of metastatic colorectal cancer patients treated with
bevacizumab-containing therapy. Additionally, angiopoietin-2 has
been associated with other diseases, including chronic kidney
disease (51) and cerebral malaria
(52).
In conclusion, the present study used a microarray
platform to detect 1,000 proteins to identify dysregulated serum
factors in RSA samples. This method was demonstrated to be
effective in investigating dynamic alterations in protein profiles,
and to select target proteins for further RSA research. The results
indicated that IGFBP-rp1/IGFBP-7, Dkk3, RAGE and angiopoietin-2
expression were downregulated in RSA patients, suggesting that they
may be important in the pathological process of RSA. Furthermore,
upregulating them may inhibit the development of RSA. Therefore,
these biomarkers represent potential predictive and diagnostic
markers for RSA due to their high sensitivity and specificity.
However, larger-scale studies are required to confirm the
diagnostic value of these markers.
Acknowledgements
The present study was supported by Beijing Municipal
Administration of Hospitals Clinical Medicine Development of
Special Funding (China; grant no. ZYLX201510). The authors would
like to thank Mr Xiangfu Ren (Beijing KeZhongZhi Biotechnology Co.,
Ltd., Beijing, China) for technical assistance and Ms. Hong Shao
(Beijing KeZhongZhi Biotechnology Co., Ltd.) for valuable
discussions.
Glossary
Abbreviations
Abbreviations:
|
RSA
|
recurrent spontaneous abortion
|
|
AUC
|
area under the curve
|
References
|
1
|
Ford HB and Schust DJ: Recurrent pregnancy
loss: Etiology, diagnosis, and therapy. Rev Obstet Gynecol.
2:76–83. 2009.PubMed/NCBI
|
|
2
|
Tang AW, Alfirevic Z, Turner MA, Drury J
and Quenby S: Prednisolone trial: Study protocol for a randomized
controlled trial of prednisolone for women with idiopathic
recurrent miscarriage and raised levels of uterine natural killer
(uNK) cells in the endometrium. Trials. 10:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen DI and Hong ZB: Research on the
pathogenesis diagnosis and treatment of female reproductive
disorders. J Shanghai Jiaotong Univ. 32:1161–1165. 2012.
|
|
4
|
Li TC, Markris M, Tomsu M, Tuckerman E and
Laird S: Recurrent miscarriage: Aetiology, management and
prognosis. Hum Reprod Update. 8:463–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Regan L: Recurrent miscarriage. BMJ.
302:543–544. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chuan Z, Jie D, Hao X, Junhua B, Mengjing
G, Liguo P, Yousheng Y, Hong L and Zhenghao H: Associations between
androgen receptor CAG & GGN repeat polymorphism & recurrent
spontaneous abortions in Chinese women. Indian J Med Res.
139:730–736. 2014.PubMed/NCBI
|
|
7
|
Shankarkumar U, Pradhan VD, Patwardhan MM,
Shankarkumar A and Ghosh K: Autoantibody profile and other
immunological parameters in recurrent spontaneous abortion
patients. Niger Med J. 52:163–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pang L, Wei Z, Li O, Huang R, Qin J, Chen
H, Fan X and Chen ZJ: An increase in vascular endothelial growth
factor (VEGF) and VEGF soluble receptor-1 (Sflt-1) are associated
with early recurrent spontaneous abortion. PLoS One. 8:e757592013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rull K, Tomberg K, Kõks S, Männik J, Möls
M, Sirotkina M, Värv S and Laan M: Increased placental expression
and maternal serum levels of apoptosis-inducing TRAIL in recurrent
miscarriage. Placenta. 34:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burska A, Boissinot M and Ponchel F:
Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm.
2014:5454932014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsangaris GT, Anagnostopoulos AK, Tounta
G, Antsaklis A, Mavrou A and Kolialexi A: Application of proteomics
for the identification of biomarkers in amniotic fluid: Are we
ready to provide a reliable prediction? EPMA J. 2:149–155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stortoni P, Cecati M, Giannubilo SR,
Sartini D, Turi A, Emanuelli M and Tranquilli AL: Placental
thrombomodulin expression in recurrent miscarriage. Reprod Biol
Endocrinol. 8:12010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao SH, Shuai W, Tong J, Wang L, Chen P
and Duan T: Increased Dickkopf-1 expression in patients with
unexplained recurrent spontaneous miscarriage. Clin Exp Immunol.
172:437–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang RP, Burkholder B, Jones Sloane V,
Jiang WD, Mao YQ, Chen QL and Shi Z: Cytokine antibody arrays in
biomarker discovery and validation. Curr Proteomics. 9:55–70. 2012.
View Article : Google Scholar
|
|
15
|
Wilson JJ, Burgess R, Mao YQ, Luo S, Tang
H, Jones VS, Weisheng B, Huang RY, Chen X and Huang RP: Antibody
arrays in biomarker discovery. Adv Clin Chem. 69:255–324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burkholder B, Huang RY, Burgess R, Luo S,
Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM and Huang RP:
Tumor-induced perturbations of cytokines and immune cell networks.
Biochim Biophys Acta. 1845:182–201. 2014.PubMed/NCBI
|
|
17
|
Liao J, Wang J, Liu Y, Li J, Duan L, Chen
G and Hu J: Modern researches on blood stasis syndrome 1989–2015: A
bibliometric analysis. Medicine (Baltimore). 95:e55332016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park B, Yun KJ, Jung J, You S, Lee JA,
Choi J, Kang BK, Alraek T, Birch S and Lee MS: Conceptualization
and utilization of blood stasis syndrome among doctors of Korean
medicine: Results of a web-based survey. Am J Transl Res.
6:857–868. 2014.PubMed/NCBI
|
|
19
|
Mahlknecht P, Stemberger S, Sprenger F,
Rainer J, Hametner E, Kirchmair R, Grabmer C, Scherfler C, Wenning
GK, Seppi K, et al: An antibody microarray analysis of serum
cytokines in neurodegenerative Parkinsonian syndromes. Proteome
Sci. 10:712012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khonina NA, Broitman EV, Shevela EY,
Pasman NM and Chernykh ER: Mixed lymphocyte reaction blocking
factors (MLR-Bf) as potential biomarker for indication and efficacy
of paternal lymphocyte immunization in recurrent spontaneous
abortion. Arch Gynecol Obstet. 288:933–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Metwally M, Preece R, Thomas J, Ledger W
and Li TC: A proteomic analysis of the endometrium in obese and
overweight women with recurrent miscarriage: Preliminary evidence
for an endometrial defect. Reprod Biol Endocrinol. 12:752014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ibrahim MI, Harb HM, Ellaithy MI,
Elkabarity RH and Abdelgwad MH: First trimester assessment of
Pentraxin-3 levels in women with primary unexplained recurrent
pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 165:37–41. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MS, Gu BH, Song S, Choi BC, Cha DH and
Baek KH: ITI-H4, as a biomarker in the serum of recurrent pregnancy
loss (RPL) patients. Mol Biosyst. 7:1430–1440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ota K, Yamagishi S, Kim M, Dambaeva S,
Gilman-Sachs A, Beaman K and Kwak-Kim J: Elevation of soluble form
of receptor for advanced glycation end products (sRAGE) in
recurrent pregnancy losses (RPL): Possible participation of RAGE in
RPL. Fertil Steril. 102:782–789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neuhouser ML, Platz EA, Till C, Tangen CM,
Goodman PJ, Kristal A, Parnes HL, Tao Y, Figg WD, Lucia MS, et al:
Insulin-like growth factors and insulin-like growth factor binding
proteins and prostate cancer risk: Results from the prostate cancer
prevention trial. Cancer Prev Res (Phila). 6:91–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bower NI and Johnston IA: Transcriptional
regulation of the igf signaling pathway by amino acids and
insulin-like growth factors during myogenesis in Atlantic salmon.
PLoS One. 5:e111002010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsumoto T, Hess S, Kajiyama H, Sakairi
T, Saleem MA, Mathieson PW, Nojima Y and Kopp JB: Proteomic
analysis identifies insulin-like growth factor-binding
protein-related protein-1 as a podocyte product. Am J Physiol Renal
Physiol. 299:F776–F784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benatar T, Yang W, Amemiya Y, Evdokimova
V, Kahn H, Holloway C and Seth A: IGFBP7 reduces breast tumor
growth by induction of senescence and apoptosis pathways. Breast
Cancer Res Treat. 133:563–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Yang Z, Zhang W, Yan B, Gu Q, Jiao
J and Yue X: Decreased expression of IGFBP7 was a poor prognosis
predictor for gastric cancer patients. Tumour Biol. 35:8875–8881.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kashani K, Al-Khafaji A, Ardiles T,
Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM,
Chawla LS, et al: Discovery and validation of cell cycle arrest
biomarkers in human acute kidney injury. Crit Care. 17:R252013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gandhi PU, Gaggin HK, Sheftel AD, Belcher
AM, Weiner RB, Baggish AL, Motiwala SR, Liu PP and Januzzi JL Jr:
Prognostic usefulness of insulin-like growth factor-binding protein
7 in heart failure with reduced ejection fraction: A novel
biomarker of myocardial diastolic function? Am J Cardiol.
114:1543–1549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dellinger TH, Planutis K, Jandial DD,
Eskander RN, Martinez ME, Zi X, Monk BJ and Holcombe RF: Expression
of the Wnt antagonist Dickkopf-3 is associated with prognostic
clinicopathologic characteristics and impairs proliferation and
invasion in endometrial cancer. Gynecol Oncol. 126:259–267. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JM, Kim MK, Chi KC, Kim JH, Lee SH
and Lee EJ: Aberrant loss of dickkopf-3 in gastric cancer: Can it
predict lymph node metastasis preoperatively? World J Surg.
39:1018–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo CC, Zhang XL, Yang B, Geng J, Peng B
and Zheng JH: Decreased expression of Dkk1 and Dkk3 in human clear
cell renal cell carcinoma. Mol Med Rep. 9:2367–2373.
2014.PubMed/NCBI
|
|
37
|
Bao MW, Cai Z, Zhang XJ, Li L, Liu X, Wan
N, Hu G, Wan F, Zhang R, Zhu X, et al: Dickkopf-3 protects against
cardiac dysfunction and ventricular remodelling following
myocardial infarction. Basic Res Cardiol. 110:252015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neeper M, Schmidt AM, Brett J, Yan SD,
Wang F, Pan YC, Elliston K, Stern D and Shaw A: Cloning and
expression of a cell surface receptor for advanced glycosylation
end products of proteins. J Biol Chem. 267:14998–15004.
1992.PubMed/NCBI
|
|
39
|
Yammani RR, Carlson CS, Bresnick AR and
Loeser RF: Increase in production of matrix metalloproteinase 13 by
human articular chondrocytes due to stimulation with S100A4: Role
of the receptor for advanced glycation end products. Arthritis
Rheum. 54:2901–2911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang H, Antoine DJ, Andersson U and Tracey
KJ: The many faces of HMGB1: Molecular structure-functional
activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol.
93:865–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher
A, Slattery T, Zhao L, Nagashima M, Morser J, et al: RAGE and
amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature.
382:685–691. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dahlmann M, Okhrimenko A, Marcinkowski P,
Osterland M, Herrmann P, Smith J, Heizmann CW, Schlag PM and Stein
U: Rage mediates S100A4-induced cell motility via MAPK/ERK and
hypoxia signaling and is a prognostic biomarker for human
colorectal cancer metastasis. Oncotarget. 5:3220–3233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo Y, Xia P, Zheng JJ, Sun XB, Pan XD,
Zhang X and Wu CZ: Receptors for advanced glycation end products
(rage) is associated with microvessel density and is a prognostic
biomarker for clear cell renal cell carcinoma. Biomed Pharmacother.
73:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nasser NW, Wani NA, Ahirwar DK, Powell CA,
Ravi J, Elbaz M, Zhao H, Padilla L, Zhang X, Shilo K, et al: Rage
mediates S100A7-induced breast cancer growth and metastasis by
modulating the tumor microenvironment. Cancer Res. 75:974–985.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang D, Li T, Ye G, Shen Z, Hu Y, Mou T,
Yu J, Li S, Liu H and Li G: Overexpression of the receptor for
advanced glycation endproducts (rage) is associated with poor
prognosis in gastric cancer. PLoS One. 10:e01226972015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu B and Cheng SY: Angiopoietin-2:
Development of inhibitors for cancer therapy. Curr Oncol Rep.
11:111–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bupathi M, Kaseb A and Janku F:
Angiopoietin 2 as a therapeutic target in hepatocellular carcinoma
treatment: Current perspectives. Onco Targets Ther. 7:1927–1932.
2014.PubMed/NCBI
|
|
48
|
Morrissey C, Dowell A, Koreckij TD, Nguyen
H, Lakely B, Fanslow WC, True LD, Corey E and Vessella RL:
Inhibition of angiopoietin-2 in LuCaP 23. 1 prostate cancer tumors
decreases tumor growth and viability. Prostate. 70:1799–1808.
2010.PubMed/NCBI
|
|
49
|
Calfee CS, Gallagher D, Abbott J, Thompson
BT, Matthay MA, et al: NHLBI ARDS Network: Plasma angiopoietin-2 in
clinical acute lung injury: Prognostic and pathogenetic
significance. Crit Care Med. 40:1731–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goede V, Coutelle O, Neuneier J,
Reinacher-Schick A, Schnell R, Koslowsky TC, Weihrauch MR, Cremer
B, Kashkar H, Odenthal M, et al: Identification of serum
angiopoietin-2 as a biomarker for clinical outcome of colorectal
cancer patients treated with bevacizumab-containing therapy. Br J
Cancer. 103:1407–1414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang FC, Lai TS, Chiang CK, Chen YM, Wu
MS, Chu TS, Wu KD and Lin SL: Angiopoietin-2 is associated with
albuminuria and microinflammation in chronic kidney disease. PLoS
One. 8:e546682013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Conroy AL, Glover SJ, Hawkes M, Erdman LK,
Seydel KB, Taylor TE, Molyneux ME and Kain KC: Angiopoietin-2
levels are associated with retinopathy and predict mortality in
Malawian children with cerebral malaria: A retrospective
case-control study*. Crit Care Med. 40:952–959. 2012. View Article : Google Scholar : PubMed/NCBI
|