Introduction
Prostate cancer (PCa) is the second most commonly
diagnosed cancer in men worldwide (1). A number of factors are critical in
the development of PCa, including genetic mutations and the tumor
microenvironment (2,3). Several reports have indicated that
genetic alterations are observed in advanced PCa; however, no
single oncogenic event by itself is sufficient to determine a large
proportion of the diseases (4–6).
Accordingly, further understanding how oncogenic lesions may
collaborate to augment the progression of PCa is likely to assist
in clinical diagnosis and treatment.
MicroRNAs (miRNAs) are RNA polymerase II-transcribed
RNAs, with a length of 18–24-nucleotides, which can negatively
regulate gene expression (7).
Altered levels of miRNAs have been observed in several solid
tumors, including PCa (8–10). Dysfunctional miRNAs can mediate
divergent cellular processes, including cellular proliferation,
migration, differentiation and apoptosis (9). Several reports have indicated that
specific miRNAs have been associated with PCa tumorigenesis and the
clinical outcome of patients (10–12).
Our previous data demonstrated that that miR-30c
represents a potential tumor suppressor gene, the expression of
which is associated with decreased oncogenic potential in PC cell
lines (13,14). Several genes, including UBC9, B-Myb
and BCL9 have also been identified as miR-30c targets in tumor
cells (15–17). As one miRNA can target multiple
genes simultaneously, it is suggested that the functional role and
mechanisms underlying the effect of miR-30c in PCa remain to be
fully elucidated.
The serine- and arginine-rich (SR) proteins, and the
heterogeneous nuclear ribonucleoproteins (hnRNPs) are two families
of mRNA processing proteins, which serve, respectively, as splicing
activators and repressors (18,19).
These RNA-binding proteins can regulate the alternative splicing of
several genes and have been associated with splicing dysregulation
in various diseases (20,21).
The prototypical SR protein, of alternative splicing
factor/splicing factor 2 (ASF/SF2; also termed SF2/ASF or SRSF1)
functions in mRNA constitutive and alternative splicing, and is
also incolved in stability, nuclear export and translation
(22). SF2/ASF has been found to
be involved in several malignancies, and can be oncogenic with
effects on cellular transformation (20,21).
Although ASF/SF2 is frequently upregulated in PCa (23), its roles in the cancer initiation
and progression remain to be fully elucidated.
In the present study, it was demonstrated that the
overexpression of ASF/SF2 was inhibited by miR-30c in PCa cells. A
luciferase reporter assay further confirmed that ASF/SF2 was a
direct target of miR-30c. The ectopic expression of miR-30c also
resulted in the inhibition of proliferation, and induced cell cycle
arrest and apoptosis by downregulating ASF/SF2. In tissue samples,
ASF/SF2 was significantly associated with the pathological stage of
PCa. Finally, the prognostic value of the combined analysis of
ASF/SF2 and miR-30c was determined in biopsies from patients with
PCa.
Materials and methods
Ethical approval
The clinical sample cohort used in the present study
was approved by the Ethical Committee of Guangzhou First People's
Hospital, Guangzhou Medical University (Guangzhou, China). All
patients provided written informed consent prior to enrollment in
the present study.
Patients and tissues samples
For RNA extraction, tumor samples were obtained from
111 patients with PCa, who underwent radical prostatectomy at
Guangzhou First People's Hospital between January 2002 and August
2012. The detailed characteristics of the patients are presented in
Table I. For immunohistochemistry,
tumor tissues and adjacent normal tissues were obtained from 20
patients with PCa. Any patients who received either radiotherapy or
hormonal treatment prior to surgery were not included in the
investigation. Biochemical recurrence (BCR) was described as
postoperative serum prostate-specific antigen (PSA) ≥0.2 ng/ml.
 | Table I.Patients and clinicopathologic
features. |
Table I.
Patients and clinicopathologic
features.
| Characteristic | Patients with PCa
(n=111) |
|---|
| Age range
(years) | 47–83 |
| Median age
(years) | 58 |
| Preoperative
prostate-specific antigena |
|
| <4
ng/ml | 17 |
| 4–10
ng/ml | 68 |
| ≥10
ng/ml | 24 |
| Gleason
scorea |
|
| 6 | 35 |
| 7 | 55 |
| 8 | 8 |
| 9 | 9 |
| Pathological
stagea |
|
|
pT2 | 69 |
|
pT3 | 30 |
|
pT4 | 7 |
| Follow-up range
(months) | 1–128 |
| Biochemical
recurrence (n) | 25 |
| 1–12
months | 12 |
| 13–24
months | 6 |
| >24
months | 7 |
Transient transfection of mature
miRNA
miR-30c mimics, negative control mimics,
anti-miR-30c inhibitors and negative control inhibitors were
purchased from GenPharma (Shanghai, China). The sequences of the
oligoribonucleotides were as follows: miR-30c mimic,
5′-CCAGUGUAGGGUAAACACCUCUCUCAGCUUGG-3′; and anti-miR-30c inhibitor,
5′-GCUGAGAGUGUAGGAUGUUUACA-3′. The miR-30c mimics, anti-miR-30c
inhibitors, and controls were transiently transfected into the
human DU145 and 22RV1 prostatic carcinoma cell lines (American Type
Culture Collection, Manassas, VA, USA) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. PCa cells were cultured
in RPMI 1640 medium (Hyclone; GE Healthcare LifeSciences, Logan,
UT, USA), supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientifc, Inc.) and 1% penicillin and streptomycin
(Invitrogen; Thermo Fisher Scientifc, Inc.). For transient
transfection, 0.5×105 cells were plated in 24-well
plates 24 h prior to transfection and maintained at 37°C in an
atmosphere of 5% CO2. Following transfection, ASF/SF2
mRNA and protein were extracted for further investigation.
Western blot analysis
Following standard protocol, total proteins were
extracted from the cells. Cells and tumors containing at least 70%
tumor cells were lysed using 200 µl M-PER Mammalian Protein
Extraction Reagent (Thermo Fisher Scientific, Inc.) and the
supernatants were collected following centrifugation at 10,000 × g
for 5 min at 4°C. Protein concentration was measured using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Equal amounts of extracted protein samples (~20–30 µg) were
boiled at 95°C for 5 min, separated by 10% SDS-PAGE (NuPAGE™ Novex
4–12% Bis-Tris Gel; cat. no. NP0322BOX; Thermo Fisher Scientific,
Inc.), and transferred onto a nitrocellulose membrane (cat no.
IPFL00010; EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skim milk in PBS containing 0.1% Tween-20, and
probed the following primary antibodies at 4°C overnight:
Anti-ASF/SF2 (cat. no. HPA061301; dilution, 1:500; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and anti-GAPDH (cat. no. 2118;
dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA). The membrane was then incubated with horseradish
peroxidase-conjugated donkey anti-rabbit immunoglobulin G secondary
antibody (cat no. SAB3700852; 1:2,000; Sigma-Aldrich; Merck KGaA)
for 1 h at room temperature. Protein bands were visualized by
enhanced chemiluminescence using the Super Signal West Pico
Chemiluminescent Substrate kit (Pierce; Thermo Fisher Scientific,
Inc.).
ASF/SF2 small interfering (si)RNA
transfection and vector construction
PCa cells (0.5×105) were plated in
24-well plates and cultured in RPMI 1640 medium (Hyclone; GE
Healthcare LifeSciences) at 37°C for 12 h prior to transfection.
siRNA reverse transfection reactions were performed using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). The siRNA
sequence targeting ASF/SF2 (Qiagen, Inc., Valencia, CA, USA) was
5′-CCAACAAGATAGAGTATAA-3′. AllStars Neg. Control siRNA (Qiagen,
Inc.) was used as a negative control. The final concentration for
control siRNA and ASF/SF2 siRNA transfection was 100 nM. The
ASF/SF2 coding sequence, without the 3′-untranslated region (UTR)
was cloned into the pGCMV/EGFP/Neo-Vector (GenePharma). A blank
vector served as the negative control. The culture medium was
replaced with fresh medium 48 h post-transfection, and the cell
lysates were prepared at 72 h for western blot analysis.
In vitro luciferase assay
Using the databases TargetScan (http://targetscan.org/), PicTar (http://pictar.mdc-berlin.de/) and miRDB (http://www.mirdb.org/miRDB/), the putative target
genes of miR-30c were investigated, and the ASF/SF2 gene was
identified among them. To amplify the 3′UTR sequence of the human
ASF/SF2 gene, primers were designed. Mlu1 and Spe1
sequences were cloned into the ends of primers (Promega
Corporation, Madison, WI, USA). According to the manufacturer's
protocol, 3′-UTR cloning was performed in a pMIR reporter vector
(Promega Corporation). The pMIR-ASF/SF2 3′-UTR reporter vector (100
ng) was cotransfected with 50 ng of β-Gal and miRNA-30c mimic or
anti-miR-30c using Lipofectamine 2000 transfection reagent. All
Stars negative control siRNA (Qiagen, Inc.) and miScript inhibitor
negative control (Qiagen, Inc.) were used as controls for mimic or
inhibitor transfection, respectively. At 24 h post-transfection,
luciferase reporter assays were performed, as described previously
(9).
Immunohistochemical staining
Immunohistochemical assays were performed to detect
the expression of ASF/SF2 in 4-cm thick formalin-fixed,
paraffin-embedded sections of PCa and normal tissues. Using the
DAKO EnVision system (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA), hematoxylin and eosin or immunohistochemical
staining were performed on the formalin-fixed paraffin-embedded
tissue sections. Endogenous peroxidase activity was quenched using
3% hydrogen peroxidase for 10 min at room temperature. Microwave
antigen retrieval was performed with EDTA buffer for 30 min. The
slides were then incubated overnight with primary antibody against
ASF/SF2 protein (cat. no. HPA061301; Sigma-Aldrich; Merck KGaA) at
a dilution of 1:200 at 4°C. Following washing with PBS, the
sections were incubated with horseradish peroxidase-labeled
polymer-conjugated secondary antibodies (DAKO EnVision system;
Dako; Agilent Technologies, Inc.) for 1 h at room temperature and
the proteins of interest were visualized following incubation with
3,3′-diaminobenzidine (Dako; Agilent Technologies, Inc.) at room
temperature for 5 min, under an Olympus AX70 microscope (Olympus
Corporation, Tokyo, Japan).
The extent and intensity of staining was evaluated
independently by two experienced pathologists in a blinded manner.
The extension was scored as the percentage of positive cells, and a
score of 0–4 was assigned (0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%;
or 4, 76–100%). The percentage of positive cells was also
calculated (0, no staining; 1, weak; 2, moderate; 3, strong
staining). The sum of the two scores was used as the final staining
score. Tumor specimens with a score of ≥3 were considered to be
positive.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR analysis was performed as previously
described (13). miRNA was
extracted from the 111 frozen PCa tissues and the two transfected
PCa cell lines (22RV1 and DU145) following transfection using a
miRNA extraction kit (BioTek China, Beijing, China). cDNA synthesis
was performed according to the manufacturer's protocol using the
All-in-One™ miRNA qRT-PCR Detection kit (GeneCopoeia, Inc.,
Rockville, MD, USA) with specific primers. The sequences of the
primers, purchased from Ambio; Thermo Fisher Scientific, Inc. were
as follows: miR-30c, forward 5′-TGTGTTTTTATTGTTTTTGTTGTCCCA-3′,
reverse 5′-GGGACAGAACAGGTTAATGGGAA-3′; RNU6B, forward
5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-AACGCTTCACGAATTTGCGT-3′;
ASF/SF2, forward 5′-TTTAGATCTCACGAGGGAGAA-3′, reverse
5′-CGTGGTGATCCTCTGCTTC-3′; and β-actin, forward
5′-GGTGGCTTTTAGGATGGCAAG-3′ and reverse
5′-ACTGGAACGGTGAAGGTGACAG-3′. RNU6B and β-actin were used as the
internal controls for miR-30c and ASF/SF2, respectively. qPCR was
performed using TaqMan Gene Expression assays (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the MyiQ™2 Two-Color Real-Time
PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Amplification conditions were as follows: Initial 1 step at
95°C for 5 min, followed by 40 cycles at 95°C for 10 sec and at
60°C for 20 sec, with a final extension step at 72°C for 20 sec.
Gene expression was quantified using the comparative Cq method
(24), with the IQ™5 Optical
System software version 2.0 (Bio-Rad Laboratories, Inc.). The
experiments were performed in triplicate.
Cell proliferation assay
For the cell proliferation assay, 2,000 cells/well
were seeded into 96-well plates, cultured in RPMI 1640 medium
(Hyclone; GE Healthcare Life Sciences) supplemented with 10% FBS
for 24, 48 and 72 h, and then incubated with 20 µl CCK-8 solution
(cat no. C0038; Beyotime Institute of Biotechnology, Haimen, China)
for 4 h at 37°C. The absorbance of each sample was measured at 495
nm using a spectrophotometer.
Cell cycle analysis
Flow cytometric analysis was performed to assess the
distribution of cells in different phases of the cell cycle.
Briefly, at 24 h post-transfection, a total of 10,000 cells were
harvested, fixed in ice-cold 70% ethanol at −20°C overnight,
treated with 0.2 mg/ml RNase A (Sigma-Aldrich; Merck KGaA) for 4 h
at 4°C, and then stained with 10 µg/ml propidium iodide (PI;
Sigma-Aldrich; Merck KGaA) for 30 min at room temperature in the
dark. The percentages of cells in the G1, S and G2 phases were
analyzed using a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). Flow cytometric data were analyzed using the
ModiFit LT software version 3.0 (Verity Software House, Inc.,
Topsham, ME, USA). The assays were performed in triplicate.
Apoptosis analysis
The percentages of apoptotic cells following SF2/ASF
(or si-ASF/SF2) and miR-30c cotransfection were determined
according to the protocol of the fluorescein isothiocyanate
(FITC)/Annexin V Apoptosis Detection kit I (BD Pharmingen; BD
Biosciences). Briefly, at 24 h post-transfection, the PCa cells
were harvested, processed according to the manufacturer's protocol,
and then analyzed on the FACScan flow cytometer (BD Biosciences).
Flow cytometric data were analyzed using the BD FACSuite™ software
(BD Biosciences). Apoptotic cells were defined as Annexin V/FITC-
and PI-positive cells.
Statistical analysis
All statistical analyses were performed using SPSS
software version 17.0. (SPSS, Inc., Chicago, IL, USA). The
statistical significance of the differences between groups was
assessed using Student's t-test for pair-wise comparisons or
one-way analysis of variance followed by a post hoc Tukey-Kramer
test for multiple comparisons. The patients were stratified in two
groups according to the median values of the relative levels for
miR-30c and SF2/ASF. The correlation between ASF/SF2 and
clinicopathologic variables was assessed using Fisher's exact test
or Pearson's χ2 test. Kaplan-Meier survival curves were
generated to evaluate the significance of the expression of miR-30c
and ASF/SF2 on the prognosis of the patients with PCa. Cox
proportional hazards regression was used to analyze the independent
factors for BCR. P<0.05 was considered to indicate a
statistically significant difference.
Results
ASF/SF2 is upregulated in PCa
tissues
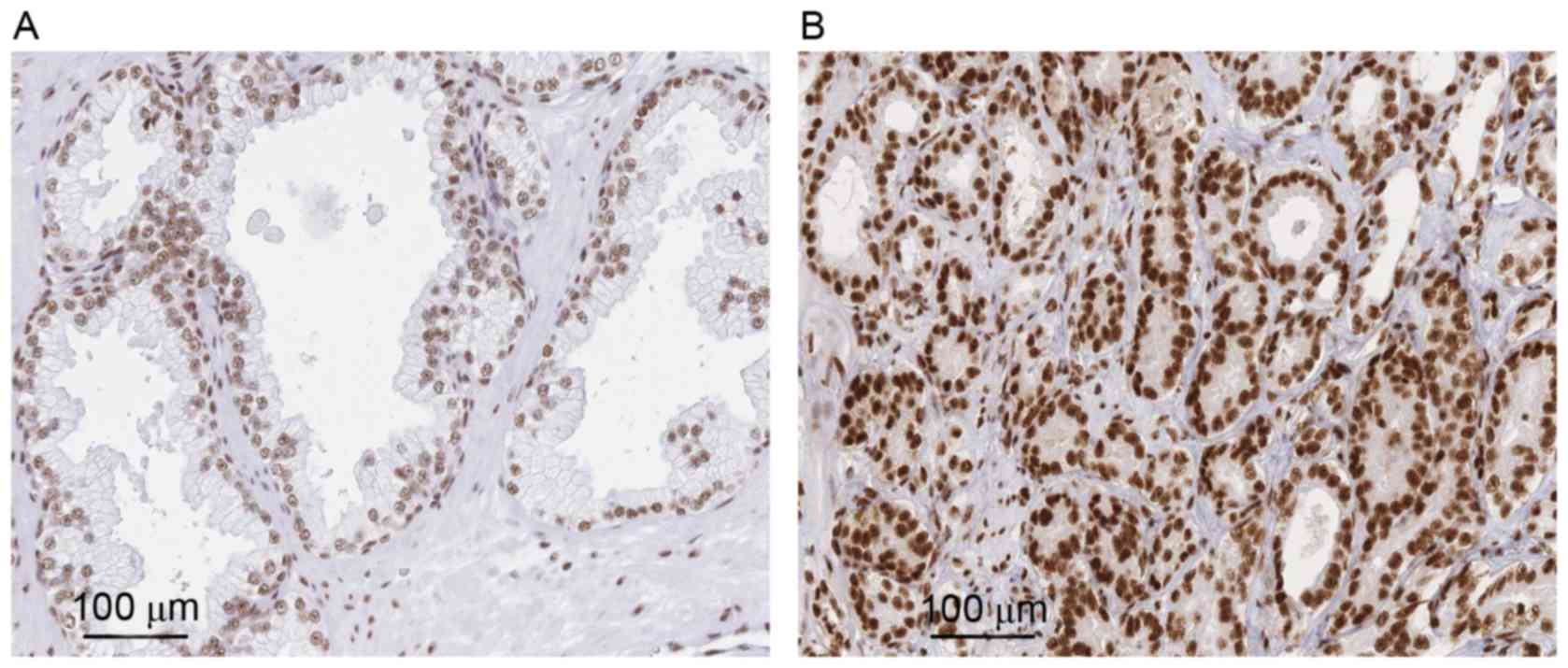
To evaluate the role of ASF/SF2 in PCa, the present
study analyzed its expression in 20 tumor and normal tissues. In
general, it was found the antibody staining was primarily in the
nuclei of the PCa cells and exhibited an evenly distributed
staining pattern. Compared with the PCa tissues, faint BCL9
staining was observed in certain sections of the normal tissues.
Statistical analysis showed that the positive expression rate in
normal tissue was significantly lower, compared with that in the
tumor counterpart (P<0.001; Fig. 1A
and B).
ASF/SF2 is a direct target of miR-30c
in PCa cells
Using the TargetScan, PicTar and miRDB databases,
the ASF/SF2 3′-UTR was found to contain one putative binding site
for miR-30c. To further determine whether miR-30c interacts
directly with the ASF/SF2 protein, a luciferase reporter assay was
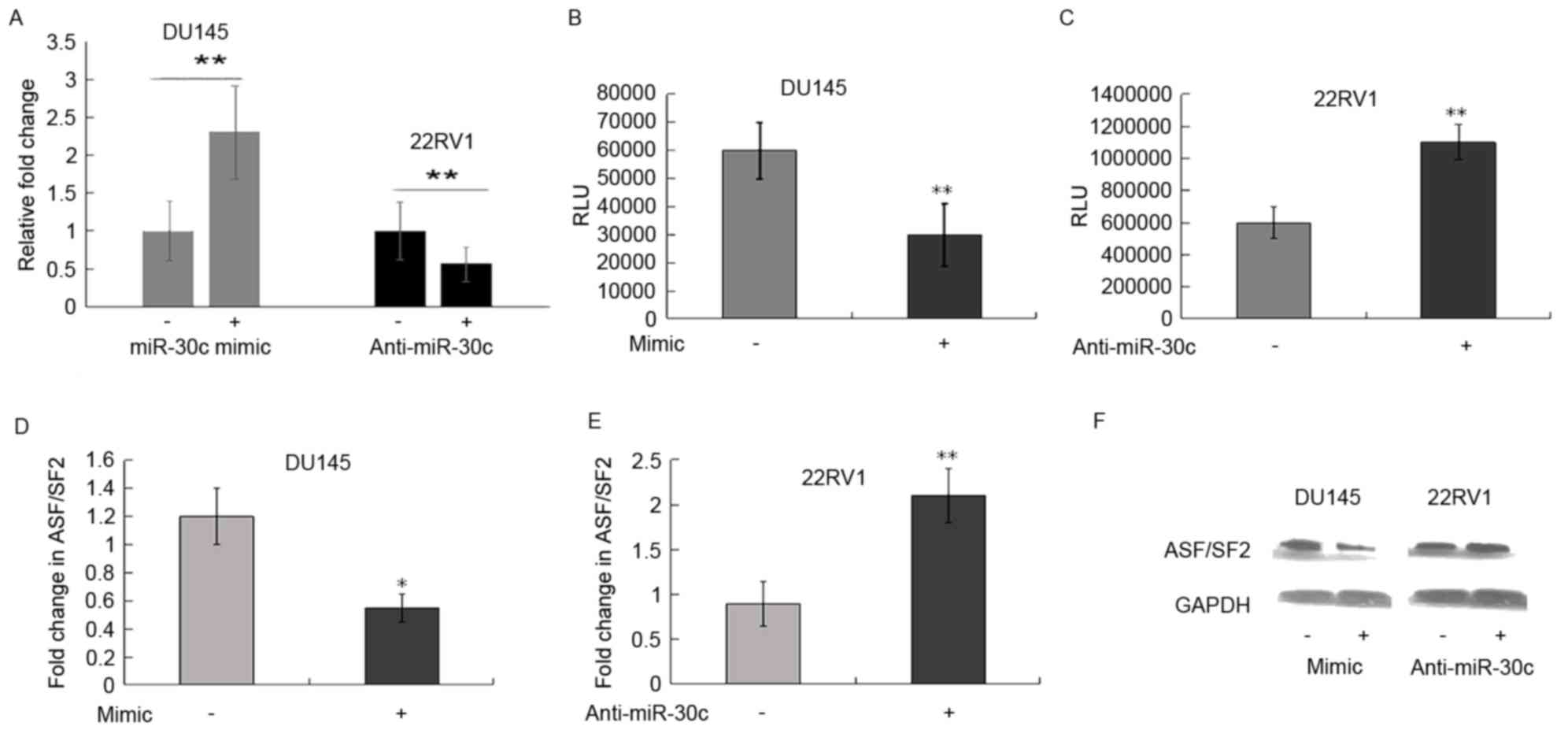
performed. The DU145 cells were transiently transfected cells with
miR-30c mimic and its control, respectively. Following
transfection, the increased expression level of miR-30c was
confirmed using RT-qPCR analysis (P<0.01; Fig. 2A). However, decreased expression of
miR-30c was observed following transfection with anti-miR-30c
(P<0.01; Fig. 2A).
To determine whether miR-30c binds to the ASF/SF2
3′-UTR, the DU145 cells were transfected with the pMIRreporter
construct containing the ASF/SF2 3′-UTR together with miR-30c
mimic, and a luciferase assay was performed. Luciferase activity
was significantly downregulated in the pMIR-ASF/SF2
3′-UTR-transfected cells, compared with that in the
scramble-transfected cells (P<0.01; Fig. 2B). By contrast, luciferase activity
was significantly upregulated when 22Rv1 cells were transfected
with the pMIR-ASF/SF2 3′-UTR construct together with anti-miR-30c
inhibitor (P<0.01; Fig. 2C).
These results indicated that miR-30c may modulate the expression of
ASF/SF2by binding to its 3′-UTR.
To further substantiate these findings, the effects
of miR-30c mimic and inhibitor on the mRNA and protein levels of
ASF/SF2 were measured. The ectopic expression of miR-30c was
associated with a reduction in the mRNA and protein expression of
ASF/SF2; however, transfection of the 22Rv1 cells with anti-miR-30c
inhibitor increased the expression of ASF/SF2 (Fig. 2D-F).
miR-30c inhibits cell proliferation
and induces apoptosis by negatively regulating ASF/SF2
The data obtained in our previous study showed that
the enforced expression of miR-30c inhibited tumorigenesis in PCa
cells (13,14). Others have reported that the
downregulation of SF2/ASF arrests cell proliferation and induces
apoptosis in lung cancer and breast tumor (25,26).
Therefore, the present study focused predominantly on whether
miR-30c mediated ASF/SF2 has a similar effect on PCa cells. The
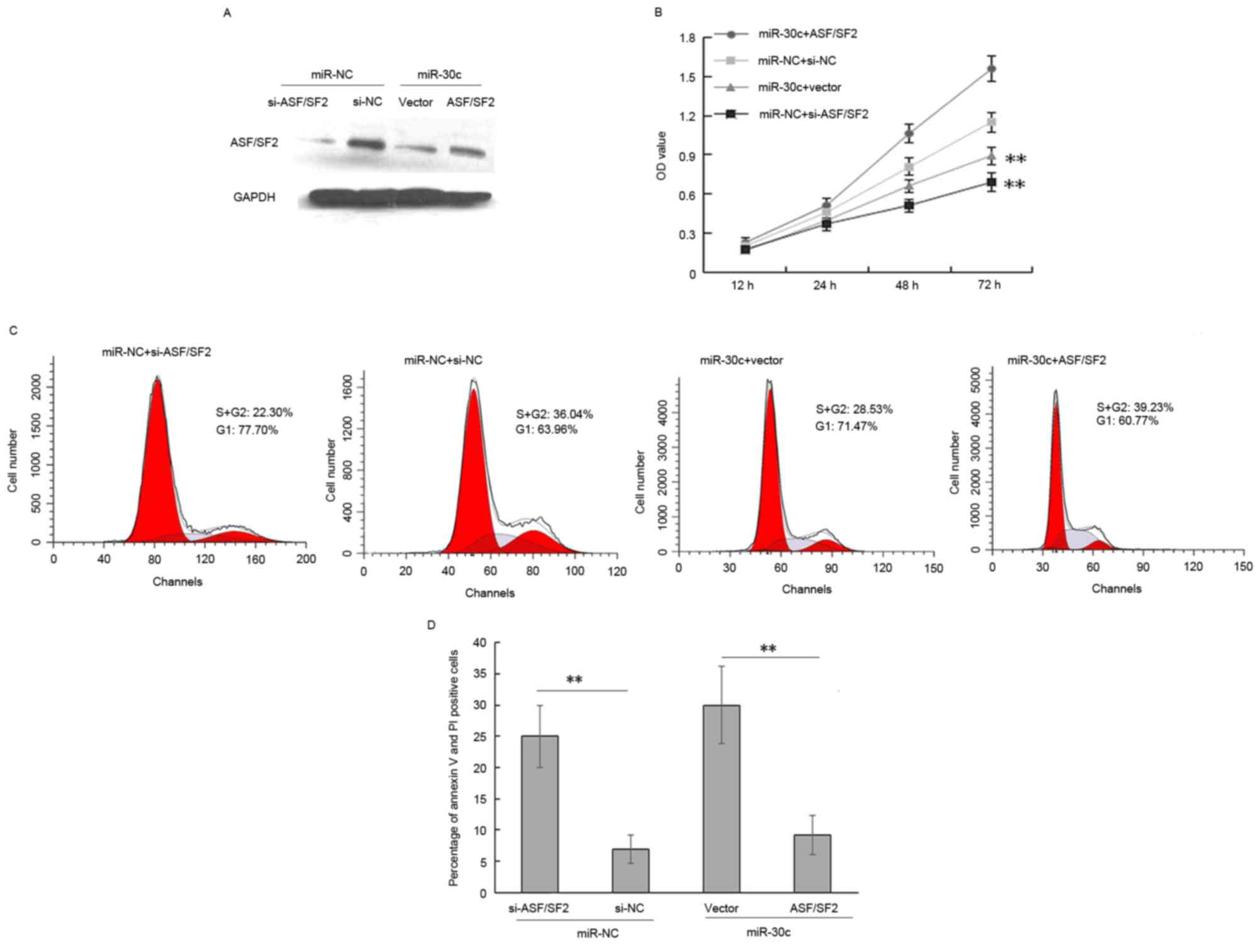
expression of ASF/SF2 in DU145 cells was knocked down via siRNA
(Fig. 3A). As shown in Fig. 3, the overexpression of ASF/SF2
eradicated the effects of miR-30c on proliferation of the DU145
cells (Fig. 3B; P<0.001). The
cell cycle of the cells was analyzed using flow cytometry to
determine whether the decrease in cell proliferation due to
SF2/ASF-knockdown was associated with cell cycle arrest. The, cell
cycle assay showed that ASF/SF2 promoted cell proliferation via an
increase in the ratio of S+G2 phase cells in miR-30c-overexpressing
cells (Fig. 3C; P<0.01).
Therefore, the data indicated that miR-30c inhibited PCa cell
proliferation and induced apoptosis, partially mediated by the
targeting of ASF/SF2. Furthermore, the detection and quantification
of apoptotic cells using flow cytometry showed a significant
increase in the percentage of apoptotic cells in the
miR-30c-overexpressing cells, whereas ASF/SF2 delayed the apoptotic
effect induced by miR-30c (P<0.01; Fig. 3D).
ASF/SF2 is associated with
clinicopathological features
The present study examined the possible correlation
between ASF/SF2 and clinicopathological features in the study
cohort. Significantly increased expression levels of ASF/SF2 were
observed in patients with PCa at an advanced pathological stage
(P=0.038) and BCR (P=0.014), as showed in Table II. However, no significant
associations were found between ASF/SF2 and other
clinicopathological features, including preoperative PSA levels,
Gleason score and surgical margin (all P>0.05).
 | Table II.Expression levels of ASF/SF2 and
clinicopathological features. |
Table II.
Expression levels of ASF/SF2 and
clinicopathological features.
|
|
| Expression of
ASF/SF2 |
|
|---|
|
|
|
|
|
|---|
| Clinical
feature | Low, n | High, n (%) | n (%) | P-value |
|---|
| Preoperative |
|
|
| 0.835 |
| PSA
(ng/ml)a |
|
<10 | 85 | 41 (48.2) | 44 (51.8) |
|
|
≥10 | 24 | 11 (45.8) | 13 (54.2) |
|
| Gleason
scorea |
|
|
| 0.450 |
| ≤6 | 32 | 16 (50.0) | 16 (50.0) |
|
| 7 | 55 | 29 (52.7) | 26 (47.3) |
|
| ≥8 | 17 | 6 (35.3) | 11 (64.7) |
|
| Pathological
stagea |
|
|
| 0.038 |
|
pT2 | 69 | 35 (50.7) | 34 (49.3) |
|
|
pT3-pT4 | 37 | 11 (29.7) | 26 (54.1) |
|
| Surgical margin
statusa |
|
|
| 0.857 |
|
Negative | 89 | 44 (49.4) | 45 (50.6) |
|
|
Positive | 17 | 8 (47.1) | 9 (52.9) |
|
| Biochemical
recurrencea |
|
|
| 0.014 |
|
Negative | 80 | 45 (56.2) | 35 (43.8) |
|
|
Positive | 25 | 7 (28.0) | 18 (72.0) |
|
miR-30c and ASF/SF2 synergistically
predict BCR
The data obtained in our previous study showed that
miR-30c was correlated with the progression PCa and may be an
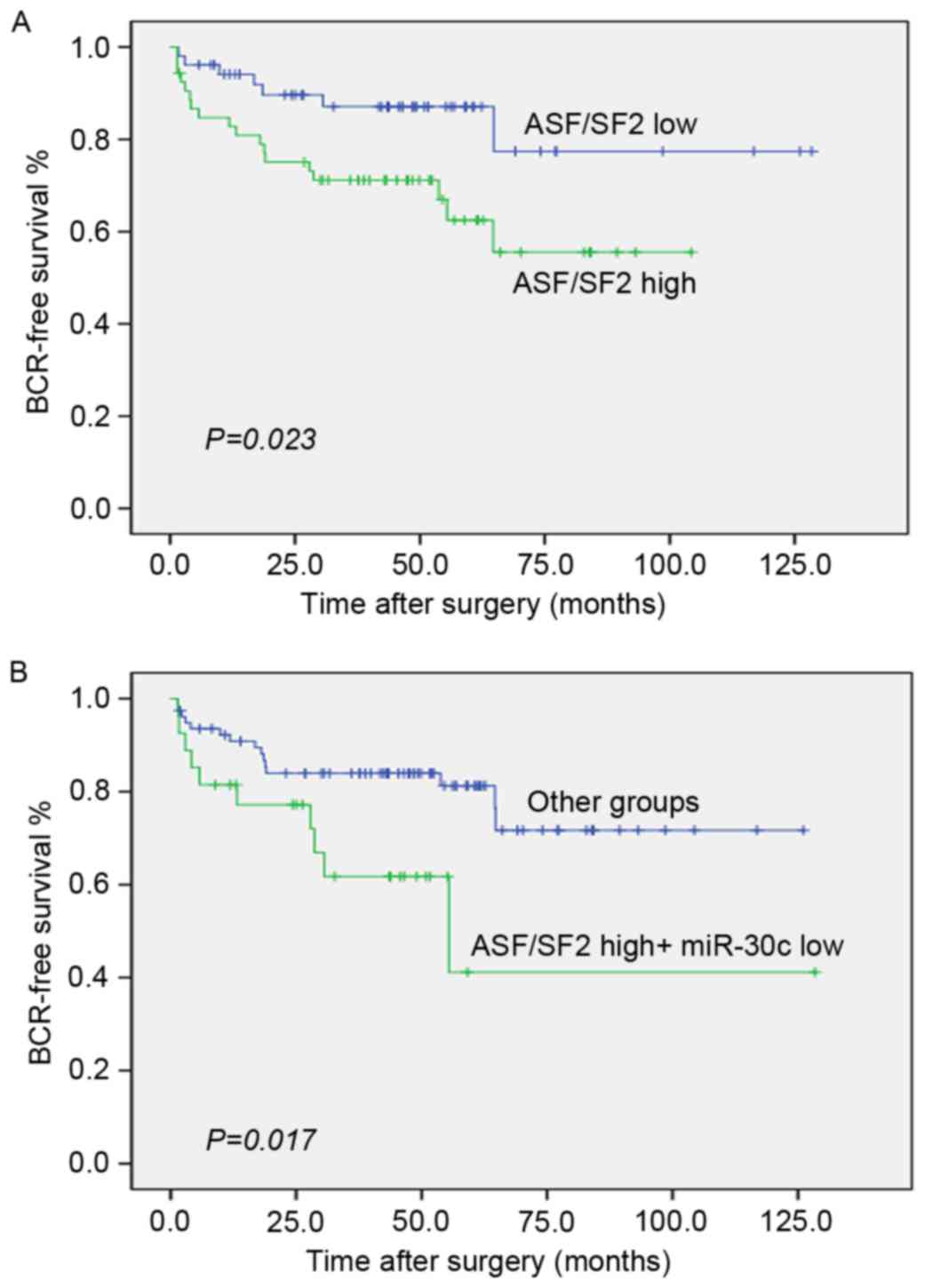
independent predictor of BCR in PCa. As shown in Fig. 4A, the tumors with levels of ASF/SF2
above the median were significantly more likely to experience early
recurrence (P=0.023; log-rank test; Fig. 4A). As ASF/SF2 was negatively
regulated by miR-30c, the combined use of these two biomarkers
merits further investigation. Of note, the group of PCa patients
with low expression levels of miR-30c and high expression levels of
ASF/SF2 had significantly lower rates of BCR-free survival compared
with other groups of patients (P=0.017; log-rank test; Fig. 4B).
The Cox proportional hazards model was used to
assess independent predictors of BCR-free survival, including
preoperative PSA, Gleason score, surgical margin, pathological
stage, and the coexpression of miR-30c and ASF/SF2. In the
multivariate analysis, a low expression of miR-30c and high
expression of ASF/SF2 (HR 2.89; P=0.024) were revealed to be
independent prognostic factors for poor BCR-free survival (Table III).
 | Table III.Univariate and multivariate analyses
using Cox proportional hazard regression model for biochemical
recurrence-free survival. |
Table III.
Univariate and multivariate analyses
using Cox proportional hazard regression model for biochemical
recurrence-free survival.
| Predictor | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Univariate |
| 0.022 |
|
miR-30c*ASF/SF2 status | 2.61
(1.15–5.90) |
|
| Gleason
score | 9.00
(4.30–18.84) | <0.001 |
|
Preoperative PSA | 1.01
(1.00–1.01) | 0.010 |
|
Pathological tumor stage | 5.83
(2.50–13.56) | <0.001 |
|
Surgical margin status | 2.28
(0.98–5.30) | 0.056 |
| Multivariate |
| 0.024 |
|
miR-30c*ASF/SF2 status | 2.89
(1.15–7.22) |
|
| Gleason
score | 5.71
(2.46–13.29) | <0.001 |
|
Preoperative PSA | 1.00
(1.00–1.03) | 0.001 |
|
Pathological tumor stage | 2.84
(1.03–7.79) | 0.043 |
Discussion
Although miR-30c has been widely regarded as a tumor
suppressor, its function in PCa remains to be elucidated as a
result of relatively fewer investigations and known direct targets.
Previous studies, including our own, have demonstrated that miR-30c
is significantly reduced in tissue and plasma samples from patients
with PCa, compared with their corresponding counterparts, and its
expression predicts the risk of BCR and survival (13,27–29).
Therefore, it is necessary to understand the role of miR-30c and
its downstream effectors to determine its contribution to PCa
tumorigenesis.
Verduci et al (30) demonstrated that two miRNAs (miR-28
and miR-505) affected mouse embryonic fibroblast senescence and
apoptosis by modulating the expression of ASF/SF2. However, the
possible functional link between ASF/SF2 and miR-30c remains to be
elucidated. In the present study, it was observed that PCa tissues
exhibited higher levels of ASF/SF2, compared with normal tissues.
The results also showed that miR-30c targeted the 3′-UTR region of
the ASF/SF2 gene, thereby decreasing the mRNA and protein levels of
ASF/SF2. Of note, a negative correlation was observed between
ASF/SF2 and miR-30c in PCa cells, wherein miR-30c was observed to
decrease cell proliferation, and increase the percentage of cells
in the G1 phase and apoptosis via the inhibition of ASF/SF2. The
differences between the results of the present study and those of
Verduci et al (30)
indicated that the specific mechanisms underlying the induction of
cell cycle arrest and apoptosis following a decrease in the
expression of ASF/SF2 may be dependent on the cell type.
Detailed information regarding the mechanism of
transformation modulated by ASF/SF2 has been already described,
primarily through inducing alternative splicing isoforms of certain
cancer-associated genes (23,31,32).
For example, Liu et al (31) found that RNA splicing of the
ASF/SF2 protein was critical in the splicing of AR pre-mRNA into AR
splice variant 7 in PCa cells. Olshavsky et al (23) also identified ASF/SF2 as a disease
relevant effector of CCND1 alternative splicing, which can promote
the allele-specific production of cyclin D1b in PCa. In other
tumors, it has been found that induction of the mammalian target of
rapamycin (mTOR) pathway by ASF/SF2 is necessary for
ASF/SF2-mediated transformation (25,26,33).
It is known that the mTOR pathway is constitutively activated in
several tumors, which affects the transformed phenotype (34). However, whether the upregulation of
mTOR signaling via the induction of ASF/SF2 in PCa cells by miR-30c
requires further investigation.
Although the functions of ASF/SF2 in the maintenance
of tumor cells have been widely described, few reports mention its
role as a novel prognostic factor. In lung cancer, high expression
levels of ASF/SF2 tend to be associated with poor prognosis
(25). In breast cancer, the
genomic region (17q23) of ASF/SF2 is commonly amplified, and this
amplification is associated with poor prognosis (35). In the present study, it was found
that the upregulation of ASF/SF2 was associated with PCa
progression. Furthermore, the expression of ASF/SF2 was
significantly correlated with BCR in patients with PCa. The results
of the univariate and multivariate analyses indicated that patients
with high levels of ASF/SF2 and low levels of miR-30c showed
earlier BCR following radical prostatectomy in patients with PCa.
These results support a hypothesis that ASF/SF2 has potential as a
biomarker for PCa, and therapeutic approaches targeting aberrant
levels of ASF/SF2 may be investigated as a potential approach to
improve clinical outcomes. The fact that the combined expression of
ASF/SF2 and miR-30c was more informative, compared with that of
AS/SF2 alone indicated that ASF/SF2 is modulated by other factors
in addition to miR-30c, and that ASF/SF2 is an important, but not
exclusive, mediator of the tumor-suppressive effects of miR-30c, as
it has been described.
In conclusion, the results of the present study
showed that the tumor suppressor miR-30c was involved in PCa
tumorigenesis, possibly by targeting ASF/SF2. Of note, the combined
analysis of the expression of ASF/SF2 and miR-30c may be a valuable
tool for the early prediction of BCR in PCa following radical
prostatectomy. Further investigations are required to validate the
clinical utility of ASF/SF2 and miR-30c as prognostic biomarkers
comprising large and multicentre PCa patient cohorts.
Acknowledgements
This study was supported by grants from the Science
and Technology Project of Guangdong (grant no. 2014A020212035), the
China Postdoctoral Science Foundation (grant no. 2016M590842) and
the Medical Scientific Research Foun-dation of Guangdong Province,
China (grant no. A2016052).
References
|
1
|
Kakehi Y: Watchful waiting as a treatment
option for localized prostate cancer in the PSA era. Jpn J Clin
Oncol. 33:1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santanam U, Banach-Petrosky W, Abate-Shen
C, Shen MM, White E and DiPaola RS: Atg7 cooperates with Pten loss
to drive prostate cancer tumor growth. Genes Dev. 30:399–407. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miftakhova R, Hedblom A, Semenas J,
Robinson B, Simoulis A, Malm J, Rizvanov A, Heery DM, Mongan NP,
Maitland NJ, et al: Cyclin A1 and P450 aromatase promote metastatic
homing and growth of stem-like prostate cancer cells in the bone
marrow. Cancer Res. 76:2453–2464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heng HH, Bremer SW, Stevens JB, Ye KJ, Liu
G and Ye CJ: Genetic and epigenetic heterogeneity in cancer: A
genome-centric perspective. J Cell Physiol. 220:538–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ulaganathan VK, Sperl B, Rapp UR and
Ullrich A: Germline variant FGFR4 p.G388R exposes a
membrane-proximal STAT3 binding site. Nature. 528:570–574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blattner M, Liu D, Robinson BD, Huang D,
Poliakov A, Gao D, Nataraj S, Deonarine LD, Augello MA, Sailer V,
et al: SPOP mutation drives prostate tumorigenesis in vivo through
coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell.
31:436–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Q, Song R, Ortogero N, Zheng H, Evanoff
R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM and Yan W:
The RNase III enzyme DROSHA is essential for microRNA production
and spermatogenesis. J Biol Chem. 287:25173–25190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kent OA and Mendell JT: A small piece in
the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
Oncogene. 25:6188–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hudson RS, Yi M, Esposito D, Watkins SK,
Hurwitz AA, Yfantis HG, Lee DH, Borin JF, Naslund MJ, Alexander RB,
et al: MicroRNA-1 is a candidate tumor suppressor and prognostic
marker in human prostate cancer. Nucleic Acids Res. 40:3689–3703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rane JK, Scaravilli M, Ylipää A, Pellacani
D, Mann VM, Simms MS, Nykter M, Collins AT, Visakorpi T and
Maitland NJ: MicroRNA expression profile of primary prostate cancer
stem cells as a source of biomarkers and therapeutic targets. Eur
Urol. 67:7–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alhasan AH, Scott AW, Wu JJ, Feng G, Meeks
JJ, Thaxton CS and Mirkin CA: Circulating microRNA signature for
the diagnosis of very high-risk prostate cancer. Proc Natl Acad Sci
USA. 113:10655–10660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Wan X, Chen H, Yang S, Liu Y, Mo W,
Meng D, Du W, Huang Y, Wu H, et al: Identification of miR-133b and
RB1CC1 as independent predictors for biochemical recurrence and
potential therapeutic targets for prostate cancer. Clin Cancer Res.
20:2312–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ling XH, Han ZD, Xia D, He HC, Jiang FN,
Lin ZY, Fu X, Deng YH, Dai QS, Cai C, et al: MicroRNA-30c serves as
an independent biochemical recurrence predictor and potential tumor
suppressor for prostate cancer. Mol Biol Rep. 41:2779–2788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling XH, Chen ZY, Luo HW, Liu ZZ, Liang
YK, Chen GX, Jiang FN and Zhong WD: BCL9, a coactivator for
Wnt/β-catenin transcription, is targeted by miR-30c and is
associated with prostate cancer progression. Oncol Lett.
11:2001–2008. 2016.PubMed/NCBI
|
|
15
|
Yu F, Deng H, Yao H, Liu Q, Su F and Song
E: Mir-30 reduction maintains self-renewal and inhibits apoptosis
in breast tumor-initiating cells. Oncogene. 29:4194–4204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinez I, Cazalla D, Almstead LL, Steitz
JA and DiMaio D: miR-29 and miR-30 regulate B-Myb expression during
cellular senescence. Proc Natl Acad Sci USA. 108:522–527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao JJ, Lin J, Zhu D, Wang X, Brooks D,
Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al: miR-30-5p
functions as a tumor suppressor and novel therapeutic tool by
targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res.
74:1801–1813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pino I, Pío R, Toledo G, Zabalegui N,
Vicent S, Rey N, Lozano MD, Torre W, García-Foncillas J and
Montuenga LM: Altered patterns of expression of members of the
heterogeneous nuclear ribonucleoprotein (hnRNP) family in lung
cancer. Lung Cancer. 41:131–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zerbe LK, Pino I, Pio R, Cosper PF,
Dwyer-Nield LD, Meyer AM, Port JD, Montuenga LM and Malkinson AM:
Relative amounts of antagonistic splicing factors, hnRNP A1 and
ASF/SF2, change during neoplastic lung growth: Implications for
pre-mRNA processing. Mol Carcinog. 41:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Miguel FJ, Sharma RD, Pajares MJ,
Montuenga LM, Rubio A and Pio R: Identification of alternative
splicing events regulated by the oncogenic factor SRSF1 in lung
cancer. Cancer Res. 74:1105–1115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anczuków O, Akerman M, Cléry A, Wu J, Shen
C, Shirole NH, Raimer A, Sun S, Jensen MA, Hua Y, et al:
SRSF1-regulated alternative splicing in breast cancer. Mol Cell.
60:105–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moore MJ, Wang Q, Kennedy CJ and Silver
PA: An alternative splicing network links cell-cycle control to
apoptosis. Cell. 142:625–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olshavsky NA, Comstock CE, Schiewer MJ,
Augello MA, Hyslop T, Sette C, Zhang J, Parysek LM and Knudsen KE:
Identification of ASF/SF2 as a critical, allele-specific effector
of the cyclin D1b oncogene. Cancer Res. 70:3975–3984. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ezponda T, Pajares MJ, Agorreta J,
Echeveste JI, López-Picazo JM, Torre W, Pio R and Montuenga LM: The
oncoprotein SF2/ASF promotes non-small cell lung cancer survival by
enhancing survivin expression. Clin Cancer Res. 16:4113–4125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anczuków O, Rosenberg AZ, Akerman M, Das
S, Zhan L, Karni R, Muthuswamy SK and Krainer AR: The splicing
factor SRSF1 regulates apoptosis and proliferation to promote
mammary epithelial cell transformation. Nat Struct Mol Biol.
19:220–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Zhang L, Yi X and Yu X:
Diagnostic and prognostic values of tissue hsa-miR-30c and
hsa-miR-203 in prostate carcinoma. Tumour Biol. 37:4359–4365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kachakova D, Mitkova A, Popov E, Popov I,
Vlahova A, Dikov T, Christova S, Mitev V, Slavov C and Kaneva R:
Combinations of serum prostate-specific antigen and plasma
expression levels of let-7c, miR-30c, miR-141, and miR-375 as
potential better diagnostic biomarkers for prostate cancer. DNA
Cell Biol. 34:189–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren Q, Liang J, Wei J, Basturk O, Wang J,
Daniels G, Gellert LL, Li Y, Shen Y, Osman I, et al: Epithelial and
stromal expression of miRNAs during prostate cancer progression. Am
J Transl Res. 6:329–339. 2014.PubMed/NCBI
|
|
30
|
Verduci L, Simili M, Rizzo M, Mercatanti
A, Evangelista M, Mariani L, Rainaldi G and Pitto L: microRNA
(miRNA)-mediated interaction between leukemia/lymphoma-related
factor (LRF) and alternative splicing factor/splicing factor 2
(ASF/SF2) affects mouse embryonic fibroblast senescence and
apoptosis. J Biol Chem. 285:39551–39563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu LL, Xie N, Sun S, Plymate S, Mostaghel
E and Dong X: Mechanisms of the androgen receptor splicing in
prostate cancer cells. Oncogene. 33:3140–3150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Busà R, Geremia R and Sette C: Genotoxic
stress causes the accumulation of the splicing regulator Sam68 in
nuclear foci of transcriptionally active chromatin. Nucleic Acids
Res. 38:3005–3018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karni R, Hippo Y, Lowe SW and Krainer AR:
The splicing-factor oncoprotein SF2/ASF activates mTORC1. Proc Natl
Acad Sci USA. 105:15323–15327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsieh AC, Liu Y, Edlind MP, Ingolia NT,
Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et
al: The translational landscape of mTOR signalling steers cancer
initiation and metastasis. Nature. 485:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sinclair CS, Rowley M, Naderi A and Couch
FJ: The 17q23 amplicon and breast cancer. Breast Cancer Res Treat.
78:313–322. 2003. View Article : Google Scholar : PubMed/NCBI
|