Introduction
Periodontitis is caused by a wide range of complex
microorganisms and is the primary cause of alveolar bone absorption
and eventual tooth loss in the adult population (1). This condition has become a major
public health issue, and the development of effective therapies to
treat this disease and regenerate periodontal tissue has become an
important goal of current medicine. It is widely accepted that both
the initiation of oral infectious diseases and the progression of
these disease states are associated with increased diversity and
richness of the microbiota. In contrast, oral health is associated
with decreased diversity and richness within the microbial
community. Furthermore, the immune response of the host to the oral
microbiome should be considered with respect to the
immunopathogenesis of periodontal disease and the immune defenses
against caries (2). The
inflammatory response fosters the growth of dysbiotic microbial
communities, and the bacterial biomass of human
periodontitis-associated biofilms has been shown to increase with
the aggravation of periodontal inflammation (3). As periodontal tissue may continue to
degrade even after being treated with conventional therapies
(4), there is a need to develop an
antimicrobial agent to protect regenerated periodontal tissue in
infectious environments.
Human β-defensins (HBDs) are epithelial-derived
antimicrobial peptides that contribute to the innate immune
responses of eukaryotes (5). In
addition to their microbicidal abilities, host defense peptides are
multifunctional mediators of inflammation that have effects on cell
proliferation, cytokine/chemokine production and chemotaxis in
epithelial and inflammatory cells (6). The expression of three HBDs (namely
HBD-1, −2 and −3) has been identified in oral mucosa, gingiva and
salivary glands (7). Among these
HBDs, HBD-3 is of particular interest for structural and functional
studies, and for potential pharmaceutical applications. The
broad-spectrum microbicidal activity of HBD-3 is effective against
multiple organisms, including fungi, bacteria and viruses. Thus,
HBD-3 plays an important role in the human body (8).
Recently, various approaches have been applied to
regenerate periodontal tissue, including the use of osteoinductive
agents and biomaterials, guided tissue regeneration and cell
therapy (9). Human periodontal
ligament cells (HPDLCs) and human bone marrow stromal cells
(HBMSCs) are useful seeding cells for periodontal cell therapies
(10,11). However, these two cell types only
secrete HBD-3 in trace amounts (12).
The present study aimed to construct a recombinant
lentiviral vector with the HBD-3 gene, and to investigate the
effects of the vector in an effort to develop a novel and suitable
treatment for periodontal inflammation to promote periodontal
tissue regeneration.
Materials and methods
Cell isolation and culture
HPDLCs and HBMSCs were received as gifts from the
Shanghai Research Institute of Stomatology (Shanghai, China). The
cells were cultured in Dulbecco's modified Eagle's medium at 37°C
in a humidified atmosphere of 95% air and 5% CO2. The
medium was replaced the following day and subsequently every 3
days. The cells were passaged with 0.25% trypsin and 0.1% EDTA upon
reaching confluence. Cells from passage three or four were used in
the subsequent experiments.
Recombinant plasmid construction
The expression vector pLV.Des3d.P/puro was purchased
from Cyagen Biosciences (Guangzhou, China). E. coli Stbl3 was used
as the host. The HBD-3 gene (code:
MRIHYLLFALLFLFLVPVPGHGGIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK)
and the green fluorescent protein (eGFP) gene were cloned into the
pLV.Des3d.P/puro vector. The recombinant plasmid
pLV.EX3d.P/puro-EF1A- Humacalx-IRES/eGFP was constructed using
Gateway Technology, as previously described (13). This technology was invented and
commercialized by Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and is a universal cloning method based on the
site-specific recombination properties of bacteriophage λ. The
recombinant plasmid pLV.EX3d.P/puro-EF1A-PTH-IRES/eGFP was
constructed without HBD-3. The constructed expression plasmids were
amplified in the E. coli strain Stbl3.
Transfection
The lentiviral vector containing HBD-3 was first
transfected into 293T packaging cells to obtain high levels of
lentiviral particles in the culture supernatant. The HPDLCs and
HBMSCs were cultured in 25-cm2 dishes until 80–90%
confluence was reached. Transfection was performed by adding
polybrene (8 µg/ml) and 20 µl each viral dilution to the cells,
thoroughly and gently mixing the solutions, and incubating the
cells in 5% CO2 at 37°C. After 18 h, the viral particles
remaining in the supernatant were removed and the medium was
replaced with fresh medium supplemented with 10% fetal bovine serum
(FBS; Gibco, Thermo Fisher Scientific, Inc.). The cells were
incubated in 5% CO2 at 37°C for an additional 72 h. The
transfection efficiency was calculated using a fluorescence
microscope.
Western blot analysis
The cells were collected from the culture dishes
with radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). Phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology) was added to the
samples, and a Bicinchoninic Acid assay was used to determine the
protein concentrations. The samples were boiled at 100°C for 5 min.
Total proteins (20 µg per lane) were separated by 5–15% SDS-PAGE
and subsequently transferred onto polyvinylidene difluoride
membranes. Membranes were blocked with 5% nonfat milk (5 g nonfat
milk powder diluted in 100 ml PBS) for 1 h at room temperature and
incubated overnight at 4°C with the following corresponding primary
antibodies: Anti-β-actin (cat. no. ab1801; 1:1,000; Abcam,
Cambridge, UK) and anti-β-defensin-3 (cat. no. ab19270; 1:1,000;
Abcam), followed by incubation with a goat anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibody (cat. no.
KC-RB-035; 1:5,000; KangChen Bio-tech, Inc., Shanghai, China) for 1
h at room temperature. After washing with TBS with Tween-20, the
membranes were developed using an EZ-enhanced chemiluminescence
detection kit according to the manufacturer's protocol (Biological
Industries Beit Haemek Ltd., Israel) and were then imaged using a
UVitec gel documentation system (UVitec Limited, Cambridge,
UK).
Enzyme-linked immunosorbent assay
(ELISA)
The amount of secreted HBD-3 in the culture
supernatant was detected using an HBD-3 ELISA kit (cat. no.
JL19214; Shanghai Jiang Lai Biotechnology Co., Ltd., Shanghai,
China) according to the manufacturer's instructions. Absorbance at
490 nm was determined with a microplate reader (BioTek ELx800;
Omega Bio-Tek, Inc., Norcross, GA, USA). Each sample was analyzed
in triplicate.
Microbial strains
Actinomyces viscosus (ATCC 19246); Candida albicans
(ATCC 10231); Rothia dentocariosa (ATCC 19426); Porphyromonas
gingivalis (ATCC 33277) and Streptococcus mutans (UA 159) were used
to test the antimicrobial activity of the cells. All the strains
were received as gifts from the Shanghai Research Institute of
Stomatology.
Antimicrobial activity as assessed by
liquid growth inhibition assay
The antimicrobial activity of HBD-3 against the five
microbial strains mentioned above was determined by a liquid growth
inhibition assay. The purified HBD-3 peptide was serially diluted
two-fold with 0.01% acetic acid and 0.2% bovine serum albumin
(Thermo Fisher Scientific, Inc.). Aliquots (10 µl) from each
dilution were transferred to a 96-well microplate, and each well
was inoculated with 100 µl suspension of mid-log bacteria
(106 CFU/ml) in brain heart infusion (BHI) broth (BBL,
Cockeysville, USA). Medium alone (BHI broth) and untreated cells
served as control groups. After the cultures were incubated at 37°C
for 24 h, microbial growth was assessed by measuring the optical
density at a wavelength of 590 nm with a microplate reader. All
experiments were performed in triplicate.
Colony-forming assay
The suspensions of the tested microorganisms were
separately cultured in BHI agar supplemented with 5% FBS at 37°C
for 48 h. Subsequently, the number of colony-forming units (CFUs)
was counted.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used for all
statistical analyses. Significant differences were calculated using
one-way analysis of variance followed by Bonferroni or Tamhane post
hoc tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
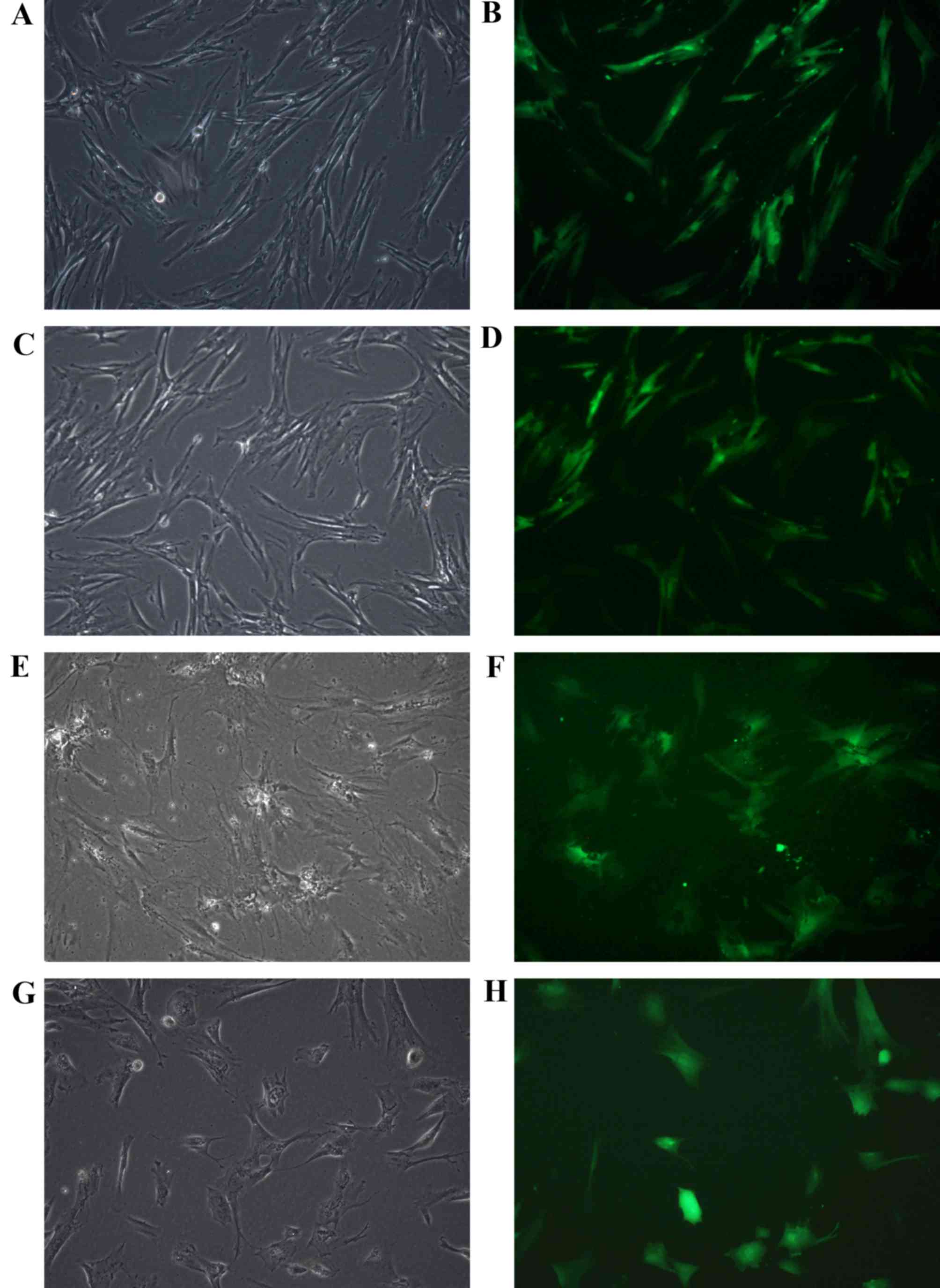
Transfection efficiency
Fusiform or polygonal transfected HPDLCs and HBMSCs
were observed adhering to the bottom of the dishes by optical
microscopy (Fig. 1). Transfection
of the recombinant plasmids into HPDLCs and HBMSCs was assessed by
detecting GFP expression using a fluorescence microscope. The
transfected HPDLCs and HBMSCs exhibited similar GFP-positive
expression throughout several repeated experiments, as observed by
fluorescence microscopy (Fig. 1).
Transfection efficiency was calculated by counting the cells that
fluoresced green. The results demonstrated that the rate of HPDLC
and HBMSC transfection with the HBD-3 and GFP genes was 79.94 and
64.81%, respectively. The rate of HPDLC and HBMSC transfection with
the GFP gene only was 75.98 and 53.71%, respectively (data not
shown).
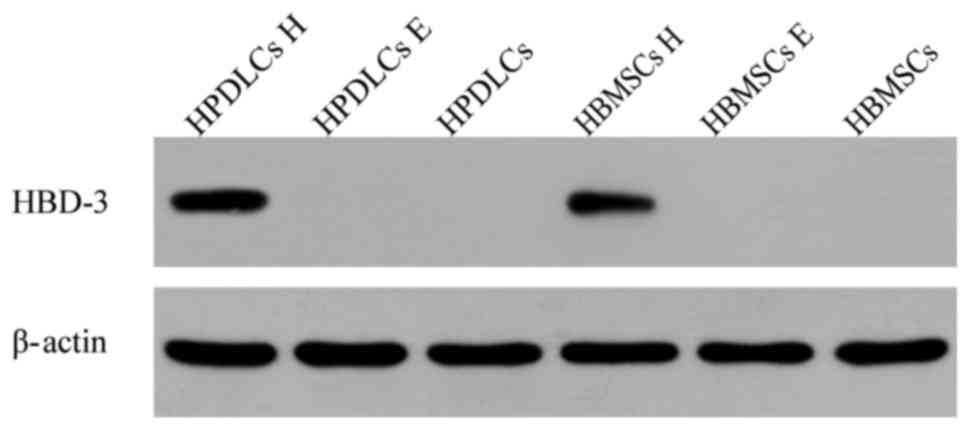
Western blot analysis
To examine the protein expression level of HBD-3 in
the transfected HPDLCs and HBMSCs, western blot analysis was
performed. Distinct positive bands were observed for HPDLCs and
HBMSCs transfected with the HBD-3 and GFP genes. However, no
positive bands were observed in the other two groups (Fig. 2).
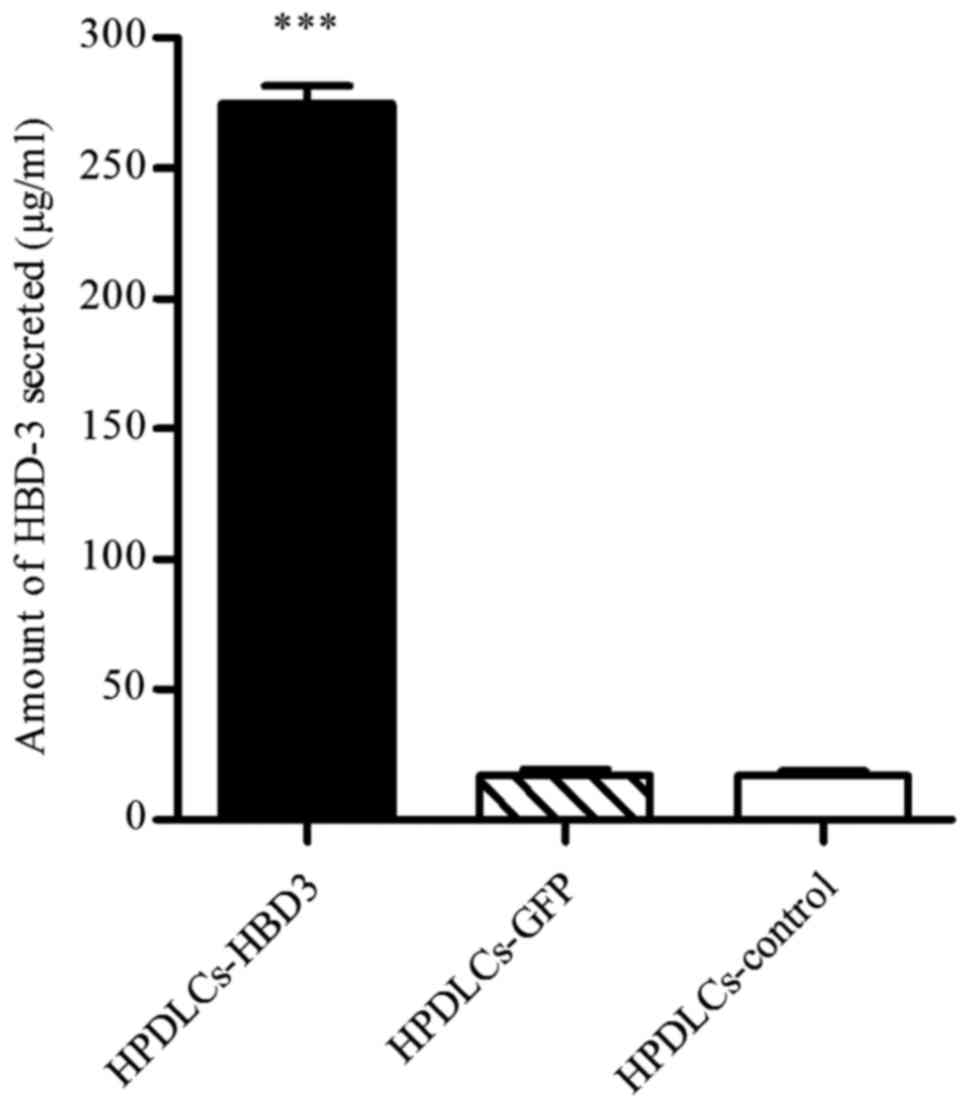
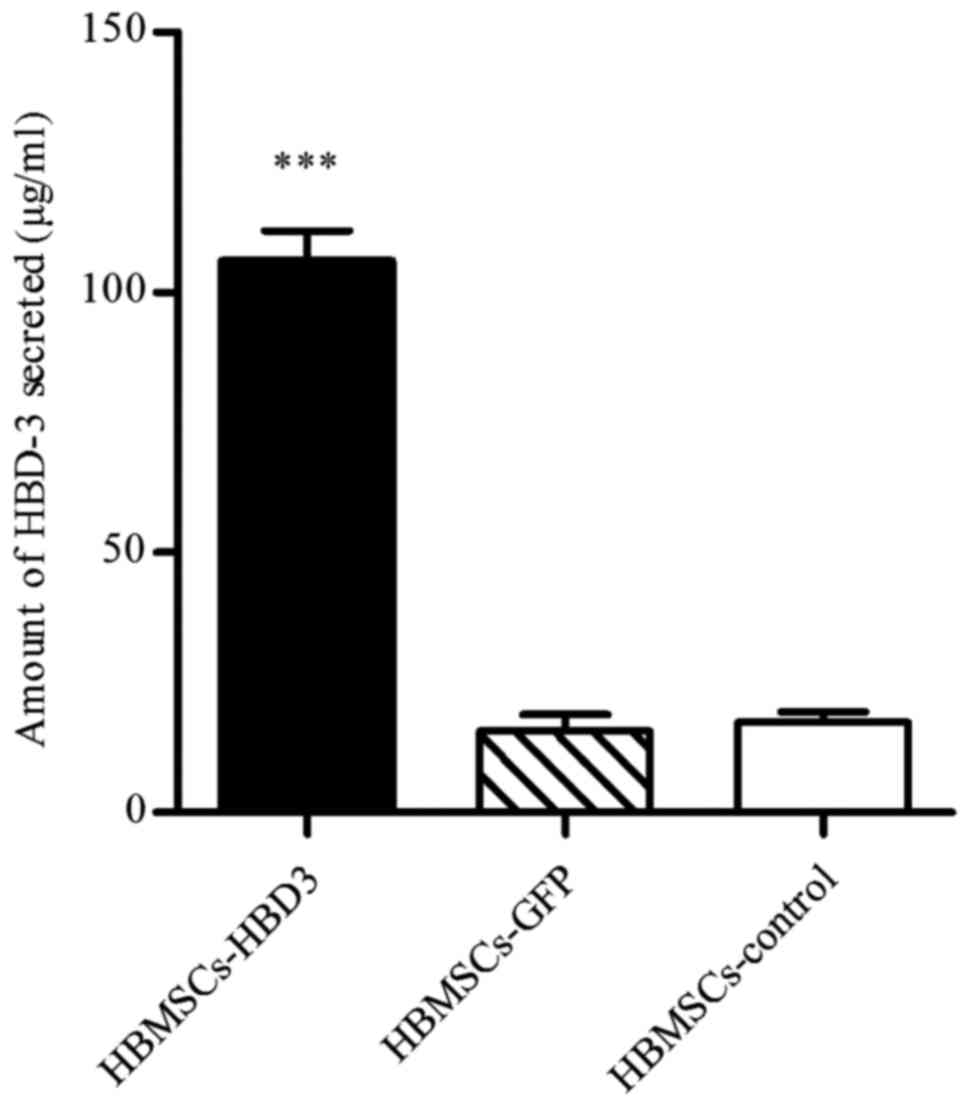
ELISA
ELISA was performed to quantify the levels of HBD-3
in HPDLCs and HBMSCs transfected with the HBD-3 and GFP genes. The
untreated cells served as a control. The results demonstrated that
the concentration of secreted HBD-3 was 274.89±6.79 µg/ml and
106.11±5.67 µg/ml in the supernatants of transfected HPDLCs
(Fig. 3) and HBMSCs (Fig. 4), respectively; these
concentrations were significantly higher than those of the
corresponding cells transfected with the GFP control vector, and
that of the untreated control group (P<0.001).
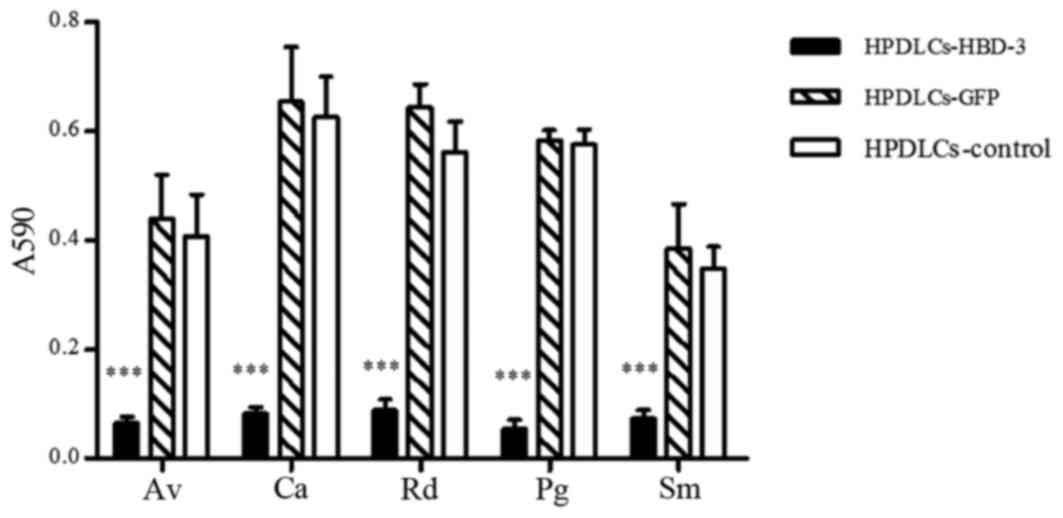
Liquid growth inhibition assay
A liquid growth inhibition assay was performed, and
the optical density of each stock suspension was measured at 590 nm
to evaluate the approximate numbers of microbes present. As
presented in Fig. 5, the HPDLCs
transfected with the HBD-3 gene and cultured with Actinomyces
viscosus, Candida albicans, Rothia dentocariosa, Porphyromonas
gingivalis and Streptococcus mutans yielded small optical density
(OD) values of 0.065±0.012, 0.081±0.013, 0.088±0.020, 0.054±0.017
and 0.073±0.016, respectively, which were significantly reduced
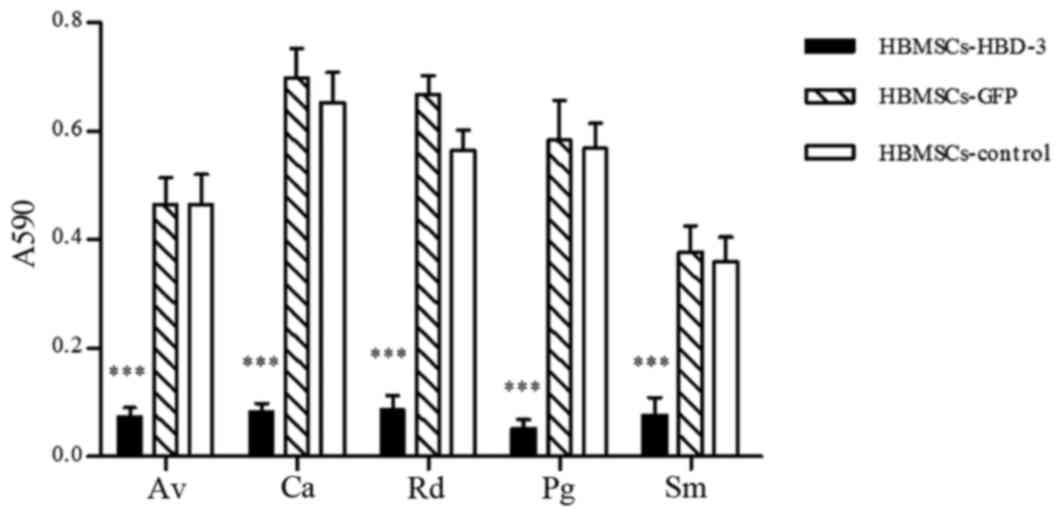
compared with those of the other groups (P<0.001). A similar
effect was observed for HBMSCs transfected with the HBD-3 gene and
cultured with the microbes; corresponding to the order listed
above, the cultures yielded OD values of 0.073±0.017, 0.082±0.016,
0.086±0.026, 0.052±0.017 and 0.076±0.033, respectively (P<0.001;
Fig. 6).
Colony-forming assay
A colony-forming assay was performed to evaluate the
effects of HBD-3 on the important antimicrobial activity of the
cells against the tested bacteria. The HPDLCs transfected with the
HBD-3 gene demonstrated significantly reduced colony counts
compared with control and GFP-transfected HPDLCs (P<0.001;
Fig. 7). Similar results were
observed in HBMSCs (P<0.001; Fig.
8).
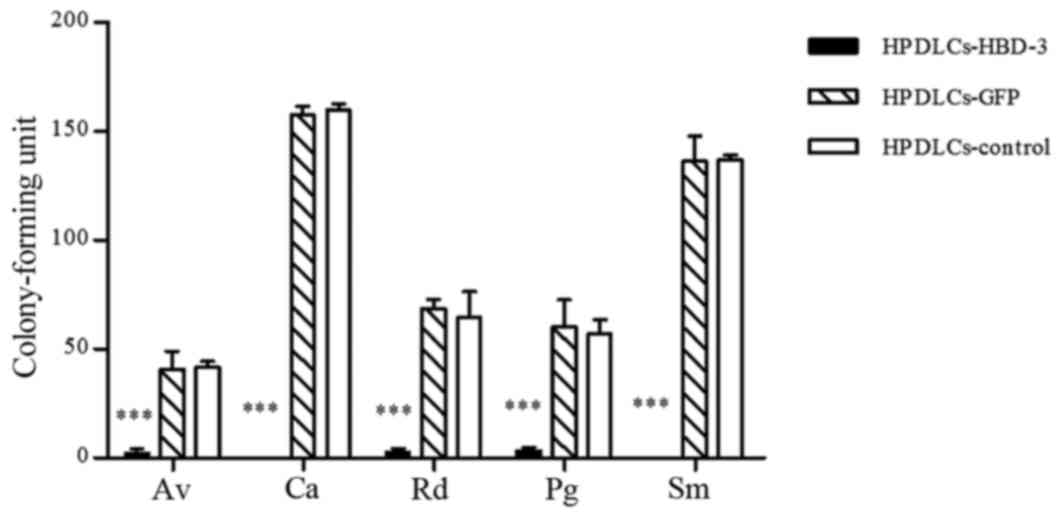 | Figure 7.Antimicrobial testing for HPDLCs
transfected with HBD-3, as determined by colony-forming assay. Data
are expressed as the mean ± standard deviation. *P<0.05,
***P<0.001 vs. control and GFP-transfected cells. HBD-3, human
β-defensin-3; GFP, green fluorescent protein; HPDLCs, human
periodontal ligament cells; Av, Actinomyces viscosus; Ca, Candida
albicans; Rd, Rothia dentocariosa; Pg, Porphyromonas gingivalis;
Sm, Streptococcus mutans. |
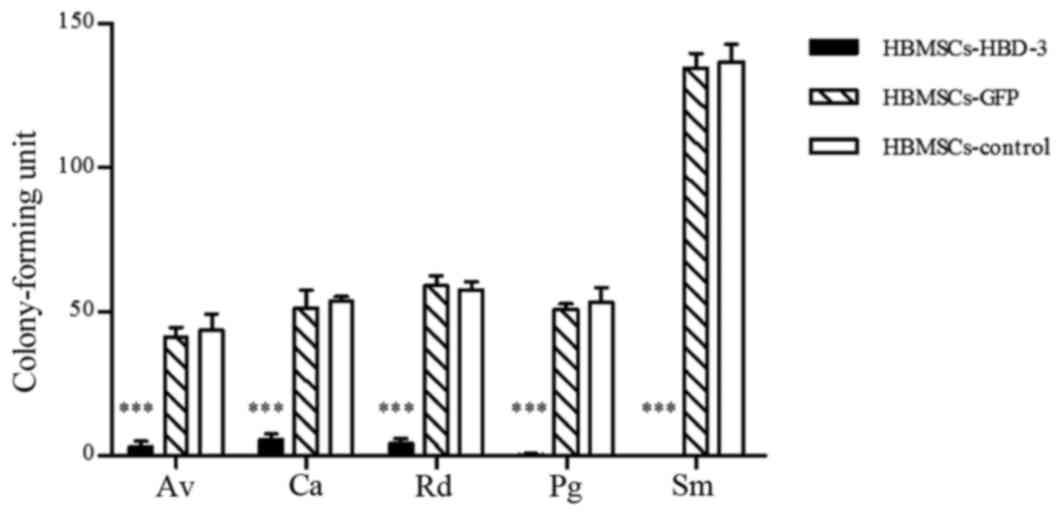 | Figure 8.Antimicrobial testing for HBMSCs
transfected with HBD-3, as determined by colony-forming assay. Data
are expressed as the mean ± standard deviation. *P<0.05,
***P<0.001 vs. control and GFP-transfected cells. HBD-3, human
β-defensin-3; GFP, green fluorescent protein; HBMSCs, human bone
marrow stromal cells; Av, Actinomyces viscosus; Ca, Candida
albicans; Rd, Rothia dentocariosa; Pg, Porphyromonas gingivalis;
Sm, Streptococcus mutans. |
Discussion
Periodontal diseases are highly prevalent and affect
<90% of the population worldwide (1). Pathogenic bacteria are widely
recognized to be a major cause of periodontal tissue destruction,
and the ultimate goals of periodontal treatments are to support
good oral hygiene and regenerate tissue integrity, which may have
been damaged by the inflammatory process (14). Currently, periodontal regeneration
is shifting towards cell- and gene-based therapies (15). The present study constructed a
recombinant lentiviral HBD-3 expression vector and investigated the
effects of an antimicrobial peptide using a combination of gene-
and cell-based therapies.
Firstly, the efficiency of HBD-3 transfection into
HPDLCs and HBMSCs was determined, and transfection was validated
using western blotting. HBD-3 protein levels in the transfected
HPDLCs and HBMSCs were sustained and were significantly higher than
those of the control group. Similar results were also obtained from
the ELISA analyses. Certain studies have reported that HPDLCs have
the capacity to function as osteoblasts or cementoblasts under
regenerative conditions, suggesting that they are the best
candidates for regeneration applications (16,17).
In addition, HBMSCs are the most widely investigated mesenchymal
stem cells, which have tremendous potential in regenerative
medicine because of their multipotency and capability of forming a
variety of tissues, including the periodontium (18). The crucial steps of gene therapy
include the efficient transfer and appropriate expression of the
target gene. Currently, the lentiviral vector is one of the most
useful methods for treating periodontal disease by virtue of its
high transduction efficiency (15). The studies mentioned above
indicated that HPDLCs and HBMSCs are promising for use as seeding
cells for cell- and gene-based therapies for periodontal disease.
In addition, the lentiviral vector with eGFP is an appropriate
expression vector system.
Furthermore, the present study detected the
antimicrobial activity of the HPDLCs and HBMSCs transfected by a
lentivirus containing the HBD-3 gene, using liquid growth
inhibition and colony-forming assays. HBD-3 is an endogenous
antibiotic and is active against both gram-positive and
gram-negative bacteria. Its ability to act against
multidrug-resistant clinical isolates of Staphylococcus aureus,
Enterococcus faecium, Pseudomonas aeruginosa, Stenotrophomonas
maltophilia, and Acinetobacter baumannii has been confirmed
(19). In the present study, the
numbers of bacteria were significantly lower in the experimental
group than in the control group. The periodontal pathogens
Actinomyces viscosus, Porphyromonas gingivalis, Rothia dentocariosa
and Candida albicans (20–22) were demonstrated to be susceptible
to the cells containing Humacalx-IRES compared with those in the
control and untreated groups. The results indicated that HBD-3 has
the capacity to inhibit microbial activity in vitro, which
is consistent with the results of our previous study (23) and with other research (24). As Candida albicans is the most
common opportunistic fungal pathogen of humans, and can cause
superficial epithelial infections and life-threatening systemic
infections, HBD-3 also demonstrated beneficial antifungal effects.
Notably, the caries-causing bacteria Streptococcus mutans (2) was also susceptible to HBD-3 in both
experimental groups. The results of the present study demonstrated
the multifunctional, broad-spectrum activity of HBD-3 against a
collection of oral microorganisms; this activity could be applied
in the treatment of oral infectious diseases.
Cells of several human tissue types can secrete
HBD-3. Previous studies have demonstrated that HBD is susceptible
to degradation and inactivation by both host and bacterial
proteases. It has also been reported that inflamed gingival tissues
express lower levels of HBD-3 mRNA than healthy tissues (7,25).
Brancatisano et al (26)
detected HBD-3 using ELISA, and demonstrated that its levels were
inversely correlated with the severity of the disease and with the
degree of colonization by combinations of bacterial species having
elevated periodontopathogenic potential. Based on this information,
it is reasonable to hypothesize that aggressive inflammation and
tissue destruction occur when the HBD-3 peptide cannot counteract
the antimicrobial activity. However, appropriate expression of HBD
peptides in states of health and disease may contribute to the
maintenance of periodontal homeostasis, potentially via the
antimicrobial effects of HBD-3 and the promotion of adaptive immune
responses (27). Therefore, the
transfection of HPDLCs and HBMSCs with HBD-3 may have favorable
effects on antimicrobial activity by complementing the low levels
of HBD-3 in aggressive periodontitis and other oral infectious
diseases.
In conclusion, application of the lentiviral vector
containing HBD-3 has great potential as a safe and efficient gene
therapy for antimicrobial activity in periodontitis. Further
research will be conducted to investigate the influence of HBD-3
transfection on HPDLCs and HBMSCs in periodontal tissue
regeneration.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81271157).
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costalonga M and Herzberg MC: The oral
microbiome and the immunobiology of periodontal disease and caries.
Immunol Lett. 162:22–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hajishengallis G: The inflammophilic
character of the periodontitis-associated microbiota. Mol Oral
Microbiol. 29:248–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holmlund A, Hänström L and Lerner UH: Bone
resorbing activity and cytokine levels in gingival crevicular fluid
before and after treatment of periodontal disease. J Clin
Periodontol. 31:475–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taggart CC, Greene CM, Smith SG, Levine
RL, McCray PB Jr, O'Neill S and McElvaney NG: Inactivation of human
beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol.
171:931–937. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niyonsaba F and Ogawa H: Protective roles
of the skin against infection: Implication of naturally occurring
human antimicrobial agents beta-defensins, cathelicidin LL-37 and
lysozyme. J Dermatol Sci. 40:157–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bissell J, Joly S, Johnson GK, Organ CC,
Dawson D, McCray PB Jr and Guthmiller JM: Expression of
beta-defensins in gingival health and in periodontal disease. J
Oral Pathol Med. 33:278–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song W, Shi Y, Xiao M, Lu H, Qu T, Li P,
Wu G and Tian Y: In vitro bactericidal activity of recombinant
human beta-defensin-3 against pathogenic bacterial strains in human
tooth root canal. Int J Antimicrob Agents. 33:237–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Yan F, Lei L, Li Y and Xiao Y:
Application of autologous cryopreserved bone marrow mesenchymal
stem cells for periodontal regeneration in dogs. Cells Tissues
Organs. 190:94–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dan H, Vaquette C, Fisher AG, Hamlet SM,
Xiao Y, Hutmacher DW and Ivanovski S: The influence of cellular
source on periodontal regeneration using calcium phosphate coated
polycaprolactone scaffold supported cell sheets. Biomaterials.
35:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Osugi M, Katagiri W, Yoshimi R, Inukai T,
Hibi H and Ueda M: Conditioned media from mesenchymal stem cells
enhanced bone regeneration in rat calvarial bone defects. Tissue
Eng Part A. 18:1479–1489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sutton JM and Pritts TA: Human
beta-defensin 3: A novel inhibitor of Staphylococcus-produced
biofilm production. Commentary on ‘Human β-defensin 3 inhibits
antibiotic-resistant Staphylococcus biofilm formation’. J Surg Res.
186:99–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartley JL, Temple GF and Brasch MA: DNA
cloning using in vitro site-specific recombination. Genome
Res. 10:1788–1795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HL, Greenwell H, Fiorellini J,
Giannobile W, Offenbacher S, Salkin L, Townsend C, Sheridan P,
Genco RJ, et al: Research, Science and Therapy Committee:
Periodontal regeneration. J Periodontol. 76:1601–1622.
2005.PubMed/NCBI
|
|
15
|
Rios HF, Lin Z, Oh B, Park CH and
Giannobile WV: Cell- and gene-based therapeutic strategies for
periodontal regenerative medicine. J Periodontol. 82:1223–1237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polimeni G, Xiropaidis AV and Wikesjö UM:
Biology and principles of periodontal wound healing/regeneration.
Periodontol 2000. 41:30–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JY, Jeon SH and Choung PH: Efficacy
of periodontal stem cell transplantation in the treatment of
advanced periodontitis. Cell Transplant. 20:271–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada Y, Ueda M, Hibi H and Nagasaka T:
Translational research for injectable tissue-engineered bone
regeneration using mesenchymal stem cells and platelet-rich plasma:
From basic research to clinical case study. Cell Transplant.
13:343–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maisetta G, Batoni G, Esin S, Florio W,
Bottai D, Favilli F and Campa M: In vitro bactericidal activity of
human beta-defensin 3 against multidrug-resistant nosocomial
strains. Antimicrob Agents Chemother. 50:806–809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grant MM, Kolamunne RT, Lock FE, Matthews
JB, Chapple IL and Griffiths HR: Oxygen tension modulates the
cytokine response of oral epithelium to periodontal bacteria. J
Clin Periodontol. 37:1039–1048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang CY, Hsueh PR, Lu CY, Tsai HY, Lee PI,
Shao PL, Wang CY, Wu TZ, Chen SW and Huang LM: Rothia dentocariosa
bacteremia in children: Report of two cases and review of the
literature. J Formos Med Assoc. 106:(3 Suppl). S33–S38. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Järvensivu A, Hietanen J, Rautemaa R,
Sorsa T and Richardson M: Candida yeasts in chronic periodontitis
tissues and subgingival microbial biofilms in vivo. Oral
Dis. 10:106–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Watanabe H, Ogita M, Ichinose S
and Izumi Y: Effect of human beta-defensin-3 on the proliferation
of fibroblasts on periodontally involved root surfaces. Peptides.
32:888–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ouhara K, Komatsuzawa H, Yamada S, Shiba
H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H and Sugai
M: Susceptibilities of periodontopathogenic and cariogenic bacteria
to antibacterial peptides, {beta}-defensins and LL37, produced by
human epithelial cells. J Antimicrob Chemother. 55:888–896. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hosokawa I, Hosokawa Y, Komatsuzawa H,
Goncalves RB, Karimbux N, Napimoga MH, Seki M, Ouhara K, Sugai M,
Taubman MA and Kawai T: Innate immune peptide LL-37 displays
distinct expression pattern from beta-defensins in inflamed
gingival tissue. Clin Exp Immunol. 146:218–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brancatisano F, Maisetta G, Barsotti F,
Esin S, Miceli M, Gabriele M, Giuca MR, Campa M and Batoni G:
Reduced human beta defensin 3 in individuals with periodontal
disease. J Dent Res. 90:241–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ebrahem MA: Expression of human beta
defensins (HBDs) 1, 2 and 3 in gingival crevicular fluid of
patients affected by localized aggressive periodontitis. Saudi Dent
J. 25:75–82. 2013. View Article : Google Scholar : PubMed/NCBI
|