Introduction
Lung cancer is a leading cause of cancer-associated
mortality worldwide (1). It is the
most common fatal cancer in males and females, and accounts for 29
and 26% of all male and female cancer-associated mortalities
worldwide, respectively (2). In
China, lung cancer is the most frequently diagnosed cancer in males
(22.14%), and is the leading cause of cancer-associated mortality
in males (27.21%) and females (21.91%) (3). Despite advances in combination
chemotherapy and surgical techniques, the prognosis of non-small
cell lung cancer (NSCLC) remains poor; the 5-year survival rate for
all stages and subtypes combined remains as low as 11% (4). Metastasis is the predominant cause of
mortality in patients with lung cancer; ~90% of patients succumb to
metastatic cancer (5). The
metastatic process is initiated by dissemination of clonal cells
from the primary tumor site, which invade the extracellular matrix
(ECM) and surrounding stroma (6).
The matrix metalloproteinase (MMP) family members
are involved in degradation of the ECM during normal physiological
processes, including embryonic development and tissue remodeling,
as well as in disease processes, including tumor metastasis
(7). MMP9 is a member of the ECM
(8–10). Overexpression of MMP9 has been
reported to facilitate metastatic spread of various cancer cells,
including lung cancer cells. A recent study demonstrated that MMP9
expression is positively correlated with lung cancer malignancy
(11), and suggested that MMP9 is
an important factor in the process of lung cancer metastasis.
Furthermore, the process of epithelial-mesenchymal transition (EMT)
is known to serve an important role in metastasis formation
(12). The series of EMT events is
predominantly activated in cancer cells acquiring invasive and
metastatic properties (13); MMP9
and EMT are critical in the processes associated with cancer
metastasis. Signal transducer and activator of transcription 3
(STAT3) is an oncogenic transcription factor known to be involved
in cancer cell proliferation and metastasis (14). In numerous types of cancer, STAT3
is constitutively active, leading to continued expression of target
genes that promote cell proliferation, survival and invasion. The
role of STAT3 in tumorigenesis and cancer cell invasion has been
well established in a wide range of human cancers, including lung
cancer (15). When activated by
upstream signaling pathways, including epidermal growth factor and
the interleukin-6/Janus kinase pathway, STAT3 is phosphorylated and
then forms homodimers or heterodimers with other members of the
STAT family. Subsequently, the activated STAT3 complex translocates
into the nucleus to initiate transcription of STAT3 target genes,
including MMP9 (16).
Fuzheng Kang-Ai (FZKA) decoction, initially
prescribed by Dr. Wanyin Wu (17),
has been used to treat patients with NSCLC at the Guangdong
Provincial Hospital of Traditional Chinese Medicine (Guangzhou,
China) for a decade, and has been shown to exert a positive impact
on patient health. Our previous study demonstrated that the
efficacy of a combination of gefitinib plus FZKA exhibited better
outcome compared with gefitinib alone (17). In addition, FZKA could enhance the
disease control rate, and prolong the progression-free survival
(PFS) and median survival time (MST) in patients with NSCLC
(18,19). Furthermore, our recent study
reported that FZKA inhibited lung cancer cell growth through
AMP-activated protein kinase α-mediated induction, and an interplay
between insulin-like growth factor-binding protein 1 and forkhead
box O3a, indicating its therapeutic effect on lung cancer (20). Metastasis is the predominant cause
of mortality in patients with lung cancer; however, the mechanism
by which FZKA affects lung cancer metastasis remains to be
elucidated. The present study identified the inhibitory effects of
FZKA on lung cancer cell invasion and migration. In addition, the
probable mechanisms by which FZKA inhibited lung cancer cell
metastasis were examined, which may provide evidence to support the
clinical usage of FZKA decoction to treat patients with NSCLC.
Materials and methods
Cells
Human A549 NSCLC cells were obtained from the Cell
Line Bank at the Laboratory Animal Center of Sun Yat-sen University
(Guangzhou, China). PC9 and H1650 cells were obtained from the
Chinese Academy of Sciences Cell Bank of Type Culture Collection
(Shanghai, China). All cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 0.5% penicillin-streptomycin sulfate, and incubated at 37°C
with 5% CO2. Cells were counted using a Countstar
automated cell counter (Inno-Alliance Biotech, Inc., Wilmington,
DE, USA).
Chemicals
Monoclonal antibodies against total STAT3 (cat no.
8232), phosphorylated (p)-STAT3 (cat. no. 4093), vimentin (cat. no.
12826), N-cadherin (cat. no. 13116) and MMP9 (cat. no. 13667) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Lipofectamine 3000 reagent was purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). The control (pCMV6-AC) and STAT3
overexpression (pCMV6-AC-STAT3) vectors were obtained from OriGene
Technologies, Inc. (Rockville, MD, USA).
FZKA decoction
FZKA decoction is a Chinese herbal medicine that has
been used to treat NSCLC at the Guangdong Provincial Hospital of
Traditional Chinese Medicine for >10 years. It consists of
Taizishen, 30 g; Atractylodes macrocephala Koidz. (Baizhu),
15 g; Astragalus membranaceus (Fisch.) Bge. (Huangqi), 30 g;
Oldenlandia diffusa (Willd.) Roxb. (Baihuasheshecao), 30 g;
Solanum nigrum L. (Longkui), 30 g; Salvia chinensis
Benth (Shi-jianchuan), 30 g; Cremastra appendiculata (D.
Don) Makino (Shancigu), 30 g; Coix lachrymal-jobi L.
(Yiyiren), 30 g; Akebia quinata (Thunb.) Decne (Bayuezha),
30 g; Rubus parviflolius L. (Shepaole), 30 g; Curcuma
kwangsiensis S.G. Lee et C.F. Liang (Ezhu), 15 g; and
Glycyrrhiza uralensis Fisch. (Gancao), 10 g (19). All of the components were soaked
together for 30 min prior to decoction. The concentrated liquid was
finally spray dried into particles by Guangdong One Pharmaceutical
Co., Ltd (Guangzhou, Guangdong, China). The FZKA particles were
dissolved in RPMI-1640 and filtered using 0.22 µm filters prior to
use. The pH value of the cultured cells in media was adjusted to
7.2–7.4 following FZKA addition.
Cell viability assay
Cells were seeded in 6-well plates at a density of
3×105 cells/well. After 24 h of culture, cells were
treated with FZKA (0, 1, 2 and 3 mg/ml) and were incubated at 37°C
for 24 h; 0 mg/ml FZKA cultured cells were used as the untreated
control cells. Subsequently, cells were collected by trypsinization
and stained with trypan blue at a concentration of 1:1. The cells
were resuspended and were then counted using a Countstar automated
cell counter. Cell viability was expressed as a percentage of
untreated cells. Data were taken from an average of three
independent experiments.
Wound-healing assay
Wound-healing assay was performed to determine the
migratory ability of cells. The cells were cultured
(4×105) in 6-well plates, and incubated until the cell
density reached 90%. Cell monolayers were wounded by scratching
with a 200-µl pipette tip, after which the plates were washed twice
with PBS to remove detached cells, and were incubated in RPMI-1640
supplemented with 2% FBS containing FZKA (0, 1 and 2 mg/ml). After
12 or 24 h at 37°C, the medium was replaced with PBS and washed
twice. The wound gap was observed and images were captured using a
fluorescence microscope (Olympus IX71; Olympus Corporation, Tokyo,
Japan; magnification, ×40). The distance of the scratch was
measured using ImageJ software (version 1.48; National Institutes
of Health, Bethesda, MD, USA). The results were obtained from three
independent experiments.
Transwell assay
A Transwell plate (Corning Incorporation, Corning,
NY, USA; diameter, 10 mm; 8 µm pore polycarbonate membrane) was
used to detect the migratory and invasive potential of the cells.
In the invasion assay, prior to experimentation, Matrigel (BD
Bioscience, San Jose, CA, USA) was diluted 8-fold using PBS and was
injected into the upper chamber. In the migration assay, this step
was omitted. To the lower chamber, 500 µl cell culture medium
supplemented with 30% FBS was added. Subsequently, cells were
diluted to 0.5×106/ml, pretreated with FZKA (0, 1 and 2
mg/ml) for 24 h at 37°C, and a 200 µl cell suspension was added
into the upper chamber. The Transwell plate was then incubated at
37°C in a 5% CO2 atmosphere for 16 h. Non-migrated cells
were removed with a cotton swab, and invaded cells were fixed in 4%
paraformaldehyde for 15 min at room temperature prior to staining
with crystal violet. Images were captured under ×100 magnification
with a fluorescence microscope (Olympus DP72; Olympus Corporation).
Subsequently, 200 µl 33% acetic acid was added to the chamber and
the eluent was removed into 96-well plates. Absorbance at 570 nm
was determined using an ELISA reader (Victor X5; Perkin Elmer,
Inc., Waltham, MA, USA). The experiment was repeated at least three
times.
MMP9 activity assay
The activity of MMP9 was measured using the
SensoLyte® 520 MMP9 assay kit (AnaSpec, Fremont, CA,
USA) according to the manufacturer's protocol. The cells were
seeded in 6-well plates at a density of 3×105 cells/well
and treated with FZKA (0, 1, 2 and 3 mg/ml) for 24 h. Subsequently,
the cell culture media supernatant was collected and centrifuged at
1,000 × g for 15 min at 4°C. The MMP containing samples were
incubated with APMA (component C) at a final concentration of 1 mM
in the assay buffer (Component D) and were incubated for 2 h at
37°C in order to activate pro-MMPs. The working solutions were then
prepared by diluting the MMP9 substrate 1:100 in assay buffer. The
reagents: 50 µl MMP9 containing sample and 50 µl MMP9 substrate
solution, were mixed in a 96-well plate by gentle agitation for 30
sec. The reactions were incubated at 37°C for 1 h and fluorescence
intensity was measured at excitation/emission=490/520 nm. The
experiment was repeated three times.
Western blot analysis
Cells were seeded in 6-well plates at a density of
3×105 cells/well. Following 24 h of culture, cells were
treated with FZKA (0, 1, 1.5 and 2 mg/ml) and were incubated at
37°C for 24 h. Then, the cells were harvested, washed and lysed
with 1X radioimmunoprecipitation assay buffer (cat. no. 9806; CST
Biological Reagents Company Limited, Shanghai, China). Protein
concentration was determined using the bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of
protein (40 µg) from cell lysates were solubilized in 5X SDS sample
buffer and were separated by 10% SDS-PAGE, prior to being
transferred onto polyvinylidene fluoride membranes. Membranes were
blocked with 5% non-fat milk in TBS containing 1% Tween-20 and were
then incubated with primary antibodies against STAT3 (cat. no.
8232; CST Biological Reagents Company Limited; dilution, 1:1,000),
p-STAT3 (cat. no. 4093; CST Biological Reagents Company Limited;
dilution, 1:1,000), vimentin (cat. no. 12826; CST Biological
Reagents Company Limited; dilution, 1:1,000), N-cadherin (cat. no.
13116; CST Biological Reagents Company Limited; dilution, 1:1,000),
MMP9 (cat. no. 13667; CST Biological Reagents Company Limited;
dilution, 1:1,000) and GAPDH (cat. no. 5174; CST Biological
Reagents Company Limited; dilution, 1:3,000) at 4°C overnight.
Subsequently, the membranes were washed and incubated with a
secondary antibody against rabbit immunoglobulin G (cat. no.
1706515; Bio-Rad Laboratories, Inc., Hercules, CA, USA; dilution,
1:10,000) for 1 h at room temperature. The membranes were then
washed and visualized using enhanced chemiluminescence solution
(Merck KGaA, Darmstadt, Germany); the blots were exposed and
scanned under the Bio-Rad ChemiDoc XRS+ Chemiluminescence imaging
system (Bio-Rad Laboratories, Inc.). The results were analyzed
using ImageJ software (version 1.48; National Institutes of
Health).
Transient transfection assay
The cells were seeded in 6-well plates
(3×105 cells/well) and were allowed to reach 50–60%
confluence. The pCMV6-AC and pCMV6-AC-STAT3 vectors were obtained
from OriGene Technologies, Inc. In each well, 2 µg pCMV6-AC control
or pCMV6-AC-STAT3 constructs were transfected into the cells using
Lipofectamine 3000 reagent for 30 h at 37°C, according to the
manufacturer's protocol. Subsequently, the cells were treated with
2 mg/ml FZKA for an additional 24 h prior to experimentation.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
statistical software (SPSS, Inc., Chicago, IL, USA). All data are
presented as the mean ± standard deviation. Differences between
groups were assessed by one-way analysis of variance and a Tukey's
post hoc test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Lung cancer cell growth is suppressed
by FZKA
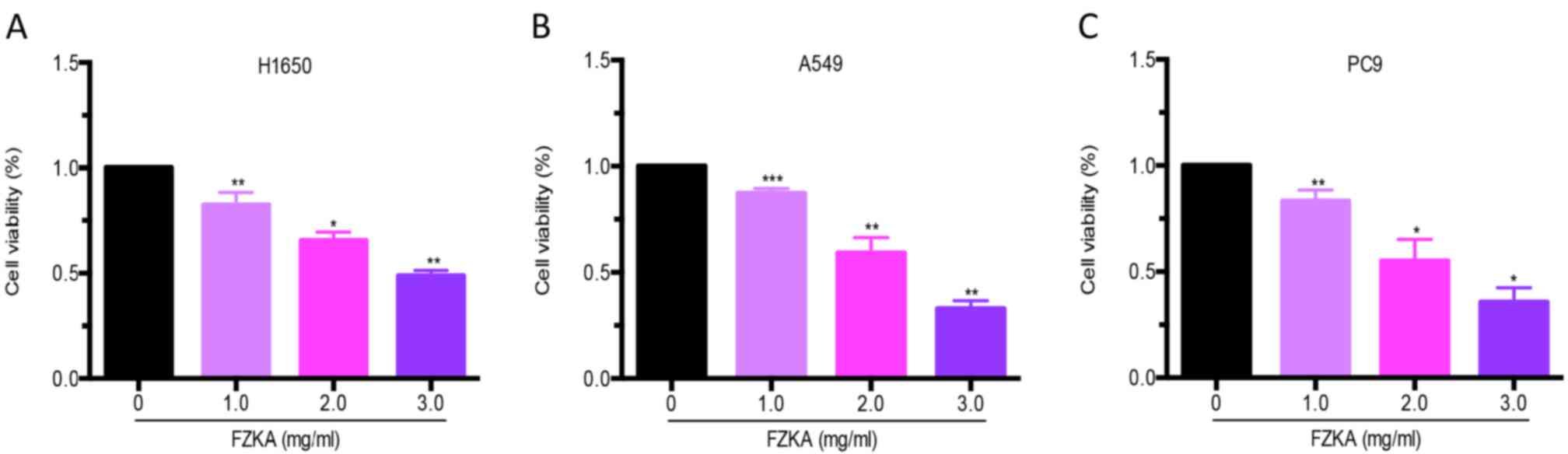
The present study detected the effects of FZKA on
lung cancer cell growth. A cell viability assay was performed using
trypan blue staining following treatment of lung cancer cells
(H1650, A549 and PC9) with various doses of FZKA for 24 h. The
results demonstrated that FZKA significantly suppressed the growth
of lung cancer cells (>50% following 3 mg/ml FZKA treatment) in
a dose-dependent manner (Fig.
1).
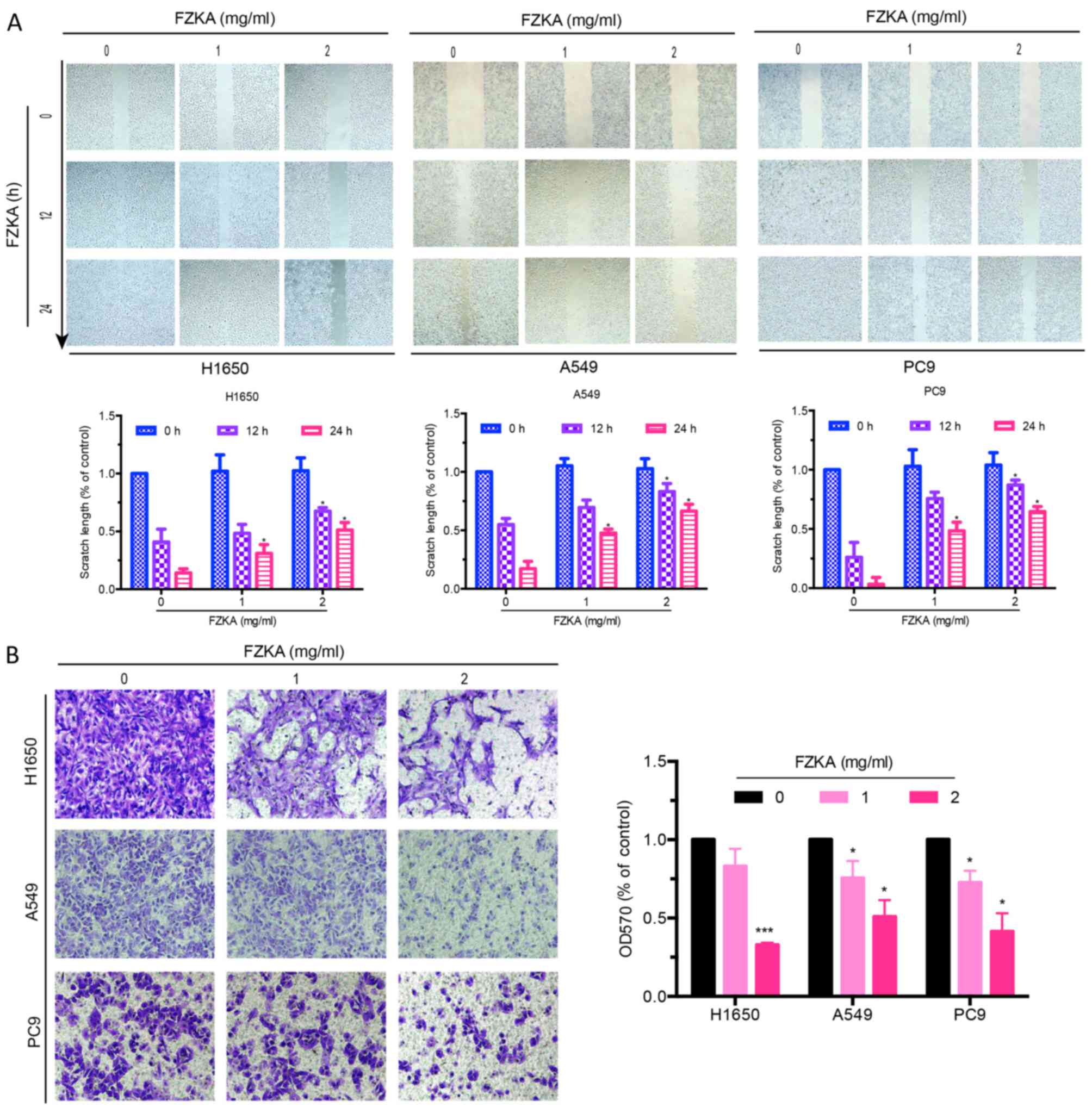
FZKA inhibits migration of lung cancer
cells in vitro
A characteristic of tumor metastasis is the
increased migratory ability of tumor cells. The present study
conducted a wound-healing assay to determine the effects of FZKA on
lung cancer cell migration. As presented in Fig. 2A, the scratch length of all three
lung cancer cells was markedly extended by FZKA treatment in a
dose-dependent manner, indicating the inhibitory effects of FZKA on
lung cancer cell migration. To further verify the inhibitory
effects of FZKA on lung cancer cell migration, a Transwell
migration assay was used. The results confirmed that FZKA inhibited
the migration of lung cancer cells in a dose-dependent manner
(Fig. 2B; ~50% decrease in
migration following treatment with 2 mg/ml FZKA). These results
suggested that FZKA decoction inhibited lung cancer cell
migration.
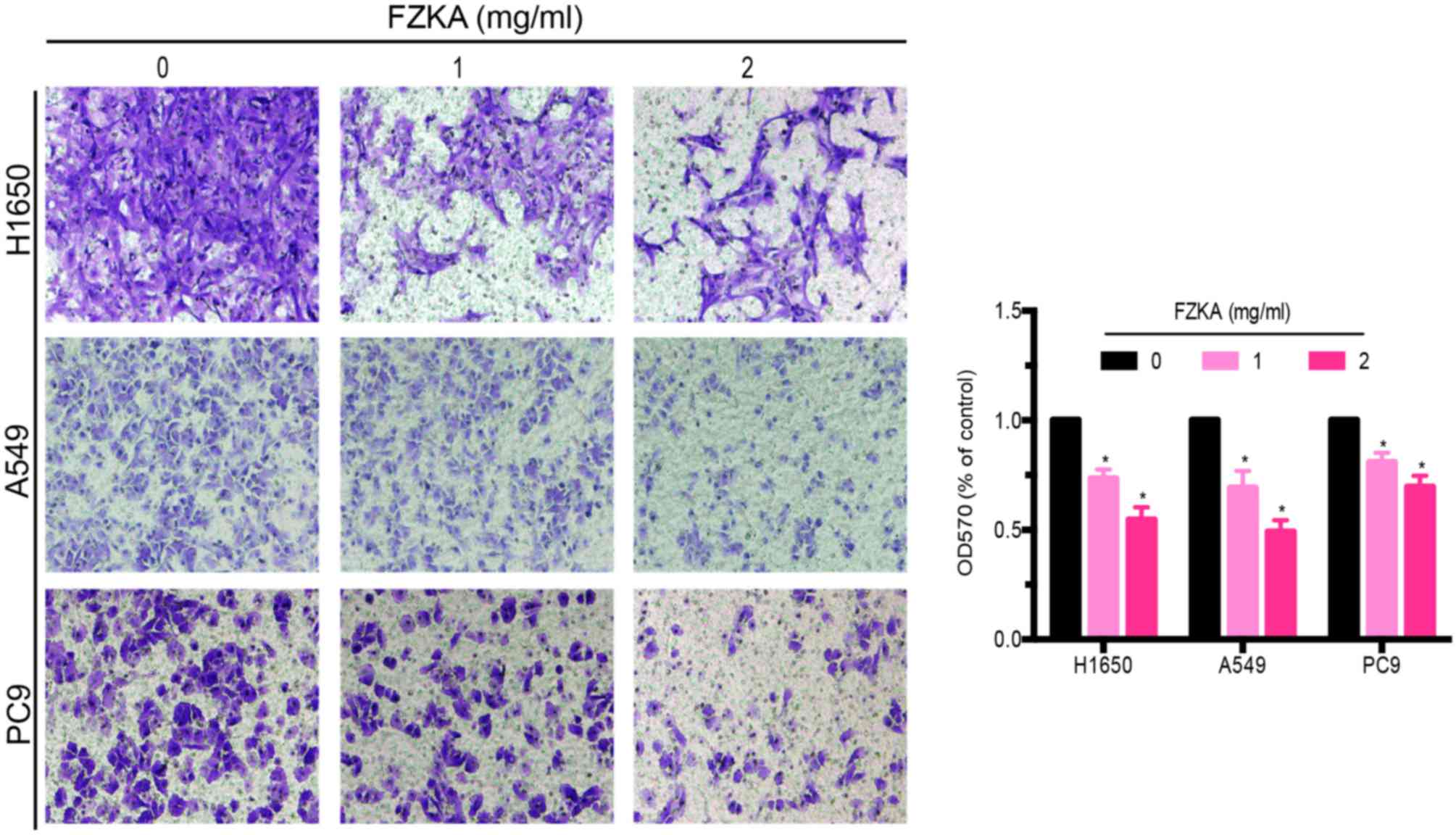
FZKA inhibits lung cancer cell
invasion in vitro
Since treatment with FZKA was able to reduce the
migratory capabilities of lung cancer cells, the present study
aimed to determine the effects of FZKA on lung cancer cell invasion
using a Transwell invasion assay. The results demonstrated that
FZKA was also able to inhibit the invasion of the three lung cancer
cell lines in a dose-dependent manner (Fig. 3; ~60% decrease in invasion
following treatment with 2 mg/ml FZKA). These findings indicated
that FZKA may act as a suppressor of lung cancer cell invasion.
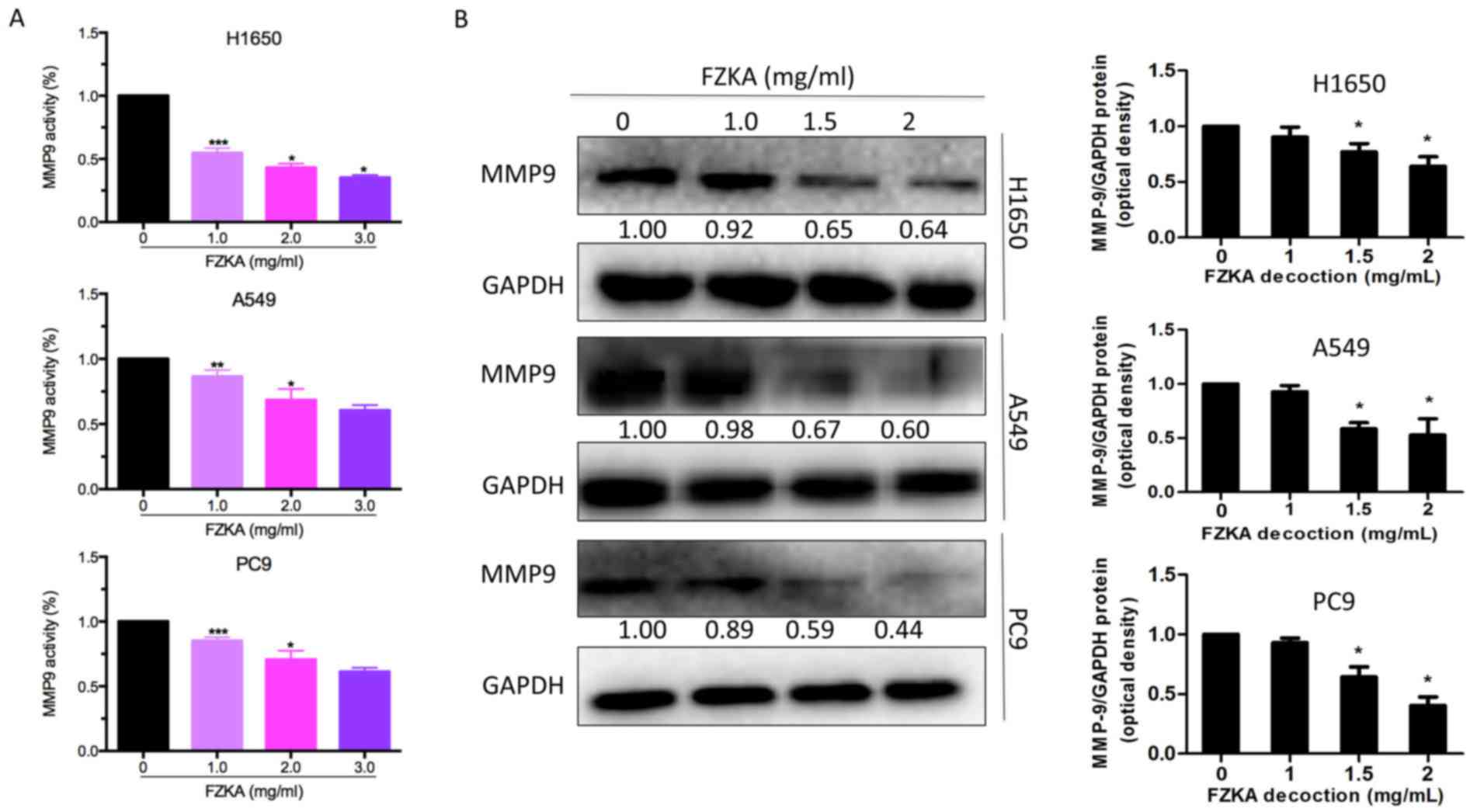
MMP9 activity and expression is
downregulated by FZKA
MMP9 is a well-known factor that facilitates cell
invasion; therefore, the present study detected MMP9 activity
following FZKA treatment using an MMP9 activity assay. The results
indicated that MMP9 activity was markedly downregulated by FZKA in
a dose-dependent manner in all three lung cancer cell lines
(Fig. 4A). Furthermore, the
protein expression levels of MMP9 were decreased in the lung cancer
cells following FZKA treatment in a dose-dependent manner (Fig. 4B). These data suggested that MMP9
served an important role in FZKA-mediated inhibition of lung cancer
cell invasion.
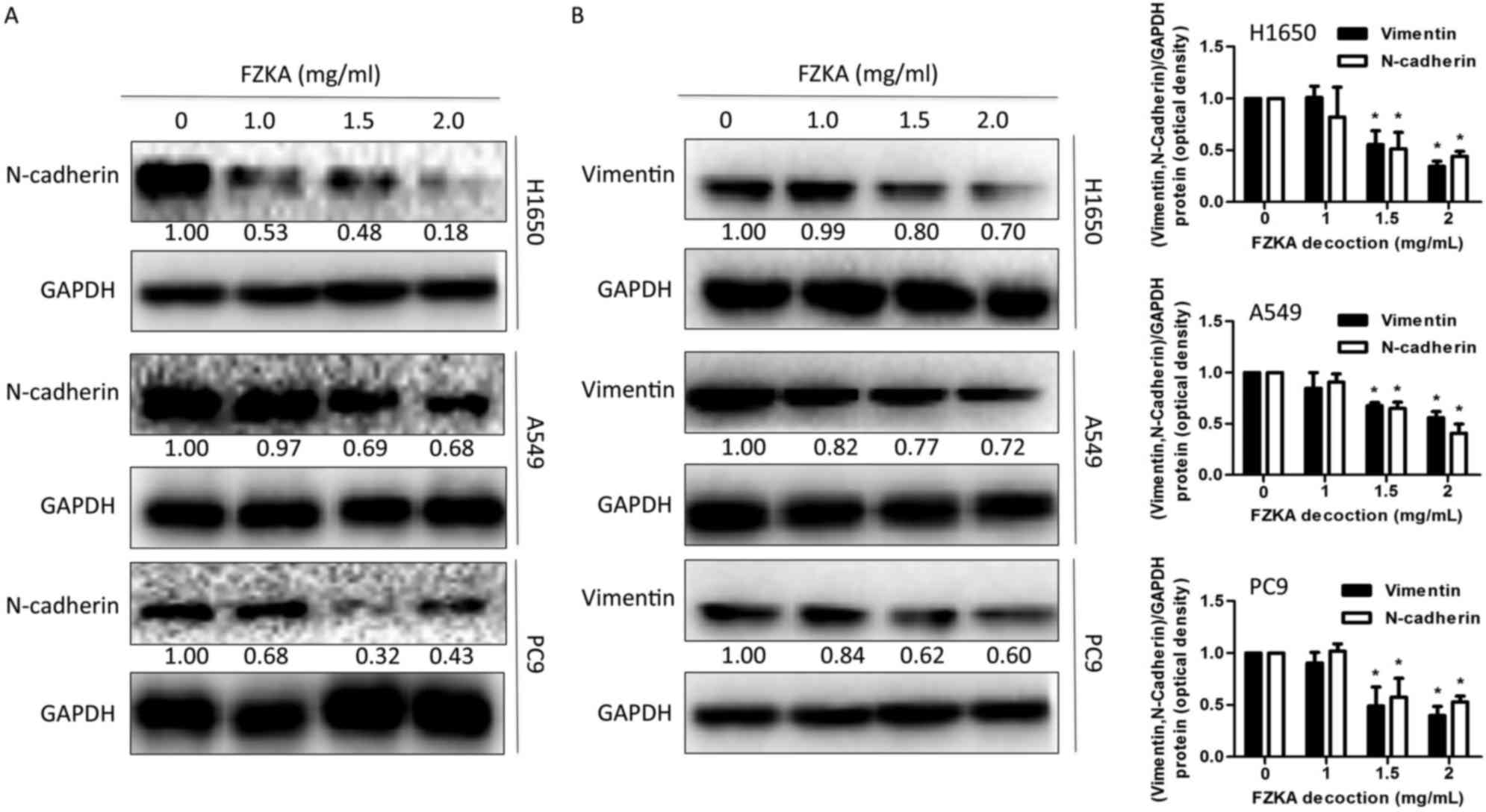
EMT is involved in FZKA-induced
inhibition of lung cancer cell metastasis
Previous studies have reported that metastasis and
invasion are associated with EMT (21,22).
To determine if EMT also mediates the effects of FZKA on lung
cancer cells, the present study detected the expression levels of
proteins involved in the EMT process following FZKA treatment. The
results indicated that the mesenchymal marker N-cadherin and the
intermediate filament protein vimentin, which are associated with
increased cell motility (23),
were downregulated by FZKA treatment in a dose-dependent manner
(Fig. 5A and B). These data
provided another potential mechanism by which FZKA affected lung
cancer metastasis.
STAT3 may mediate the effects of FZKA
on lung cancer cell metastasis
STAT3 is an oncogenic transcription factor, which
leads to cell proliferation and invasion. Its activation can induce
tumor cell growth, invasion and mesenchymal transition (24). In addition, MMP9 has been reported
to be a target of STAT3 (25). In
the present study, STAT3 activation was inhibited in a
time-dependent manner in lung cancer cells following treatment with
FZKA (Fig. 6A). Furthermore,
overexpression of STAT3 was able to suppress the FZKA-mediated
inhibition of MMP9 activity (Fig. 6B
and C), indicating that STAT3 may be an upstream factor of
MMP9, which is affected by FZKA treatment in lung cancer cells.
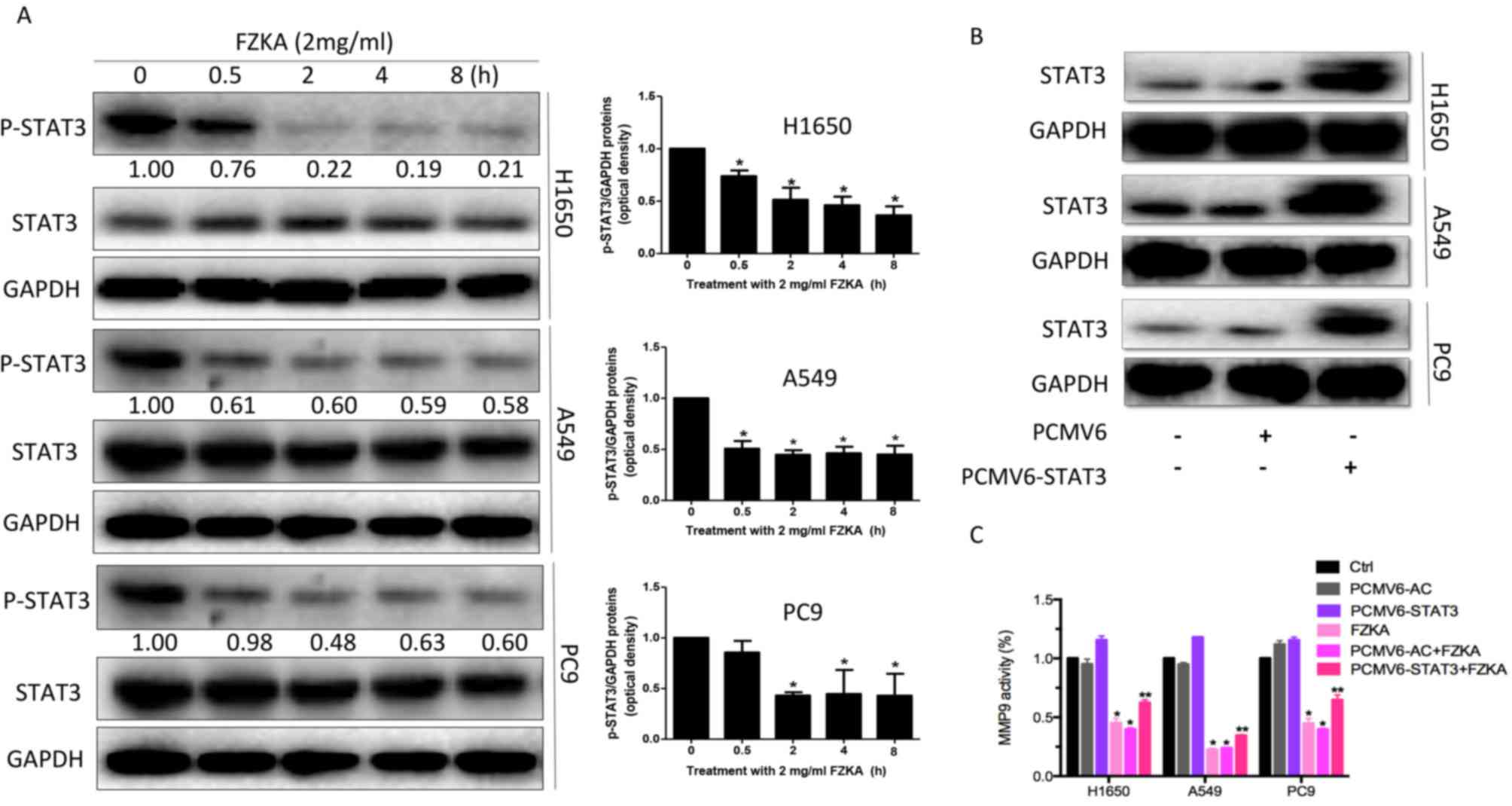 | Figure 6.STAT3 regulates MMP9 activity in lung
cancer cells treated with FZKA. (A) Protein expression levels of
STAT3 were reduced following treatment with FZKA (2 mg/ml; 0, 0.5,
2, 4 and 8 h). *P<0.05 vs. 0 h. (B) To overexpress STAT3, cells
(H1650, A549 and PC9) were seeded into 6-well plates, and
transfected with pCMV6-AC (negative control) and pCMV6-AC-STAT3 DNA
constructs, prior to treatment with FZKA. STAT3 protein expression
was then measured by western blot analysis. (C) MMP9 activity was
increased by STAT3 overexpression. Following treatment with FZKA,
the FZKA-mediated inhibition of MMP9 activity was significantly
suppressed by STAT3 overexpression. *P<0.05 and **P<0.01 vs.
control (Ctrl). FZKA, Fuzheng Kang-Ai; MMP9, matrix
metalloproteinase 9; STAT5, signal transducer and activator of
signaling 3; Ctrl, control. |
Discussion
Although advances have been made in the treatment of
human cancers, cancer remains a leading cause of human mortality
each year. More effective therapies are therefore required for the
treatment of patients with cancer. Traditional Chinese medicine
popular in Chinese and East Asian societies and serves an active
role in modern healthcare systems, including in the treatment of
patients with cancer, and therefore may be considered a potential
effective strategy for the treatment of human cancers.
The present study was based on our previous clinical
and fundamental findings, which indicated that FZKA could sensitize
the effects of gefitinib on patients with late stage lung cancer,
and prolong the PFS and MST in patients with NSCLC (17–20).
In addition, FZKA was reported to inhibit lung cancer cell growth
in vivo and in vitro (17–20).
The present study aimed to determine the role and mechanisms of
FZKA decoction on the process of lung cancer cell metastasis.
Initially, the inhibitory role of FZKA in lung cancer cell growth
was identified. In addition, the results demonstrated that FZKA
significantly inhibited lung cancer cell migration and invasion, as
determined by wound-healing and Transwell assays. While the three
lung cancer cell lines used in the present study responded to
different extents to FZKA decoction, the overall effects of FZKA
were consistent in all of the cell lines suggesting that the FZKA
decoction had substantial inhibitory effects on human lung cancer
cells.
MMP9, which is closely associated with the invasive
and metastatic potential of numerous types of solid cancer
(26), is critical during the
process of FZKA-induced inhibition of lung cancer cell invasion.
The present study demonstrated that MMP9 secretion was inhibited by
FZKA treatment, as determined using an MMP9 activity assay. MMPs
are able to degrade various components of the ECM and basement
membrane (27). Once MMP9 is
activated, it is able to degrade collagen in the ECM, which
increases the metastasis of cancer cells (28). Therefore, the FZKA-induced
inhibition of MM9 activity and expression may be considered an
important mechanism by which FZKA inhibits lung cancer cell
metastasis. Furthermore, STAT3 activation was inhibited by FZKA
treatment. Notably, in cells overexpressing STAT3, as mediated by
transient transfection, the FZKA-mediated inhibition of MMP9
activity was suppressed to some extent. Aberrant activation of
STAT3 contributes to cancer progression in human malignances
(29). Therefore, inhibition of
STAT3 activation by FZKA may hinder tumor progression. Furthermore,
the STAT3/MMP9 pathway has been demonstrated to participate in
colon cancer cell invasion (30).
The present study obtained similar results suggesting that the
STAT3/MMP9 pathway may mediate the inhibitory effects of FZKA
decoction on lung cancer cell metastasis.
The present study detected the expression levels of
N-cadherin and vimentin, two important proteins involved in the EMT
process, both of which were downregulated by FZKA treatment. EMT is
characterized by the loss of epithelial characteristics and the
acquisition of mesenchymal characteristics. The induction of
mesenchymal markers, including N-cadherin and vimentin, are
hallmark early- and late-stage events of EMT, respectively
(31). The present study
demonstrated that FZKA inhibited EMT, as indicated by a decrease in
the protein expression levels of N-cadherin and vimentin. EMT is a
well-known molecular mechanism associated with cancer metastasis
(32). Therefore, FZKA-induced
inhibition of EMT in lung cancer cells may be a potential mechanism
underlying the effects of FZKA treatment on patients with lung
cancer. Since EMT can also be induced by MMPs (33), the mesenchymal markers may be
downstream proteins of the STAT3/MMP9 pathway. However, further
study is required to verify this.
In conclusion, the present study identified a
probable mechanism by which FZKA decoction inhibits lung cancer
cell metastasis via the STAT3/MMP9 pathway, thus indicating that
FZKA decoction may be considered as a potential effective strategy
for the treatment of patients with lung cancer. Briefly, in the
present study, FZKA decoction inhibited lung cancer cell migration
and invasion in vitro. In addition, the results demonstrated
that the STAT3/MMP9 pathway and EMT may mediate the inhibitory
effects of FZKA on lung cancer metastasis. These findings provide
valid experimental evidence for the clinical usage of FZKA
decoction in treating patients with late stage lung cancer.
Acknowledgements
This work was supported by a grant from the National
Natural Science Foundation of China (grant nos. 81273965 and
81272614), the Canadian Terry Fox Run Foundation for Cancer
Research (grant no. YN2014TF01), the Science and Technology
Planning Project of Guangdong Province (grant nos. 2016A020226035
and 2014A020221024), and the Administration of Traditional Chinese
Medicine of Guangdong Province in China (grant no. 20141104).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
4
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L, Kunkler I, et al: EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000–02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas G: Furin at the cutting edge: From
protein traffic to embryogenesis and disease. Nat Rev Mol Cell
Biol. 3:753–766. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rhee JS and Coussens LM: RECKing MMP
function: Implications for cancer development. Trends Cell Biol.
12:209–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang R, Ke ZF, Wang F, Zhang WH, Wang YF,
Li SH and Wang LT: GOLPH3 overexpression is closely correlated with
poor prognosis in human non-small cell lung cancer and mediates its
metastasis through upregulating MMP-2 and MMP-9. Cell Physiol
Biochem. 35:969–982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmad R, Shihab PK, Jasem S and Behbehani
K: FSL-1 induces MMP-9 production through TLR-2 and NF-κB/AP-1
signaling pathways in monocytic THP-1 cells. Cell Physiol Biochem.
34:929–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang CQ, Li W, Li SQ, Li J, Li YW, Kong
SX, Liu RM, Wang SM and Lv WM: MCP-1 stimulates MMP-9 expression
via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth
muscle cells. Cell Physiol Biochem. 34:266–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng X, Yang Y, Fan Z, Yu L, Bai H, Zhou
B, Wu X, Xu H, Fang M, Shen A, et al: MKL1 potentiates lung cancer
cell migration and invasion by epigenetically activating MMP9
transcription. Oncogene. 34:5570–5581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sreekumar R, Sayan BS, Mirnezami AH and
Sayan AE: MicroRNA control of invasion and metastasis pathways.
Front Genet. 2:582011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia H and Hui KM: MicroRNAs involved in
regulating epithelial-mesenchymal transition and cancer stem cells
as molecular targets for cancer therapeutics. Cancer Gene Ther.
19:723–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of Stat3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F, Zhang T, Zou S, Jiang B and Hua D:
B7-H3 promotes cell migration and invasion through the
Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med
Rep. 12:5455–5460. 2015.PubMed/NCBI
|
|
17
|
Yang XB, Wu WY, Long SQ, Deng H, Pan ZQ,
He WF, Zhou YS, Liao GY, Li QP, Xiao SJ and Cai JZ: Fuzheng Kang'ai
decoction combined with gefitinib in advanced non-small cell lung
cancer patients with epidermal growth factor receptor mutations:
Study protocol for a randomized controlled trial. Trials.
16:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu WY, Yang XB, Deng H, Long SQ, Sun LS,
He WF, Zhou YS, Liao GY, Chan SM and Shan SP: Treatment of advanced
non-small cell lung cancer with extracorporeal high frequency
thermotherapy combined with Chinese medicine. Chin J Integr Med.
16:406–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang XB, Wu WY, Long SQ, Deng H and Pan
ZQ: Effect of gefitinib plus Chinese herbal medicine (CHM) in
patients with advanced non-small-cell lung cancer: A retrospective
case-control study. Complement Ther Med. 22:1010–1018. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng F, Wu J, Li X, Tang Q, Yang L, Yang
X, Wu W and Hann SS: Chinese Herbal Medicine Fuzheng Kang-Ai
Decoction inhibited lung cancer cell growth through AMPKα-mediated
induction and interplay of IGFBP1 and FOXO3a. Evid Based Complement
Alternat Med. 2016:50607572016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan L, Pantel K and Kang Y: Tumor
metastasis: Moving new biological insights into the clinic. Nat
Med. 19:1450–1464. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development and disease. J Cell Biol. 172:973–981. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lui VW, Wong EY, Ho Y, Hong B, Wong SC,
Tao Q, Choi GC, Au TC, Ho K, Yau DM, et al: STAT3 activation
contributes directly to Epstein-Barr virus-mediated invasiveness of
nasopharyngeal cancer cells in vitro. Int J Cancer. 125:1884–1893.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo K, Ma Q, Li J, Wang Z, Shan T, Li W,
Xu Q and Xie K: Interaction of the sympathetic nerve with
pancreatic cancer cells promotes perineural invasion through the
activation of STAT3 signaling. Mol Cancer Ther. 12:264–273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Badrawy MK, Yousef AM, Shaalan D and
Elsamanoudy AZ: Matrix metalloproteinase-9 expression in lung
cancer patients and its relation to serum mmp-9 activity,
pathologic type, and prognosis. J Bronchology Interv Pulmonol.
21:327–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vilen ST, Salo T, Sorsa T and Nyberg P:
Fluctuating roles of matrix metalloproteinase-9 in oral squamous
cell carcinoma. ScientificWorldJournal. 2013:9205952013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Backstrom JR and Tökés ZA: The 84-kDa form
of human matrix metalloproteinase-9 degrades substance P and
gelatin. J Neurochem. 64:1312–1318. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ao N and Liu Y, Bian X, Feng H and Liu Y:
Ubiquitin-specific peptidase 22 inhibits colon cancer cell invasion
by suppressing the signal transducer and activator of transcription
3/matrix metalloproteinase 9 pathway. Mol Med Rep. 12:2107–2113.
2015.PubMed/NCBI
|
|
31
|
Shirakihara T, Saitoh M and Miyazono K:
Differential regulation of epithelial and mesenchymal markers by
deltaEF1 proteins in epithelial mesenchymal transition induced by
TGF-beta. Mol Biol Cell. 18:3533–3544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orlichenko LS and Radisky DC: Matrix
metalloproteinases stimulate epithelial-mesenchymal transition
during tumor development. Clin Exp Metastasis. 25:593–600. 2008.
View Article : Google Scholar : PubMed/NCBI
|