Introduction
Gastric cancer (GC) is one of the most common
malignant tumors worldwide and is globally the second leading cause
of cancer-associated mortality, being particularly prevalent in
China (1–3). Despite a steadily declining incidence
of GC, it was still estimated that ~498,000 Chinese individuals
would succumb to GC in 2015 (3). A
detailed molecular understanding of GC pathogenesis is required, in
order to improve the prognosis of patients with this complex
disease (4). In addition, an
improved understanding of GC pathogenesis would be advantageous for
translating molecular findings into clinical use, helping to
identify novel biomarkers and treatment targets, and developing
personalized therapies for individual patients with GC in the
future (4).
Noncoding RNAs (ncRNAs) are defined as RNAs that do
not encode proteins, these include ribosomal RNAs, transfer RNAs
and small nuclear RNAs, as well as the more recently discovered
microRNAs (miRNAs), long noncoding RNAs (lncRNAs) and circular RNAs
(circRNAs), which have critical regulatory roles in cancer biology
(5–7). CircRNAs represent a specific type of
ncRNA that is present in the cytoplasm of eukaryotic cells and is
predominantly produced by pre-mRNAs through variable shearing
processes (8–11).
CircRNAs are a novel type of RNA that, unlike linear
RNA, forms a covalently closed continuous loop, and in some cases
is highly represented in the eukaryotic transcriptome (8–11).
The majority of circRNAs are composed of exonic sequences, which
are conserved in various species, and often exhibit
tissue/developmental-stage-specific expression (8–11).
Since circRNAs are not sensitive to digestion by RNases, they are
more conserved and stable than linear RNA (10,11),
which highlights clear advantages in using circRNAs as novel
diagnostic markers (10,11). In addition, recent studies have
indicated that circRNAs may act as competitive endogenous RNAs
(ceRNAs) to sequester miRNAs of a particular family, thereby
serving as competitive inhibitors that suppress the ability of a
miRNA to bind its mRNA targets (12,13).
Therefore, it has been hypothesized that circRNAs potentially
regulate disease progression by sequestering a miRNA associated
with a particular disease (12,13).
Previous studies have demonstrated that circRNAs
serve an important role in the regulation of various cancer
pathways (8–11). Ghosal et al (14) performed a Gene Ontology (GO)
enrichment analysis on a set of protein-coding genes in the
miRNA-circRNA interactome for individual diseases, in order to
study the enrichment of genes associated with particular biological
processes. The results demonstrated that 194 and 68 genes involved
in various biological processes were associated with cervical
cancer and GC, respectively. Li et al (15) discovered that hsa_circ_002059 was
significantly downregulated in GC and was potentially involved in
GC development. However, no direct biological evidence has
indicated that circRNAs are associated with GC.
To explore the underlying molecular regulation of
circRNAs in GC, the present study examined circRNA expression using
a microarray analysis, in order to acquire circRNA profiles in GC
and adjacent normal tissues. Subsequently, a quantitative
polymerase chain reaction (qPCR) analysis was conducted to confirm
the results. In addition, a bioinformatics analysis was performed
to predict the biological functions of the differentially expressed
circRNAs in GC.
Materials and methods
Patient specimens
Three sets of primary GC tissue samples (the GC
group) and paired, adjacent noncancerous tissues (the control
group), which were ≥5 cm away from cancerous tissue, were obtained
from patients who had undergone curative surgical resection at the
Affiliated Hospital of Hainan Medical University (Haikou, China)
from June 2014 to July 2014. Histological diagnoses were made from
formalin-fixed, paraffin-embedded tissues by two pathologists. The
clinicopathological characteristics were obtained from medical
records, including gender, age, tumor size, tumor location,
histological type, Lauren classification, differentiation grade and
surgical record. None of the patients received neoadjuvant therapy.
Written informed consent was obtained from the patients for the use
of their samples for research, and the research protocols were
approved by the Ethics Committee of the Affiliated Hospital of
Hainan Medical University.
RNA isolation
A total of 100 mg tissue was taken from each GC and
paired normal mucosal tissues, and were homogenized using a
TissueLyser II BioRobot Universal system (Qiagen GmbH, Hilden,
Germany). Total RNA was isolated using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA was purified
using the RNeasy Mini kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Subsequently, RNA
quality and quantity were measured using a NanoDrop
spectrophotometer (ND-1000; NanoDrop; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA), and RNA integrity was determined by gel
electrophoresis.
CircRNA microarray
The Arraystar Human Circular RNA Microarray V2.0
(Arraystar, Inc., Rockville, MD, USA) is designed for global
profiling of human circRNAs. This microarray is comprised of
~13,617 circRNAs with stringent experimental support, which was
carefully and comprehensively collected from circRNA studies and
landmark publications (10,11).
Each circRNA is represented by a circular splice junction probe,
which can reliably and accurately detect the circRNA, even in the
presence of its linear counterparts. A random primer-based labeling
system is coupled with RNase R sample pretreatment to ensure
specific and efficient labeling of circRNAs. RNA spike-in controls
were included to monitor labeling and hybridization efficiencies.
Microarray hybridization and bioinformatic analysis were performed
by KangChen Bio-tech, Inc. (Shanghai, China).
Labeling and hybridization
RNA from three pairs of selected samples was
subjected to microarray analysis according to the manufacturers
protocol (Arraystar, Inc.). Briefly, total RNA was digested with
RNase R (Epicentre; Illumina, Inc., Madison, WI, USA) to remove
linear RNAs and enrich for circRNAs. Subsequently, the enriched
circRNAs were amplified and transcribed into fluorescent cRNA using
a random priming method (Arraystar Super RNA Labeling kit;
Arraystar, Inc.). The Cy3-labeled cRNAs were purified using the
RNeasy Mini kit (Qiagen GmbH). Each labeled cRNA (1 µg) was
fragmented by adding 5 µl 10X blocking agent and 1 µl 25X
fragmentation buffer, after which the mixture was incubated at 60°C
for 30 min and was then diluted in 25 µl 2X hybridization buffer. A
total of 50 µl hybridization solution was dispensed into the gasket
slide, which was assembled onto the circRNA expression microarray
slide. The slides were incubated for 17 h at 65°C in a
hybridization oven (Agilent Technologies, Inc., Santa Clara, CA,
USA). The hybridized arrays were washed, fixed and scanned using an
Agilent Microarray Scanner system (catalog no. G2505C; Agilent
Technologies, Inc.).
Data analysis
Scanned images were imported into Agilent Feature
Extraction software version 11.0.1.1 (Agilent Technologies, Inc.)
for raw data extraction. Quantile normalization of raw data and
subsequent data processing were performed using the R software
package (www.arraystar.com/arraystar-human-circular-rna-microarray).
Following quantile normalization of the raw data, low-intensity
filtering was performed and circRNAs having the ‘P’ or ‘M’ flags
(‘All Targets Value’) in ≥3 out of 6 samples were retained for
further analyses. Subsequently, samples were clustered
hierarchically with Cluster software version 2.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm)
to evaluate the robustness of the formed clusters, using the
correlation-centered metric and average linkage-clustering
algorithm. When comparing profile differences between the GC and
control groups, the fold-change between the groups for each circRNA
was computed. The statistical significance of each difference was
estimated by Student's t-test. CircRNAs exhibiting a ≥2-fold change
in expression (P<0.05) were considered to be significantly
differentially expressed.
Biomathematical analyses
The present study initially identified circRNAs that
exhibited a >2-fold change in expression by microarray analysis.
Subsequently, circRNA/miRNA interactions were predicted using
Arraystar in-house generated miRNA target-prediction software
(Arraystar, Inc.), based on TargetScan (16) and miRanda (17). Differentially expressed circRNAs
were used as seeds to enrich for a circRNA-miRNA-gene network,
based on a cutoff value determined using miRNA support-vector
regression (mirSVR), as described by Wang et al (18). The predicted gene functions in the
networks were annotated using GO (http://www.geneontology.org/) and the Database for
Annotation, Visualization and Integrated Discovery (DAVID;
https://david.ncifcrf.gov/).
Validation by reverse transcription
(RT)-qPCR
Total RNA from three GC specimens and matched,
adjacent normal tissues were reverse transcribed to cDNA using the
FastQuant RT kit with gDNase (Tiangen Biotech Co., Ltd., Beijing,
China) in 20-µl reactions. Triplicate qPCR assays were performed in
20-µl reactions using the FastFire qPCR PreMix (SYBR-Green) kit
(Tiangen Biotech Co., Ltd.) according to the manufacturer's
protocol. The thermal cycling conditions were as follows: Initial
denaturation at 95°C for 60 sec, 40 cycles of amplification at 95°C
for 20 sec, annealing and extension at 60°C for 30 sec. GAPDH was
used as an internal control for PCR amplification. The sequences of
circRNAs were obtained from the circBase database (http://circbase.org/), and PCR primers were designed
in divergent orientation, in order to be capable of amplifying the
reverse splice site of the circRNA, using Primer Premier software
version 6.0 (Premier Biosoft International, Palo Alto, CA, USA) and
were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China)
(Table I). The data were analyzed
using the 2−ΔΔCq method (19). Validation was performed by
determining the ratio of differential cellular
expression/differential microarray expression, and amplification
products were analyzed by 1.5% agarose gel electrophoresis and
stained with 0.1% GeneGreen Nucleic Acid gel stain (Tiangen Biotech
Co., Ltd.) for band size consistency. The qPCR data were analyzed
by Student's t-test using SPSS software version 18.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
 | Table I.Quantitative polymerase chain
reaction primers and product sizes. |
Table I.
Quantitative polymerase chain
reaction primers and product sizes.
| Amplicon | Primers | Accession no. | Genomic
location | Gene symbol | Tm (°C) | Length (bp) |
|---|
| GAPDH(human) | F:
5′GGGAAACTGTGGCGTGAT3′ | NM_001289746 | Chr 12 | GAPDH | 60 | 299 |
|
| R:
5′GAGTGGGTGTCGCTGTTGA3′ |
|
|
|
|
|
|
hsa_circ_0000144 | F:
5′GAAGGTAGGACAAATGGAAGTT3′ | NM_052931 | Chr 1 | SLAMF6 | 60 | 136 |
|
| R:
5′GAATCTGCTTAGTTCTACCTCTC3′ |
|
|
|
|
|
|
hsa_circ_0023642 | F:
5′ATGACAAACTGACGGAAAAGGAG3′ | NM_003369 | Chr 11 | UVRAG | 60 | 64 |
|
| R:
5′AACCAAGGGCAACAGCAATG3′ |
|
|
|
|
|
|
hsa_circ_0032821 | F:
5′AGATAGAAAGGCAGGAGCAG3′ | NM_152446 | Chr 14 | CEP128 | 60 | 146 |
|
| R:
5′TGTTCAGTCTCCAAGCAAAG3′ |
|
|
|
|
|
|
hsa_circ_0005529 | F:
5′AGTCCCTGCGCCTCATCTTG3′ | NM_018668 | Chr 15 | VPS33B | 60 | 101 |
|
| R:
5′CGCCGCTCTAGCACCTTTCT3′ |
|
|
|
|
|
|
hsa_circ_0061274 | F:
5′CAGCCTTCTCAATTTTCTTTC3′ | NM_003489 | Chr 21 | NRIP1 | 60 | 96 |
|
| R:
5′AGTCTTCAGATTCCCTGTCCT3′ |
|
|
|
|
|
|
hsa_circ_0000026 | F:
5′CCATCCCCTTATTCAGCACAT3′ | NM_001198803 | Chr 1 | EIF4G3 | 60 | 132 |
|
| R:
5′TCCAAACTTCAGTTTCCTCATCA3′ |
|
|
|
|
|
|
hsa_circ_0040039 | F:
5′CAGGATACTTGTTCAGGGTTGC3′ | NM_006750 | Chr 16 | SNTB2 | 60 | 201 |
|
| R:
5′TTGGTGCTGTTCTGGTGTTTT3′ |
|
|
|
|
|
|
hsa_circ_0041732 | F:
5′GCTCACATGCCCACCCATTA3′ | NM_019013 | Chr 17 | FAM64A | 60 | 127 |
|
| R:
5′CAGCCACTTGGTGCCACTTT3′ |
|
|
|
|
|
|
hsa_circ_0068610 | F:
5′GACAATGCTGCTTTCCCTTTC3′ | NM_003234 | Chr 3 | TFRC | 60 | 154 |
|
| R:
5′CCAGTAACCGGATGCTTCACA3′ |
|
|
|
|
|
|
hsa_circ_0005927 | F:
5′TGAATTTGGAGGTTCTATCTACCAG3′ | NM_001135694 | Chr 8 | VDAC3 | 60 | 162 |
|
| R:
5′CCTTCAATTTCCCACTCTTCTTT3′ |
|
|
|
|
|
Results
circRNA microarray analysis
The present study investigated the alterations in
circRNA expression profiles between the GC group and the control
group. To obtain consistent biological information, paired samples
from 3 patients with similar clinical data were selected for
microarray analysis. All GC cases were of the diffuse type (Lauren
classification), male, stage IIIA (TNM staging system), and aged
55–58 (average age, 56.7 years). Total RNA was subsequently
extracted, and circRNA expression in the groups was analyzed using
an Arraystar microarray.
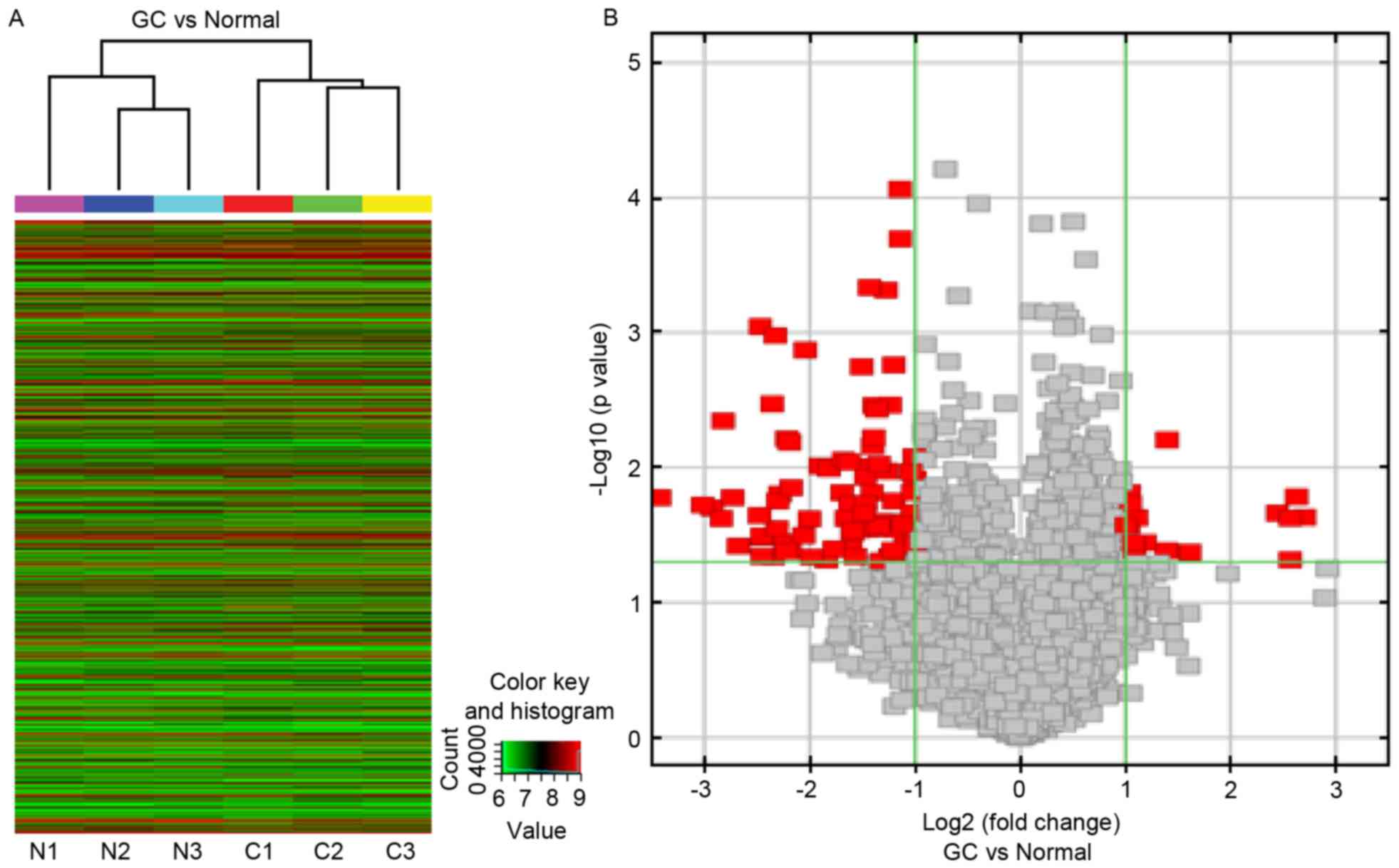
To determine whether circRNA profiles were
informative with regards to tissue type, an unsupervised,
hierarchical cluster analysis was conducted based on the circRNA
expression levels in 3 GC specimens and adjacent normal tissues.
The results of hierarchical clustering showed distinguishable
circRNA expression profiling among 6 samples. Two main clusters
were formed, the GC group cluster and the control group cluster
(Fig. 1A). These data indicated
that circRNAs have a different expression pattern in GC compared
with in normal gastric mucosa.
The circRNA chip detected >2,000 circRNAs
expressed in GC tissues and adjacent normal tissues, and 100
circRNAs (86 exonic circRNAs, 12 intronic circRNAs and 2 antisense
circRNAs) that were ≥2-fold differentially expressed between the GC
and the matching, adjacent normal tissues (Fig. 1B), which were distributed across
all 22 autosomes. A total of 16 circRNAs were significantly
upregulated and 84 were significantly downregulated in the GC
group, compared with the control group. The circRNAs were ranked
according to fold-changes in expression between the groups. The top
5 upregulated and downregulated circRNAs are presented in Table II.
 | Table II.Biological information regarding the
top 5 upregulated and downregulated circRNAs. |
Table II.
Biological information regarding the
top 5 upregulated and downregulated circRNAs.
| Aliasa | Fold change | P-value | circRNA type | Chr | Best
transcript | Gene symbol |
|---|
| Upregulated |
|
hsa_circ_0023642 | 6.450 | 0.023 | Exonic | chr11 | uc009yuh.1 | UVRAG |
|
hsa_circ_0000144 | 6.16 | 0.016 | Antisense | chr1 | NM_001184714 | SLAMF6 |
|
hsa_circ_0061274 | 5.92 | 0.023 | Exonic | chr21 | uc002yjx.2 | NRIP1 |
|
hsa_circ_0032821 | 5.91 | 0.048 | Exonic | chr14 | uc001xux.2 | CEP128 |
|
hsa_circ_0005529 | 5.43 | 0.022 | Exonic | chr15 | uc002bqp.1 | VPS33B |
| Downregulated |
|
hsa_circ_0040039 | 10.78 | 0.017 | Exonic | chr16 | uc002ewu.3 | SNTB2 |
|
hsa_circ_0000026 | 7.62 | 0.020 | Exonic | chr1 | uc001bec.3 | EIF4G3 |
|
hsa_circ_0041732 | 7.16 | 0.024 | Exonic | chr17 | uc002gcu.2 | FAM64A |
|
hsa_circ_0005927 | 7.08 | 0.004 | Exonic | chr8 | uc022aul.1 | VDAC3 |
|
hsa_circ_0092341 | 6.66 | 0.017 | Intronic | chr6 | NM_001164446 | C6orf132 |
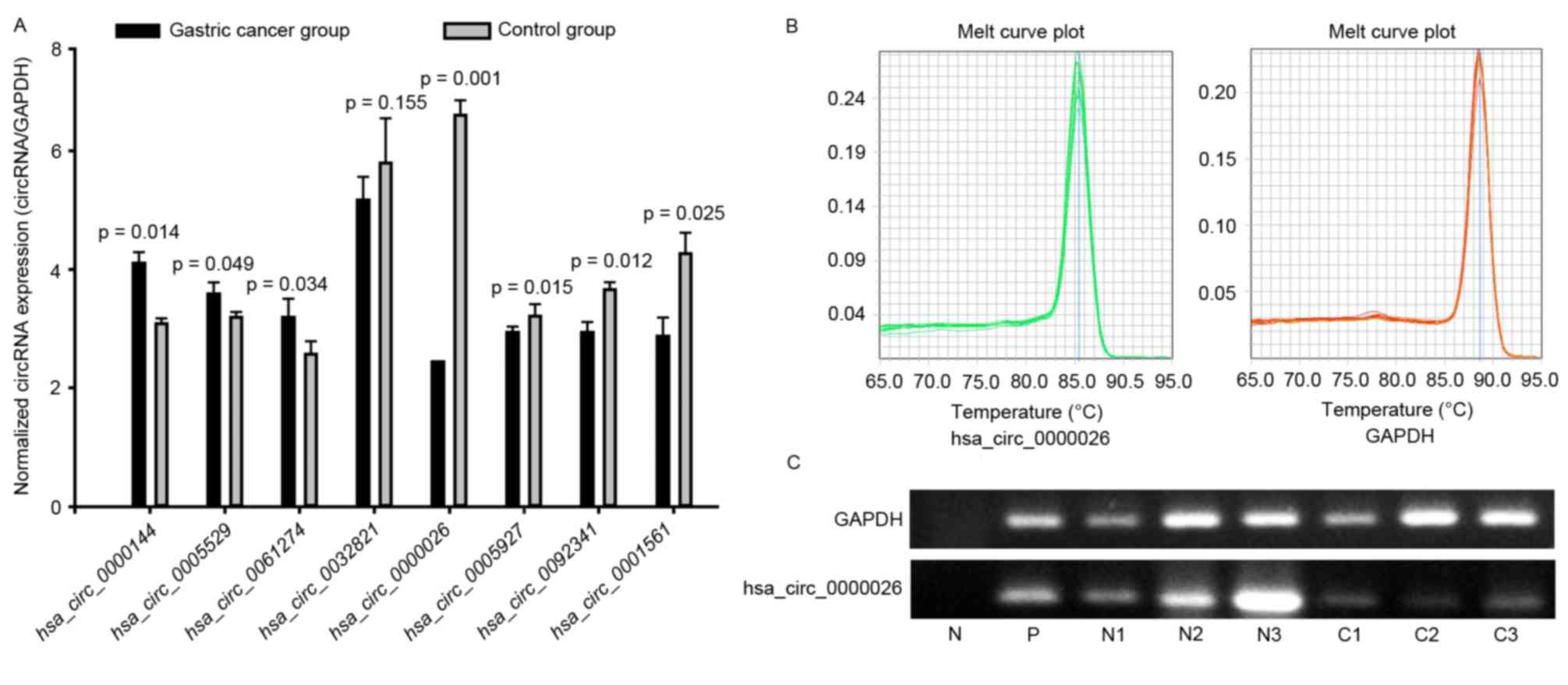
Microarray validation by RT-qPCR
To validate the expression profiles of circRNAs in
three matched pairs of GC and normal specimens, the top 5 down- and
upregulated circRNAs were further studied by qPCR (Fig. 2). The primers were specifically
capable of amplifying the back splice sites of each circRNA, and
the PCR results were validated by gel electrophoresis and
melt-curve analysis (Fig. 2B and
C); melting curves for qPCR assays of circRANs showed a single
peak indicated the specificity of the PCR results, and the sizes of
the PCR products were in accordance with the anticipated sizes,
based on the primer designs. The expression levels of 7 selected
circRNAs were consistent with those measured by microarray
analysis; however, 2 circRNAs were not detected, perhaps due to the
extremely low expression levels in the 6 samples, and differential
expression was not detected with 1 circRNA (P>0.05; Fig. 2A). Of these, only circRNA-0026
expression was significantly different between the GC and control
samples (2.8-fold, P=0.001), and all other differences were less
than 1.5 fold.
Biomathematical analyses
It has previously been indicated that circRNAs
regulate gene expression by targeting miRNAs and blocking their
biological functions (7,10). In the present study, miRNA and
circRNA sequences were aligned, and 5 miRNAs with the highest
mirSVR were identified for each differentially expressed circRNA
using miRNA target-prediction software, based on TargetScan and
miRanda. All differentially expressed circRNAs were annotated in
detail, with the circRNA/miRNA interaction information. As a
result, 327 miRNAs (including 221 miRNA families) were identified
and ranked according to their predicted number of interactions with
different circRNAs, as described by Wang et al (18); the top 10 miRNA families consisted
of the miR-29, miR-30, miR-15, miR-146, miR-16, miR-181, miR-135,
miR-23, miR-19 and miR-let-7 families. Bioinformatics analysis
revealed that these miRNAs were abnormally expressed in various
malignant tumors, including GC (20), colon cancer (21), breast cancer (22), lung cancer (23) and others (24–26),
thus suggesting their involvement in the occurrence and development
of malignant tumors. Subsequently, candidate target genes were
identified based on miRNA/mRNA sequence pairing (mRNA-dependent
cutoff value, −0.50) using TargetScan online tools, and a
circRNA-0026-targeted circRNA-miRNA-mRNA/gene network was
constructed. It was demonstrated that 13 miRNAs and 578 genes were
targeted in the network (miRNA-dependent cutoff value, −0.14;
mRNA-dependent cutoff value, −0.50). To investigate the functions
of the predicted network genes, GO analysis was performed using the
DAVID tool. Genes in the top 10 annotated clusters were involved in
regulating transcription, including RNA metabolic processes, gene
expression, gene silencing and other biological functions (Table III).
 | Table III.Gene Ontology term enrichment in the
circular RNA0026-microRNA-mRNA/gene network. |
Table III.
Gene Ontology term enrichment in the
circular RNA0026-microRNA-mRNA/gene network.
| Term | Gene count | P-value | Fold
enrichment |
|---|
| Regulation of RNA
metabolic process | 67 |
8.0×10−8 | 1.9 |
| Regulation of
transcription, DNA-dependent | 65 |
1.90×10−7 | 1.9 |
| Regulation of
transcription | 84 |
3.4×10−7 | 1.7 |
| Transcription | 69 |
3.9×10−6 | 1.7 |
| Negative regulation
of gene expression | 21 |
1.5×10−3 | 2.2 |
| Negative regulation
of transcription | 17 |
1.5×10−2 | 1.9 |
| Negative regulation
of macromolecule biosynthetic process | 19 |
1.7×10−2 | 1.8 |
| Negative regulation
of cellular biosynthetic process | 19 |
2.1×10−2 | 1.8 |
| Negative regulation
of biosynthetic process | 19 |
2.5×10−2 | 1.7 |
| Negative regulation
of nucleobase, nucleoside, nucleotide and nucleic acid metabolic
process | 17 |
3.5×10−2 | 1.7 |
| Negative regulation
of nitrogen compound metabolic process | 17 |
3.9×10−2 | 1.7 |
| Regulation of
protein kinase cascade | 10 |
4.9×10−2 | 2.1 |
| Negative regulation
of transcription from RNA | 10 |
6.8×10−2 | 2 |
| Polymerase II
promoter |
| Negative regulation
of transcription, DNA-dependent | 12 |
7.8×10−2 | 1.8 |
| Negative regulation
of RNA metabolic process | 12 |
8.4×10−2 | 1.7 |
| Cell
activation | 10 |
9.7×10−2 | 1.8 |
| Anti-apoptosis | 8 |
9.8×10−2 | 2 |
Discussion
Despite a steadily declining incidence, GC remains
the second leading cause of cancer-associated mortality in China
due to its highly malignant nature (1,4).
Over the last decade, the understanding of the molecular
pathogenesis of GC has advanced considerably; however, much remains
unclear (1,4). In recent years, mounting evidence has
suggested that ncRNAs serve important roles in cellular metabolism
and in regulatory processes, including development, proliferation,
differentiation and apoptosis (18,25).
Aberrantly expressed ncRNAs, such as lncRNA, miRNAs and circRNAs,
serve key roles in tumor pathogenesis by regulating numerous tumor
signaling pathways, including epidermal growth factor receptor
(EGFR) (26,27), Notch (28), mammalian target of rapamycin (mTOR)
(29), nuclear factor (NF)-κB
(30) and Wnt (31).
CircRNAs represent a class of widespread ncRNAs that
are considered ceRNAs, since they are capable of regulating each
other by competing for binding to shared miRNAs (32,33).
Previous studies have demonstrated that circRNAs serve crucial
roles in fine-tuning miRNA-mediated regulation of gene expression
by sequestering miRNAs (6,33), and their aberrant expression may be
associated with human diseases (6,12–15,32).
For example, the circRNA ciRS-7 contains >60 miR-7-binding
sites, thereby acting as an endogenous miRNA “sponge” to adsorb and
thereby quench normal miR-7 functions (33). Considering the widespread
involvement of miR-7 as a key regulator of various cancer pathways,
including EGFR (34–36), Raf1 (36), NF-κB (37), mTOR (38,39),
AKT (34,39) among others (6,40),
ciRS-7 may serve as a crucial factor in tumor development (6,39).
Increasing evidence has indicated that the aberrant expression of
circRNAs may promote cancer pathogenesis by adsorbing
cancer-associated miRNAs (6,40).
Some synthetic circRNAs have exhibited marked anticancer effects
(41,42), indicating that circRNAs have
diagnostic and therapeutic potential (6,40–42).
Recent findings have reported that circRNAs are
potentially involved in GC (14–15).
Ghosal et al (14)
performed a GO enrichment analysis on the protein-coding genes in
the miRNA-circRNA interactome of individual diseases, in order to
study the enrichment of genes associated with particular biological
processes; a total of 68 genes involved in various biological
processes were revealed to be associated with GC. Li et al
(15) demonstrated that
hsa_circ_002059 was significantly downregulated in GC, suggesting
its potential as a novel and stable biomarker for GC diagnosis.
The present study identified 100 circRNAs with
≥2-fold differential expression between matched GC and normal
tissues, as determined by circRNA chip analysis. The qPCR results
confirmed the abnormal expression of these circRNAs in GC. To
further analyze the role of these circRNAs in GC pathogenesis,
bioinformatics analysis was performed to identify the miRNAs with
the highest mirSVRs for each differentially expressed circRNA,
based on predicted base pairing between circRNAs and miRNAs. A
total of 327 candidate miRNAs (from 221 miRNA families) were
identified, and the top 10 miRNA families in terms of the number of
potential circRNA interactions were miR-29, miR-30, miR-15,
miR-146, miR-16, miR-181, miR-135, miR-23, miR-19 and miR-let-7.
Previous studies have provided evidence that these miRNAs are
abnormally expressed in various types of cancer, including GC
(20), colorectal cancer (21), breast cancer (22), lung cancer (23) and others (24–26),
and are involved in tumor cell proliferation and drug resistance,
as well as the occurrence, development, invasion and metastasis of
tumors (20–26). In agreement, the results of the
present study demonstrated that these differentially expressed
circRNAs may be involved in the pathogenesis of GC.
Although >7,000 circRNAs have been identified in
human tissues (43), and
sponge-like activity is the main function of some circRNAs
(33), to date only ciRS-7
(6) and circRNA transcripts from
the sex determining region Y gene (33,44)
have been reported to function as molecular sponges against their
target miRNAs. In the present study, circ_000026 was significantly
downregulated in GC tissues compared with paired, adjacent
noncancerous tissues (P=0.001), as determined using a circRNA chip
and qPCR analysis. To further understand the biological function of
circ_000026, the top 5 miRNAs with the highest mirSVRs were
identified (including miR-23a, miR-23b, miR-581, miR-146a and
miR-450a), and the circ_000026-miRNA-gene network was predicted
using TargetScan and miRanda. The DAVID tool was used to enrich for
GO terms associated with circRNA-producing genes. The analysis
revealed that the circ_000026-targeted miRNA-mRNA network may
regulate transcription, RNA metabolism, gene expression and gene
silencing, among other functions. These results suggested that
circRNAs not only act as miRNA sponges, but also may potentially
regulate RNA and protein production, which is in agreement with the
findings from previous studies (45,46).
In conclusion, the present study demonstrated that
circRNAs are aberrantly expressed in GC. Significant downregulation
of circ_000026 expression in GC tissues was confirmed, suggesting
its potential involvement in GC tumorigenesis and its potential use
as a novel biomarker for GC diagnosis and targeted therapy. In the
future, a longitudinal study is required to investigate the
potential of circRNAs as biomarkers for GC diagnosis and targeted
therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant no. 81260321). circRNA microarray
results were deposited in the National Center for Biotechnology
Information Gene Expression Omnibus database under accession number
GSE78092.
Glossary
Abbreviations
Abbreviations:
|
ceRNA
|
competitive endogenous RNA
|
|
circRNA
|
circular RNA
|
|
DAVID
|
Database for Annotation, Visualization
and Integrated Discovery
|
|
GC
|
gastric cancer
|
|
GO
|
Gene Ontology
|
|
lncRNA
|
long noncoding RNA
|
|
miRNA
|
microRNA
|
|
mirSVR
|
miRNA support vector regression
|
|
ncRNA
|
non-coding RNA
|
|
qPCR
|
quantitative polymerase chain
reaction
|
References
|
1
|
Tan P and Yeoh KG: Genetics and molecular
pathogenesis of gastric adenocarcinoma. Gastroenterology.
149:1153–1162.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McLean MH and El-Omar EM: Genetics of
gastric cancer. Nat Rev Gastroenterol Hepatol. 11:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilusz JE: Long noncoding RNAs: Re-writing
dogmas of RNA processing and stability. Biochim Biophys Acta.
1859:128–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen TB, Kjems J and Damgaard CK:
Circular RNA and miR-7 in cancer. Cancer Res. 73:5609–5612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrett SP, Wang PL and Salzman J:
Circular RNA biogenesis can proceed through an exon-containing
lariat precursor. Elife. 4:e075402015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Li C, Tan C and Liu X: Circular
RNAs: A new frontier in the study of human diseases. J Med Genet.
53:359–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghosal S, Das S, Sen R, Basak P and
Chakrabarti J: Circ2Traits: A comprehensive database for circular
RNA potentially associated with disease and traits. Front Genet.
4:2832013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
18
|
Wang YH, Yu XH, Luo SS and Han H:
Comprehensive circular RNA profiling reveals that circular
RNA100783 is involved in chronic CD28-associated CD8(+)T cell
ageing. Immun Ageing. 12:172015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Tan X and Fu SW: May Circulating
microRNAs be gastric cancer diagnostic biomarkers. J Cancer.
6:1206–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas J, Ohtsuka M, Pichler M and Ling H:
MicroRNAs: clinical relevance in colorectal cancer. Int J Mol Sci.
16:28063–28076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inamura K and Ishikawa Y: MicroRNA in lung
cancer: Novel biomarkers and potential tools for treatment. J Clin
Med. 5:pii: E36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heneghan HM, Miller N and Kerin MJ: MiRNAs
as biomarkers and therapeutic targets in cancer. Curr Opin
Pharmacol. 10:543–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Song YX and Wang ZN: Non-coding
RNAs in gastric cancer. Gene. 560:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng G and Sui G: Noncoding RNA in
oncogenesis: A new era of identifying key players. Int J Mol Sci.
14:18319–18349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pak MG, Lee CH, Lee WJ, Shin DH and Roh
MS: Unique microRNAs in lung adenocarcinoma groups according to
major TKI sensitive EGFR mutation status. Diagn Pathol. 10:992015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng N, Cai W, Ren S, Li X, Wang Q, Pan
H, Zhao M, Li J, Zhang Y, Zhao C, et al: Long non-coding RNA UCA1
induces non-T790M acquired resistance to EGFR-TKIs by activating
the AKT/mTOR pathway in EGFR-mutant non-small cell lung cancer.
Oncotarget. 6:23582–23593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji J, Tang J, Deng L, Xie Y, Jiang R, Li G
and Sun B: LINC00152 promotes proliferation in hepatocellular
carcinoma by targeting EpCAM via the mTOR signaling pathway.
Oncotarget. 6:42813–42824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajbhandari R, McFarland BC, Patel A,
Gerigk M, Gray GK, Fehling SC, Bredel M, Berbari NF, Kim H, Marks
MP, et al: Loss of tumor suppressive microRNA-31 enhances
TRADD/NF-κB signaling in glioblastoma. Oncotarget. 6:17805–17816.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S and
Purow B: microRNA-7 inhibits the epidermal growth factor receptor
and the Akt pathway and is down-regulated in glioblastoma. Cancer
Res. 68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suto T, Yokobori T, Yajima R, Morita H,
Fujii T, Yamaguchi S, Altan B, Tsutsumi S, Asao T and Kuwano H:
MicroRNA-7 expression in colorectal cancer is associated with poor
prognosis and regulates cetuximab sensitivity via EGFR regulation.
Carcinogenesis. 36:338–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Webster RJ, Giles KM, Price KJ, Zhang PM,
Mattick JS and Leedman PJ: Regulation of epidermal growth factor
receptor signaling in human cancer cells by microRNA-7. J Biol
Chem. 284:5731–5741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen
GF, Zhou JF, Li T, Hu SJ, Zhou L, et al: MicroRNA-7/NF-κB signaling
regulatory feedback circuit regulates gastric carcinogenesis. J
Cell Biol. 210:613–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Liu J, Liu C, Naji A and Stoffers
DA: MicroRNA-7 regulates the mTOR pathway and proliferation in
adult pancreatic β-cells. Diabetes. 62:887–895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Yang J, Zhou P, Le Y, Zhou C, Wang
S, Xu D, Lin HK and Gong Z: Circular RNAs in cancer: Novel insights
into origins, properties, functions and implications. Am J Cancer
Res. 5:472–480. 2015.PubMed/NCBI
|
|
41
|
Bak RO, Hollensen AK and Mikkelsen JG:
Managing microRNAs with vector-encoded decoy-type inhibitors. Mol
Ther. 21:1478–1485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haraguchi T, Ozaki Y and Iba H: Vectors
expressing efficient RNA decoys achieve the long-term suppression
of specific microRNA activity in mammalian cells. Nucleic Acids
Res. 37:e432009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Capel B, Swain A, Nicolis S, Hacker A,
Walter M, Koopman P, Goodfellow P and Lovell-Badge R: Circular
transcripts of the testis-determining gene Sry in adult mouse
testis. Cell. 73:1019–1030. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilusz JE and Sharp PA: Molecular biology.
A circuitous route to noncoding RNA. Science. 340:440–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martineau Y, Derry MC, Wang X, Yanagiya A,
Berlanga JJ, Shyu AB, Imataka H, Gehring K and Sonenberg N:
Poly(A)-binding protein-interacting protein 1 binds to eukaryotic
translation initiation factor 3 to stimulate translation. Mol Cell
Biol. 28:6658–6667. 2008. View Article : Google Scholar : PubMed/NCBI
|