Introduction
Xp11.2 translocation renal cell carcinomas (RCCs)
are the most common subtype of pediatric RCCs, in addition to
affecting ~1.5% of adults (1,2).
Xp11.2 translocation RCCs were first identified to be a novel,
genetically distinct disease in the 2010 World Health Organization
renal tumor classification (3).
Xp11.2 translocation RCCs are characterized by chromosomal
translocations involving the Xp11.2 breakpoint, resulting in
transcription factor E3 (TFE3) gene fusion. TFE3 fusion with other
regulatory elements and TFE3 overexpression is a notable feature of
Xp11.2 translocation RCCs (4).
TFE3 is a member of the basic helix-loop-helix
leucine zipper family of transcription factors. TFE3 interacts with
transcriptional regulators, including E2F transcription factor 3
(E2F3), SMAD family member 3 and lymphoid enhancer-binding factor
1, and serves an important role in cell growth and cell
proliferation (1). In addition,
translocations/TFE3 fusions have been directly implicated in
tumorigenesis (5). However, the
association between growth-related factors and the cell
cycle-related factors that regulate the signal transduction
pathways of TFE3-overexpressing cells has not been
investigated.
Although Xp11.2 translocation RCCs predominantly
occur in children and adolescents, the prognosis for adult
translocation RCC is particularly poor (6). The TFE3-mediated direct
transcriptional upregulation of the Met tyrosine kinase receptor
triggers the activation of downstream signaling pathways, such as
the phosphatidylinositol 3-kinase (PI3K)/AKT serine/threonine
kinase 1 (AKT)/mammalian target of rapamycin (mTOR) pathway and the
Ras/mitogen-activated protein kinase (MAPK) pathway (7). Temsirolimus directly interferes with
the PI3K/AKT/mTOR signaling pathway by acting on mTOR and
decreasing the activity of effector molecules, including
phosphorylated ribosomal protein S6 (p-rpS6). Parikh et al
(8) reported the successful
treatment of adult Xp11.2 translocation RCC via temsirolimus.
However, Choueiri et al (9)
reported that certain patients with advanced translocation RCC
develop progressive disease following temsirolimus treatment.
Therefore, the role of mTOR in Xp11.2 translocation RCCs requires
further investigation.
In the present study, renal adenocarcinoma ACHN
cells were infected with the lentivirus LV-TFE3 to produce a stable
TFE3-overexpressing cell line. Subsequently, the effects of TFE3
overexpression on cell proliferation, plate clone formation, cell
cycle distribution and the activation of the mTOR signaling pathway
were examined.
Materials and methods
Cell culture
Human embryonic kidney 293T cells (293T) and human
renal adenocarcinoma ACHN cells were purchased from the Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). The cell lines were cultured at 37°C with 5%
CO2 and saturated humidity. When the cells grew to 75%
confluence, they were harvested for further analysis.
Human TFE3 overexpression (OE)
lentivirus package
The packaging GV341 plasmid (Shanghai GeneChem Co.,
Ltd., Shanghai, China) was transfected into 293T cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The cells were placed
onto complete medium and cultured for 48 h prior to the supernatant
being collected, and the titer was detected via titration analysis.
The human TFE3 gene (National Center for Biotechnology Information
GenBank accession no. NM_006521) fused with a FLAG-tag was
synthesized by Shanghai GeneChem Co., Ltd. (Shanghai, China), to
construct the LV-TFE3 OE vector. The empty vector was used for the
negative control (NC) group. The lentiviral vector also contained
the green fluorescent protein (GFP) gene. The lentivirus titer was
2×108 transducing units (TU)/ml in the OE group and
1×109 TU/ml in the NC group. ACHN cells were seeded at a
concentration of 4×104 cells/well in 6-well culture
plates, cultivated in the incubator for 12 h, and subsequently
infected with OE or NC lentivirus at a multiplicity of infection of
10. The two lentiviruses in the OE and NC groups contained a
puromycin-resistant cassette, which confers puromycin resistance to
eukaryotic cells. The puromycin resistance gene is routinely used
as a selectable marker for stably-transformed mammalian cell lines.
The infected cells were cultivated for a further 72 h, and 2 µg/ml
puromycin was added to each well. Following 48 h in culture, an
inverted fluorescence microscope was used to analyze efficiency of
overexpression (magnification, ×100).
Western blot analysis
Total protein was isolated from cells using
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). A bicinchoninic acid assay was used to
measure the total protein concentration. Equal amounts of protein
(20 µg) were separated by 10% SDS-PAGE and subsequently transferred
onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica,
MA, USA). The membranes were blocked in TBS-Tween 20 with 5% skim
milk at room temperature for 1 h. The membranes were incubated with
murine antibody against FLAG-tag (1:3,000; cat. no. F1804;
Sigma-Aldrich; Merck KGaA), rabbit polyclonal antibody against mTOR
(1:3,000; cat. no. 2936-1; Epitomics; Abcam, Cambridge, UK), rabbit
polyclonal antibody against p-rpS6 (1:1,000; cat. no. 4858; Cell
Signaling Technology, Inc., Danvers, MA, USA) and murine antibody
against human GADPH (1:2,000; cat. no. SC-32233; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. The
membranes were subsequently incubated with appropriate
peroxidase-conjugated secondary antibody mouse immunoglobulin G
(1:5,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.).
Antibody binding was visualized using enhanced chemiluminescence
solution (Pierce; Thermo Fisher Scientific, Inc.). GADPH was used
as an endogenous control.
MTT assay
The cells were seeded into 96-well plates (2,000
cells/well) and incubated for 24 h, and subsequently treated with
20 µl 5 g/l MTT (Gen-View Scientific, Inc., El Monte, FL, USA) and
incubated at 37°C for 4 h. The supernatants were removed and 150 µl
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added to each
well. The absorbance at 450 nm was measured using a microplate
reader. The optical density value was measured each day for 5 days,
and growth curves were produced.
Plate colony formation assay
Cells were seeded in 6-well plates; each group had
three replicate wells and contained 500 cells/well. After 14 days,
the cell colonies were visible to the naked eye. The cells were
stained with Giemsa (cat. no. AR-0752; Shanghai Dingguo Biotech
Co., Ltd., Shanghai, China) and the number of visible colonies
containing >50 cells were quantified.
Analysis of cell cycle distribution
using flow cytometry
Following 5 days of transduction, the cells were
trypsinized, washed with PBS, and fixed in 70% ethanol at 4°C
overnight. Cell cycle distribution analysis was performed using a
cell cycle detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
The cells were incubated with propidium iodide at 37°C for 30 min
in the dark. The cell cycle was analyzed using BD FACSArray™
software (v6.0) on a BD FACSVerse flow cytometer (BD
Biosciences).
Statistical analysis
Each experiment was repeated at least three times to
ensure experimental repeatability. Data were expressed as the mean
± standard error of the mean, and analyses were performed using
SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). One-way
analysis of variance followed by the Tukey test was used to compare
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
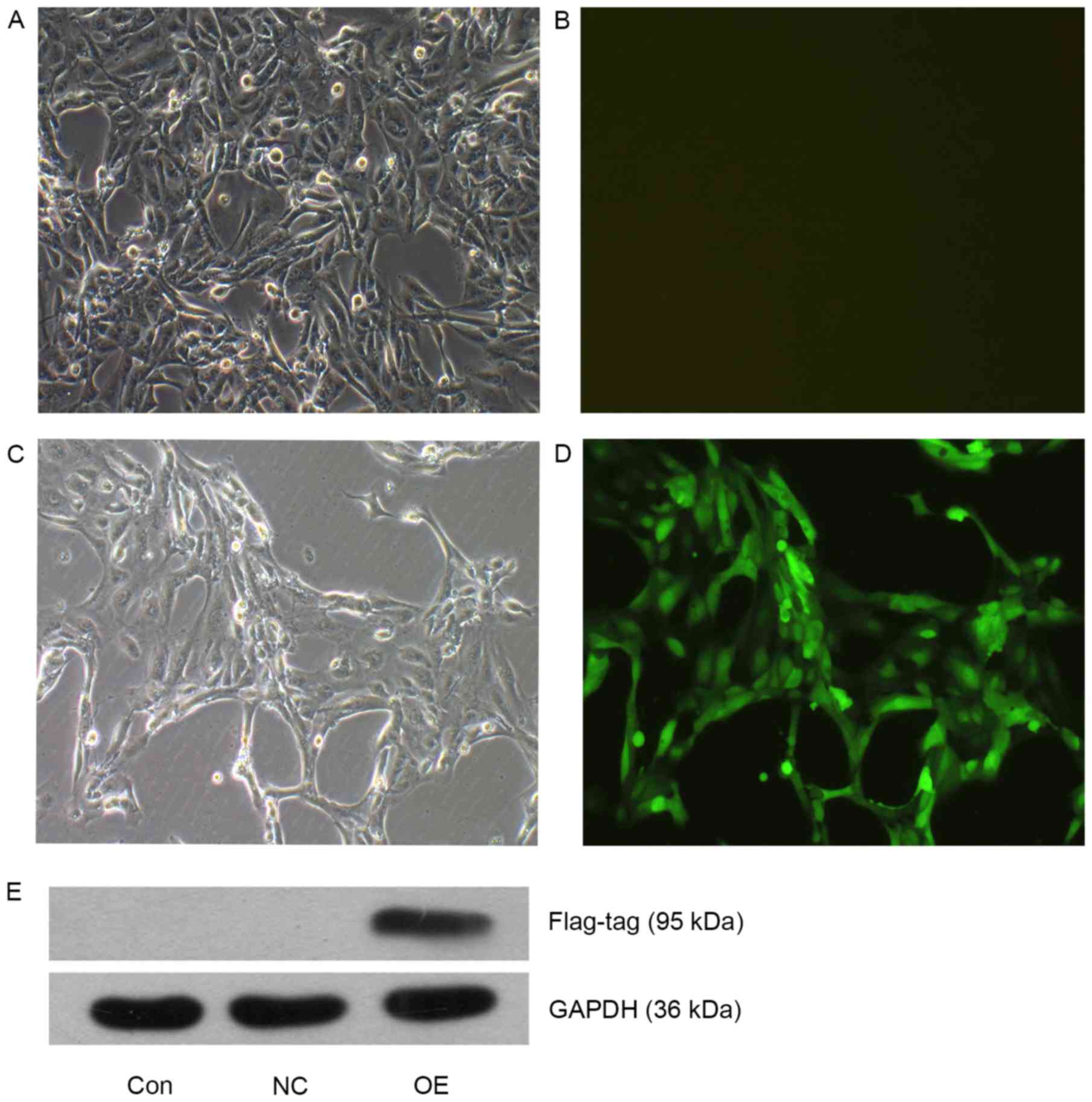
Overexpression of TEF3 in ACHN
cells
ACHN cells in the OE (TFE3-overexpressing
lentivirus) were cultured to 90% confluence and GFP fluorescence
was observed under an inverted fluorescence microscope (Fig. 1A-D). Following puromycin selection,
the majority of the cells in the OE group were positive for GFP
fluorescence, suggesting a stable overexpressing cell line was
successfully constructed by the lentivirus transduction. The
protein expression levels of TFE3 were also detected using western
blot analysis (Fig. 1E), and TFE3
overexpression in the OE cells was confirmed.
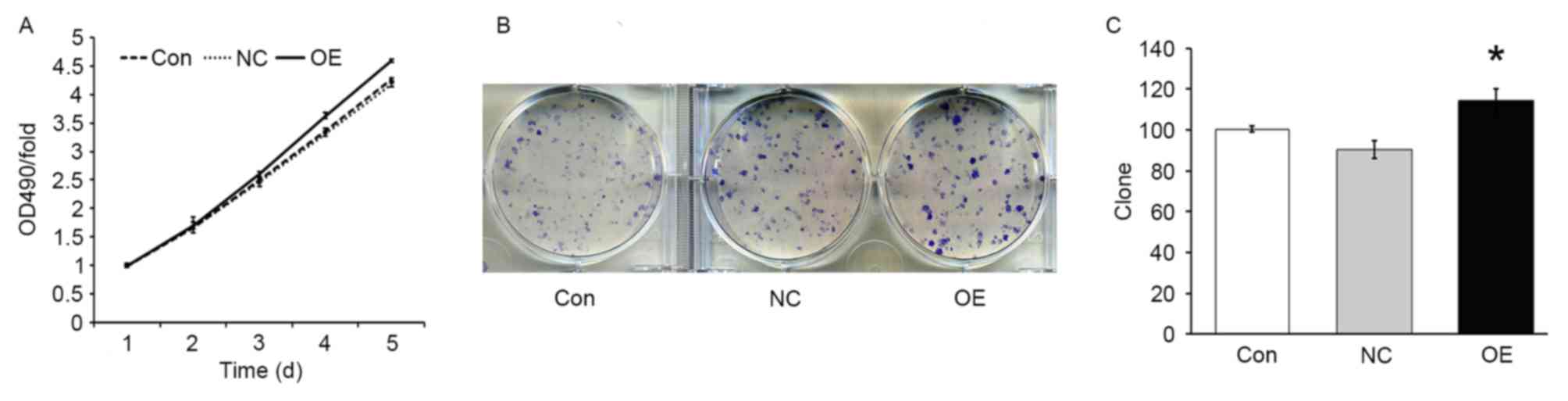
TFE3 overexpression enhances ACHN cell
growth and proliferation
In order to evaluate the effect of TFE3
overexpression on the proliferation of ACHN cells, an MTT assay was
performed. The results demonstrated that, under the same growth
conditions, the growth rate was increased in the OE group compared
with the NC group (P<0.05; Fig.
2A), indicating that overexpression of TFE3 promoted the growth
of ACHN cells. In addition, the colony formation assay demonstrated
that the number of colonies in the NC and OE groups were 90±5 and
114±6, respectively. The colony formation ability was significantly
increased in the OE group compared with the NC group (P<0.05;
Fig. 2B and C).
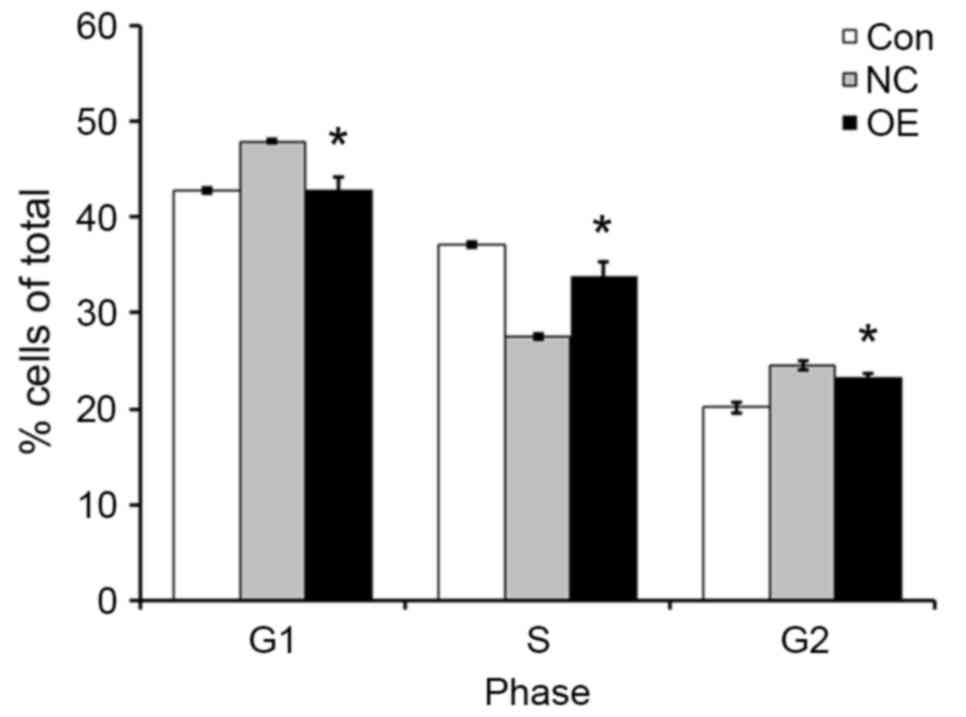
TFE3 overexpression promotes the cell
cycle progression of ACHN cells
In order to further investigate how TFE3
overexpression enhanced the proliferation of ACHN cells, cell cycle
profiles were examined by flow cytometry. Compared with the NC
group, the OE group exhibited decreased percent of cells in the G1
and G2 phases of the cell cycle, and increased percent of cells in
the S phase (Fig. 3). The results
of the present study indicated that TEF3 promoted ACHN cell cycle
progression.
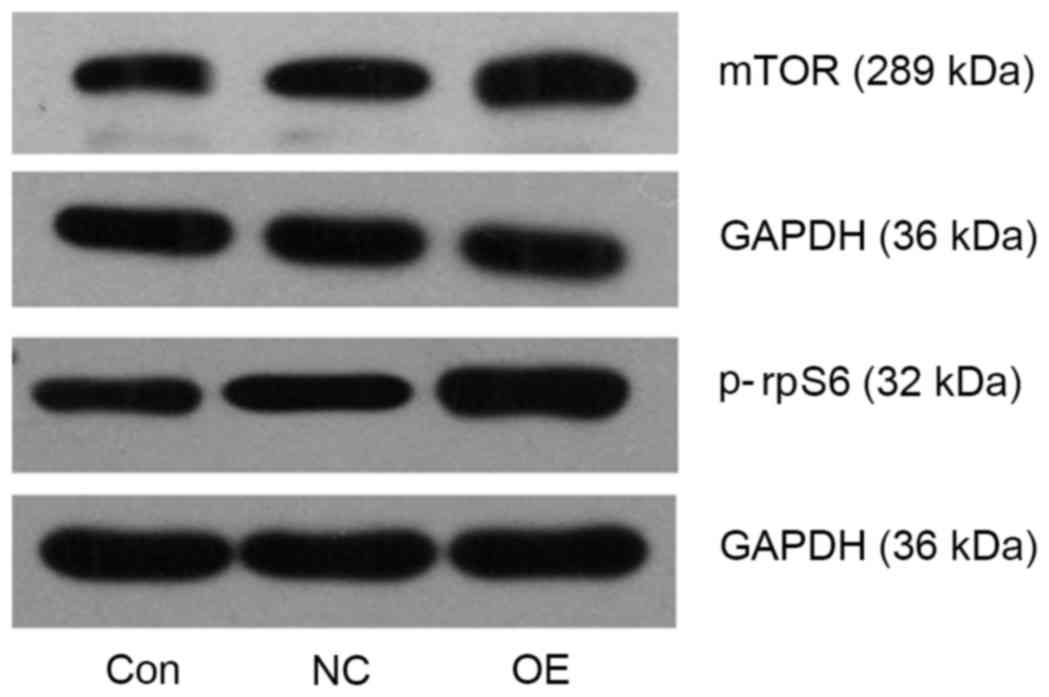
TFE3 overexpression activates the mTOR
pathway in ACHN cells
mTOR and p-rpS6 protein expression levels were
detected using western blot analysis. Compared with the NC group,
mTOR and p-rpS6 levels were upregulated in the OE group (Fig. 4). The results of the present study
suggested that TFE3 may be involved in activating the mTOR pathway
in ACHN cells.
Discussion
Xp11.2 translocation RCCs have been increasingly
demonstrated to be a subset of RCC characterized by a variety of
chromosomal translocations involving the TFE3 gene with a
breakpoint at Xp11.2, resulting in fusion with a number of
translocation partners, including papillary renal cell carcinoma,
ASPSCR1 UBX domain containing tether for SLC2A4, splicing factor
proline and glutamine rich, clathrin heavy chain and non-POU domain
containing octamer-binding (10).
TFE3 protein is constitutively overexpressed in Xp11.2
translocation RCCs (4,11). Rao et al (12) suggested that TFE3-positive
pediatric RCCs may be associated with poorer outcomes and higher
stages (III/IV), compared with TFE3-negative RCCs. In addition,
patients with Xp11.2 translocation RCCs frequently present at an
advanced stage and demonstrate a more invasive clinical course and
poorer prognosis, compared with patients with non-Xp11.2
translocation RCCs (13). However,
the cytological mechanisms by which TFE3 overexpression may
regulate the proliferation and cell cycle distribution in a renal
adenocarcinoma model have not been reported.
In the present study, the TFE3 gene sequence was
introduced in the human renal adenocarcinoma ACHN cells via a
recombinant lentivirus. Puromycin was used to select and enrich for
the infected cells and the fluorescence emitted from the GFP marker
was observed under an inverted fluorescence microscope. The present
study established TFE3-overexpressing cells and observed, through
growth curves, that the TFE3-overexpressing cells grew more rapidly
compared with the negative control group. In addition, the plate
colony-forming assays demonstrated that TFE3 overexpression
resulted in increased colony formation capacity compared with the
negative control group. The results of the present study indicated
that TFE3 overexpression promoted ACHN cell proliferation. Ploper
et al (14) reviewed the
role of micropthalmia-associated transcription factor (MITF) in
lysosomal biogenesis, and how cancers overexpressing MITF,
transcription factor EB or TFE3 are able to reorganize the
lysosomal pathway and inhibit cellular senescence. The cell cycle
analysis performed in the present study demonstrated that TFE3
overexpression promoted DNA synthesis and accelerated the cell
cycle progression from G1 to S phase. The results of the present
study may help to explain how TFE3 overexpression promotes ACHN
cell proliferation. Giangrande et al (15) identified the TFE3 transcription
factor to be a specific partner for E2F3, which is able to rescue
retinoblastoma associated protein (Rb)-mediated growth arrest,
providing a functional link between TFE3 and the Rb/E2F pathway.
Leone et al (16) proposed
that E2F3 activity serves an important role in the cell cycle of
proliferating cells, controlling the expression of genes whose
products limit the initiation of DNA replication, imparting a more
marked control of S phase than may otherwise be achieved by
post-transcriptional regulation alone. The results of the present
study demonstrated that TFE3 overexpression likely promoted ACHN
cell proliferation through cell cycle regulation, which is
consistent with previous studies.
Transcription factors and cell signaling pathways
serve important roles in carcinogenesis (17,18).
The activation of membrane growth factor receptors drives cell
proliferation signals through at least two biochemical pathways:
the PI3K/AKT/mTOR and Ras/MAPK pathway. Overt activation of the
PI3K/Akt/mTOR pathway has been observed in various types of human
cancer, including RCCs (19).
Previous investigations have demonstrated that rpS6, the
best-characterized downstream effector of mTOR complex 1, is
upregulated in primary renal tumors with metastasis (19,20).
Immunoreactivity for rpS6 may be used as a measure of the
activation of the mTOR pathway, which promotes cell growth. In
addition, rpS6 may be an important tool for stratifying patients
with metastatic RCC into different risk groups and improving
patient selection for mTOR-targeted therapies (21). Argani et al (22) demonstrated that targeting the mTOR
signaling pathway may be an effective way to treat Xp11.2
translocation RCCs. The present study demonstrated that the mTOR
and p-rpS6 levels were increased in the OE group compared with the
NC group, suggesting that TFE3 overexpression improved the
proliferative capacity of ACHN cells through the activation of the
mTOR pathway.
In conclusion, the results of the present study
indicated that an increase in TFE3 expression resulted in malignant
phenotypes in renal adenocarcinoma ACHN cells. TFE3 may serve an
important role in the regulation of the cell cycle and cell
proliferation. Additionally, the results suggested that mTOR and
rpS6 may be effective therapeutic targets for Xp11.2 translocation
RCCs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372743, to
X.J.Z.).
References
|
1
|
Ramphal R, Pappo A, Zielenska M, Grant R
and Ngan BY: Pediatric renal cell carcinoma: Clinical, pathologic,
and molecular abnormalities associated with the members of the mit
transcription factor family. Am J Clin Pathol. 126:349–364. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Komai Y, Fujiwara M, Fujii Y, Mukai H,
Yonese J, Kawakami S, Yamamoto S, Migita T, Ishikawa Y, Kurata M,
et al: Adult Xp11 translocation renal cell carcinoma diagnosed by
cytogenetics and immunohistochemistry. Clin Cancer Res.
15:1170–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez-Beltran A, Cheng L, Vidal A,
Scarpelli M, Kirkali Z, Blanca A and Montironi R: Pathology of
renal cell carcinoma: An update. Anal Quant Cytopathol Histpathol.
35:61–76. 2013.PubMed/NCBI
|
|
4
|
Argani P, Lal P, Hutchinson B, Lui MY,
Reuter VE and Ladanyi M: Aberrant nuclear immunoreactivity for TFE3
in neoplasms with TFE3 gene fusions: A sensitive and specific
immunohistochemical assay. Am J Surg Pathol. 27:750–761. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Argani P, Antonescu CR, Illei PB, Lui MY,
Timmons CF, Newbury R, Reuter VE, Garvin AJ, Perez-Atayde AR,
Fletcher JA, et al: Primary renal neoplasms with the ASPL-TFE3 gene
fusion of alveolar soft part sarcoma: A distinctive tumor entity
previously included among renal cell carcinomas of children and
adolescents. Am J Pathol. 159:179–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argani P, Olgac S, Tickoo SK, Goldfischer
M, Moch H, Chan DY, Eble JN, Bonsib SM, Jimeno M, Lloreta J, et al:
Xp11 translocation renal cell carcinoma in adults: Expanded
clinical, pathologic, and genetic spectrum. Am J Surg Pathol.
31:1149–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castellvi J, Garcia A, Ruiz-Marcellan C,
Hernández-Losa J, Peg V, Salcedo M, Gil-Moreno A, Ramon Y and Cajal
S: Cell signaling in endometrial carcinoma: Phosphorylated
4E-binding protein-1 expression in endometrial cancer correlates
with aggressive tumors and prognosis. Hum Pathol. 40:1418–1426.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parikh J, Coleman T, Messias N and Brown
J: Temsirolimus in the treatment of renal cell carcinoma associated
with Xp11.2 translocation/TFE gene fusion proteins: A case report
and review of literature. Rare Tumors. 1:e532009.PubMed/NCBI
|
|
9
|
Choueiri TK, Lim ZD, Hirsch MS, Tamboli P,
Jonasch E, McDermott DF, Dal Cin P, Corn P, Vaishampayan U, Heng DY
and Tannir NM: Vascular endothelial growth factor-targeted therapy
for the treatment of adult metastatic Xp11.2 translocation renal
cell carcinoma. Cancer. 116:5219–5225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao Q, Williamson SR, Zhang S, Eble JN,
Grignon DJ, Wang M, Zhou XJ, Huang W, Tan PH, Maclennan GT and
Cheng L: TFE3 break-apart FISH has a higher sensitivity for Xp11.2
translocation-associated renal cell carcinoma compared with TFE3 or
cathepsin K immunohistochemical staining alone: Expanding the
morphologic spectrum. Am J Surg Pathol. 37:804–815. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dickson BC, Brooks JS, Pasha TL and Zhang
PJ: TFE3 expression in tumors of the microphthalmia-associated
transcription factor (MiTF) family. Int J Surg Pathol. 19:26–30.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao Q, Guan B and Zhou XJ: Xp11.2
Translocation renal cell carcinomas have a poorer prognosis than
non-Xp11.2 translocation carcinomas in children and young adults: A
meta-analysis. Int J Surg Pathol. 18:458–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu L, Yang R, Gan W, Chen X, Qiu X, Fu K,
Huang J, Zhu G and Guo H: Xp11.2 translocation renal cell
carcinomas in young adults. BMC Urol. 15:572015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ploper D and De Robertis EM: The MITF
family of transcription factors: Role in endolysosomal biogenesis,
Wnt signaling, and oncogenesis. Pharmacol Res. 99:36–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giangrande PH, Hallstrom TC, Tunyaplin C,
Calame K and Nevins JR: Identification of E-Box factor TFE3 as a
functional partner for the E2F3 transcription factor. Mol Cell
Biol. 23:3707–3720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leone G, Degregori J, Yan Z, Jakoi L,
Ishida S, Williams RS and Nevins JR: E2F3 activity is regulated
during the cell cycle and is required for the induction of S phase.
Genes Dev. 12:2120–2130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Wang Y, Yu Y, Nishizawa M, Nakajima
T, Ito S and Kannan P: The human transcription factor activation
protein-2 gamma (AP-2gamma): Gene structure, promoter and,
expression in mammary carcinoma cell lines. Gene. 301:43–51. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Wang Y, Li M and Kannan P:
Tumorigenic effect of transcription factor hAP-2alpha and the
intricate link between hAP-2alpha activation and squelching. Mol
Carcinog. 34:172–179. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furuya N, Kamai T, Shirataki H, Yanai Y,
Fukuda T, Mizuno T, Nakamura F, Kambara T, Nakanishi K, Abe H and
Yoshida K: Serum interferon alpha receptor 2 mRNA may predict
efficacy of interferon alpha with/without low-dose sorafenib for
metastatic clear cell renal cell carcinoma. Cancer Immunol
Immunother. 60:793–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
21
|
Pantuck AJ, Seligson DB, Klatte T, Yu H,
Leppert JT, Moore L, O'Toole T, Gibbons J, Belldegrun AS and Figlin
RA: Prognostic relevance of the mTOR pathway in renal cell
carcinoma: Implications for molecular patient selection for
targeted therapy. Cancer. 109:2257–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Argani P, Hicks J, De Marzo AM, Albadine
R, Illei PB, Ladanyi M, Reuter VE and Netto GJ: Xp11 translocation
renal cell carcinoma (RCC): Extended immunohistochemical profile
emphasizing novel RCC Markers. Am J Surg Pathol. 34:1295–1303.
2010. View Article : Google Scholar : PubMed/NCBI
|