Introduction
Endotoxins cause acute lung injury (ALI), which is
an inflammatory derangement associated with the recruitment and
activation of inflammatory cytokines and neutrophils in lungs
(1). Thus, early prevention and
regulation of inflammatory responses, including attenuation of
inflammatory mediator expression and upregulation of
anti-inflammatory factors, is critical to ALI treatment. Heat shock
proteins (HSPs), a class of functionally related proteins, protect
cells against a variety of stressful conditions. On the basis of
recent research, HSPs may serve a crucial role in the protection of
ALI by its anti-inflammatory function, antioxidant activity and
antiapoptotic effect (2,3). Wischmeyer et al (4) demonstrated that, following
intravenous infusion of glutamine, HSP70 expression increased
significantly in liver, lung and renal tissues in rats with sepsis
induced by LPS, thereby depressing the inflammatory reaction and
improving outcomes. This indicated that glutamine's effect on the
inflammatory response was due to enhanced HSP70 expression.
However, the data favoring the administration timing
of glutamine (Gln) remains controversial. Many previous studies
investigated the effect of Gln given following the initiation of
sepsis and got different results (4–6).
Besides, Singleton et al (7,9),
Singleton and Wischmeyer (8,10),
and another study (11) applied
Gln several days prior to the initiation of stress reaction via
oral administration or intravenous injection and indicated that Gln
may enhance tissue HSP expression and improve survival rates.
However, this way of Gln administration is not in conformity with
the clinical practice and may increase the costs of healthcare,
thus imposing a burden on patients.
In a latest international randomized controlled
study, Heyland et al (12)
and his colleagues pointed out that giving intravenous Gln within
24 h of presentation to the ICU may actually be harmful for
patients, which represented that in-hospital mortality and 6 month
mortality were significantly increased in patients given
intravenous Gln, compared with those given placebo. Therefore,
whether Gln should be applied to Gln to critical ill patients, as
well as the timing of Gln use are difficult to understand. In the
present study, the authors tried to investigate Gln administrations
just prior to and 1 h following LPS injection, in order to reveal
the best time of Gln administration.
Nevertheless, as Gln is thermally unstable in
solution and generates toxic ammonium ions, its use is restricted.
Without these obvious limitations, Ala-Gln, the synthetic
glutamine-containing dipeptide that may be hydrolyzed into
equimolar amount of Ala and glutamine immediately following
intravenous infusion, exhibits good thermal and aqueous stability
to withstand conditions of sterilization and significant ranges in
pH. Therefore, Ala-Gln may be a suitable source of free glutamine
to treat critically ill patients in clinic. A few previous studies
have investigated whether using Ala-Gln before or after ALI would
have the same protective effect on endotoxin-induced lung injury.
For this reason, in the current study, the aim was to evaluate the
protective effect of Ala-Gln on the expression of HSP70 in rats
with LPS-induced ALI and tried to elucidate which was the best time
to apply Ala-Gln.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of Guangxi Medical University (Nanning, China). All
animals received humane care in compliance with the Principles of
Laboratory Animal Care and the Dutch Law on Experimental Animal
Care.
Animals and groups
Endotoxemia was induced by an intravenous infusion
of Escherichia coli endotoxin. A total of 60 healthy adult
Wistar rats weighing 220–250 g were randomly assigned into 6 groups
(n=10): Control group (C group), LPS-induced shock group (LPS
group), pre-Ala-Gln treated group (A1 group), pre-Gln
treated group (G1 group), post-Ala-Gln treated group
(A2 group) and post-Gln treated group (G2
group). All rats were purchased from Guangxi Medical University
Laboratory Animal Centre (Nanning, China). A total of 30 male and
30 female rats were randomly selected for the present study and
were housed at a temperature of 22±1°C and 55% humidity under a
12-h light/dark cycle with free access to food and water. The C
group was given an intravenous infusion of 28 ml/kg Lactated
Ringer's solution (LR; 07091426; Zhejiang Juneng Lesi
Pharmaceutical Industry Co., Ltd., Zhejiang, China); the LPS group
was injected with 3 ml/kg (6 mg/kg) LPS (L-2880; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) immediately following
administration of 25 ml/kg LR; the A1 and G1
groups were respectively received 4.5% Dipeptiven (25 ml/kg,
equaling 0.75 g/kg glutamine) and 3% Gln 25 ml/kg (0.75 g/kg)
immediately before LPS; the A2 group and G2
groups were separately administered with 4.5% Dipeptiven (25 ml/kg,
equaling 0.75 g/kg glutamine) and 3% Gln 25 ml/kg (0.75 g/kg) 1 h
following LPS. All rats were anesthetized with an intraperitoneal
injection of 30 mg sodium pentobarbital (Nembutal; 50 mg/ml; Abbott
Pharmaceutical Co., Ltd., Lake Bluff, IL, USA) per kg of body
weight, and a supplemental dose (15 mg/kg) was injected, if
necessary. Following anesthesia induced by pentobarbital sodium, an
Insyte Autoguard Shielded IV catheter (BD Biosciences, Franklin
Lakes, NJ, USA) was placed into the right femoral vein for infusion
of fluids and all fluids were infused by micro-pump at the rate of
0.5 ml/min.
Blood samples (1 ml) were taken for analysis, and
cytokine levels were measured before LPS injection (T0)
and 6 h after LPS infusion (T1). Survival rate was
observed at 6 h following administration of LPS. In order to
explore the early protective effect of Ala-Gln on LPS-induced ALI
in rats, surviving animals were euthanized for humane reasons. At
the end of the current study, the rats were anaesthetized with
pentobarbital and sacrificed at T1, and the samples of
pulmonary tissues were harvested. Plasma concentrations of tumor
necrosis factor (TNF)-α, interleukin (IL)-1β and IL-8 at
T0 and T1 were detected by ELISA, and the
lung wet/dry weight ratio and the content of protein in the
bronchoalveolar lavage fluid (BALF) were determined. The extent and
location of cell apoptosis in the bronchus and lung tissues and the
expression of HSP70 were studied by terminal deoxynucleotidyl
transferase dUTP nick-end labeling (TUNEL) technique and western
blot analysis, respectively.
Preparation of alanyl-glutamine
Glutamine was provided as a glutamine-containing
dipeptide, L-alanyl-L-glutamine (Dipeptiven). Dipeptiven (20%,
TD1602; Fresenius SE & Co., KGaA, Bad Homburg, Germany),
containing 20 g N(2)-L-alanyl-L-glutamine, was diluted into
a 4.5% solution with LR. Ala-Gln was yielded 0.75 g/kg per dose of
glutamine. The solutions were filtered with a 0.45 µm filter prior
to administration.
Assessment of histopathological
changes in LPS-induced ALI rats
Tissues from the right side of the lung were fixed
in 4% paraformaldehyde at room temperature for 24 h, embedded in
paraffin and cut into 5 µm slices. The tissues were then stained
with hematoxylin for 5 min and eosin for 2 min at room temperature.
A modified scoring system (13)
was used to assess lung injury by two pathologists (from the
Department of Pathology, Nanfang Hospital, Southern Medical
University, Guangzhou, Guangdong, China), in a blind-control
manner. For each section, 10 random areas of the right lung tissue
were examined at a magnification of ×100. Lung injury was scored
according to the degree of interstitial cellular infiltration,
alveolar protein exudation and tissue hemorrhage. The assessment of
each slide was graded as follows: 0, no injury; 1, mild injury; 2,
moderate injury; 3, severe injury. The sum of each category was
calculated by adding the individual scores from 10 different
microscopic fields. The total lung injury score of each rat was
denoted as the sum of the three individual scores, consisting of
alveolar cellularity, protein exudation and tissue hemorrhage.
Apoptosis detection
The separated tissues (the right upper lungs) were
immersed in 4% formalin for 4 h. Following this, the formalin-fixed
sections were embedded in paraffin wax, and cut into 5 µm sections
by an ultramicrotome. A TUNEL detection kit (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) was used to detect apoptosis, according
to the manufacturer's instructions. Briefly, tissues from the right
upper lungs were fixed in 4% paraformaldehyde at room temperature
for 6 h and permeabilized with proteinase K (10 µg/ml) at 37°C for
10 min. The samples were then transferred into 50 µl reaction
buffer (TdT enzyme 5 µl + labeling safe buffer 45 µl) for a 1 h
incubation at 37°C. Finally, the labeling procedure was stopped by
washing with a PBS solution. These were then stained with
hematoxylin and examined with a light microscope. The dark brown
nuclei indicate TUNEL-positive nuclei, while TUNEL-negative nuclei
were stained blue. Quantitative assessment of apoptotic index (AI)
was calculated by randomly counting 100 cells on each slide
(magnification, ×400). The right lower pulmonary lobes were
harvested, fixed with 4% formalin, embedded in paraffin and cut.
The 5 µm sections were stained with hematoxylin and eosin for light
microscope observation (magnification, ×100).
Wet/dry weight ratio detection
A total of 6 h following LPS or saline
administration, all rats were killed. The left upper lobe of each
lung was weighed and then dried to constant weight at 70°C for 24 h
in an oven. The ratio of lung wet/dry weight was calculated.
Total albumin concentration
determination
The entire right lung was slowly infused three times
by using 4°C saline (1st time, 4 ml; 2nd time, 3 ml; 3rd time, 3
ml; total 10 ml) and withdrawn to obtain BALF. The fluid was
centrifuged at 800 × g and 4°C) to obtain plasma, and the
supernatant was collected. The plasma TNF-α (cat no. E-EL-M0021),
IL-1β (cat no. E-EL-M0037) and IL-8 levels were measured by ELISA
kits (cat no. E-EL-M0045; Shang Bo Science & Technology Co.,
Ltd., Beijing, China) according to manufacturer instructions.
Western blot analysis
The cells were lysed in lysis buffer for 15 sec to
extract proteins. Following centrifuging tissue lysates at 10,000 ×
g at 4°C for 60 min, supernatant was collected and the protein
concentration was measured using a Bio-Rad Protein Assay kit (cat
no. 20010EDU; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Laemmli gel loading buffer was added to 40 µg of protein and boiled
for 5 min, following which proteins were separated on 12% SDS-PAGE
gels, and then blotted onto a polyvinylidene difluoride transfer
membrane (Bio-Rad Laboratories, Inc.) overnight. The blot was
blocked in Tris-buffered saline (TBS) containing 0.1% Tween-20 and
5% dry milk at 37°C for 1 h. The membrane was then incubated in
primary antibody (cat no. BY3624W; 1:400; Shanghai Ke Min
Biotechnology Co., Ltd., Shanghai, China) at 4°C overnight. After
being washed three times in TBS-Tween-20 buffer, a secondary
antibody conjugated to horseradish peroxidase (cat no. BY6276R;
1:10,000; Shanghai Ke Min Biotechnology Co., Ltd.) was added and
agitated at room temperature for 1 h. Proteins were detected via
chemiluminescence ECL reagent (cat no. BY4297R; Shanghai Ke Min
Biotechnology Co., Ltd.) and the signal on the blot was exposed to
an x-ray film. Digital images were captured, and analyzed using the
Bio-Rad Gel-Doc system (Gel Doc 2000™ Documentation system and
Quantity One software version 4.2; Bio-Rad Laboratories, Inc.) to
quantify the results in terms of optical density in densitometry
(equals mean optical density × band area). All blots were
normalized against β-actin to control for protein loading.
Statistical analysis
Data are expressed as mean ± standard deviation and
were analyzed by SPSS software (version, 11.0; SPSS, Inc., Chicago,
IL, USA). A one-way analysis of variance and Student-Newman-Keuls
post hoc test were used to evaluate the differences between the 4
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
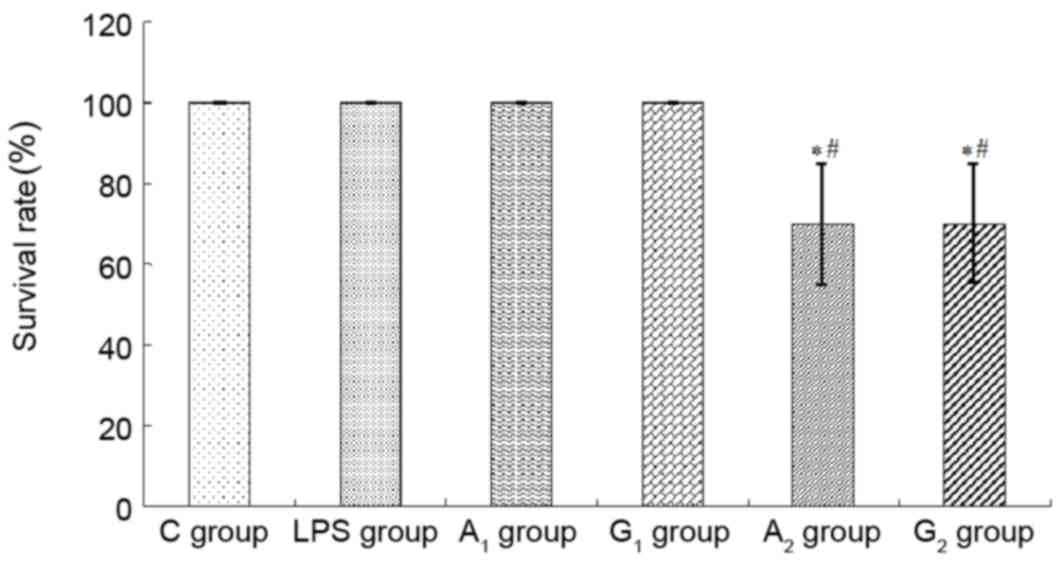
Survival rates
Compared with the C group, the survival rate
presented no significant difference among LPS, A1 and
G1 groups (100%; P>0.05), but it decreased markedly
in A2 and G2 groups (P<0.05). In contrast
with the LPS group, no significant difference was observed between
A1 and G1 groups (P>0.05), but was
obviously decreased in A2 and G2 groups
(P<0.05; Fig. 1).
Pathomorphological changes within rat
lungs
Microscopic findings indicated that the structural
integrity in the lungs did appear not be impaired in the C group.
In the A1 and G1 groups, the morphological
changes including fluid in the alveolar space, protein accumulation
and the infiltration of inflammatory cells and red blood cells,
were less severe than those observed in the LPS, A2 and
G2 groups (Fig. 2).
Table I presents the
semi-quantitative analysis of the rat lung histopathological
scores.
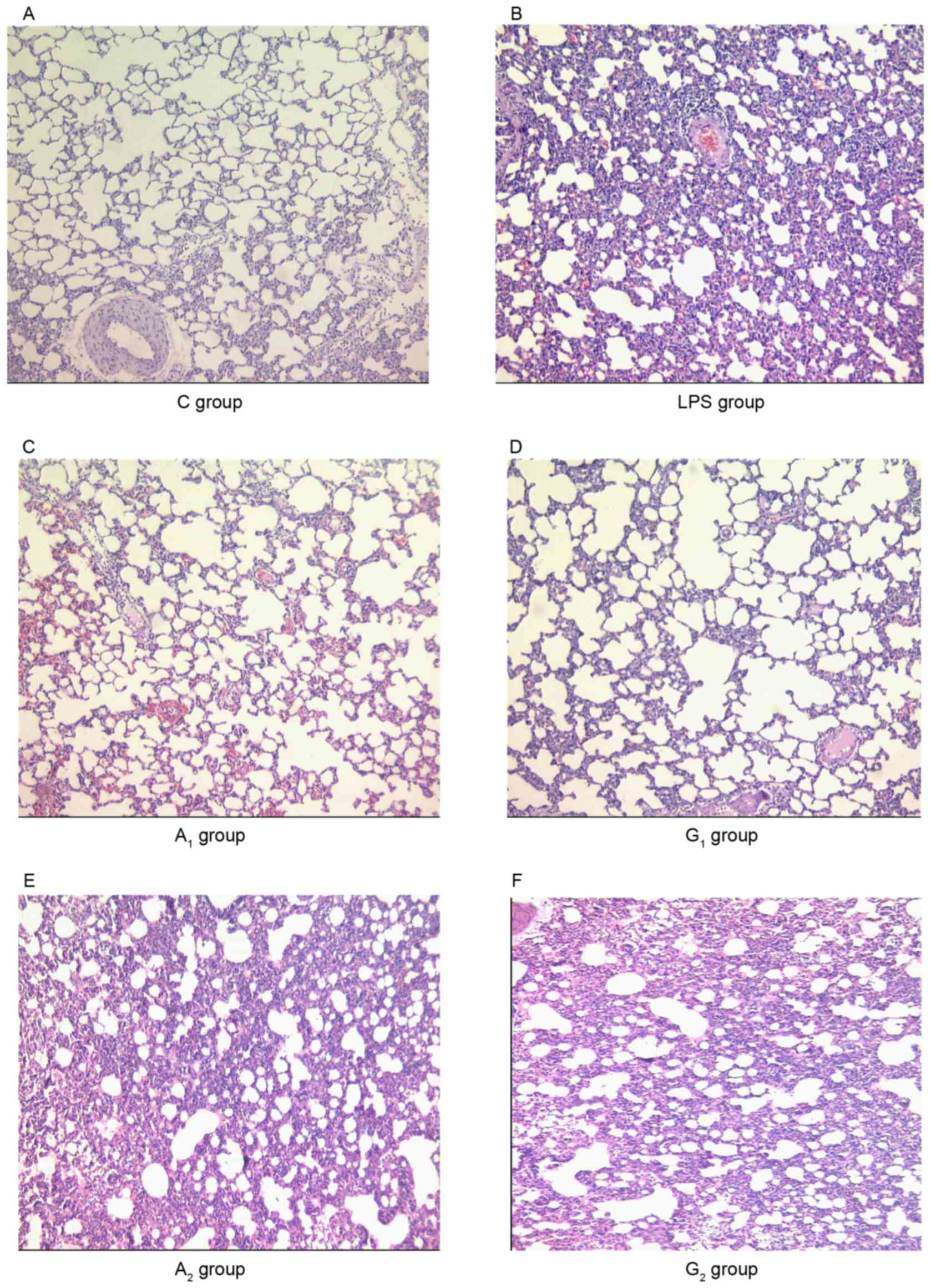 | Figure 2.Histological alterations of the left
lung (magnification, ×100). (A) The pulmonary alveoli presented
normal fine structure; (B, E and F) presented more extensive
pulmonary interstitial edema, alveolar septa damage, inflammatory
cell infiltration, and capillary congestion and hemorrhage. (B-D)
presented mild congestion and telangiectasia. C group, control
group; LPS group, LPS-induced shock group; A1 group,
pre-Ala-Gln treated group; G1 group, pre-Gln treated
group; A2 group, post-Ala-Gln treated group;
G2 group, post-Gln treated group. Data are expressed as
the mean ± standard deviation. LPS, lipopolysaccharide; Ala-Gln,
alanyl-glutamine. |
 | Table I.Effect of glutamine and
alanyl-glutamine on the histopathological scores in
lipopolysaccharide-induced acute lung injury. |
Table I.
Effect of glutamine and
alanyl-glutamine on the histopathological scores in
lipopolysaccharide-induced acute lung injury.
| Group | Cellularity
score | Protein exudation
score | Hemorrhage
score | Total score |
|---|
| C |
6.9±1.2 |
4.0±0.5 |
7.9±2.1 |
18.7±3.5 |
| LPS |
19.0±0.5a |
11.3±0.6a |
17.4±1.3a |
47.2±2.3a |
| A1 |
7.3±1.0b |
4.7±0.8b |
8.8
±0.5b |
20.7±2.2b |
| G1 |
7.1±0.9b |
4.9±1.0b |
8.5±0.3b |
20.6±2.1b |
| A2 |
17.5±0.8 |
11.5±1.0 |
16.9±1.5 |
45.8±3.2 |
| G2 |
17.7±0.7 |
11.2±0.8 |
17.1±1.4 |
46.0±2.9 |
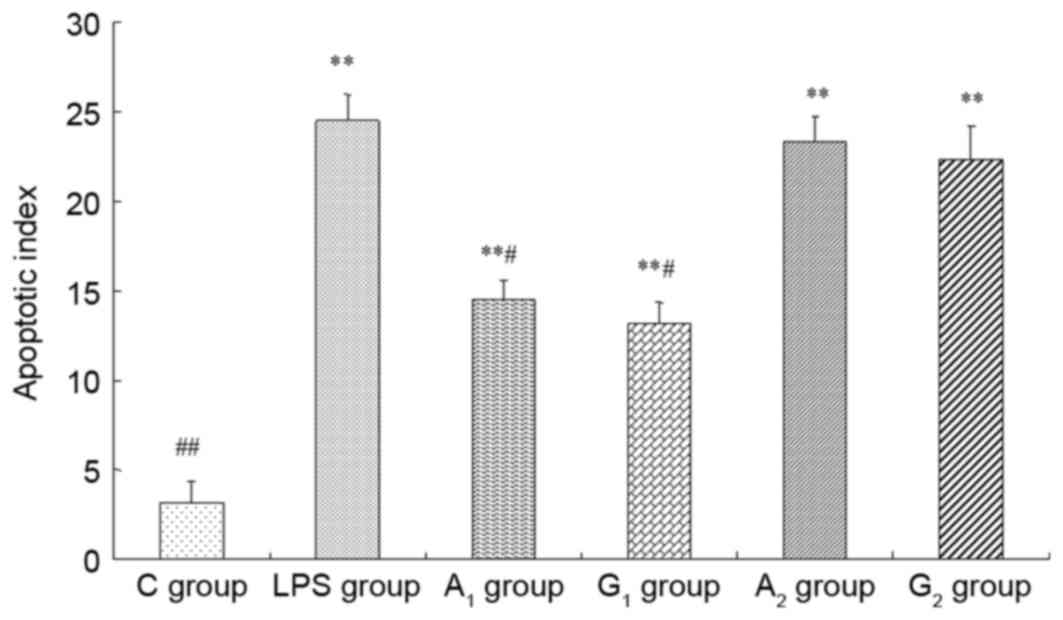
Apoptosis
Under the light microscope, apoptotic cells that
presented pronounced nuclear condensation and the TUNEL-positive
nuclei were deep brown. The AI of LPS, A1,
A2, G1 and G2 groups were
significantly increased, when compared with that of the C group
(P<0.01). However, AI markedly declined in A1 and
G1 groups, when compared with the LPS group (P<0.05).
There was no significant difference in A2 and
G2 groups (P>0.05; Fig.
3).
Wet/dry weight ratio and protein
concentrations in BALF
The wet/dry weight ratio and the total albumin
concentrations obviously increased in A1, G1,
A2 and G2 groups, compared with the C group
(P<0.05 or P<0.01). W/D and protein concentration
significantly decreased when compared with the LPS group
(P<0.05), but no statistical difference between A2
and G2 group was observed (P>0.05; Figs. 4 and 5).
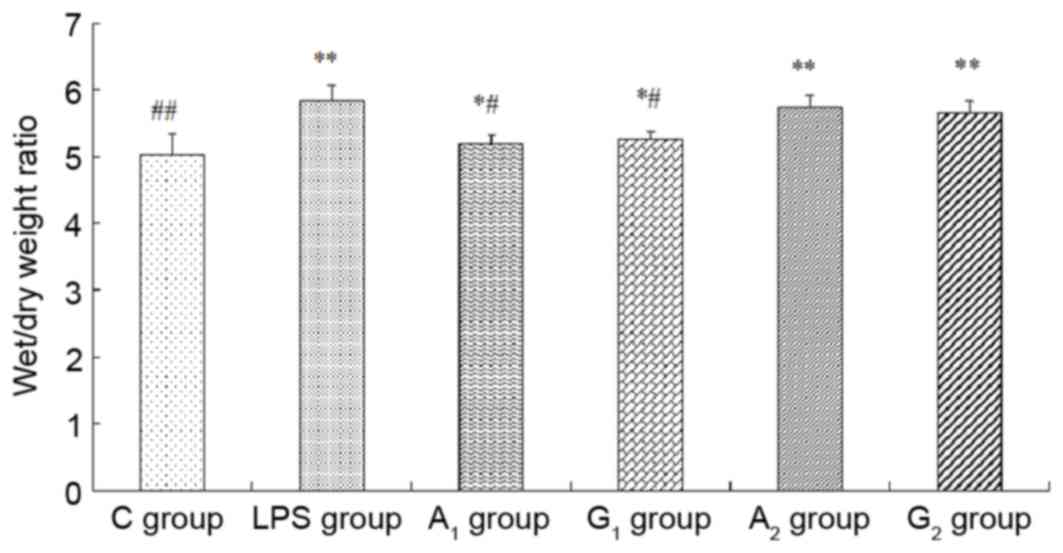 | Figure 4.Examination of wet/dry weight ratio. C
group, Control group; LPS group, LPS-induced shock group;
A1 group, pre-Ala-Gln treated group; G1
group, pre-Gln treated group; A2 group, post-Ala-Gln
treated group; G2 group, post-Gln treated group. Data
are expressed as the mean ± standard deviation. *P<0.05,
**P<0.01 vs. C group; #P<0.05,
##P<0.01 vs. LPS group. LPS, lipopolysaccharide;
Ala-Gln, alanyl-glutamine. |
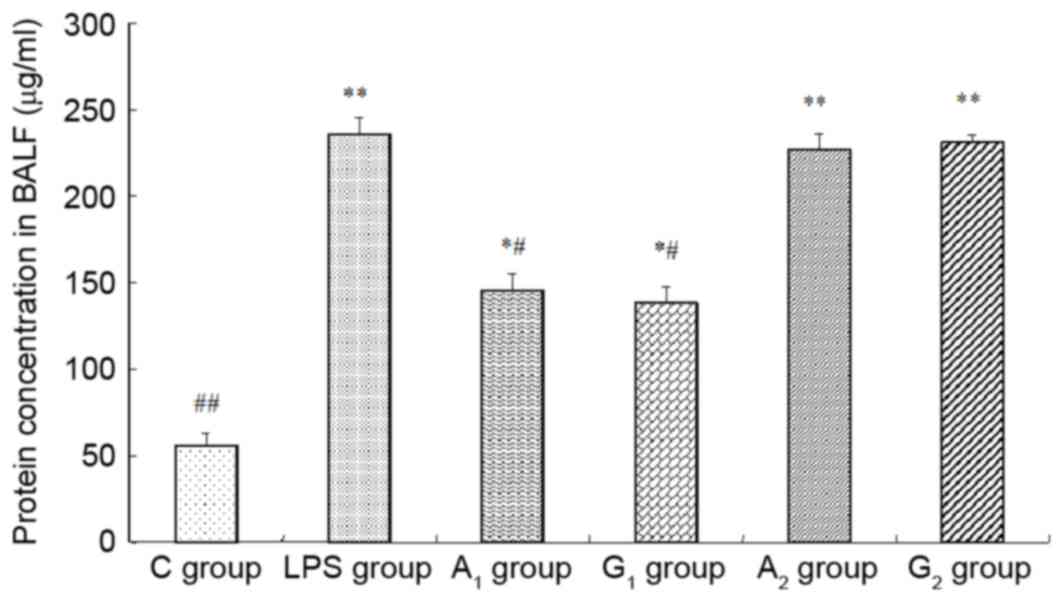 | Figure 5.Protein concentrations in BALF. C
group, control group; LPS group, LPS-induced shock group;
A1 group, pre-Ala-Gln treated group; G1
group, pre-Gln treated group; A2 group, post-Ala-Gln
treated group; G2 group, post-Gln treated group. Data
are expressed as the mean ± standard deviation. *P<0.05,
**P<0.01 vs. C group; #P<0.05,
##P<0.01 vs. LPS group. BALF, bronchoalveolar lavage
fluid; LPS, lipopolysaccharide; Ala-Gln, alanyl-glutamine. |
Plasma concentrations of TNF-α, IL-1β
and IL-8
There was no significant difference in the four
groups at T0 (P>0.05). At 6 h following LPS
administration, the plasma TNF-α, IL-1β, IL-8 levels were increased
except the control group (P<0.01). Ala-Gln pretreatment
significantly decreased the plasma concentrations of TNF-α, IL-1β
and IL-8 in comparison with the LPS and G2 group at
T1 (P<0.05). There was no difference observed between
the LPS group and A2 group 6 h following LPS
administration (P>0.05; Figs.
6–8).
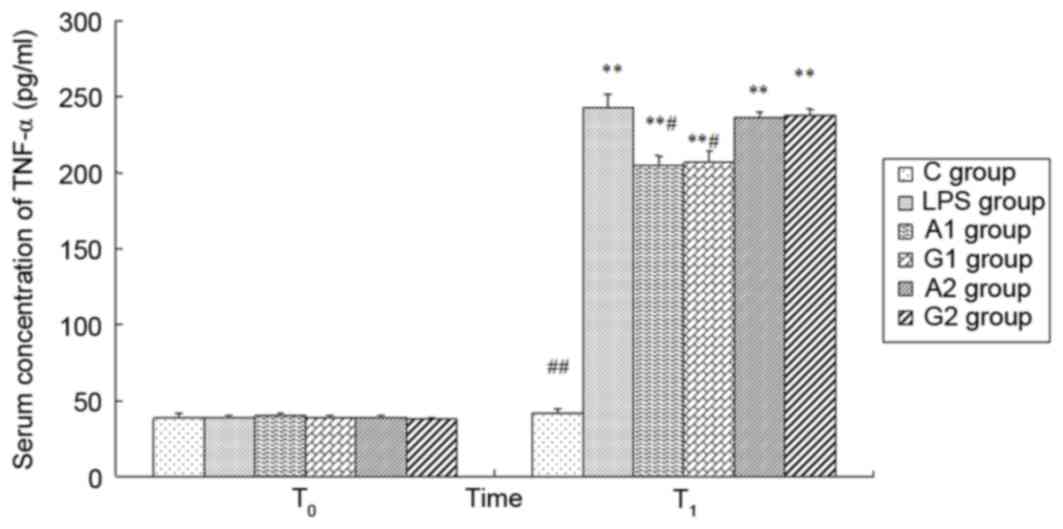 | Figure 6.Plasma concentrations of TNF-α at
different times. T0 represents a time just prior to LPS
administration; T1 represents a time 6 h following LPS
administration. C group, control group; LPS group, LPS-induced
shock group; A1 group, pre-Ala-Gln treated group;
G1 group, pre-Gln treated group; A2 group,
post-Ala-Gln treated group; G2 group, post-Gln treated
group. Data are expressed as the mean ± standard deviation.
**P<0.01 vs. C group; #P<0.05,
##P<0.01 vs. LPS group. TNF-α, tumor necrosis
factor-α; LPS, lipopolysaccharide; Ala-Gln, alanyl-glutamine. |
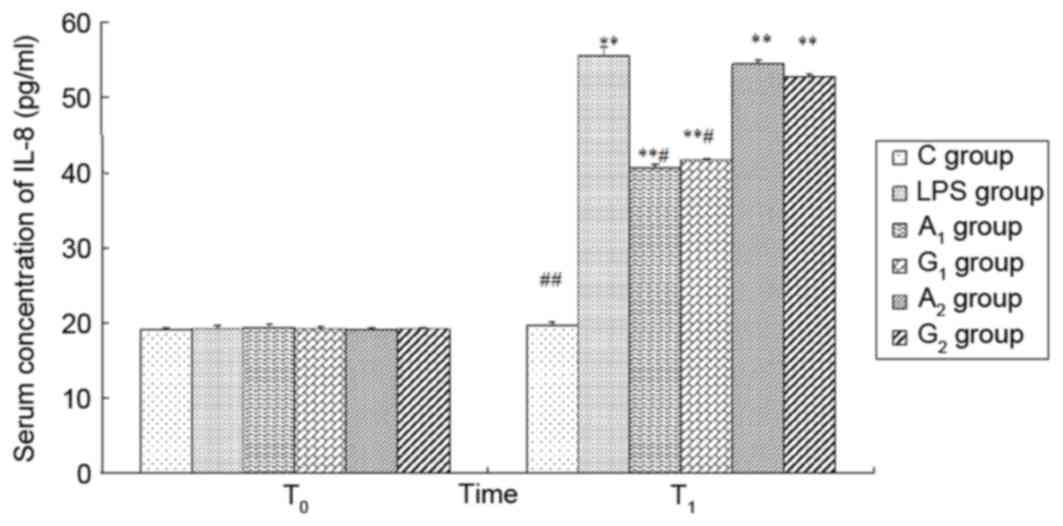 | Figure 8.Plasma concentration of IL-8 at
different times. T0 represents a time just prior to LPS
administration; T1 represents a time 6 h following LPS
administration. C group, control group; LPS group, LPS-induced
shock group; A1 group, pre-Ala-Gln treated group;
G1 group, pre-Gln treated group; A2 group,
post-Ala-Gln treated group; G2 group, post-Gln treated
group. Data are expressed as the mean ± standard deviation.
**P<0.01 vs. C group; #P<0.05,
##P<0.01 vs. LPS group. IL, interleukin; LPS,
lipopolysaccharide; Ala-Gln, alanyl-glutamine. |
Expression of HSP70
Western blot analysis indicated that, compared with
the control group, the optical density of the A1 treated
group increased (P<0.05), yet decreased significantly in LPS
group and A2 treated group (P<0.05). The optical
density was almost the same in LPS group and A2 treated
group (P>0.05; Fig. 9).
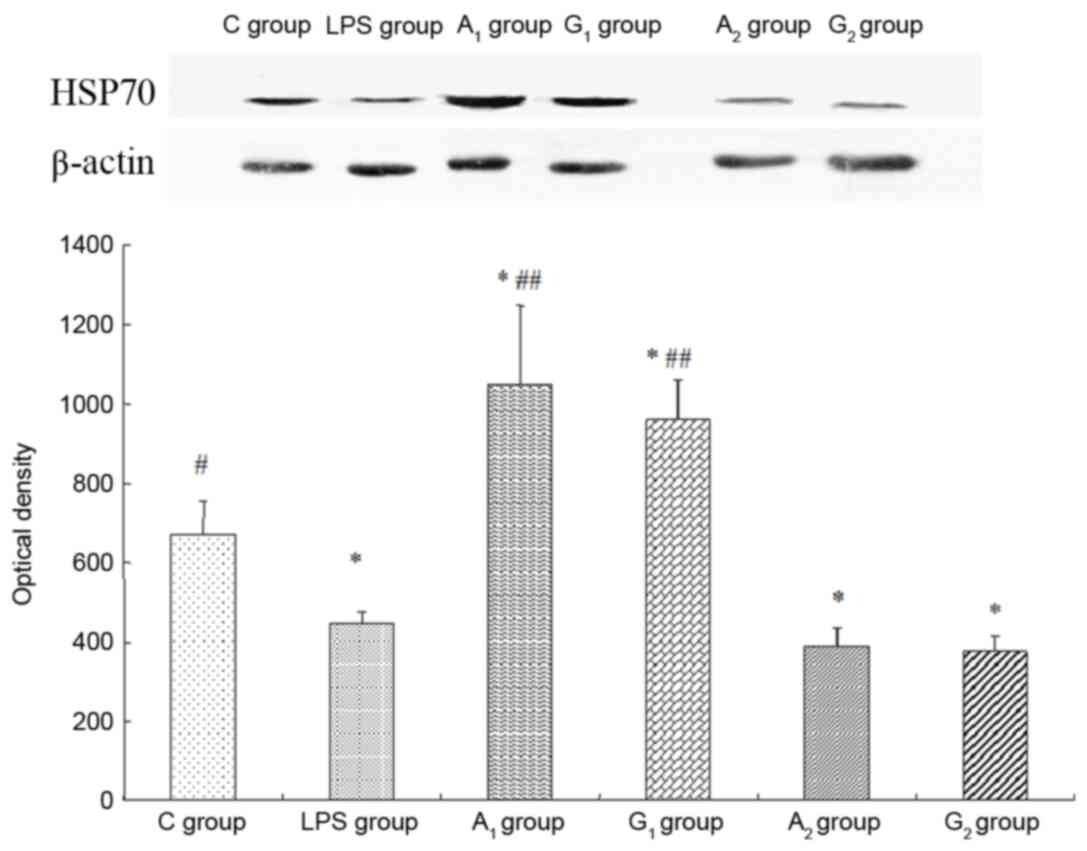 | Figure 9.Expression of HSP70 detected by
western blot analysis. Intravenous injection of Ala-Gln prior to
LPS administration upregulates lung HSP70 expression. Optical
density is expressed as the mean ± standard deviation. The upper
band and the lower graph represented western blotting of HSP70 and
the optical density of HSP70 in the six groups. All blots were
normalized against β-actin to control for protein loading. C group,
control group; LPS group, LPS-induced shock group; A1
group, pre-Ala-Gln treated group; G1 group, pre-Gln
treated group; A2 group, post-Ala-Gln treated group;
G2 group, post-Gln treated group. *P<0.05 vs. C
group; #P<0.05, ##P<0.01 vs.
LPS-induced shock group. HSP, heat shock protein; Ala-Gln,
alanyl-glutamine; LPS, lipopolysaccharide. |
Discussion
In the present paper, a single intraperitoneal
injection of Escherichia coli endotoxin was used to establish an
ALI model and Ala-Gln was infused for ultra-early intervention.
Results from the current study demonstrated that administering
Ala-Gln immediately prior to an LPS injection significantly
improved the inflammatory response in septic shock, and the
mechanisms of protection may be related to the increase in HSP70
expression and the attenuation of plasma IL-8, TNF-α and IL-1β
concentrations.
The results of the current study demonstrated that
pretreatment of Ala-Gln significantly improved vascular response to
catecholamine vasoconstrictors and that the effect of Ala-Gln is
associated with its capacity to induce HSP70 expression, attenuate
release of pro-inflammatory cytokines and oxidize species
production following septic shock.
The acute inflammatory reaction in lungs depends on
nuclear transcription factor (NF-κB) with the release of cytokines
(TNF-α, IL-1β and IL-8) and chemokines. NF-κB, a nuclear
transcription factor involved in the control of immune and
inflammatory reactions, developmental processes, cellular growth
and apoptosis, integrates into the specific NF-κB binding sites in
promoters to activate pro-inflammatory genes, thereby enhancing the
transcription of many inflammatory proteins (14). HSP70, a cellular protective
protein, attenuates the inflammatory response mainly via decreased
NF-κB activation to reduce pro-inflammatory cytokine expression
involving TNF-α, IL-1β and IL-8. Malhotra and Wong (15) demonstrated that HSP70 degraded the
activation of NF-κB by enhancing the expression of I-κB (inhibitory
proteins that regulate the activity of NF-κB) and by inhibiting the
phosphorylation of I-κB. The potential mechanisms of how HSP70
inhibits NF-κB lie in three aspects: Depression of the
phosphorylation of I-κB and the activation of IKK; induction of
expression of I-κB at the mRNA level; competitive inhibition of
NF-κB entering the nucleus through the nuclear pore to depress the
expression of TNF-α, IL-1β and IL-8 genes. Thus, as a consequence
of suppression of NF-κB activity, the inflammatory response may
decrease.
According to the present research, the more HSP70
that is expressed, the less inflammatory cytokines (TNF-α, IL-1β,
IL-8) and less lung damage (pathologic changes and apoptosis) is
exhibited in pre-Ala-Gln and pre-Gln treated groups, when compared
with LPS, A2 and G2 groups (P<0.05). However, there was no
significant difference among the LPS, A2 and G2 groups. It seems
that intravenous infusion of Ala-Gln or Gln 1 h following LPS could
not give enough protection to prevent aggravation of ALI.
Conversely, injection Ala-Gln or Gln immediately before LPS
administration may inhibit the inflammatory reaction, in addition
to contributing to the protection of rats against ALI by enhancing
the expression of HSP70. Until recently, whether the increase in
HSP expression following the onset of acute lung injury exerts a
protective effect is still not known. So far, there has been
relatively little research in this area. Following the initiation
of endotoxemia produced by LPS, Chu et al (16) immediately applied heat stress to
rats and demonstrated that 12 h following sepsis, the survival rate
of the heated group is significantly higher than the LPS group,
which indicated that HSP induced after the onset of endotoxemia may
provide protection. Nevertheless, DeMeester et al (17) suggested that induction of a
subsequent heat stress in cells damaged by inflammation can
precipitate cell death by apoptosis. Bai et al (18) administered Gln 1 h following LPS
injection and observed the ultrastructural changes in lung tissue
under a transmission electron microscope 4 h later. They
demonstrated that there was no obvious improvement in lung
ultrastructure, indicating that infusion of Gln after the onset of
inflammation presented on protective effect. In the current study,
injecting Ala-Gln or Gln 1 h after LPS could not increase the
expression of HSP70, inhibit the release of TNF-α, IL-1β and IL-8
or reduce apoptosis in injured cells. Therefore, applying Ala-Gln
or Gln after sepsis cannot be involved in a protective role in ALI.
The difference between pre- and post Ala-Gln treated groups may
depend on the time of Ala-Gln usage. Researchers identified that
inhibition of NF-κB at the onset of inflammation resulted in a
decreased inflammatory response (19), yet suppression of NF-κB during
inflammation may protract the inflammatory reaction. The reasons
for the difference occurring between the two groups may be related
to the idea that the inflammatory reaction may be increased before
intravenous injection of Ala-Gln, thus the expression of HSP70 was
depressed by sepsis and then the activity of NF-κB could not be
degraded. Nevertheless, Scharte et al (20) had a reverse result in their
investigation. They indicated that Ala-Gln treated sheep had a
greater increase in myocardial HSPs following endotoxemia. The
difference between the present study and that of Scharte may be
related to the dose of Ala-Gln and the time of HSP detection.
Therefore, the mechanisms of whether the inflammatory reaction can
suppress the expression of HSP70 require further investigation.
Glutamine, the most common non-essential amino acid
in the human body, is an important nitrogen donor for the formation
of urea and purines (which are essential to make DNA and RNA)
(21). When confronted with any
type of physical stress, patients tend to be hyper-metabolic and
use up the body's store of glutamine. In these severe conditions,
glutamine has been suggested to beneficially activate the heat
shock factors in order to induce the expression of the
cytoprotective HSP70 to increase anti-inflammatory reactivity
following endotoxemia (22–25).
As yet, blood and tissue levels of glutamine are rapidly depleted
under catabolic events and the body is therefore unable to create
enough glutamine to meet its needs to initiate immune function,
enhance the synthesis of protein and reduce the loss of amino
acids. Thus, glutamine has to be supplemented via intravenous
infusion. However, glutamine is thermally unstable in solution,
easily breaks down and generates free ammonium ions, which are very
toxic to cells (26). As a result,
glutamine has been proven difficult to use in the clinic. Without
these obvious limitations, Ala-Gln, a highly soluble and stable
glutamine dipeptide that can withstand conditions of sterilization
and significant rages in pH, is a commonly used substrate to take
the place of glutamine in treating critical ill patients (27).
ALI is usually caused by a stimulus of local or
systemic inflammation, primarily sepsis. Increase of microvascular
permeability, leakage of protein-rich fluid, infiltration of
neutrophils and formation of a hyaline membrane are major
pathological features of ALI. Thus, early prevention or
intervention of the excessive inflammatory reaction is crucial for
ALI treatment. In summary, intravenous administration of Ala-Gln
before LPS has protective effects on ALI via enhancing the
expression of HSP70 to alleviate excessive inflammatory reaction.
However, the actual mechanisms of how Ala-Gln protects rats against
ALI still needs more study.
Pre-administration of Ala-Gln just before LPS can
effectively protect the lung by enhancing HSP70 expression, but
delayed administration cannot protect LPS induced lung injury.
Acknowledgements
This research was supported by the Department of
Science and Technology of Guangxi Zhuang Autonomous Region Basic
Research Fund (grant no. 0236022).
References
|
1
|
Bernard GR, Artigas A, Brigham KL, Carlet
J, Falke K, Hudson L, Lamy M, Legall JR, Morris A and Spragg R: The
American-European Consensus Conference on ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Margulis BA, Sandler S, Eizirik DL, Welsh
N and Welsh M: Liposomal delivery of purified heat shock protein
hsp70 into rat pancreatic islets as protection against interleukin
1 beta-induced impaired beta-cell function. Diabetes. 40:1418–1422.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White DJ, Carlson D, Ordway GA and Horton
JW: Protective role of heat stress in burn trauma. Crit Care Med.
32:1338–1345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wischmeyer PE, Kahana M, Wolfson R, Ren H,
Musch MM and Chang EB: Glutamine induces heat shock protein and
protects against endotoxin shock in the rat. J Appl Physiol (1985).
90:2403–2410. 2001.PubMed/NCBI
|
|
5
|
Jing L, Wu Q and Wang F: Glutamine induces
heat-shock protein and protects against Escherichia coli
lipopolysaccharide-induced vascular hyporeactivity in rats. Crit
Care. 11:R342007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliveira GP, Oliveira MB, Santos RS, Lima
LD, Dias CM, Saber AM Ab', Teodoro WR, Capelozzi VL, Gomes RN,
Bozza PT, et al: Intravenous glutamine decreases lung and distal
organ injury in an experimental model of abdominal sepsis. Crit
Care. 13:R742009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singleton KD, Beckey VE and Wischmeyer PE:
Glutamine prevents activation of NF-kappaB and stress kinase
pathways, attenuates inflammatory cytokine release, and prevents
acute respiratory distress syndrome (ARDS) following sepsis. Shock.
24:583–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singleton KD and Wischmeyer PE:
Glutamine's protection against sepsis and lung injury is dependent
on heat shock protein 70 expression. Am J Physiol Regul Integr Comp
Physiol. 292:R1839–R1845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singleton KD, Serkova N, Beckey VE and
Wischmeyer PE: Glutamine attenuates lung injury and improves
survival after sepsis: Role of enhanced heat shock protein
expression. Crit Care Med. 33:1206–1213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singleton KD and Wischmeyer PE: Oral
glutamine enhances heat shock protein expression and improves
survival following hyperthermia. Shock. 25:295–299. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H
and Matsuda H: Preoperative glutamine administration induces
heat-shock protein 70 expression and attenuates cardiopulmonary
bypass-induced inflammatory response by regulating nitric oxide
synthase activity. Circulation. 106:2601–2607. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heyland D, Muscedere J, Wischmeyer PE,
Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG, et al:
Canadian Critical Care Trials Group: A randomized trial of
glutamine and antioxidants in critically ill patients. N Engl J
Med. 368:1489–1497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristof AS, Goldberg P, Laubach V and
Hussain SN: Role of inducible nitric oxide synthase in
endotoxin-induced acute lung injury. Am J Respir Crit Care Med.
158:1883–1889. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun D, Chen D, Du B and Pan J: Heat shock
response inhibits NF-kappaB activation and cytokine production in
murine Kupffer cells. J Surg Res. 129:114–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malhotra V and Wong HR: Interactions
between the heat shock response and the nuclear factor-kappaB
signaling pathway. Crit Care Med. 30:(1 Supp). S89–S95. 2002.
View Article : Google Scholar
|
|
16
|
Chu EK, Ribeiro SP and Slutsky AS: Heat
stress increases survival rates in lipopolysaccharide-stimulated
rats. Crit Care Med. 25:1727–1732. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeMeester SL, Buchman TG and Cobb JP: The
heat shock paradox: Does NF-kappaB determine cell fate? FASEB J.
15:270–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai T, Sun YH and Wang JK: Effect of
glutamine administered at different times on endotoxin-induced
acute lung injury in rats. J China Med Univ. 36:418–420. 2007.
|
|
19
|
Kaplan J, Nowell M, Chima R and Zingarelli
B: Pioglitazone reduces inflammation through inhibition of NF-κB in
polymicrobial sepsis. Innate Immun. 20:519–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scharte M, Baba HA, Van Aken H, Schulzki
C, Meyer J, Goeters C and Bone HG: Alanyl-glutamine dipeptide does
not affect hemodynamics despite a greater increase in myocardial
heat shock protein 72 immunoreactivity in endotoxemic sheep. J
Nutr. 131:1433–1437. 2001.PubMed/NCBI
|
|
21
|
Bonet A and Grau T: Glutamine, an almost
essential amino acid in critically ill patient. Med Intensiva.
31:402–406. 2007.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al:
Essential role of the mitochondrial apoptosis-inducing factor in
programmed cell death. Nature. 410:549–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmad S, White CW, Chang LY, Schneider BK
and Allen CB: Glutamine protects mitochondrial structure and
function in oxygen toxicity. Am J Physiol Lung Cell Mol Physiol.
280:L779–L791. 2001.PubMed/NCBI
|
|
24
|
Roth E, Oehler R, Manhart N, Exner R,
Wessner B, Strasser E and Spittler A: Regulative potential of
glutamine-relation to glutathione metabolism. Nutrition.
18:217–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armeni T, Ghiselli R, Balercia G, Goffi L,
Jassem W, Saba V and Principato G: Glutathione and ultrastructural
changes in inflow occlusion of rat liver. J Surg Res. 88:207–214.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tritsch GL and More GE: Spontaneous
decomposition of glutamine in cell culture media. Exp Cell Res.
28:360–364. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto Y, Kume M and Yamaoka Y:
Implications of heat shock proteins during liver surgery and liver
perfusion. Recent Results Cancer Res. 147:157–172. 1998. View Article : Google Scholar : PubMed/NCBI
|