Introduction
Prostate cancer is the most common diagnosed male
cancer in western countries (1)
and big variation of the prostate cancer incidence exists among
different populations (2,3). Although prostate cancer has
relatively low mortality compared with other cancers, its high
incidence makes prostate cancer the second leading cause of
cancer-related death in USA (3,4).
Patients have multiple active treatment options for prostate
cancer, such as radical prostatectomy, radiotherapy, vaccine
treatment and androgen deprivation therapy (ADT) (3,5).
Using these methods, sound effects have been achieved in early
stage prostate cancer, showing apparently extended survival time.
However, for late stage or metastatic prostate cancer, ADT drug
resistance and recurrence often occur. In these cases, chemotherapy
becomes a common option (6).
However, more clinical interventions are required due to moderate
efficacy of chemotherapies.
Immunotherapy for cancers obtained increased
attention due to the knowledge that tumor cells can be control by
immune response and positive effects that were observed in some
cancers (7). It has been widely
accepted that activated host cytotoxic CD8+ T cells can
eliminate tumor cells once infiltrate into tumor tissue.
Consistently, CD8+ tumor-infiltrating lymphocytes and a
high CD8+/regulatory T (Treg) cell ratio are associated with
positive prognosis in cancers, such as ovarian cancer (8), andcolorectal cancer (9), while CD8+ T cell
impairment and increase of Treg cells are associated with poor
prognosis of cancer patients (10). However, in tumor tissue, effective
T cell immune response is usually absent while abnormally increased
activity of inhibitory immune checkpoint signaling pathways are
widely observed. Increased expression of programmed death-1
(PD-1)/PD-ligand 1 (PD-L1) and PD-L2 axisand cytotoxic lymphocyte
antigen (CTLA-4)/CD28 system depressed CD8+ T cell
activity in tumor microenvironment (7). Thus, refining T cell immune response
in tumor microenvironment could be a potential way of prostate
cancer treatment.
Several drugs targeting the T cell inhibitory
checkpoint signaling pathways have been used in clinic application
and promising effects have been observed. Pembrolizumab and
ipilimumab, the respective antibodies targeting PD-1 and CTLA-4,
have been approved by the US Food and Drug Administration for
treating advanced melanoma patients (7,11,12).
Around 20% of advanced melanoma patients got extended survival time
of at least 3 years after ipilimumab treatment (13). What's more, combination of
conventional chemotherapies were considered as a synergistic
approach of immune checkpoint blockades (14–16).
However, whether immune checkpoint blockades can benefit prostate
cancer patients is still unclear. Here, we aimed to evaluate the
therapeutic values of immune checkpoint blockades and potential
synergistic effects of chemotherapy immune checkpoint blockade in
pre-clinical prostate cancer models.
Materials and methods
Cell culture
We purchased mouse prostate cancer cell lines,
PTEN-CaP8 and PNEC30, and human prostate cancer cell lines, DU145
and PC3 from ATCC. PTEN-CaP8 cells were cultured using DMEM medium
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St.
Louis, MO, USA), 100 U/ml of penicillin and 100 µg/ml of
streptomycin, 25 µg/ml bovine pituitary extract (BPE), 5 µg/ml
bovine insulin and 6 ng/ml recombinant epidermal growth factor
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). PNEC30 cells
were cultured using Neural Progenitor Basal Medium (NPBM)
supplemented with 10% FBS, 100 U/ml of penicillin and 100 µg/ml of
streptomycin, 0.3% BPE, and additives that are supplied with the
NPMM Bullet Kit (Lonza, Verviers, Belgium). DU145 and PC3 cells
were cultured by DMEM medium supplemented with 10% FBS and 100 U/ml
of penicillin and 100 µg/ml of streptomycin. When the cells grew to
85% confluent, they were sub-cultured.
Patient samples
A total number of 100 prostate patients who got
surgery treatment during January 2001 to December 2004 in Tianjin
Nankai Hospital were included in our study. We collected the
formalin-fixed, paraffin-embedded (FFPE) tumor tissues ofall cases
with informed consents assigned by each patient. All patients
didn't take any radiotherapy or chemotherapy before surgery. This
study was approved by the ethics committee of Tianjin Nankai
Hospital. Clinicopathological characteristics of these samples were
summarized in Table I. AJCC cancer
staging manual 7th edition was used as the criteria for TNM
classification. Gleason gradewas used to evaluate the
differentiation of tumor tissues. Follow-up of these patients
started from the date of surgery and ended at December 2014 and
overall survival was the interval between the date of death and the
date of surgery. Patients died due to other reasons than prostate
cancer were excluded from this study.
 | Table I.Relationship between the number of
CD8+ T cells and clinicopathological features of
prostate cancer. |
Table I.
Relationship between the number of
CD8+ T cells and clinicopathological features of
prostate cancer.
|
| CD8+ T
cells |
|
|---|
|
|
|
|
|---|
| Features | Low (≤7) (%) | High (>7)
(%) | P-value |
|---|
| Age |
|
| 0.542 |
|
<65 | 33 (62.3) | 20 (37.7) |
|
|
≥65 | 32 (68.1) | 15 (31.9) |
|
| Gleason
scoring |
|
| 0.723 |
|
<6 | 50 (64.1) | 28 (35.9) |
|
| ≥6 | 15 (68.2) | 7 (31.8) |
|
| T Stage |
|
| 0.304 |
|
T1+T2 | 8 (53.3) | 7 (46.7) |
|
|
T3+T4 | 57 (67.1) | 28 (32.9) |
|
| Lymph node |
|
| 0.402 |
|
N0-N2 | 43 (62.3) | 26 (37.7) |
|
|
N3-N4 | 22 (71.0) | 9 (29.0) |
|
| Metastasis |
|
| 0.295 |
|
Negative | 63 (64.3) | 35 (35.7) |
|
|
Positive | 2 (100.0) | 0 (0.0) |
|
| TNM stage |
|
| 0.013 |
|
I+II | 24 (52.2) | 22 (47.8) |
|
|
III+IV | 41 (75.9) | 13 (24.1) |
|
| Survival
status |
|
| 0.002 |
|
Alive | 25 (50.0) | 25 (50.0) |
|
|
Dead | 40 (80.0) | 10 (20) |
|
Immunohistochemistry
Immunohistochemistry (IHC) was performed to evaluate
the expression of CD8 in the tumor tissue of prostate cancer
patients. All the procedures followed the standard IHC procedures.
Briefly, FFPE tissue samples were deparaffinized within xylene and
rehydrated in gradient ethanol. Then tissue samples wereincubated
in 1X Reveal Decloaker (Biocare Medical, LLC., Concord, CA, US) at
120°C for 45 min for antigen retrieval and reducing non-specific
background staining. After washing the slides with PBST, 3%
hydrogen peroxide was added for incubation in dark to quench the
endogenous peroxidase within the cells. Then, washed the slides
again for 3 times using PBST and block them at room temperature for
15 minusing 5% bovine serum albumin. Subsequently, mouse monoclonal
anti-human CD8 antibody (1:100; Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA) was added for incubation overnight at 4°C.
Then horseradish peroxidase (HRP) linked secondary antibody
(1:1,000; Abcam, Cambridge, UK) was added for incubation at room
temperature for 1 h. Staining was completed by incubation with DAB
for 5 min. Then sample slides were mounted immediately and detected
under light microscope. Five fields of each slide were selected for
observation, and the final score was determined based on the
average number (AN) of positive cells in each field: High
expression if AN>7, low expression if AN≤7.
Cytokine assay
We detected the expression of cytokines (IFN-γ,
PD-L1, IL-2, CXCL-12, TGF-β and IL-10) in xenograft mouse prostate
tumor tissue using cytokine-specificbead-based assay (BioLegend,
Inc., London, UK). Prostate tumor tissue from xenograft mouse model
treated with different drugs (saline, oxaliplatin, anti-PD-1
antibody (Ab), or the combination of Oxaliplatinand anti-PD-1 Ab)
were cut, minced and filtered for cell precipitation. Then, protein
extraction of these cell precipitations was performed using RIPA
buffer under the presence of protein inhibitor. Protein
concentration was measured using BCA protein assay followed by
protein concentration normalization. The subsequent processes of
bead-based assays were performed following the manufacturer's
instruction. Triple measurements were conducted for each sample and
the mean values were used for final analysis.
Fluorescence-activated cell sorter
(FACS) analysis
FACS analysis was used to measure the number of
CD8+ T cells (CD3+, CD4−,
CD8+), Treg cells (CD3+, CD4+,
CD8− and CD25+) and expression of
calreticulin (CRT) and staining of DAPI. All of the fluorescence
conjugated primary antibodies were purchased from BioLegend, Inc.
and Abcam. After harvested, the cells were incubated with primary
antibodies for 20 min at 4°C. After washed with PBS for three
times, the stained cells were analyzed on BDFACSCanto II equipment
(BD Biosciences, San Diego, CA, USA). Data visualization was
performed using FlowJo software.
Xenograft mouse model
A subcutaneous prostate cancer model was established
using five-week-old female NOD/SCID nude mice (18–20 g; Shanghai
SLAC Laboratory Animal Co., Ltd.) with PNEC30 to analyze the in
vivo activity of different treatments. All mice were kept under
specific pathogen-free environment with a 12-h light-dark cycle,
standard food, and free access to autoclaved water. A total of
5×106 PNEC30 cells were inoculated subcutaneously to
flanks of the mice. All treatments started one week later of tumor
seeding. Oxaliplatin (Oxa) and anti-PD-1 antibody were injected
intraperitoneally once a week for 3 weeks (2.5 and 3 mg/kg,
respectively). Tumor size and body weight were measured weekly.
Tumor volume was calculated according to the following formula:
tumor volume = length × width2 × p/6. Development of more than 20%
body weight loose, serious ulceration and any other symptoms of
distress were considered as death.
Cell viability
Cell viability assay was performed using Cell
Counting kit 8 (CCK-8; Sigma-Aldrich). A total number of
1.0×104 cells were seeded in each well of 96-well plate.
The cells of different groups were culture with 100 µl appropriate
medium accordingly for 24 h, followed by different treatments for
24 h. Then, 10 µl of CCK-8 solution was added to each plate for
incubation of 1 h. At last, the absorbance at 450 nm was measured
by MRX II microplate reader (Dynex Technologies, West Sussex, UK).
The final results were calculated by normalizing each OD value to
that of the control group.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was
performed to measure the expression of high mobility group box 1
(HMGB1) protein in prostate cancer cell lines PETN-Cap8 and PNEC30
using ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
Every procedure was performed following the manufacturer's
instruction. The total protein normalization is based on the BCA
assay. Two people read one case blindly without knowing the clinic
data.
Statistical analysis
GraphPad Prism software (GraphPad Software, Inc., La
Jolla, CA, USA) and SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) were used for statistical analysis and data visualization.
Statistical difference of comparisons was analyzed by t-test,
Chi-square test, or one-way ANOVA appropriately according to the
data characteristics. Bonferroni's pairwise comparison was used to
analyze the difference between different treatments. Kaplan-Meier
method and log-rank test were used for survival analysis and
evaluating the difference between different cohorts, respectively.
Multivariate Cox regression model was used for determine the
independent predictors of survival of prostate cancer patients. The
detail is as reported before (17). A two-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
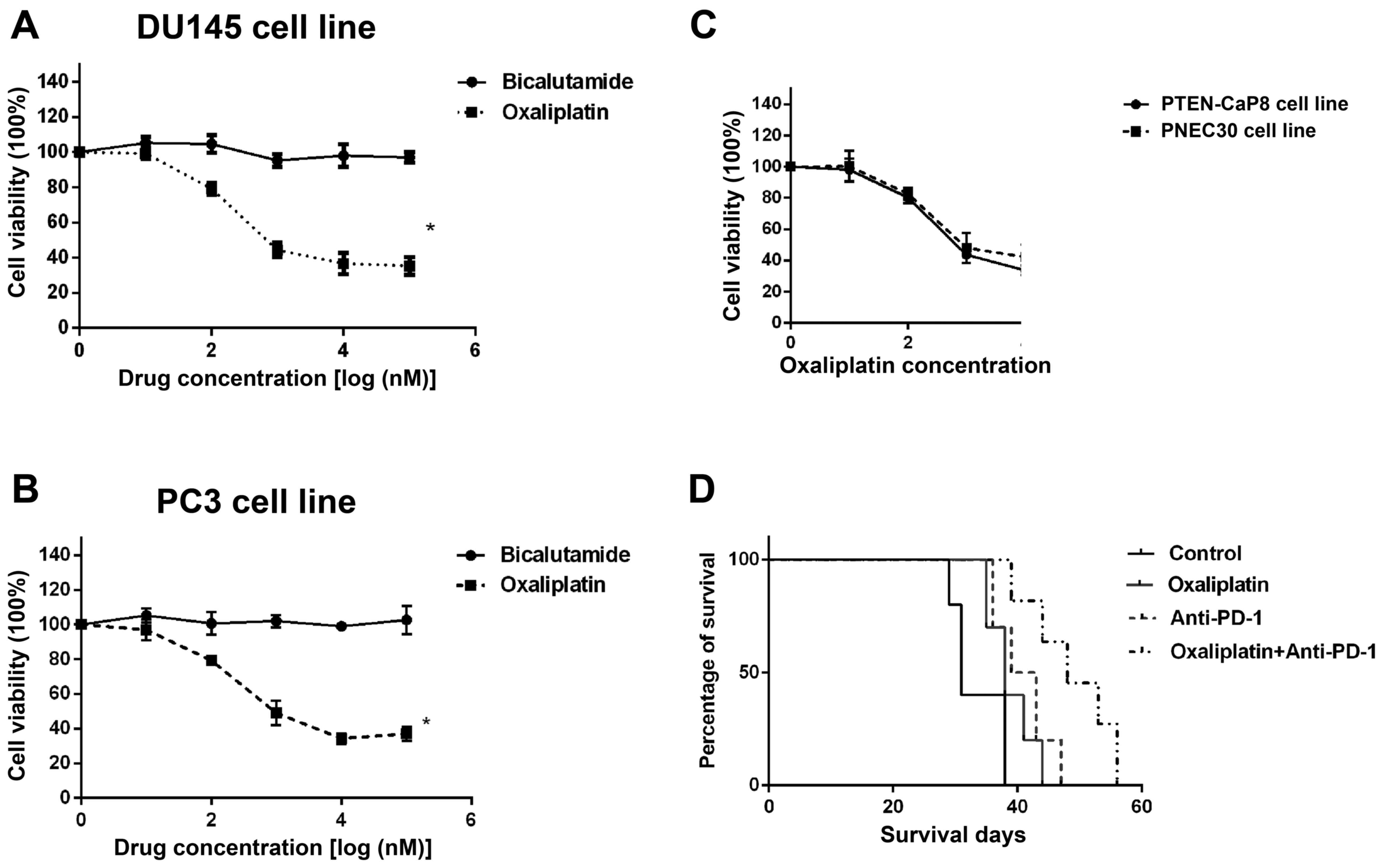
Oxaliplatin sensitizes the prostate
cancer cells to immune checkpoint blockade therapy
ADT therapy is a classic therapy for prostate
cancer, but some patients got resistance to it. Chemotherapy is
often chosen to treat these patients with high ADT resistance. Here
we treated two human prostate cancer cell lines DU145 and PC3 with
bicalutamide andoxaliplatin. As shown in Fig. 1A and B, these two human prostate
cancer cell lines were highly resistant to Bicalutamide, but
sensitive to oxaliplatin treatment. This indicates that
chemotherapy might be a choice for the castration-resistant
prostate cancer patients. Then we also confirmed the effect of
oxaliplatinin two murine prostate cancer cell lines PTEN-Cap8 and
PNEC30 (Fig. 1C). In prostate
cancer xenograft mouse model, we used oxaliplatin treatment in
combination with anti-PD-1 Ab. Interestingly, the mice treated by
oxaliplatin plus anti-PDAb got the best survival compared with the
mice accepted Oxa or anti-PD Ab single treatment (Fig. 1D), suggesting that the chemotherapy
might sensitize the prostate cancer to anti-PD-1 treatment.
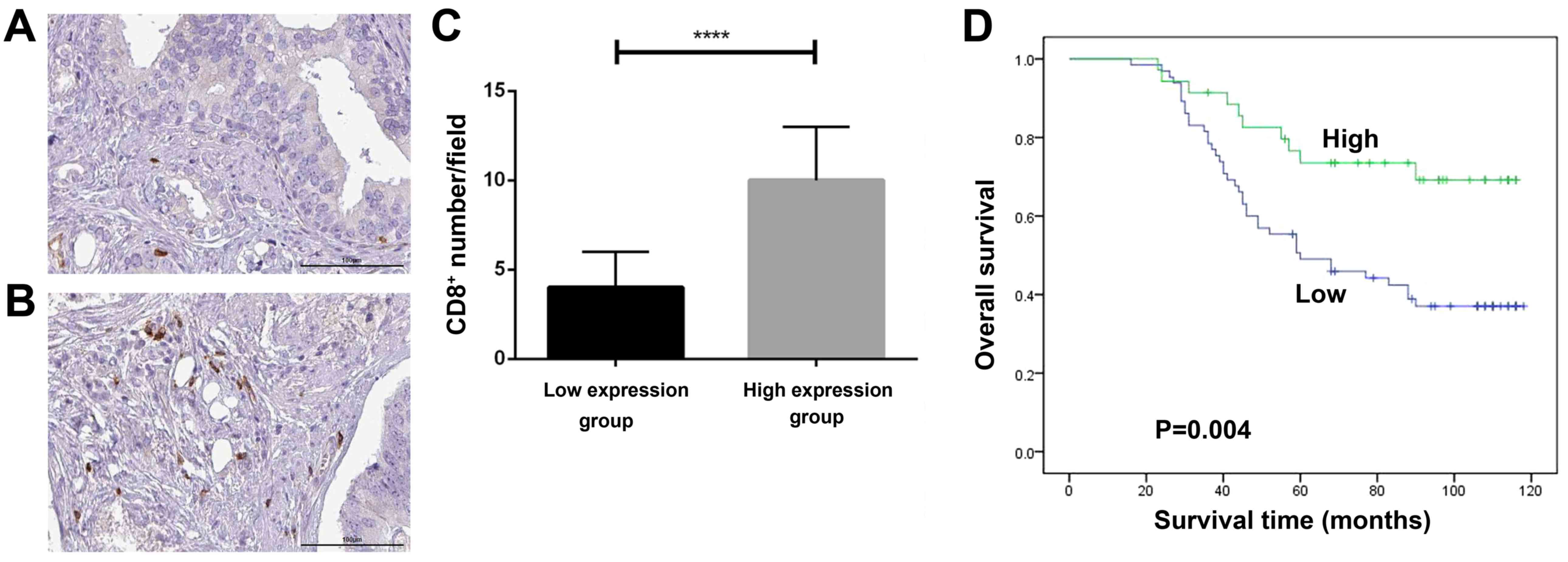
High expression of CD8 is an
independent predictor of good prognosis of prostate cancer
patients
The data shown in Fig.
1 suggested that immune checkpoint blockade therapy is
effective in prostate cancer especially in combination with
oxaliplatin. CD8+ T cells are the basis of immune
checkpoint blockade therapies. For this reason, we collected 100
prostate cancer patient tissue samples and evaluated the clinical
significance of CD8+ T cells in prostate cancer. All the
clinic pathological features of these patients and their
relationship to the CD8+ T cells number were summarized
in Table I. Patients with higher
CD8+ T cells number have lower proportion of advanced
TNF stages (III+IV) (P=0.013). Importantly, patients with higher
CD8+ T cells number have better survivalrate (P=0.002).
Furthermore, as shown in Fig. 2D,
the survival analysis indicated that patients with high
CD8+ T cells number had longer survival than those with
low number (P=0.004). Consistently, Cox regression model analysis
proved that high CD8+ T cells number is an independent
favorable predictor of prostate cancer patients (P=0.047, HR=0.482,
95% CI: 0.235–0.991), while high Gleason score and high TNM stage
are adverse independent predictor of these patients with P=0.002
(HR=2.548, 95% CI: 1.424–4.560) and 0.001 (HR=3.231, 95% CI:
1.622–6.437), respectively (Table
II).
 | Table II.Multivariate COX regression model
analysis of the overall survival of prostate cancer patients. |
Table II.
Multivariate COX regression model
analysis of the overall survival of prostate cancer patients.
| Factors | P-value | HR (95% CI) |
|---|
| Age (≥65 vs.
<65) | 0.530 | 1.204
(0.675–2.149) |
| Gleason scoring (≥6
vs. <6) | 0.002 | 2.548
(1.424–4.560) |
| TNM stage (III-IV
vs. I-II) | 0.001 | 3.231
(1.622–6.437) |
| T stage (T3-T4 vs.
T1-T2) | 0.915 | 1.051
(0.422–2.618) |
| Lymph node (N3-N4
vs. N0-N2) | 0.594 | 1.181
(0.640–2.180) |
| Metastasis (Yes vs.
No) | 0.247 | 2.484
(0.532–11.604) |
| CD8+ T cells (High
vs. Low) | 0.047 | 0.482
(0.235–0.991) |
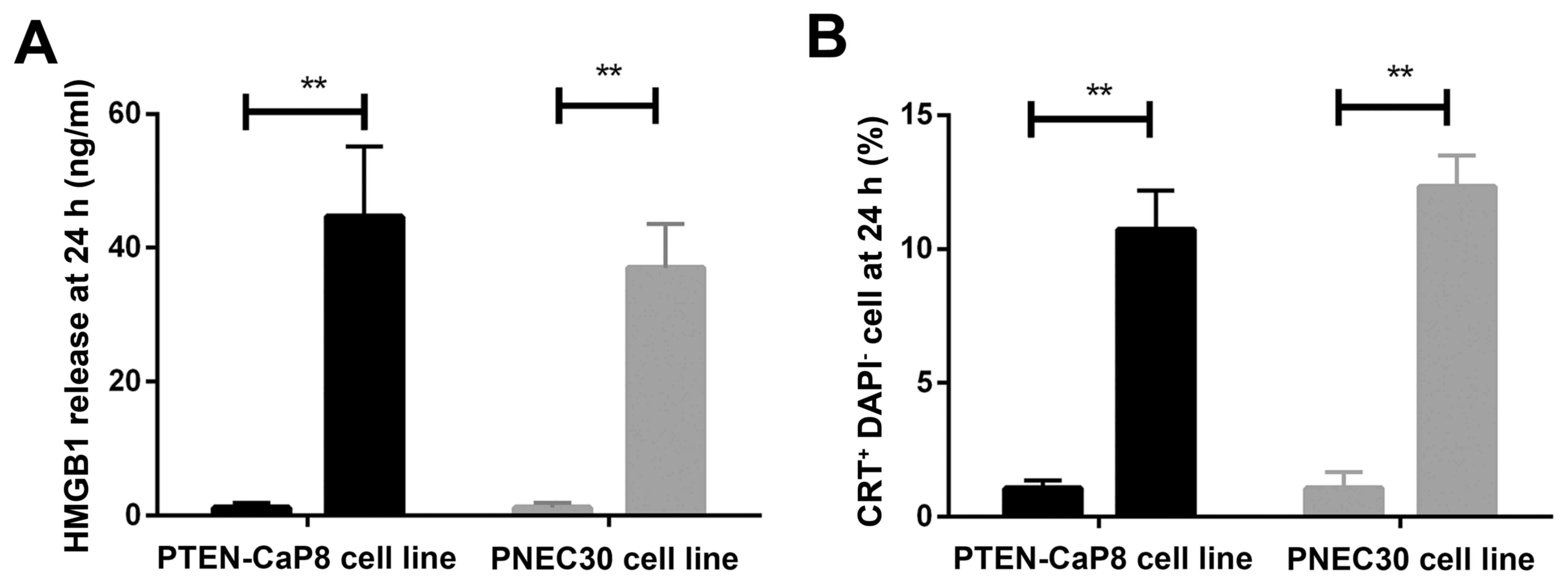
Oxaliplatin induces immunogenic
phenotype in prostate cancer cells
Based on the evidence mentioned above, it is highly
likely that immune response was activated by the combination of
oxaliplatinand anti-PD-1 therapy. Thus, we tested the exposure of
two the immunogenic makers in prostate cancer cell lines PTEN-CaP8
and PNEC30 treated by oxaliplatin: HMGB1 and calreticulin (CRT). As
shown in Fig. 3, after oxaliplatin
treatment for 24 h, the exposure of both HMGB1 and CRT were
increased significantly. This suggested that oxaliplatin could
activate the immunogenic phenotype of prostate cancer cells.
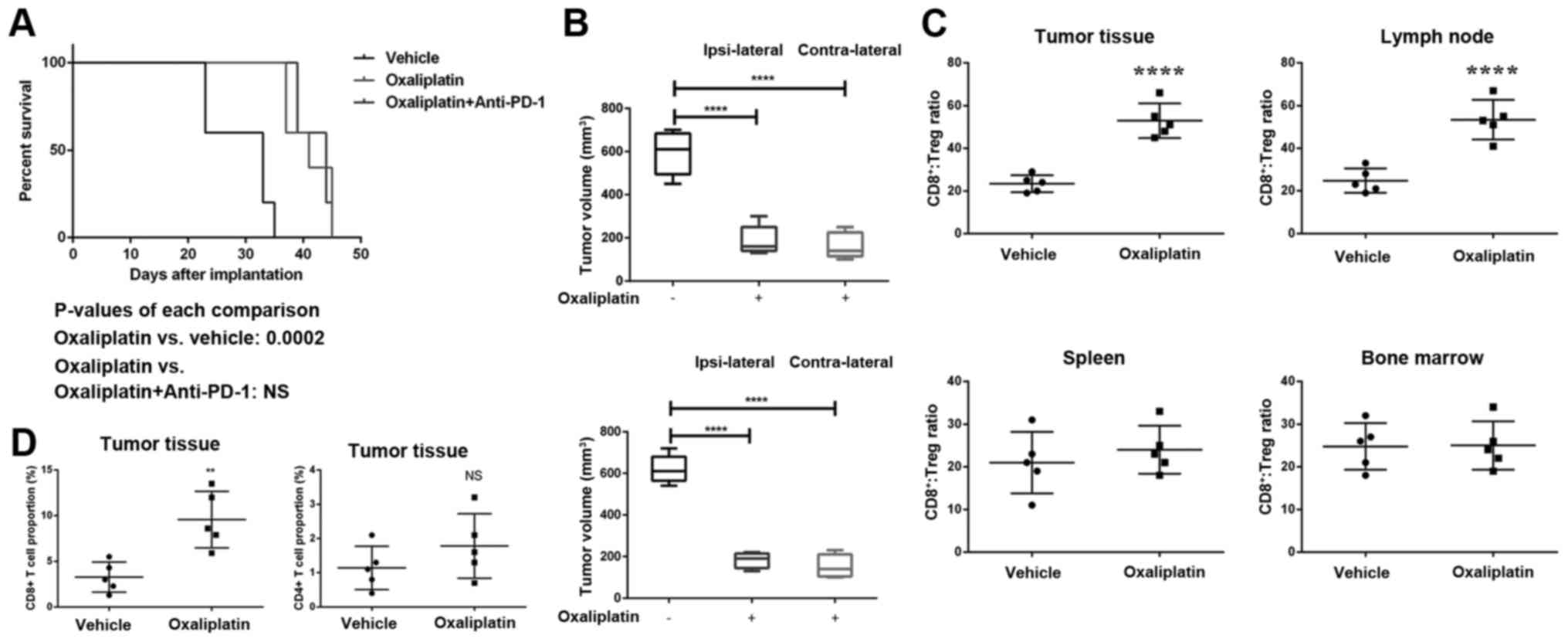
Oxaliplatin sensitizes prostate cancer
cell to immune checkpoint blockade through inducing systemic immune
response
To further explore the mechanisms by which
oxaliplatin sensitizes prostate cancer cell to immune checkpoint
blockade, we established prostate cancer xenograft mouse model
using NOD-SCID mice, wide type Balb/c mice andPNEC30 cell lines.
Fig. 4A showed the survival
analysis of NOD-SCID bearing xenograft prostate cancer treated by
oxaliplatin and/or anti-PD-1 therapy. Interestingly, even though
the survival time of both oxaliplatin and oxaliplatin + anti-PD-1
Ab treated groups has been extended vs. PBS control, there is no
difference between these two treatments. This suggested that the
anti-PD-1 Ab synergistic effects of oxaliplatin rely on complete
adaptive immune system. We further measured the growth of xenograft
prostate cancer in wild type Balb/c mice which were vaccinated by
oxaliplatin-killed prostate cancer cells. As shown in Fig. 4B, in wild type Balb/c mice, the
tumor in control groups grew much quicker than that of oxaliplatin
treated group. This indicated that oxaliplatin-killed prostate
cancer cells vaccination can induce tumor growth inhibition. What's
more, as both ipsilateral and contralateral tumor growth were
inhibited by oxaliplatin treatment, it was proposed that not local
immune response but systemic response caused by vaccination induced
the antitumor effect. Mechanistically, we found that oxaliplatin
treatment altered the ratio of CD8+ T cells to Treg
cells in tumor tissue and tumor draining lymph nodes (Fig. 4C). Further, the Oxaliplatin
treatment increased CD8+ T cell infiltration in the
tumor tissue but not CD4+ T cells (Fig. 4D). Taken together, oxaliplatin
sensitizes anti-PD-1 Ab treatment through activating systemic tumor
immune response.
Combination of oxaliplatin and
anti-PD-1 Ab changes the cytokine expression in prostate
cancer
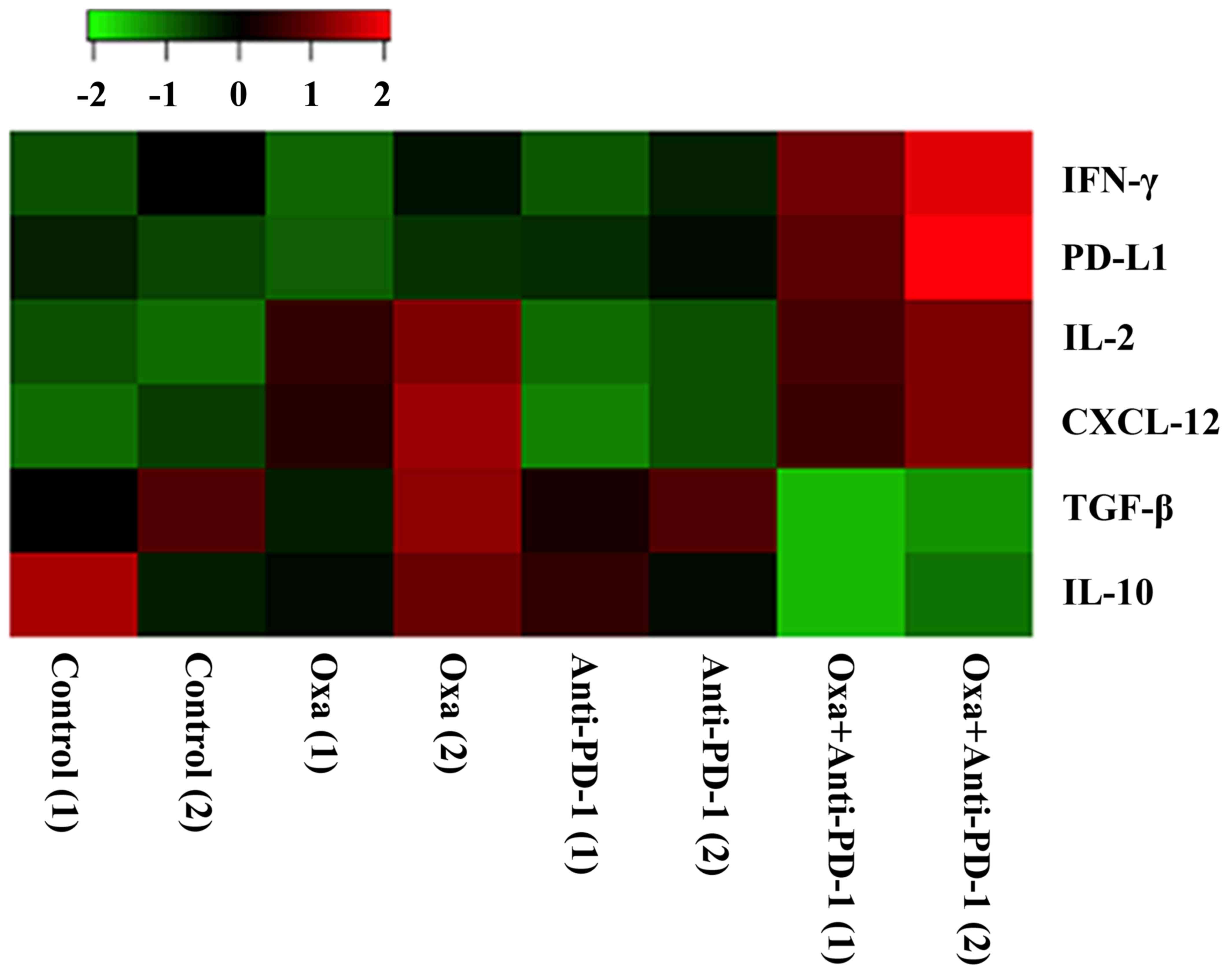
Immune response is regulated by cytokine network.
Thus, we measured the expression of some key regulatory cytokines
(IFN-γ, PD-L1, IL-2, CXCL-12, TGF-β, and IL-10) in the tumor tissue
of xenograft mouse model. As shown in Fig. 5, the combination treatment of
oxaliplatin and anti-PD-1 Ab increased the expression of IFN-γ,
PD-L1, IL-2 and CXCL-12; while inhibited the expression of TGF-β,
IL-10. This cytokine network changes is favorable for T cell
activation and antigen presenting. Therefore, it is reasonable to
conclude that oxaliplatin sensitizes prostate cancer to immune
checkpoint blockade by inducing pro-immune-responsephenotype.
Discussion
Immune checkpoints are molecules in the immune
system that either boost up a signal, such CD28 and CD137 or turn
down a signal, such as PD-1 and CTLA-4 (18–21).
Many cancers protect themselves from the immune system by
inhibiting the T cell signal via dampening co-stimulatory signals
and enhancing inhibitory signals (7). From 2010, inhibitors of inhibitory
immune checkpoints, referred as immune checkpoint blockades,
started to be approved for the treatment of multiple cancers
(12,22,23).
However, a great proportion of solid tumors are resistant to immune
checkpoint blockades monotherapy due to low tumor immunogenicity
and heavy immunosuppression (7).
Recently studies showed that chemotherapies are sensitizers of
immune checkpoint blockades in certain types of tumors. Here, we
investigated the role of chemotherapy drug oxaliplatin in
sensitizing anti-PD-1 Ab in prostate cancer.
Chemotherapy is a common option for ADT insensitive
and late stage castration resistant prostate cancer patients
(24–26). As a chemotherapy drug, oxaliplatin
is used in many cancers, such as colorectal cancer and lung cancer
(27,28). In recent years, it's also actively
researched in castration resistant prostate cancer patients
(29,30). Our data indicated that even cancer
cells are resistant to ADT, they can be sensitive to chemotherapy,
such as oxaliplatin due to its non-targeted cell death induction
roles (31). In our syngeneic
animal model of prostate cancer, either oxaliplatin or anti-PD-1 Ab
increased overall survival marginally. However, when combined with
oxaliplatin, anti-PD-1 Ab showed an obvious prolonged overall
survival, suggesting that anti-PD-1 Ab treatment was sensitized by
oxaliplatin.
For dissecting the antitumor mechanism of
oxaliplatin sensitizing anti-PD-1 Ab, we found that this process
involves in modulation of adaptive immunity. In line with the in
vitro data that oxaliplatin induced immunogenic phenotype of
prostate cancer cells, our animal data confirmed these mechanisms
by using oxaliplatin killed tumor cells as tumor vaccine.
Additional studies revealed that oxaliplatin treatment regulated T
cell subpopulation in tumor and draining lymphnodes. The ratio of
cytotoxic T cells to Treg cells was increase dramatically meaning
an immunogenic tumor microenvironment (8,10).
The antitumor functions of tumor specific T cells rely on positive
regulation of cytokines and chemokines. Our data showed that
oxaliplatin and anti-PD-1 Ab combination enhance dsecretion of
cytokines, such as IFN-γ, IL-2 and CXCL-12, which are related to
promoteantigen presentation, activate immune cells, recruit
leukocytes and induce inflammation (32–34).
Whereas the decreased cytokines are related to inhibiting antigen
presentation, depressing co-stimulatory molecules and inducing Treg
cells differentiation (35). As a
negative feedback mechanism of anti-PD-1 treatment, PD-L1
expression level was also elevated in combination treatment group.
All these data supported the idea that oxaliplatin and anti-PD-1 Ab
combination induced prostate cancer regression via systematic
activation of adaptive immune response.
In conclusion, as far as we knew, our study showed
that oxaliplatin sensitizes anti-PD-1 Ab treatment in prostate
cancer for the first time. The chemotherapy drug oxaliplatin
stimulated immunogenic potential of prostate cancer, induced
systemic antitumor immune response and therefore enhanced anti-PD-1
Ab effects in prostate cancer pre-clinical model. Further clinical
studies based on these pre-clinical results are highly wanted.
Acknowledgements
We thank Basic Medical Research Center of Tianjin
Nankai Hospital for her excellent technical assistance and helpful
criticism of the manuscript.
Glossary
Abbreviation
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
References
|
1
|
Porche D: Prostate cancer: Overview of
screening, diagnosis and treatment. Adv NPs PAs. 2:18–21; quiz 22.
2011.PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Mbalawa C Gombe, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer incidence in five
continents: Inclusion criteria, highlights from volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grönberg H: Prostate cancer epidemiology.
Lancet. 361:859–864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hussain S, Haidar A, Bloom RE, Zayouna N,
Piper MH and Jafri SM: Bicalutamide-induced hepatotoxicity: A rare
adverse effect. Am J Case Rep. 15:266–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato E, Olson SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating lymphocytes and a
high CD8+/regulatory T cell ratio are associated with favorable
prognosis in ovarian cancer. Proc Natl Acad Sci USA.
102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naito Y, Saito K, Shiiba K, Ohuchi A,
Saigenji K, Nagura H and Ohtani H: CD8+ T cells infiltrated within
cancer cell nests as a prognostic factor in human colorectal
cancer. Cancer Res. 58:3491–3494. 1998.PubMed/NCBI
|
|
10
|
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B,
Zhang Z, Yang H, Zhang H, Zhou C, et al: Increased regulatory T
cells correlate with CD8 T-cell impairment and poor survival in
hepatocellular carcinoma patients. Gastroenterology. 132:2328–2339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-programmed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schadendorf D, Hodi FS, Robert C, Weber
JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM and Wolchok JD:
Pooled analysis of long-term survival data from Phase II and Phase
III trials of ipilimumab in unresectable or metastatic melanoma. J
Clin Oncol. 33:1889–1894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minn AJ and Wherry EJ: Combination cancer
therapies with immune checkpoint blockade: Convergence on
interferon signaling. Cell. 165:272–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galsky MD, Noah H, Starodub A, Hauke RJ,
Twardowski P, Fleming M, Qi J, Sonpavde G, Patel M, Zhu J, et al:
Impact of chemotherapy alone, and chemotherapy plus ipilimumab, on
circulating immune cells in patients with metastatic bladder
cancer. J Immunother Cancer. 3:(Suppl 2). P2572015. View Article : Google Scholar :
|
|
16
|
Zamarin D and Postow MA: Immune checkpoint
modulation: Rational design of combination strategies. Pharmacol
Ther. 150:23–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao X, He Y, Gao J, Fan L, Li Z, Yang G
and Chen H: Caveolin-1 expression level in cancer associated
fibroblasts predicts outcome in gastric cancer. PLoS One.
8:e591022013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eastwood D, Findlay L, Poole S, Bird C,
Wadhwa M, Moore M, Burns C, Thorpe R and Stebbings R: Monoclonal
antibody TGN1412 trial failure explained by species differences in
CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol.
161:512–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mittler RS, Foell J, McCausland M,
Strahotin S, Niu L, Bapat A and Hewes LB: Anti-CD137 antibodies in
the treatment of autoimmune disease and cancer. Immunol Res.
29:197–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolar P, Knieke K, Hegel JK, Quandt D,
Burmester GR, Hoff H and Brunner-Weinzierl MC: CTLA-4 (CD152)
controls homeostasis and suppressive capacity of regulatory T cells
in mice. Arthritis Rheum. 60:123–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Philips GK and Atkins M: Therapeutic uses
of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 27:39–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: Updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galsky MD and Vogelzang NJ:
Docetaxel-based combination therapy for castration-resistant
prostate cancer. Ann Oncol. 21:2135–2144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh RP and Agarwal R: Prostate cancer
chemoprevention by silibinin: Bench to bedside. Mol Carcinog.
45:436–442. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boxberger F, Albrecht H, Konturek PC,
Reulbach U, Maennlein G, Meyer T, Hohenberger W, Hahn EG and Wein
A: Neoadjuvant treatment with weekly high-dose 5-fluorouracil as a
24 h-infusion, folinic acid and biweekly oxaliplatin in patients
with primary resectable liver metastases of colorectal cancer:
Long-term results of a phase II trial. Med Sci Monit. 16:CR49–CR55.
2010.PubMed/NCBI
|
|
28
|
Zhang K, Qin H, Pan F, Liu E, Liang H and
Ruan Z: Nedaplatin or oxaliplatin combined with paclitaxel and
docetaxel as first-line treatment for patients with advanced
non-small cell lung cancer. Med Sci Monit. 20:2830–2836. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Droz JP, Muracciole X, Mottet N, Kaci M
Ould, Vannetzel JM, Albin N, Culine S, Rodier JM, Misset JL,
Mackenzie S, et al: Phase II study of oxaliplatin versus
oxaliplatin combined with infusional 5-fluorouracil in hormone
refractory metastatic prostate cancer patients. Ann Oncol.
14:1291–1298. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dorff TB, Tsao-Wei DD, Groshen S, Boswell
W, Goldkorn A, Xiong S, Quinn DI and Pinski JK: Efficacy of
oxaliplatin plus pemetrexed in chemotherapy pretreated metastatic
castration-resistant prostate cancer. Clin Genitourin Cancer.
11:416–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan B, Ma F, Wang M and Xu X:
Down-regulating receptor interacting protein kinase 1 (RIP1)
promotes oxaliplatin-induced Tca8113 cell apoptosis. Med Sci Monit.
21:3089–3094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horwitz DA, Zheng SG, Wang J and Gray JD:
Critical role of IL-2 and TGF-beta in generation, function and
stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 38:912–915. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nanki T and Lipsky PE: Stimulation of
T-Cell activation by CXCL12/stromal cell derived factor-1 involves
a G-protein mediated signaling pathway. Cell Immunol. 214:145–154.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whitmire JK, Tan JT and Whitton JL:
Interferon-gamma acts directly on CD8+ T cells to increase their
abundance during virus infection. J Exp Med. 201:1053–1059. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu S, Zhang N, Yopp AC, Chen D, Mao M,
Chen D, Zhang H, Ding Y and Bromberg JS: TGF-beta induces Foxp3 +
T-regulatory cells from CD4+ CD25- precursors. Am J Transplant.
4:1614–1627. 2004. View Article : Google Scholar : PubMed/NCBI
|