Introduction
Osteoarthritis (OA) is a degenerative disease. It is
characterized by articular cartilage loss, osteophyte formation,
synovium alterations and functional changes of entire joints
(1). Injury, impaired muscle
function, sex and obesity are modifiable risk factors in the
pathophysiology of OA (2).
Obesity, which is considered to be one of the major risk factors
for OA, may induce cartilage damage by enhancing the mechanical
pressure on the weight-bearing joints, including the hip and knee
(3). However, the association
between obesity and OA in non-weight bearing joints, including the
wrists and fingers, has also been reported (4), and a previous study suggested that
additional cytokines produced by white adipose tissue (WAT), termed
adipokines, may also be involved during the progression of OA
(5). In addition, it has been
demonstrated that metabolic surplus and nutrient overload can
increase metabolic inflammation. Adipokines, may be involved in the
progression of OA by increasing the expression of degradative
enzymes and pro-inflammatory cytokines, leading to synovial
hyperplasia and cartilage extracellular matrix degradation
(6). Of these adipokines, leptin
is considered to be the most important. The synovial fluid
concentration of leptin has been identified to be significantly
associated with the progression of OA (7) and joint pain (8). Leptin has also been shown to induce
the expression of metabolic and inflammatory factors, including a
disintegrin and metalloprotease with thrombospondin motifs
(ADAMTs), matrix metalloproteinases (MMPs), nitric oxide (NO),
prostaglandin E2, cyclooxygenase-2 (COX-2) and interleukin-1β
(IL-1β) (9–11), by activating the nuclear factor
(NF)-κB and mitogen-activated protein kinase signaling pathways
(12,13).
Visceral adipose tissue-derived serine protease
inhibitor (vaspin) is a novel cytokine, which was first identified
in the visceral adipose tissue of Zucker fatty rats in 2005
(14). Vaspin has been identified
in several human tissues, including serum, omental adipose tissue
and placenta (15–17), and increased serum levels of vaspin
in diabetes and obesity in humans have been reported (18). Vaspin exerts an insulin-sensitizing
effect, which improves insulin sensitivity and inhibits the gene
expression levels of tumor necrosis factor (TNF)-α and leptin,
which are associated with insulin resistance (14). Vaspin is considered to be a novel
adipokine with anti-inflammatory properties, and links between
vaspin and arthritis have been demonstrated (19,20).
Senolt et al (19)
identified that the synovial fluid levels of vaspin were higher in
patients with rheumatoid arthritis (RA), compared with those with
OA. Furthermore, investigations by Maijer et al (20) identified that serum levels of
vaspin may assist in predicting the progression of RA in
autoantibody-positive individuals. These findings suggest a
possible role of vaspin during the development of arthritis, on
which further investigations are required.
In our previous studies, it was identified that the
joint tissues in OA, including synovium, cartilage and osteophytes,
exhibited protein expression of vaspin (21). It was also demonstrated that vaspin
suppressed the IL-1β-induced production of inflammatory and
catabolic factors in chondrocytes (22). Vaspin and leptin are important
during the pathophysiology of OA; however, the interaction between
vaspin and leptin in chondrocytes remains to be fully elucidated.
The present study examined the effect of vaspin on the
leptin-induced gene expression of leptin receptor (OB-Rb),
ADAMTS-4, ADAMTS-5, MMP-2 and MMP-9, and the effect of vaspin on
leptin-induced production of NO and TNF-α in rat chondrocytes. In
addition, the present study investigated whether the NF-κB
signaling pathway was involved in the process.
Materials and methods
Cell culture and treatment
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital, School of Medicine,
Zhejiang University, Hangzhou, China. Normal articular cartilage
was isolated from the knee and femoral head of four-week-old
Sprague-Dawley rats (160–180 g), which were obtained from the
Animal Center of Zhejiang University (Hangzhou, China). A total of
6 rats were used in the present study, and rats were anesthetized
with sodium pentobarbital (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany; 40 mg/kg body weight; intraperitoneal injection) and
sacrificed by cervical dislocation after anesthetization for
cartilage harvest. The harvested cartilage samples were then cut
into 1 mm3 cubes, followed by digestion with 0.2%
pronase (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min, and
subsequent digestion with 0.1% collagenase at 37°C for 4 h. The
cells from one rat were centrifuged at 1,000 × g for 5 min, then
resuspended and plated in a 25 cm2 culture flask
(Corning Incorporated, Corning, NY, USA) in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (100 U/ml penicillin; Invitrogen;
Thermo Fisher Scientific, Inc.) and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). The chondrocytes were
cultured in a 5% CO2 atmosphere at 37°C, designated
passage 0 (P0). The confluent primary chondrocytes were passaged at
a ratio of 1:3, designated P1, P2 and P3. The P3 chondrocytes were
used in the subsequent experiments.
The rat chondrocytes were plated at a density of
1×105 cells/well in a 6-well plate (serum-free)
overnight. The cells were then pre-treated with different
concentrations of vaspin (0, 10, 50, 100 and 300 ng/ml) for 1 h
prior to treatment with leptin (100 ng/ml) for 24 h at 37°C. The
culture medium was collected for NO and TNF-α measurement, and the
cells were harvested for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analyses.
For the analysis of NF-κB, 5×106 rat
chondrocytes were plated in serum-free medium for 24 h, treated
with various concentrations of vaspin (0, 10, 50, 100 and 300
ng/ml) for 1 h prior to treatment with leptin (100 ng/ml) for 24 h.
The cells were collected for analysis of the NF-κB signaling
pathway.
RT-qPCR analysis of the expression
levels of OB-Rb, ADAMTS-4, ADAMTS-5, MMP-2 and MMP-9
Total RNA was isolated from the samples using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. A total of 1 µg RNA was used for the
synthesis of first strand cDNA, using the PrimeScript-RT reagent
kit (Takara Bio, Inc., Otsu, Japan). The qPCR procedure was then
performed using SYBR Premix Ex Taq (Takara Bio, Inc.). The primers
used for the target genes were as follows: ADAMTS4, forward
5′-GCCAGCAACCGAGGTCCCATA-3′ and reverse
5′-CCACCAGTGTCTCCACGAATCTAC; ADAMTS5, forward
5′-GGGGTCAGTGTTCTCGCTCTTG-3′ and reverse GCCGTTAGGTGGGCAGGGTAT-3′;
MMP-2, forward 5′-AGGATGGAGGCACGATTGG-3′ and reverse
5′-CTTGATGATGGGCGACGGT-3′; MMP-9, forward
5′-ACCCCATGTATCACTACCACGAG-3′ and reverse
5′-TCAGGTTTAGAGCCACGACCAT-3′; 18S, forward 5′-TTGACGGAAGGGCACCA-3′
and reverse 5′-CAGACAAATCGCTCCACCAA-3′ (NR046237.1) (Table I). Parallel amplification of 18S
was performed as an internal control. The RT-qPCR program was 95°C
for 1 min, then 45 cycles of 95°C for 10 sec and 63°C for 25 sec.
The melt curve analysis of amplification products was done at the
end of each PCR reaction to confirm that only one PCR product was
amplified and detected. The relative PCR data were analyzed using
the 2−ΔΔCq method (23).
 | Table I.Primers of target genes for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers of target genes for reverse
transcription-quantitative polymerase chain reaction analysis.
| Target gene | Sequence
(5′-3′) |
|---|
| MMP-2 | F:
AGGATGGAGGCACGATTGG |
|
| R:
CTTGATGATGGGCGACGGT |
| MMP-9 | F:
ACCCCATGTATCACTACCACGAG |
|
| R:
TCAGGTTTAGAGCCACGACCAT |
| ADAMTS-4 | F:
GCCAGCAACCGAGGTCCCATA |
|
| R:
CCACCAGTGTCTCCACGAATCTAC |
| ADAMTS-5 | F:
GGGGTCAGTGTTCTCGCTCTTG |
|
| R:
GCCGTTAGGTGGGCAGGGTAT |
| 18S | F:
TTGACGGAAGGGCACCA |
|
| R:
CAGACAAATCGCTCCACCAA |
ELISA analysis of the levels of NO and
TNF-α in culture medium
The rat chondrocytes were pre-treated with different
concentrations of vaspin (0, 10, 50, 100 and 300 ng/ml) for 1 h
prior to treatment with leptin (100 ng/ml) for 24 h. The culture
medium was collected and then analyzed using commercially available
ELISA kits according to the manufacturer's protocols (R&D
Systems, Inc., Minneapolis, MN, USA).
Western blot analysis
All cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and harvested, following which the
cytoplasmic proteins were isolated using an extraction kit
(Beyotime Institute of Biotechnology, Jiangsu, China). The proteins
were resolved using 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (60 µg in each lane), transferred onto
nitrocellulose membranes and then probed with 1:1,000 dilution of
primary antibodies against phosphorylated (p-)NF-κB (CST 3033),
p-inhibitor of NF-κB (IκB)-α (CST 2859), p-IκB kinase (IKK)-α and
p-IKK-β (CST 2078; all from Cell Signaling Technology, Inc.,
Dallas, TX, USA) overnight at 4°C. The membranes were then washed
and incubated for 1 h at room temperature with 1:10,000 of goat
anti-rabbit/mouse secondary antibody (31460 and 32230; Invitrogen;
Thermo Fisher Scientific, Inc.), followed by visualization with
enhanced chemiluminescence using a commercially available kit (Cell
Signaling Technology, Inc.). The density of bands was measured by
densitometry using Quantity One version 4.6.2 software (Bio-Rad
Laboratories Inc., Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA) for Windows. Statistical
significance was assessed using Student's t-test and one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
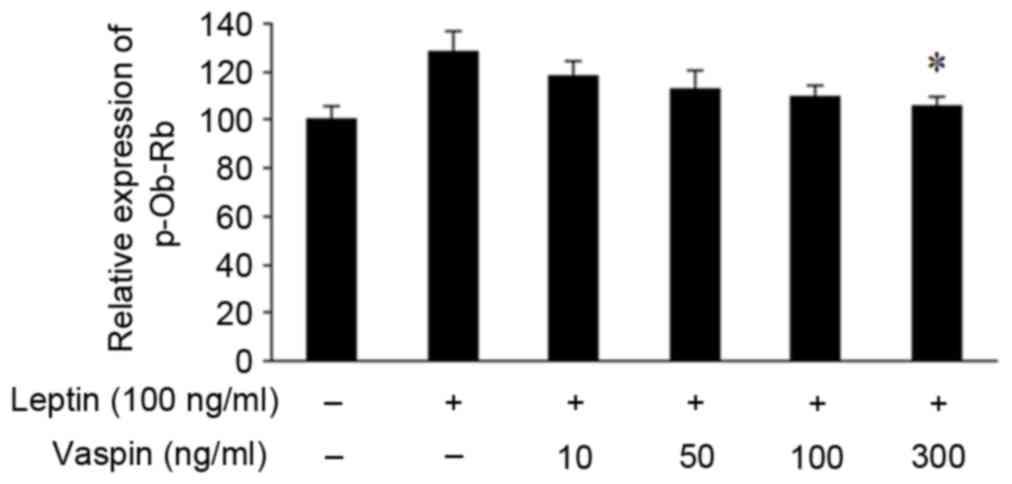
Effects of vaspin on leptin-induced
mRNA expression of OB-Rb in rat chondrocytes
Stimulation with leptin for 24 h resulted in
upregulated mRNA expression of p-OB-Rb in chondrocytes. Vaspin at
various concentrations inhibited the gene expression of p-OB-Rb in
a dose-dependent manner (Fig.
1).
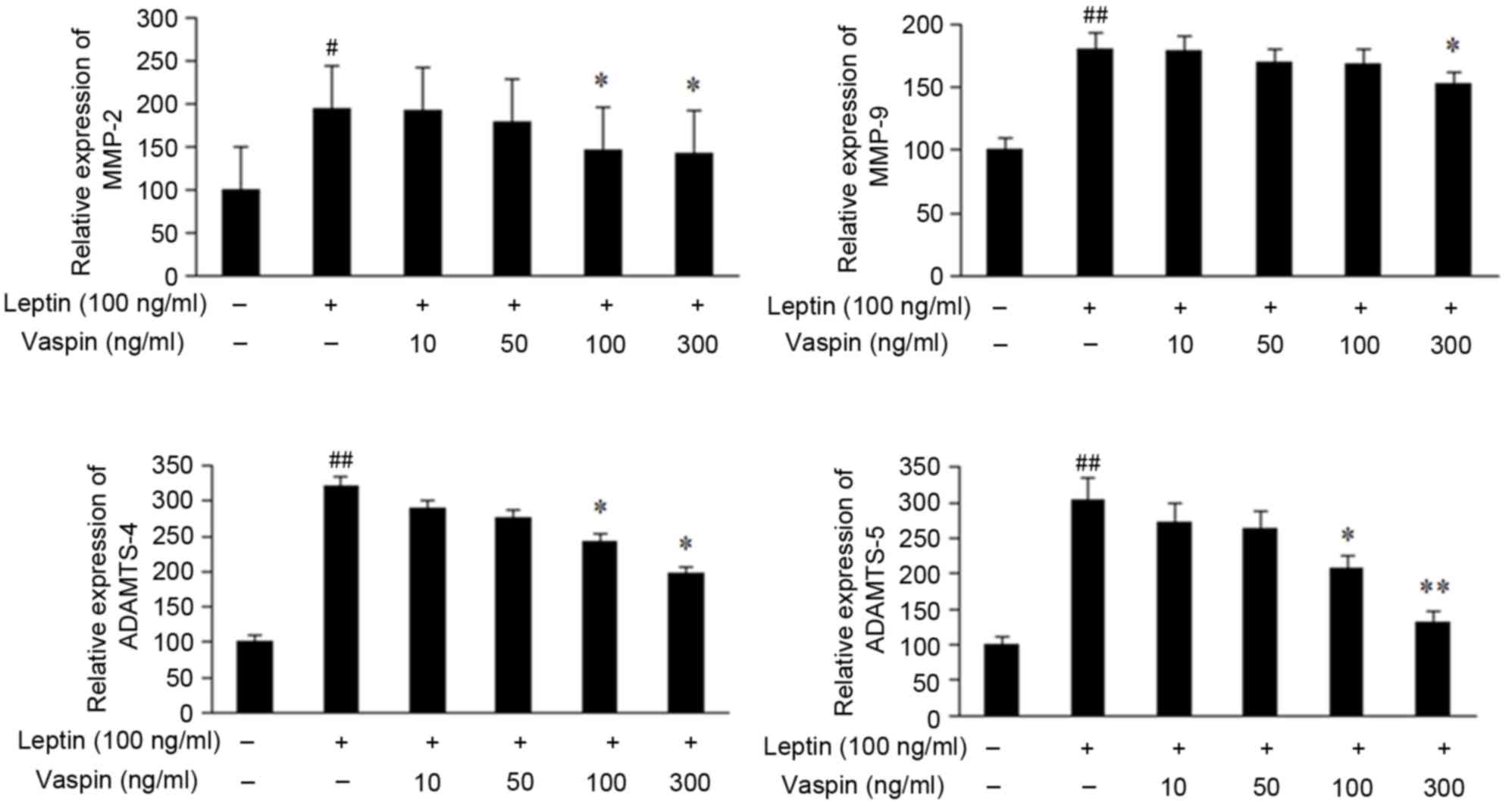
Effects of vaspin on leptin-induced
mRNA levels of ADAMTS-4, ADAMTS-5, MMP-2 and MMP-9 in rat
chondrocytes
As demonstrated in Fig.
2, the chondrocytes not exposed to leptin exhibited low gene
expression levels of ADAMTS-4, ADAMTS-5, MMP-2 and MMP-9. Leptin
significantly induced the expression of these genes, whereas this
induction was inhibited by vaspin.
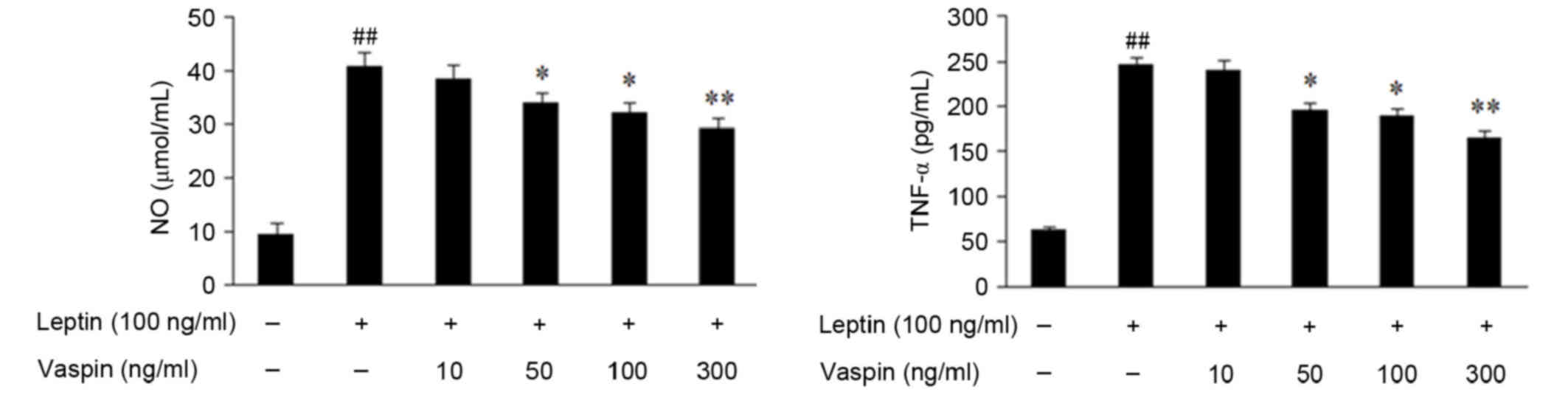
Effects of vaspin on leptin-induced secretion of NO
and TNF-α in rat chondrocytes. The secretion of NO and TNF-α were
increased in chondrocytes treated with leptin for 24 h, which was
significantly inhibited by vaspin in a dose-dependent manner
(Fig. 3).
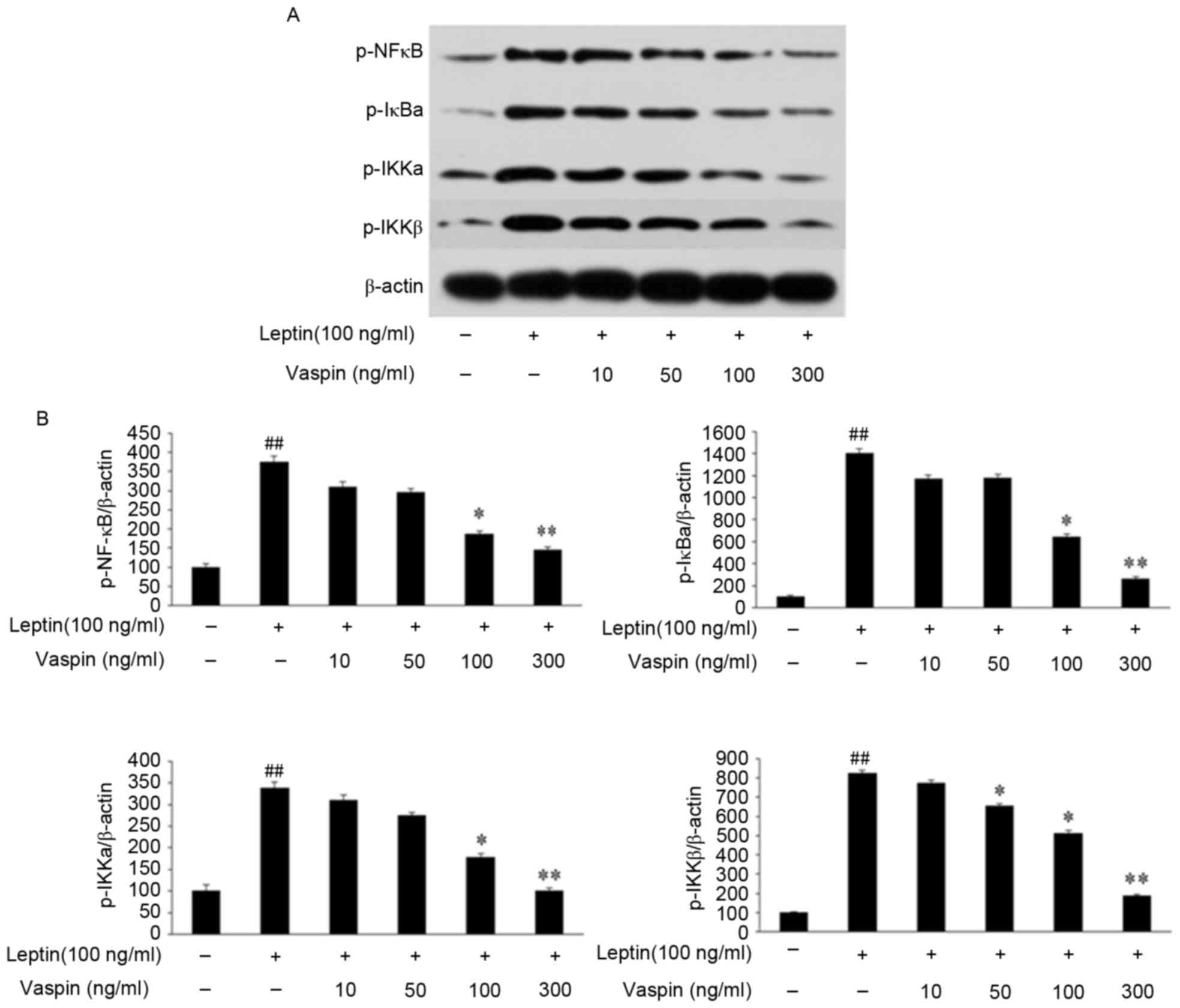
Effects of vaspin on expression of the
NF-κB signaling pathway stimulated by leptin in rat
chondrocytes
As demonstrated in Fig.
4A and B, the results of the western blot analysis demonstrated
that the phosphorylation levels of NF-κB, IκBα, IKKα and IKKβ were
markedly increased following stimulation with leptin. Vaspin
pretreatment inhibited the leptin-induced phosphorylation of NF-κB,
IκBα, IKKα and IKKβ in a dose-dependent manner, and this was
significant at concentrations of 100 ng/ml (P<0.05) and 300
ng/ml (P<0.01).
Discussion
Adipokines are cytokines secreted by WAT and include
adiponectin, leptin, resistin, vaspin and apelin. These adipokines
have been demonstrated to be involved in insulin sensitivity, lipid
metabolism, angiogenesis, immunity and inflammation (24,25).
Adipokines can damage cartilage homeostasis by regulating local
inflammatory processes or inducing the structural degradation of
joints (6). Leptin, the first
adipokine to be identified (26),
is considered to have pro-inflammatory effects, and has been
demonstrated to correlate with clinical and biological measurements
of RA activity (27). Wang et
al (6) identified that leptin
was positively correlated with body mass index, fat mass and body
weight among individuals with OA. Furthermore, our previous study
suggested that the overexpression of leptin and its receptor
induced the production of MMPs and IL-1β (28). These results confirmed that leptin
has a catabolic and pro-inflammatory role during the pathological
process of OA.
Vaspin, which has been identified to improve glucose
tolerance and insulin sensitivity in the pathogenesis of metabolic
syndrome (29), also possesses
anti-inflammatory effects (30–32).
Evidence has demonstrated that vaspin can attenuate the
cytokine-induced gene expression of adhesion molecules in vascular
smooth muscle cells (30) and
vascular endothelial cells (31).
In addition, vaspin has been shown to inhibit the expression of
TNF-α, resistin and leptin in chronic hepatitis (32). The results of our previous study
demonstrated that vaspin also reduces the IL-1β-induced
inflammatory and catabolic responses (22). As vaspin can improve insulin
sensitivity, reverse the expression of leptin (14) and suppress the expression of leptin
in chronic hepatitis (32), the
present study hypothesized that vaspin may have interactions with
leptin, and suppress the inflammatory factors induced by leptin in
chondrocytes.
OB-R can recruit cytoplasmic kinases, including
Janus kinase 2 to initiate leptin signaling upon leptin binding
(33). OB-R contains six
alternatively spliced isoforms, which have the same extracellular
binding domains, but different length cytoplasmic domains,
including one long isoform (OB-Rb), four short isoforms (OB-Ra,
OB-Rc, OB-Rd and OB-Rf), and one soluble isoform (OB-Re). However,
OB-Rb is the only isoform containing the full intracellular domain
able to transduce leptin-binding signals to the nucleus (34). The present study suggested that
vaspin suppressed the leptin signaling pathways via inhibiting the
expression of OB-Rb induced by leptin, which attenuated the
catabolic effect of leptin on rat chondrocytes. Further
investigations are required to elucidate the exact mechanism
underlying the regulation of OB-Rb by vaspin.
The present study identified that leptin
significantly induced the gene expression of ADAMTS-4, ADAMTS-5,
MMP-2 and MMP-9, which was consistent with our previous results
(28). ADAMTS-4 and ADAMTS-5 are
two important aggrecanases, which lead to structural damage during
the progression of joint OA (35).
By contrast, high levels of MMP-2 and MMP-9 were identified in OA,
suggesting they may contribute to cartilage destruction when
activated (36). The results also
demonstrated that vaspin significantly inhibited the leptin-induced
mRNA expression of ADAMTS-4, ADAMTS-5, MMP-2 and MMP-9 in a
dose-dependent manner, which suggested that vaspin may have an
anticatabolic effect on rat chondrocytes stimulated with leptin,
and prevent the progression of OA by inhibiting the expression of
MMPs and ADAMTS.
The production of NO and TNF-α were increased in the
chondrocytes treated with leptin, and this was significantly
inhibited by vaspin. NO and TNF-α are two important inflammatory
factors in the progression of OA. OA cartilage can produce high
levels of NO, and the increased levels of nitrites identified in
the synovial fluid and serum can upregulate NO synthesis (37). NO has also been demonstrated to
enhance MMP activity and be involved in joint pain in OA (38). TNF-α is considered to be a trigger
of the inflammatory cascade, which can induce a series of
inflammatory responses (39).
TNF-α has also been shown to decrease the synthesis of major
extracellular matrix composition and suppress anabolic activity
during chondrocyte metabolism (40). Therefore, the results suggested
that vaspin had anti-inflammatory effects on the rat chondrocytes
treated with leptin.
The NF-κB signaling pathway is a key regulator of
inflammatory cytokine-induced catabolic metabolism in chondrocytes,
the activation of which elevates the expression levels of IL-1,
nitric oxide synthase 2, MMPs and COX2 (41), resulting in cartilage degradation
in OA. Cytoplasmic NF-κB proteins exist in an inactive state
binding to IκB, and the NF-κB/IκB complex cannot translocate to the
nucleus. However, upon stimulation with proinflammatory leptin, IKK
is phosphorylated and activated, following which IκB is
phosphorylated and subsequently degraded. The released NF-κB
proteins are phosphorylated and then translocated into the nucleus,
where they can bind to the promoter of several target genes
(42). The present study
demonstrated that vaspin inhibited the degradation of IκB and
inhibited the NF-κB pathway, which was consistent with the results
obtained in smooth muscle cells (30) and endothelial cells (31). However, the effects of vaspin on
other signaling pathways, including AMP-activated protein kinase
and Wnt/β-catenin, remain to be elucidated and require further
investigations.
In conclusion, the results of the present study
suggested that vaspin inhibited the leptin-induced expression of
OB-Rb, ADAMTS-4, ADAMTS-5, MMP-2 and MMP-9, and also inhibited the
production of NO and TNF-α induced by leptin. These results
suggested that vaspin has anti-inflammatory and anticatabolic
effects in chondrocytes. These effects, at least in part, are
likely due to inhibition of the NF-κB pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81401824).
References
|
1
|
Mobasheri A and Batt M: An update on the
pathophysiology of osteoarthritis. Ann Phys Rehabil Med.
59:333–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roos EM and Arden NK: Strategies for the
prevention of knee osteoarthritis. Nat Rev Rheumatol. 12:92–101.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Radin EL, Paul IL and Rose RM: Role of
mechanical factors in pathogenesis of primary osteoarthritis.
Lancet. 1:519–522. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carman WJ, Sowers M, Hawthorne VM and
Weissfeld LA: Obesity as a risk factor for osteoarthritis of the
hand and wrist: A prospective study. Am J Epidemiol. 139:119–129.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poonpet T and Honsawek S: Adipokines:
Biomarkers for osteoarthritis? World J Orthop. 5:319–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Hunter D, Xu J and Ding C:
Metabolic triggered inflammation in osteoarthritis. Osteoarthritis
Cartilage. 23:22–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ku JH, Lee CK, Joo BS, An BM, Choi SH,
Wang TH and Cho HL: Correlation of synovial fluid leptin
concentrations with the severity of osteoarthritis. Clin Rheumatol.
28:1431–1435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lübbeke A, Finckh A, Puskas GJ, Suva D,
Lädermann A, Bas S, Fritschy D, Gabay C and Hoffmeyer P: Do
synovial leptin levels correlate with pain in end stage arthritis?
Int Orthop. 37:2071–2079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otero M, Reino JJ Gomez and Gualillo O:
Synergistic induction of nitric oxide synthase type II: In vitro
effect of leptin and interferon-gamma in human chondrocytes and
ATDC5 chondrogenic cells. Arthritis Rheum. 48:404–409. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otero M, Lago R, Lago F, Reino JJ and
Gualillo O: Signalling pathway involved in nitric oxide synthase
type II activation in chondrocytes: Synergistic effect of leptin
with interleukin-1. Arthritis Res Ther. 7:R581–R591. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yaykasli KO, Hatipoglu OF, Yaykasli E,
Yildirim K, Kaya E, Ozsahin M, Uslu M and Gunduz E: Leptin induces
ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by
mitogen-activated protein kinases and NF-κB signaling pathways in
human chondrocytes. Cell Biol Int. 39:104–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lam QL and Lu L: Role of leptin in
immunity. Cell Mol Immunol. 4:1–13. 2007.PubMed/NCBI
|
|
13
|
Scotece M and Mobasheri A: Leptin in
osteoarthritis: Focus on articular cartilage and chondrocytes. Life
Sci. 140:75–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hida K, Wada J, Eguchi J, Zhang H, Baba M,
Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, et al:
Visceral adipose tissue-derived serine protease inhibitor: A unique
insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci
USA. 102:10610–10615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeger J, Ziegelmeier M, Bachmann A,
Lössner U, Kratzsch J, Blüher M, Stumvoll M and Fasshauer M: Serum
levels of the adipokine vaspin in relation to metabolic andrenal
parameters. J Clin Endocrinol Metab. 93:247–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan BK, Heutling D, Chen J, Farhatullah S,
Adya R, Keay SD, Kennedy CR, Lehnert H and Randeva HS: Metformin
decreases the adipokine vaspin in overweight women with polycystic
ovary syndrome concomitant with improvement in insulin sensitivity
and a decrease in insulin resistance. Diabetes. 57:1501–1507. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caminos JE, Bravo SB, Garcés MF, González
CR, Cepeda LA, González AC, Nogueiras R, Gallego R,
García-Caballero T, Cordido F, et al: Vaspinand amylin are
expressed in human and rat placenta and regulated by nutritional
status. Histol Histopathol. 24:979–990. 2009.PubMed/NCBI
|
|
18
|
Blüher M: Vaspin in obesity and diabetes:
Pathophysiological and clinical significance. Endocrine.
41:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Senolt L, Polanská M, Filková M, Cerezo
LA, Pavelka K, Gay S, Haluzík M and Vencovsky J: Vaspin and
omentin: New adipokines differentially regulated at the site of
inflammation in rheumatoid arthritis. Ann Rheum Dis. 69:1410–1411.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maijer KI, Neumann E, Müller-Ladner U,
Drop DA, Ramwadhdoebe TH, Choi IY, Gerlag DM, de Hair MJ and Tak
PP: Serum Vaspin Levels Are Associated with the Development of
Clinically Manifest Arthritis in Autoantibody-Positive Individuals.
PLoS One. 10:e01449322015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao JP, Jiang LF, Chen WP, Hu PF and Wu
LD: Expression of vaspin in the joint and the levels in the serum
and synovial fluid of patients with osteoarthritis. Int J Clin Exp
Med. 7:3447–3453. 2014.PubMed/NCBI
|
|
22
|
Bao JP, Jiang LF, Li J, Chen WP, Hu PF and
Wu LD: Visceral adipose tissue-derived serine protease inhibitor
inhibits interleukin-1β-induced catabolic and inflammatory
responses in murine chondrocytes. Mol Med Rep. 10:2191–2197.
2014.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dozio E, Corsi MM, Ruscica M, Passafaro L,
Steffani L, Banfi G and Magni P: Adipokine actions on cartilage
homeostasis. Adv Clin Chem. 55:61–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hutcheson J: Adipokines influence the
inflammatory balance in autoimmunity. Cytokine. 75:272–279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toussirot É, Michel F, Binda D and
Dumoulin G: The role of leptin in thepathophysiology of rheumatoid
arthritis. Life Sci. 140:29–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bao JP, Chen WP, Feng J, Hu PF, Shi ZL and
Wu LD: Leptin plays a catabolic role on articular cartilage. Mol
Biol Rep. 37:3265–3272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wada J: Vaspin: A novel serpin with
insulin-sensitizing effects. Expert Opin Investig Drugs.
17:327–333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Peng W, Zhuang J, Lu Y, Jian W, Wei
Y, Li W and Xu Y: Vaspin attenuates high glucose-induced vascular
smooth muscle cells proliferation and chemokinesis by inhibiting
the MAPK, PI3K/Akt and NF-κB signaling pathways. Atherosclerosis.
228:61–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jung CH, Lee MJ, Kang YM, Lee YL, Yoon HK,
Kang SW, Lee WJ and Park JY: Vaspin inhibits cytokine-induced
nuclear factor-kappa B activation and adhesion molecule expression
via AMP-activated protein kinase activation in vascular endothelial
cells. Cardiovasc Diabetol. 13:412014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kukla M, Mazur W, Buldak RJ and
Zwirska-Korczala K: Potential role of leptin, adiponectin and three
novel adipokines-visfatin, chemerin and vaspin-in chronic
hepatitis. Mol Med. 17:1397–1410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghilardi N and Skoda RC: The leptin
receptor activates janus kinase 2 and signals for proliferation in
a factor-dependent cell line. Mol Endocrinol. 11:393–399. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinha MK, Sturis J, Ohannesian J, Magosin
S, Stephens T, Heiman ML, Polonsky KS and Caro JF: Ultradian
oscillations of leptin secretion in humans. Biochem Biophys Res
Commun. 228:733–738. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song RH, Tortorella MD, Malfait AM, Alston
JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in human
articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lipari L and Gerbino A: Expression of
gelatinases (MMP-2, MMP-9) in humanarticular cartilage. Int J
Immunopathol Pharmacol. 26:817–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pelletier JP, Martel-Pelletier J and
Abramson SB: Osteoarthritis, an inflammatory disease: Potential
implication for the selection of new therapeutic targets. Arthritis
Rheum. 44:1237–1247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murrell GA, Jang D and Williams RJ: Nitric
oxide activates metalloprotease enzymes in articular cartilage.
Biochem Biophys Res Commun. 206:15–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miller RE, Miller RJ and Malfait AM:
Osteoarthritis joint pain: The cytokine connection. Cytokine.
70:185–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blasioli DJ and Kaplan DL: The roles of
catabolic factors in the development of osteoarthritis. Tissue Eng
Part B Rev. 20:355–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-kappaB signaling: Multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|