Introduction
Endothelial progenitor cells (EPCs), also called
angioblast, are precursor of endothelial cells. Under the stimulus
of physiological or pathological factors, EPCs mobilize from bone
marrow to peripheral blood for participating in the repair of
damaged blood vessels. The EPCs play a crucial role in
re-endothelialization and repairing the injured endothelium
(1). They take part in many
diseases, however, there are few EPCs in peripheral blood, and they
do not proliferate readily even are dysfunctional (2), so expanding EPCs levels and enhancing
EPCs viability may benefit patients. Furthermore, transplantation
of EPCs is applied to the treatment of ischemic diseases, but how
to optimize the EPCs proliferation and amplification is still a
problem.
In the process of stem cell proliferation and
differentiation, cell signaling pathways play a regulatory role,
especially Notch signaling pathway and Akt signaling pathway. Akt
signaling pathway has a close relationship with cell viability and
migration. Some studies showed that it was involved in
progesterone-induced EPC viability (3). Notch signaling pathway is highly
conserved, and plays a key role in neovascularization and
angiogenesis process. It participates in progenitor cells
differentiation, proliferation (4)
and tube formation (5). We assumed
that the two signaling pathways were involved in the effect of
velvet antler (VA) on EPCs.
VA is common and precious medicinal material of
traditional Chinese medicine (TCM) which is called Lu Rong in
China, and it has been widely used as food supplement or
therapeutic drug generally in China, Japan, Russia, New Zealand and
Southeast Asia. Many researches have been carried out to study the
constituents as well as pharmacology of VA, however, like many TCM
supplements, its mechanism has not been clearly interpreted from
western viewpoint. VA has an inherent property that is the ability
of regeneration that are structural and functional replicates
(6,7). It can quickly grows into a tissue
which has rich blood vessels, nerves, fur, flesh and blood within
60 days. Some research showed that VA regeneration is a stem
cell-based epimorphic regeneration (8), so this property has drawn many
scholars' attention and was related with organ grafting and stem
cells differentiation (9), but
there is rarely research in this filed.
Some studies have revealed that polypeptides and
proteins are the main bioactive components of VA (10), promoting neurite outgrowth
(11), the proliferation of
epidermal cells and NIH3T3 cells (12), enhancing wound healing (13). Therefore, in this study we
extracted VA proteins (VA-pro) using freeze-drying technology,
water solvent and ultrasonic wave method, and determined the effect
of VA-pro on rat bone marrow-derived EPCs, as well as the possibly
molecular signaling pathway mechanisms.
Materials and methods
Velvet antler preparation
Fresh VAs were the fast-growing antlers of
4-year-old male deer (14)
(Cervus Nippon Temminck), which was bred in Jilin Province
of China. VAs were identified by Professor Chunsheng Liu, the
director of the Department of Chinese Medicine Identification at
the TCM Institute, Beijing University of Chinese Medicine. Blood
was drawn from the VAs with a vacuum instrument for 6 h in 4°C. VAs
were sliced and freeze-dried by lyophilizer (−60°C and 12 Pa,
LABCONCO) until quality change was less than 0.1 g. With the
superfine grinding technology, freeze-dried VAs were superfinely
comminuted in cryogenic environment. 54.08 g lyophilized VA powder
was created, and the dehydration percentage of VA was 67.02%. The
powder was stockpiled in sealed tubes and numbered XX-DGF01 to
XX-DGF04.
Lyophilized VA powder (XX-DGF01) was mixed with
ultrapure water (1:10) and blended through vortex oscillation (5
s/15 s). Using homogenate and immersion methods, the VA was
ultrasonically extracted for 2 h in an ice-bath. The supernate was
obtained in a centrifuge tube and the precipitate was removed by
centrifugation (12,000 rpm, 30 min). The supernate was stored at
−80°C for at least 12 h, and then get freeze-dried by the
lyophilizer for 72 h. The precipitate was processed according to
the above steps for 3 times. All freeze-dried VA-pro were mixed
together and were numbered from XX-VApro01 to XX-VApro03, being
stored at −80°C. The VA-pro mass concentration was 465.56 µg/mg,
which was measured by a BCA protein assay kit (Thermo Fisher
Scientific).
Nano LC-MS/MS analysis
We digested VA-pro with trypsin (30:1) for 24 h at
37°C, and the desalinization were taken with C18 column
(Phenomenex Strata™). High performance liquid chromatography (HPLC)
was performed and the desalted peptide mixture was separated with a
reversed phase C18 column (75 µm ×10 cm, 5 µm, 300 Ǻ,
Agela Technologies) at 400 nl/min constant flow rate. Peptides were
eluted with a gradient of 5–80% (v/v) and the acetonitrile in 0.1%
formic acid over 65 min. The eluates were directly entered into a
Q-Exactive Orbitrap Mass Spectrometer (Thermo Fisher Scientific),
which was set in positive ion mode, the scan range was 350–2000 m/z
and scan resolution was at 17,500. 1E+5 was the minimum signal
threshold and isolation width was at 2 Da. Ion source voltage was
set at 1800 V. 2 MS/MS acquisition modes and higher collision
energy dissociation were employed to evaluate the performance of
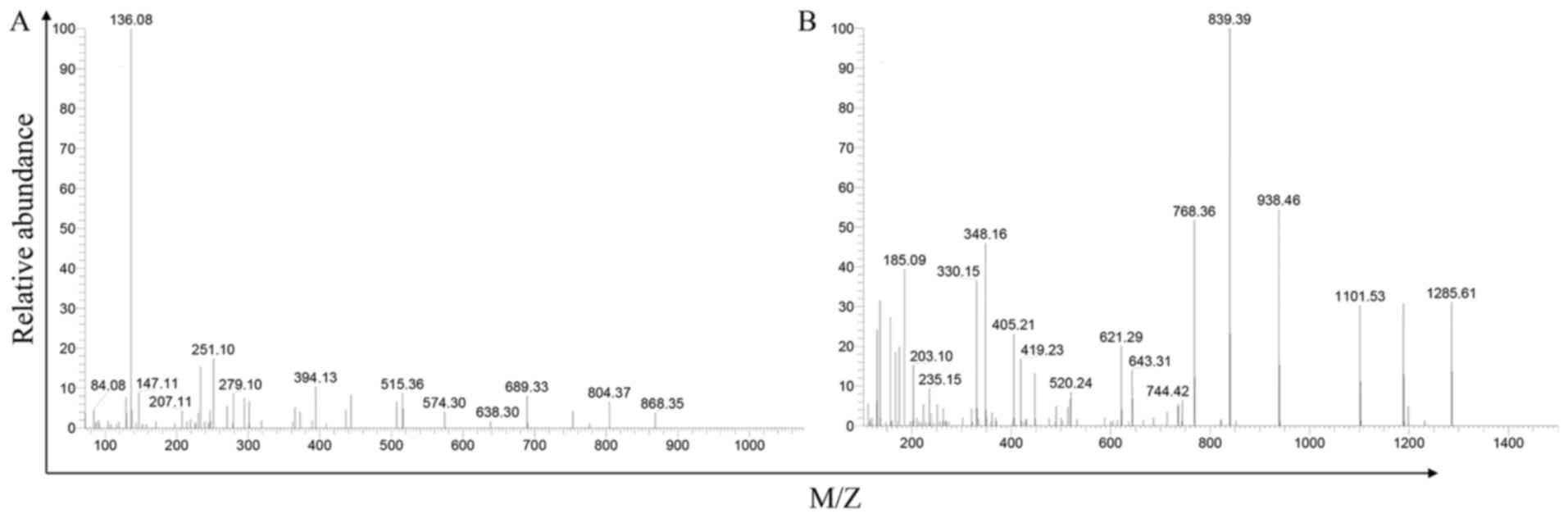
this MS on the iTRAQ-labeled samples (Fig. 1 The ensample of VA mass
spectrometric patterns).
MS data analysis
After Nano LC-MS/MS measurement, the raw data were
filtered by the PD (Proteome Discoverer 1.3, Thermo Fisher
Scientific). The collected data files were sent to a MASCOT server
(Version 2.3.0, Matrix Science) for peptide identification with the
Ruminantia (Number of sequences: 93869) taxonomy
constraint.
386 VA-pro were identified. Their molecular weight
(MW) range and isoelectric point (pI) range were showed in Tables I and II.
 | Table I.VA-pro molecular weight. |
Table I.
VA-pro molecular weight.
| MW(kDa) | <10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | 60–70 | 70–80 | 80–100 | 100–200 | >200 |
| N | 5 | 63 | 51 | 46 | 42 | 39 | 24 | 20 | 20 | 49 | 24 |
 | Table II.VA-pro isoelectric point. |
Table II.
VA-pro isoelectric point.
| pI | 4–5 | 5–6 | 6–7 | 7–8 | 8–9 | 9–10 | 10–11 | 11–12 |
| N | 50 | 128 | 84 | 29 | 49 | 20 | 16 | 7 |
EPCs primary culture
Bone marrow-derived mononuclear cells (BM-MNCs) were
isolated from 2-week-old male SD rats femur by the density gradient
centrifugation method, using lymphocyte separation medium (TBD
Science). BM-MNCs were seeded on a 35 mm culture dish and incubated
in complete endothelial basal medium (EBM-2 medium, Lonza) under
standard cell culture condition. After 72 h, first medium change
was performed, and non-adherent cells were removed, while the
adherent cells were continuously cultured for 14 days, then the
cells were identified by CD133 (Novus) and VEGFR2 (15) (Abcam).
Immunofluorescence
In order to characterize EPCs, cells were fixed for
15 min in 4% paraformaldehyde at room temperature. Then they were
deal with 0.3% triton-100/PBS for 10 min, blocked with 3% BSA/PBS
for 1 h. cells were incubated with primary antibodies overnight at
4°C, followed by immunolabelling with Alexa 488-conjugated
secondary antibody (1:200, CST), for 1 h at room temperature in the
dark. The nuclei were stained by DAPI for 10 min. The cells were
imaged and counted under an Inversed Fluorescent Microscope
(Nikon). Digital images were prepared using NIS Elements AR
Analysis 4.10.00.
Cell proliferation assay
EPCs proliferation was detected with MTS kit (the
Cell Titer 96® Aqueous One Solution Reagent). EPCs were
cultured in 96-well plates with different treatment. Then each well
was added 100 µl EBM-2 medium and 20 µl reagent and cells were
cultured for 3 h. The optical density (OD) was measured by
Microplate Spectrophotometer (Spectra MR).
Tubule formation assay
Tubule Formation Assay was determined with Matrigel
(BD Biosciences). After thawing for 3 h at 4°C, 100 µl Matrigel was
added into every well of 48-well plate and was put into an
incubator to solidify for 30 min. 2×104 EPCs were seeded
on the top of the Matrigel layer and were treated with different
concentrations of VA-pro. The net structure was observed under a
bright field microscope (Leica Microsystems) within 24 h and it was
calculated in four random fields using NIS Elements AR Analysis
4.10.00.
Wound healing assay
4×105 EPCs were cultured in 12-well
plates for 24 h. The wound was made by scratching confluent cell
monolayers with a P200 pipette tip, three parallel ‘wounds’ were
created in each well with floating cells being washed away. After
cells were incubated with different treatment, the migrated cells
were photographed and the mean distance between the two ends of the
scratch was quantified by manual measurements with Prism 5.0
program (GraphPad Inc.).
Transwell assay
Cell transwell assay was performed using invasion
chambers (BD Biosciences). 150 µl cell suspension (1×105
cells/ml, FBS-free medium) was placed to the inserts, while medium
containing VA-pro were added to the bottom chamber. After 24 h
culture, the cells still in the upper side of inserts were cleared
with cotton swabs and cells that had penetrated through the
polyethylene terephthalate membrane were fixed and stained using
mounting medium with DAPI (ZSGB-BIO). The cells were counted in 10
random microscopic fields.
Western blotting
Treated cells were washed and lysed with RIPA lysis
buffer, containing PMSF (Beyotime) and phosphatase inhibitors
(sigma-Aldrich). Protein concentration was detected using a
bicinchoninic acid protein assay kit (BCA, Thermo Fisher
Scientific). Proteins were separated on 8% polyacrylamide gel
SDS-PAGE for 110 min (Notch1, NICD, mTOR and p-mTOR) or 12%
polyacrylamide gel SDS-PAGE for 50 min (Hes1, Akt and p-Akt), and
they were transferred to a nitrocellulose membrane using 350 mA for
50 min (Hes1, Akt and p-Akt) or 3.5 h (Notch1, NICD, mTOR and
p-mTOR). Membranes were blocked with 5% nonfat dry milk and washed
with PBS. Then they were cut into 3 sections according to markers.
Each section of membrane was incubated overnight at 4°C with the
antibodies against Notch1, NICD, Hes1, Akt, p-Akt, mTOR, p-mTOR or
β-actin (CST, 1:1,000). Membranes were then washed and further
incubated for 1 h at 37°C with goat anti-mouse IgG or goat
anti-rabbit IgG antibody (1:5,000, Santa cruz), which conjugated
with HRP and was diluted in TBST containing 5% nonfat dry milk.
Membranes were soaked with super ECL plus (1:1, Millipore) and
wrapped with plastic wrap in the dark. The results were detected by
an ECL luminescent detection system. Band density was analyzed
using Image-Pro Plus 6.0 (Media Cybernetics).
Statistics
Experiments were repeated at least three times.
Quantitative Data were presented as means ± SD and were analyzed
using SPSS 17.0 software by ANOVA followed by Tukey's or Dunnett's
posttest. P<0.05 was considered as significant.
Results
EPCs characterization
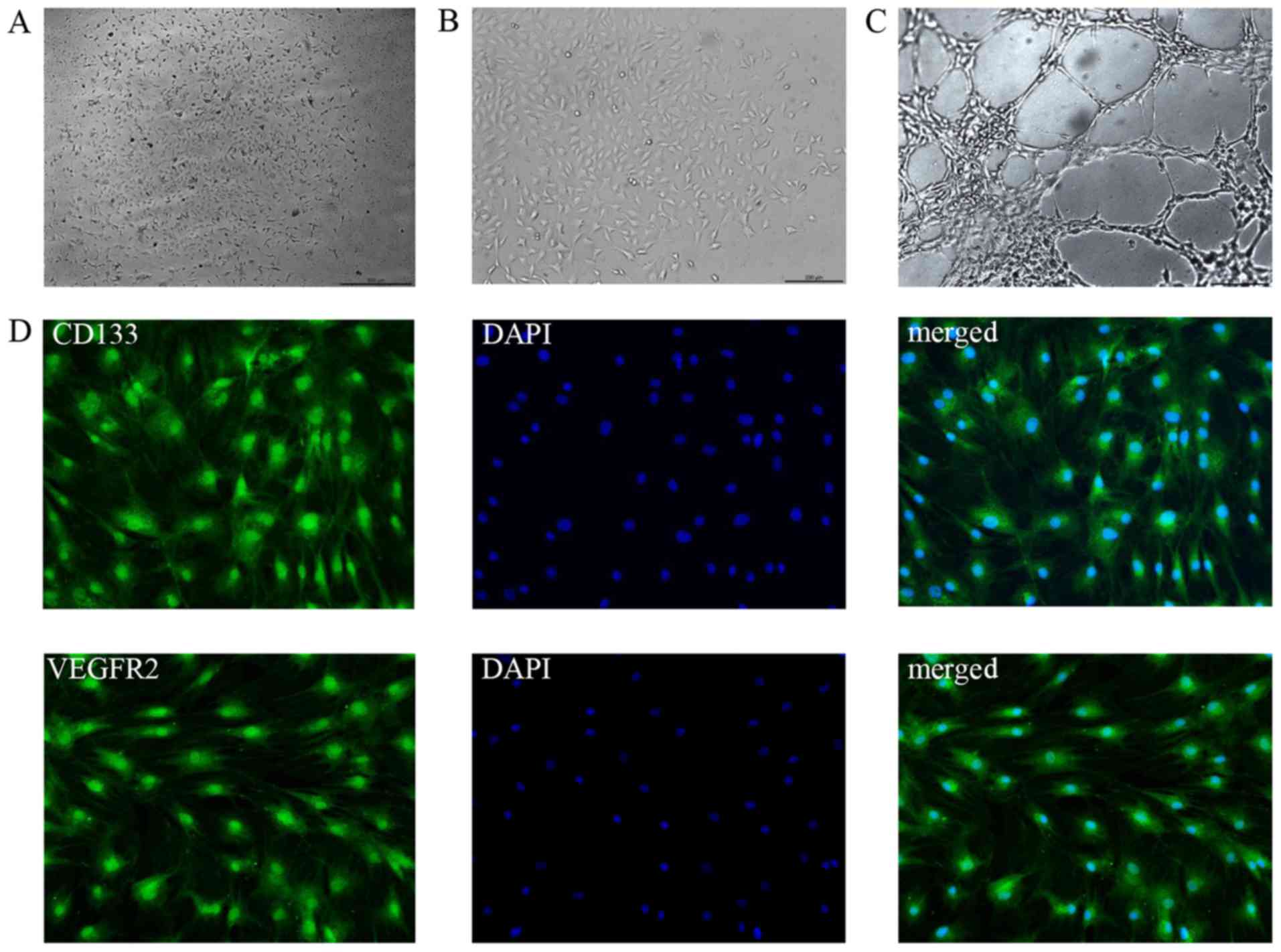
EPCs were cultured from rat bone marrow for 7 days,
the adherent cells were active and colonies were appeared (Fig. 2A, B). The cells potential to form
tubes was tested (Fig. 2C). The
stem cell marker CD133 and surface marker VEGFR2 were positive
(Fig. 2D). All above manifested
that BM-MNCs were induced into EPCs successfully.
The effect of VA-pro on EPCs
proliferation, tube formation
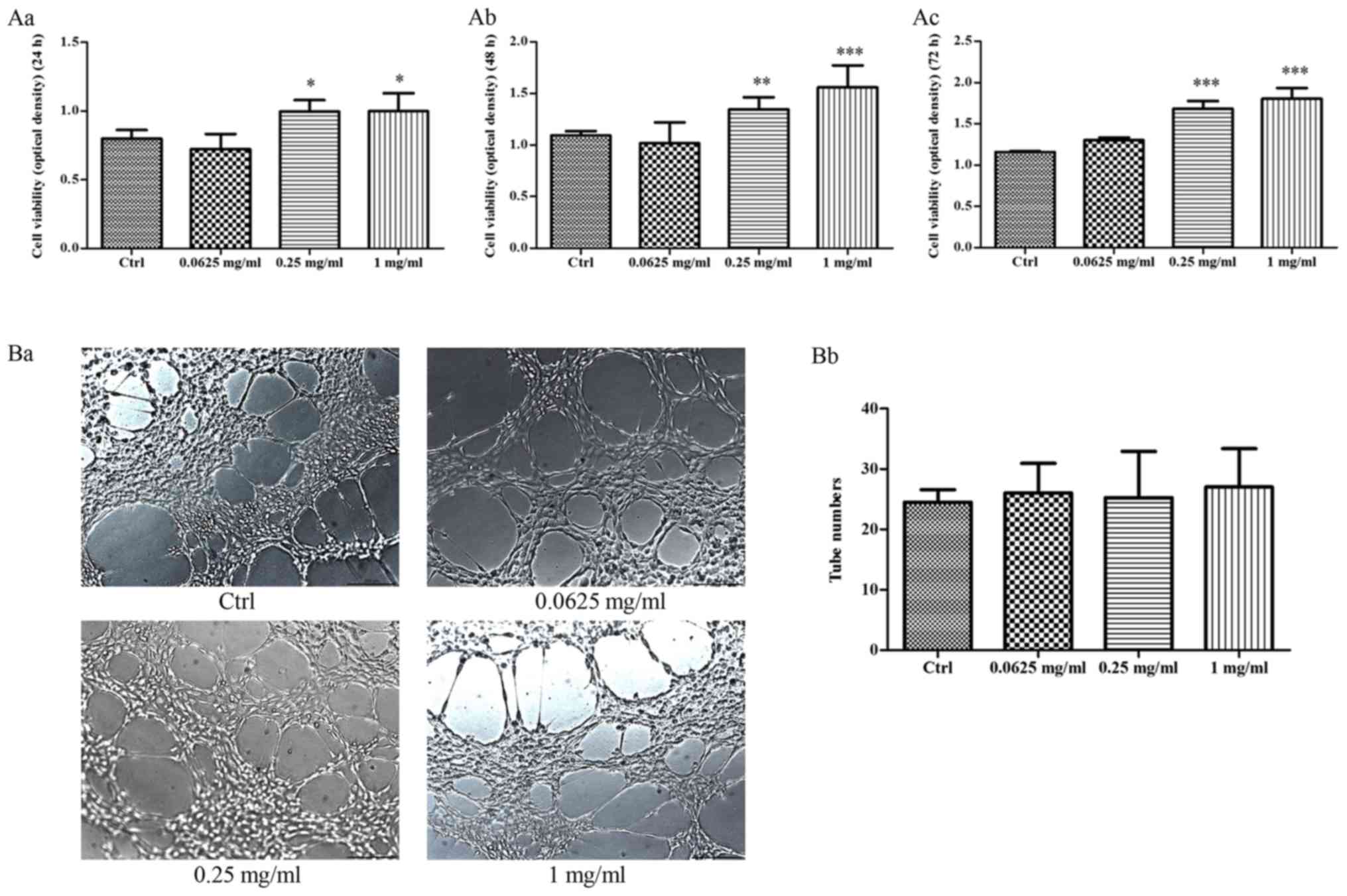
EPCs of the control group were cultured in complete
EBM-2 medium, while cells in other groups were cultured with
0.0625, 0.25 and 1 mg/ml VA-pro. We detected the cells
proliferation at 24, 48 and 72 h, respectively. 0.25 and 1 mg/ml
VA-pro treatment resulted in a promoting of EPCs proliferation in
different degree at 3 time points, cell proliferation of 0.0625
mg/ml group has no difference compared with the control group
(Fig. 3Aa, b, c). These data
suggested that VA-pro can promote EPCs proliferation. A Matrigel
tube formation assay was used to evaluate the effect of VA-pro on
EPCs tube formation. As shown in Fig.
3Ba-b, VA-pro had no effect on EPCs ability of tube
formation.
The effect of VA-pro on EPCs
migration
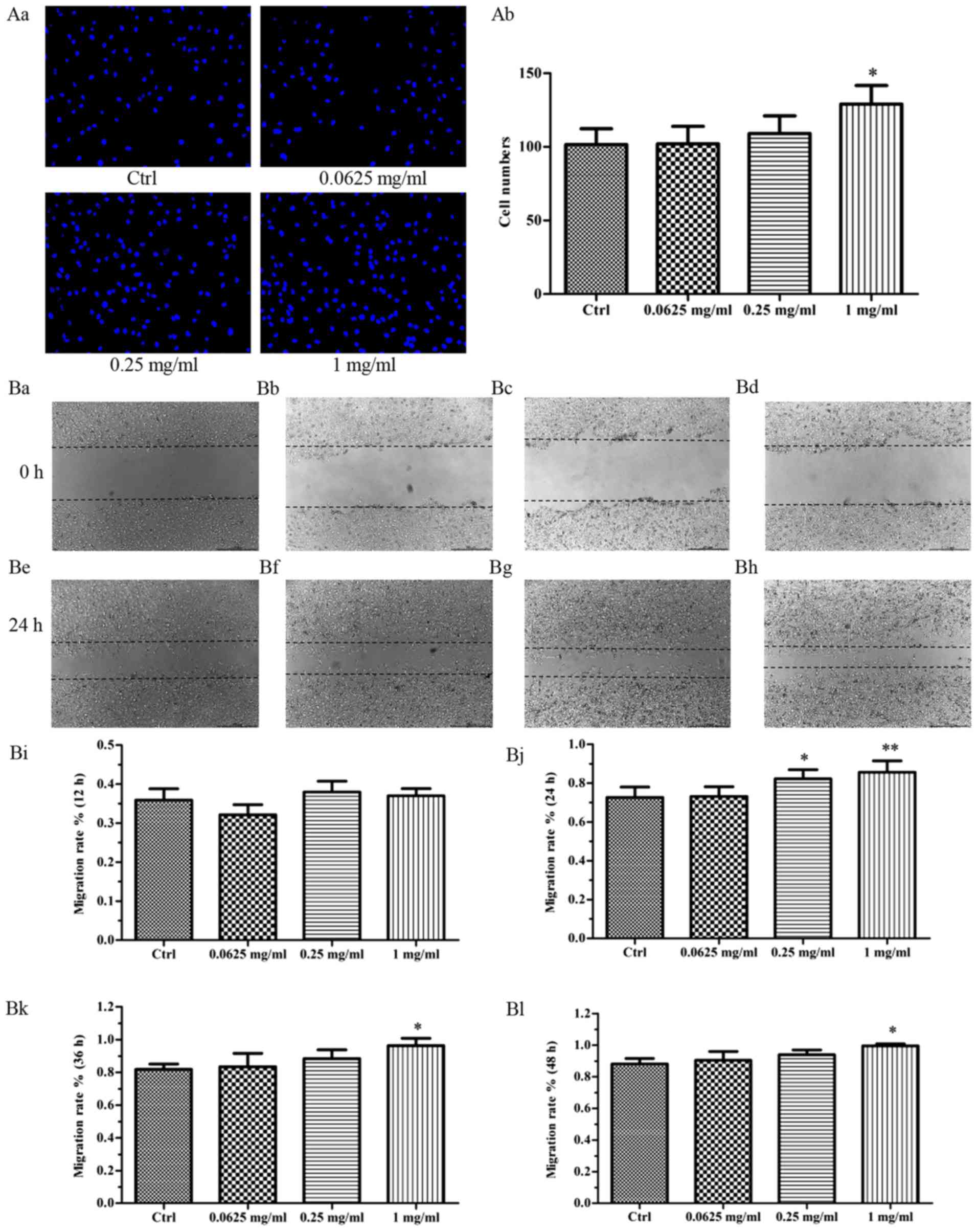
For transwell assay, after cultured for 24 h, the
migration of EPCs treated with 1 mg/ml VA-pro was different from
the control group (Fig. 4Aa).
These data indicated that VA-pro promoted the migration of EPCs,
especially the 1 mg/ml concentration (Fig. 4Ab). For the wound healing assay,
after scratched the confluent cell monolayers, EPCs were photograph
every 12 h, the average width of scratches was measured and the
EPCs migration rate was calculated. Results showed that 1 mg/ml
VA-pro treatment led to better cell migration at 24, 36 and 48 h
compared to the control group. Besides, 0.25 mg/ml VA-pro caused a
significant difference compared to the control group only at 24 h
(Fig. 4Bi-l). We chose the photos
at the time of 24 h to show the differences between groups
(Fig. 4Ba-h).
Effect of VA-pro on the activation
level of Notch and Hes1, and the phosphorylation level of Akt and
mTOR in EPCs
Notch1 pathway inhibitor DAPT (10 µM) and Akt
pathway inhibitor LY294002 (10 µM) were added to the medium
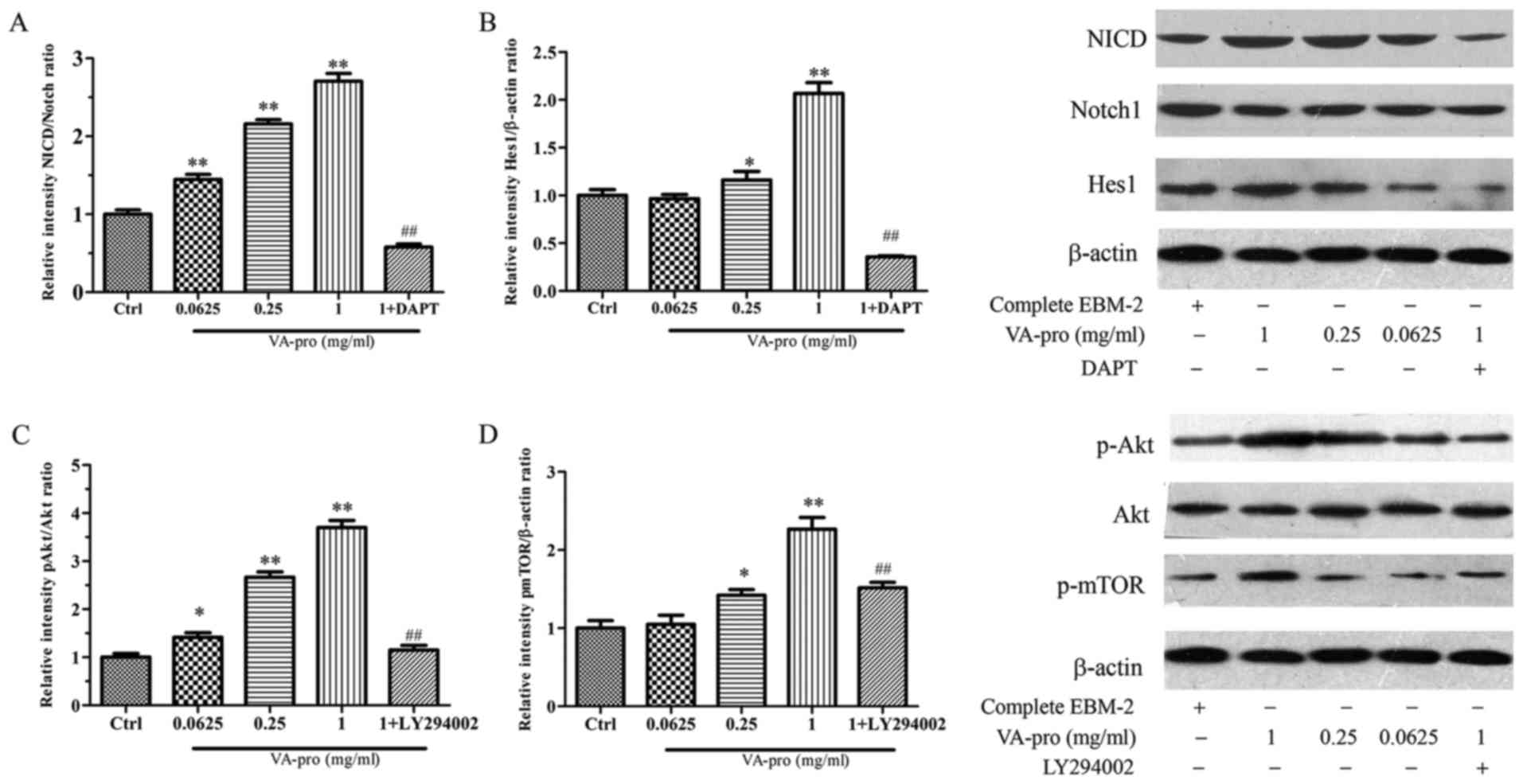
containing 1 mg/ml VA-pro. As shown in Fig. 5, there was no obvious differences
in the total amount of Notch1, but the activation level of Notch1
Intracellular Domain (NICD) significantly increased along with
VA-pro stimulation concentration-dependently, and Hes1 activation
level also significantly increased. However DAPT inversed all. The
ratio of NICD to the total Notch1 and Hes1 to the β-actin were
calculated to evaluate the activation of Notch1 and Hes1 (Fig. 5A, B).
Compared to the control group, VA-pro increased the
phosphorylation level of Akt in a concentration-dependent manner,
and mTOR phosphorylation level also significantly increased.
However LY294002 inversed this outcome, it suppressed the effects
of VA-pro on p-Akt and p-mTOR expression (Fig. 5C, D).
These indicated that VA-pro affected EPCs possibly
through activating the Notch pathway and Akt/mTOR pathway.
Discussion
EPC has been researched as a hotspot in recent
years, not only has it been linked to the development of many
diseases, but plays a key role in treating certain diseases. EPCs
have been engaged to replace damaged endothelial cells and increase
neovascularization in many animal models (16,17).
Whether stem cells transplantation is more good than
harm or not has been controversial, but some researches show that
stem cells have good curative effects on the treatment of diseases.
Better applications of this method are worth to be explored. Chen
et al transplanted autologous EPCs into cerebral ischemia
rabbits models, after 14 days, most of EPCs binded to UEA-1 lectin
were incorporated into capillaries, resulting in an increase of
microvascular density in the ischemia area, the infarction area
reducing and brain function improving (18). Other research (19) has shown that for MODS pigs,
autologous transplantation of EPCs migrated to the injured organs
and induced angiogenesis for replying the blood supply of vital
organs as well as improving their function. Furthermore, the
clinical research about therapeutic angiogenesis by cell
transplantation (TACT) reported that doctors injected the
peripheral mononuclear cells which were collected from patient's
right subclavian vein into local muscle for the treatment of ulcer
causing by occlusive arterial sclerosis, the patient's general
condition was not good and other treatment no curative effect on
ulcer. 2 weeks postoperative, skin temperature increased. 1 month
later, ulcer's blood flow improved and the ulcer completely healed
by 6 months later. This suggested that transplantation may offer a
suitable alternative rescue therapy for patients with critical limb
ischemia (20). Earlier, a TACT
program in Japan was also succeeded in 2003, the research about
allogeneic EPCs transplantation also suggested that EPCs
transplantation was very effective on vascular diseases. Meanwhile,
it indicated that EPCs immunogenicity was not very high.
Now, how to optimize the EPCs proliferation and
amplification is a great task for transplantation. Many scholars
try to make the EPCs culture system better. They have tried to use
autologous serum (21), autologous
culture system (22) and
osteopontin (23). Therefore, this
filed is interesting and ponderable. In our experiment, we used the
freeze-drying technology for better maintenance of proteins
biological activity, VA-pro was extracted by water solvent and
ultrasonic wave method (24). The
experimental results provided that VA-pro can promote EPCs
proliferation and migration.
Notch signaling pathway promotes proliferative
signaling ways during neurogenesis and gets involved in heart
development, neovascularization and angiogenesis process (25,26).
The key link of Notch1 signaling pathway is the activation of
Notch1 Intracellular Domain (NICD). Notch1 combines with the
ligands, and then NICD is releaseed into the cytoplasm and gets
into nucleus, it binds to CSL (C-promoter binding protein-1, CBF-1)
and a complex is formed to activate the genes of transcription
inhibitory factor family, such as Hes, Hey or Herp (27). To be expected, it is related to
EPCs proliferation, differentiation (4) and tube formation (5). EPCs activity reduces when Notch mRNA
is inhibited (28). Hes1 is the
direct downstream target of Notch signaling pathway and plays an
essential role in the development of blood vessel and heart, etc.
Our data revealed that the level of activatory NICD and Hes1 were
increased after intervention with VA-pro, which suggested that
Notch signaling pathway may be involved in the promoting effect of
VA-pro on EPCs proliferation and migration. Akt/mTOR signaling
pathway is directly related to cell proliferation, quiescence and
longevity via regulating the cell cycle. One research showed that
it was involved in progesterone-induced EPC viability (3). Interestingly, VA-pro elevated the
phosphorylation level of Akt and mTOR in our research. Thus, the
effect of VA-pro on EPCs may be associated with the Akt/mTOR
signaling pathway.
Actually, there is much crosstalk between Notch and
Akt signaling pathways (29,30).
Previous studies on Notch and Akt signaling pathway suggested that
Notch signaling pathway could stimulate the Akt signaling pathway
by decreasing PTEN (phosphatase and tensin homology deleted on
chromosome 10) levels (31). PTEN
can stop all downstream effects of Akt signaling pathway by
reducing the AKT activation, and it affects a variety of cellular
functions widely. Hes1 can inhibit PTEN effectively via binding to
its promoter, PTEN transcription will increase when shRNA silence
of Hes1 is performed (31,32).
Interactions between Notch and Akt signaling
pathways provide the basis for the similar results of NICD, Hes1,
p-Akt and p-mTOR affected by VA-pro in this study, Research of the
signaling pathway in this study just remind a possible molecular
mechanism of VA-pro on EPCs, but it assists our main purpose, which
is to find the effective components of VA-pro preferably. We aim to
prove that VA-pro really work on EPCs primarily, and then we
confirm the effective components of VA-pro gradually, based on VA
characteristic of regeneration and in the hope of subsequent
artificial extraction or synthetic.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant no. 81573777) and Beijing
National Science Foundation (grant no. 7162172).
Glossary
Abbreviations
Abbreviations:
|
VA
|
velvet antler
|
|
VA-pro
|
velvet antler proteins
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto encyclopedia of genes and
genomes
|
|
pI
|
Protein isoelectric point
|
|
MW
|
proteins molecular weight
|
|
CMECs
|
cardiac microvascular endothelial
cells
|
|
IH
|
ischemia hypoxia
|
|
MMP
|
mitochondrial membrane potential
|
|
CD31
|
Platelet endothelial cell adhesion
molecule-1
|
|
vWF
|
von Willebrand factor
|
|
CVD
|
cardiovascular disease
|
References
|
1
|
Miller-Kasprzak E and Jagodziński PP:
Endothelial progenitor cells as a new agent contributing to
vascular repair. Arch Immunol Ther Exp (Warsz). 55:247–259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbrig K, Haensel S, Oelschlaegel U,
Pistrosch F, Foerster S and Passauer J: Endothelial dysfunction in
patients with rheumatoid arthritis is associated with a reduced
number and impaired function of endothelial progenitor cells. Ann
Rheum Dis. 65:157–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu P, Zhang Z, Li S, Wen X, Quan W, Tian
Q, Chen J, Zhang J and Jiang R: Progesterone modulates endothelial
progenitor cell (EPC) viability through the CXCL12/CXCR4/PI3K/Akt
signalling pathway. Cell Prolif. 49:48–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortegano I, Melgar-Rojas P, Luna-Zurita
L, Siguero-Álvarez M, Marcos MA, Gaspar ML and de la Pompa JL:
Notch1 regulates progenitor cell proliferation and differentiation
during mouse yolk sac hematopoiesis. Cell Death Differ.
21:1081–1094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karcher JR, Hoffmann BR, Liu P, Liu Y,
Liang M and Greene AS: Genome-wide epigenetic and proteomic
analysis reveals altered Notch signaling in EPC dysfunction.
Physiol Rep. 3:pii:e123582015. View Article : Google Scholar
|
|
6
|
Price J, Faucheux C and Allen S: Deer
antlers as a model of mammalian regeneration. Curr Top Dev Biol.
67:1–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Price JS, Allen S, Faucheux C, Althnaian T
and Mount JG: Deer antlers: A zoological curiosity or the key to
understanding organ regeneration in mammals? J Anat. 207:603–618.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Zhao HP, Liu Z and McMahon C: Deer
antler-a novel model for studying organ regeneration in mammals.
Int J Biochem Cell Biol. 56:111–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Pearson A and McMahon C:
Morphogenetic mechanisms in the cyclic regeneration of hair
follicles and deer antlers from stem cells. Biomed Res Int.
2013:6436012013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sui Z, Zhang L, Huo Y and Zhang Y:
Bioactive components of velvet antlers and their pharmacological
properties. J Pharm Biomed Anal. 87:229–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pita-Thomas W, Nieto-Sampedro M, Maza RM
and Nieto-Diaz M: Factors promoting neurite outgrowth during deer
antler regeneration. J Neurosci Res. 88:3034–3047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan SW, Duan LX, Li YY, Wang BX and Zhou
QL: A novel polypeptide from Cervus nippon Temminck proliferation
of epidermal cells and NIH3T3 cell line. Acta Biochim Pol.
53:395–397. 2006.PubMed/NCBI
|
|
13
|
Zha E, Gao S, Pi Y, Li X, Wang Y and Yue
X: Wound healing by a 3.2 kDa recombinant polypeptide from velvet
antler of Cervus nippon Temminck. Biotechnol Lett. 34:789–793.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon B, Kim S, Lee S, Park P, Sung S, Kim
J and Moon S: Effect of antler growth period on the chemical
composition of velvet antler in sika deer (Cervus nippon). Mamm
Biol. 5:374–380. 2009. View Article : Google Scholar
|
|
15
|
Dong Y, Sun Q, Liu T, Wang H, Jiao K, Xu
J, Liu X, Liu H and Wang W: Nitrative stress participates in
endothelial progenitor cell injury in hyperhomocysteinemia. PLoS
One. 11:e01586722016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue S, Zhang HT, Zhang P, Luo J, Chen ZZ,
Jang XD and Xu RX: Functional endothelial progenitor cells derived
from adipose tissue show beneficial effect on cell therapy of
traumatic brain injury. Neurosci Lett. 473:186–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu
J, Chen Y, Su H, Young WL and Yang GY: Endothelial progenitor cell
transplantation improves long-term stroke outcome in mice. Ann
Neurol. 67:488–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen ZZ, Jiang XD, Zhang LL, Shang JH, Du
MX, Xu G and Xu RX: Beneficial effect of autologous transplantation
of bone marrow stromal cells and endothelial progenitor cells on
cerebral ischemia in rabbits. Neurosci Lett. 445:36–41. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tianhang L, Bo W, Zhengmao L, Tao P, Hong
Z, Xuchao X, Jianwei B, Hui Z and Guoen F: Autologous
transplantation of endothelial progenitor cells to prevent multiple
organ dysfunction syndromes in pig. J Trauma Acute Care Surg.
74:508–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugihara S, Yamamoto Y, Matsubara K,
Ishida K, Matsuura T, Ando F, Igawa G, Narazaki G, Miake J, Tajima
F, et al: Autoperipheral blood mononuclear cell transplantation
improved giant ulcers due to chronic arteriosclerosis obliterans.
Heart Vessels. 21:258–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shumiya T, Shibata R, Shimizu Y, Ishii M,
Kubota R, Shintani S and Murohara T: Evidence for the therapeutic
potential of ex vivo expanded human endothelial progenitor cells
using autologous serum. Circ J. 74:1006–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon SH, Kim SM, Park SJ, Kim H, Bae D,
Choi YS and Chung HM: Development of a xeno-free autologous culture
system for endothelial progenitor cells derived from human
umbilical cord blood. PLoS One. 8:e752242013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vaughan EE, Liew A, Mashayekhi K, Dockery
P, McDermott J, Kealy B, Flynn A, Duffy A, Coleman C, O'Regan A, et
al: Pretreatment of endothelial progenitor cells with osteopontin
enhances cell therapy for peripheral vascular disease. Cell
Transplant. 21:1095–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Zhao Y, Li Q and Shen M:
Extraction and immune activity of soluble proteins from velvet
antler of Cervus nippon Temminck. J Northeast Fore Univ.
42:158–163. 2014.
|
|
25
|
Niessen K and Karsan A: Notch signaling in
cardiac development. Circ Res. 102:1169–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de la Pompa JL: Notch signaling in cardiac
development and disease. Pediatr Cardiol. 30:643–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paola R, Lucio M and Roberto F: The Notch
pathway: A crossroad between the life and death of the endothelium.
Eur Heart J. 34:2504–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ii M, Takeshita K, Ibusuki K, Luedemann C,
Wecker A, Eaton E, Thorne T, Asahara T, Liao JK and Losordo DW:
Notch signaling regulates endothelial progenitor cell activity
during recovery from arterial injury in hypercholesterolemic mice.
Circulation. 121:1104–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cornejo MG, Mabialah V, Sykes SM, Khandan
T, Lo Celso C, Lopez CK, Rivera-Muñoz P, Rameau P, Tothova Z, Aster
JC, et al: Crosstalk between NOTCH and AKT signaling during murine
megakaryocyte lineage specification. Blood. 118:1264–1273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZJ, Xiao M, Balint K, Soma A, Pinnix
CC, Capobianco AJ, Velazquez OC and Herlyn M: Inhibition of
endothelial cell proliferation by Notch1 signaling is mediated by
repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J.
20:1009–1011. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palomero T, Sulis ML, Cortina M, Real PJ,
Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, et
al: Mutational loss of PTEN induces resistance to NOTCH1 inhibition
in T-cell leukemia. Nat Med. 13:1203–1210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palomero T, Dominguez M and Ferrando AA:
The role of the PTEN/AKT pathway in NOTCH1-induced leukemia. Cell
Cycle. 7:965–970. 2008. View Article : Google Scholar : PubMed/NCBI
|