Introduction
The thyroid hormones (THs), including
triiodothyronine (T3) and thyroxine (T4), are essential for the
prefrontal cortex (PFC) structure and function (1). Insufficiency of THs in the adulthood
affected performance of prefrontal learning and memory (2), in which synaptic transmission among
the neurons and synaptic plasticity may be involved. Synaptic
transmission is conducted by neurotransmitters released from
presynaptic nerve terminals by means of Ca2+-dependent
exocytosis of synaptic vesicles, and its regulation is believed to
be one of the important mechanisms of synaptic plasticity
underlying learning and memory (3). One neurotransmitter that may be
involved is acetylcholine (ACh), whose action is dependent on its
metabolizing enzyme acetylcholinesterase (AChE). Several
presynaptic proteins have been implicated in presynaptic regulation
of the Ca2+-dependent neurotransmission, including
synaptotagmin-1 (syt-1) and synaptosomal protein of 25 kDa
(SNAP-25) (4–6). Syt-1, well established as a primary
Ca2+ sensor for rapid and regulated vesicle release from
neurons and neurosecretory cells (7,8),
directly interacts with SNAP-25 on the pre-synaptic membrane to
facilitate neurotransmitter release. Thyroid dysfunction has been
demonstrated to influence the cholinergic system and synaptic
proteins in adult rats (9–14).
Thyroxine (T4) replacement therapy is, at present,
the first choice for patients with hypothyroidism (15). However, previous clinical studies
have indicated that patients with hypothyroidism who were properly
treated with T4 have not always fully recovered from the symptoms
affecting the central nervous system, such as the cognitive
functioning and well-being (16–18).
Previous animal studies of the authors have revealed that
hypothyroidism induces significant expression changes of several
synaptic proteins in the brain regions related to learning and
memory, whereas some of the proteins were not fully restored to
control values by T4 treatment (13,14,19).
Furthermore, in previous morphological studies, Madeira et
al (20,21) discovered that hypothyroidism
induces reductions in the number of pyramidal cells in the
hippocampal CA1 region and granule cells in the dentate gyrus,
which were also not restored following the normalization of thyroid
hormone levels in adult rats. These studies indicate that it is
still necessary to discover new alternative therapeutic methods for
hypothyroidism.
Donepezil (DON), as one cholinesterase inhibitor
(AChEI), was primarily administered for the treatment of mild to
moderate cognitive impairment. On the other hand, it was confirmed
the independent neuroprotective effects in recent years (22,23),
which have been demonstrated in a number of models, including
protection of cortical neurons in models of glutamate-induced
toxicity and oxygen-glucose deprivation, and protection against the
effects of hippocampal mitochondrial dysfunction in transgenic
mouse models of Alzheimer's disease (AD) (24). Therefore, it is of great interest
to know whether DON has neuroprotective effects on neuronal injury
induced by adult hypothyroidism.
In the present study, a hypothyroid rat model was
induced by adding 0.05% propylthiouracil (PTU) to their drinking
water according to the authors' previous study (14). The authors observed the
ultrastructure, content of ACh and the activity of AChE, as well as
the expression of syt-1 and SNAP-25 in the PFC of adult hypothyroid
rats, and evaluated the efficiency of T4 and DON administered
separately, or in combination on the above changes in adult
hypothyroid rats.
Materials and methods
Experimental animals and general
protocol
The present study was conducted in strict accordance
with the Animal Care and Use Committee of Anhui Medical University
(Anhui, China). The protocol was approved by the Committee on the
Ethics of Animal Experiments of Anhui Medical University (Anhui,
China). All efforts were made to minimize suffering. Male
Sprague-Dawley rats (SPF grade, ten-week-old, weighed 230–260 g)
were purchased from the Experimental Animal Center of Anhui Medical
University (Hefei, China). A total of ~120 rats were used for
various experimental approaches in the current study. The rats were
housed in groups (four to five rats per cage). All rats received a
standard rodent diet and tap water ad lib. A constant temperature
of 21–23°C and humidity of 50±5% were maintained. Following feeding
for one week, the rats were randomly separated into five groups,
with 24 rats in each group: i) Rats in the hypothyroid group (Hypo
group) were induced by including propylthiouracil
(6-n-propyl-2-thiouracil, PTU; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in the drinking water (at a concentration of
0.05% w/v) for six weeks; ii) Rats in the donepezil group (DON
group) were treated with PTU for six weeks as described above, and
0.005% (w/v) donepezil (Sigma-Aldrich; Merck KGaA) was added to the
tap drinking water every day from the fifth week; iii) Rats in the
thyroxine group (T4 group) were treated with PTU for six weeks, and
were treated with daily intraperitoneal injection of T4 (dissolved
in saline solution, 6 µg/100 g body weight) from the fifth week;
iv) A further 20 rats in the thyroxine plus donepezil group (T4 +
DON group) were treated according to the same protocols for six
weeks as the T4 group beside adding 0.005% (w/v) DON to the
drinking water from the fifth week; v) A total of 20 control rats
in the control group (CON group) were treated with saline solution
for six weeks. The total treatment time was two weeks. The dose of
T4 was selected based on previous studies (14,19),
and the dosage was adjusted according to the rats' weights weighed
weekly.
Thyroid hormones
All rats were anesthetized using chloral hydrate
(350 mg/kg body weight), following the delivery of the last dose.
The blood collected from the abdominal aorta (1.5 ml) was separated
by centrifuging at 14,000 × g for 15 min (25), and then the serum was collected and
rapidly frozen at −20°C for subsequent analysis. Serum T3 and T4
levels were obtained using a radioimmunoassay kit (Beijing North
Institute of Biological Technology, Beijing, China). All sample
measurements were run in duplicate.
Sample preparation
Following the blood collection and the rats were
sacrificed, the prefrontal tissues of 4 rats of each group were
sampled and in 4% paraformaldehyde solution for the transmission
electron microscopy (TEM) observation. The PFC of another 10 rats
of each group was isolated and placed at −80°C, for subsequent
determining the content of ACh and activity of AChE. The PFC of the
rest 10 rats of each group was quickly isolated and placed at
−80°C, for western blot analysis.
TEM observation
The tissues of the PFC were cut into small pieces,
~1 mm3, fixed with 2.5% glutaraldehyde at 4°C for 4–6 h,
then fixed with l% osmium tetroxide for 1 h, following ethanol
dehydration and epoxy resin (Epon812) embedding, the tissues were
prepared the ultrathin sections, and soaked in the uranyl acetate
solution and the lead citrate solution for the staining. Following
rinsing, the ultrastructures were observed and photographed using a
JEM-1230 TEM (Nissan Chemical Industries, Ltd., Tokyo, China).
Determination of ACh content and AChE
activity
The materials and methods for determination of ACh
content and AChE activity were as previously described (14). ACh levels and AChE activity were
expressed as µg per mg of prefrontal protein. The protein
concentration of the prefrontal homogenates was determined using a
Pierce BCA Protein assay kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Western blot analysis
The prefrontal tissues were placed in a glass
homogenizer, added radioimmunoprecipitation assay lysis buffer and
phenylmethylsulfonyl fluoride, then homogenized on ice to extract
the total proteins. The homogenate was then centrifuged at 15,000 ×
g and 4°C for 15 min, the total protein concentration of the
supernatant was then determined with the lorry method: Following
quantification, 2X loading buffer (1:1) was added to the sample,
proteins were denatured at 98°C for 10 min, packaged and
cryopreserved at −80°C. A total of 20 µg sample of each group was
subject to SDS-PAGE for 2.5 h, then transferred onto a
polyvinylidene fluoride membrane for overnight blocking with 5%
skim milk. The blots were then incubated with rabbit anti-syt-1
polyclonal antibody (cat. no. ab82414; dilution, 1:1,000; Abcam,
Cambridge, MA, USA), rabbit anti-SNAP-25 polyclonal antibody (cat.
no. ab5666; dilution, 1:10,000; Abcam) and anti-GAPDH monoclonal
antibody (cat. no. ab181602; dilution, 1:4,000; Abcam) at room
temperature for 2 h, followed by rinsing with 0.05% PBS-Tween 20
(PBS-T) solution three times, 10 min for each time. Membranes were
then incubated with secondary antibody (cat. no. AS014; dilution,
1:200,000; horseradish peroxidase-conjugated goat-anti-rabbit IgG;
ABclonal Biotech Co., Ltd., Wuhan, China) at room temperature for
1–2 h. The membrane was then rinsed with 0.05% PBS-T. Finally, the
enhanced chemiluminescence solution (SuperSignal™ West Femto
Maximum Sensitivity Substrate; Thermo Fisher Scientific, Inc.) was
added and a Fine-do X6 visualizer was used for the photographing
(Tanon Science and Technology Co., Shanghai, China). GAPDH was used
as the internal reference, the ratio of optical densities of the
target protein and GAPDH was then calculated using Image-Pro Plus
densitometric image analysis software (version, 5.0; Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Values were expressed as means ± standard error of
the mean. The data were analyzed by one-way analysis of variance
using least-significant difference for post hoc analysis. P<0.05
was used to indicate a statistically significant difference. All
analyses were conducted by statistical software, SPSS (version,
13.0; SPSS, Inc., Chicago, IL, USA).
Results
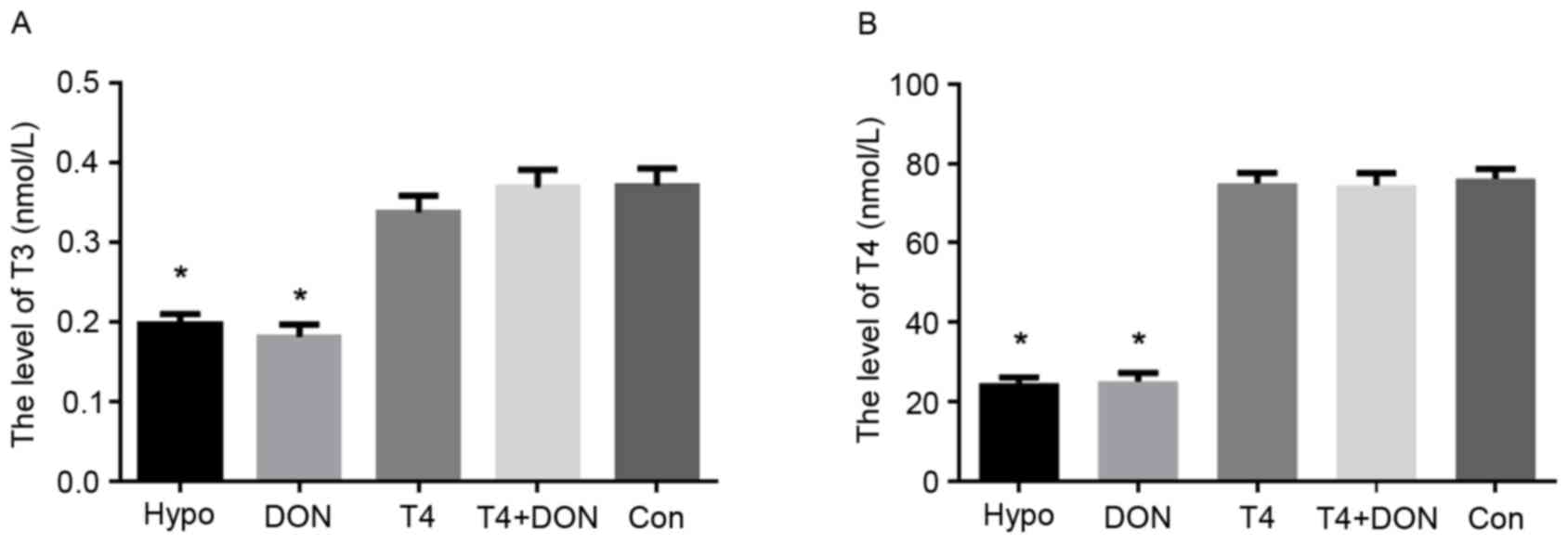
Serum TH levels in rats
The serum TH levels of the five groups are presented
in Fig. 1. Hypothyroidism was
confirmed by measuring plasma T3 and T4 levels at the end of the
experiment. Compared with the CON group, the serum T3 and T4 levels
in the Hypo and the DON groups were significantly decreased
(P<0.001). However, no significant differences in the T3 and T4
levels were observed in the T4 and the T4 + DON groups when
compared with the CON group (P>0.05).
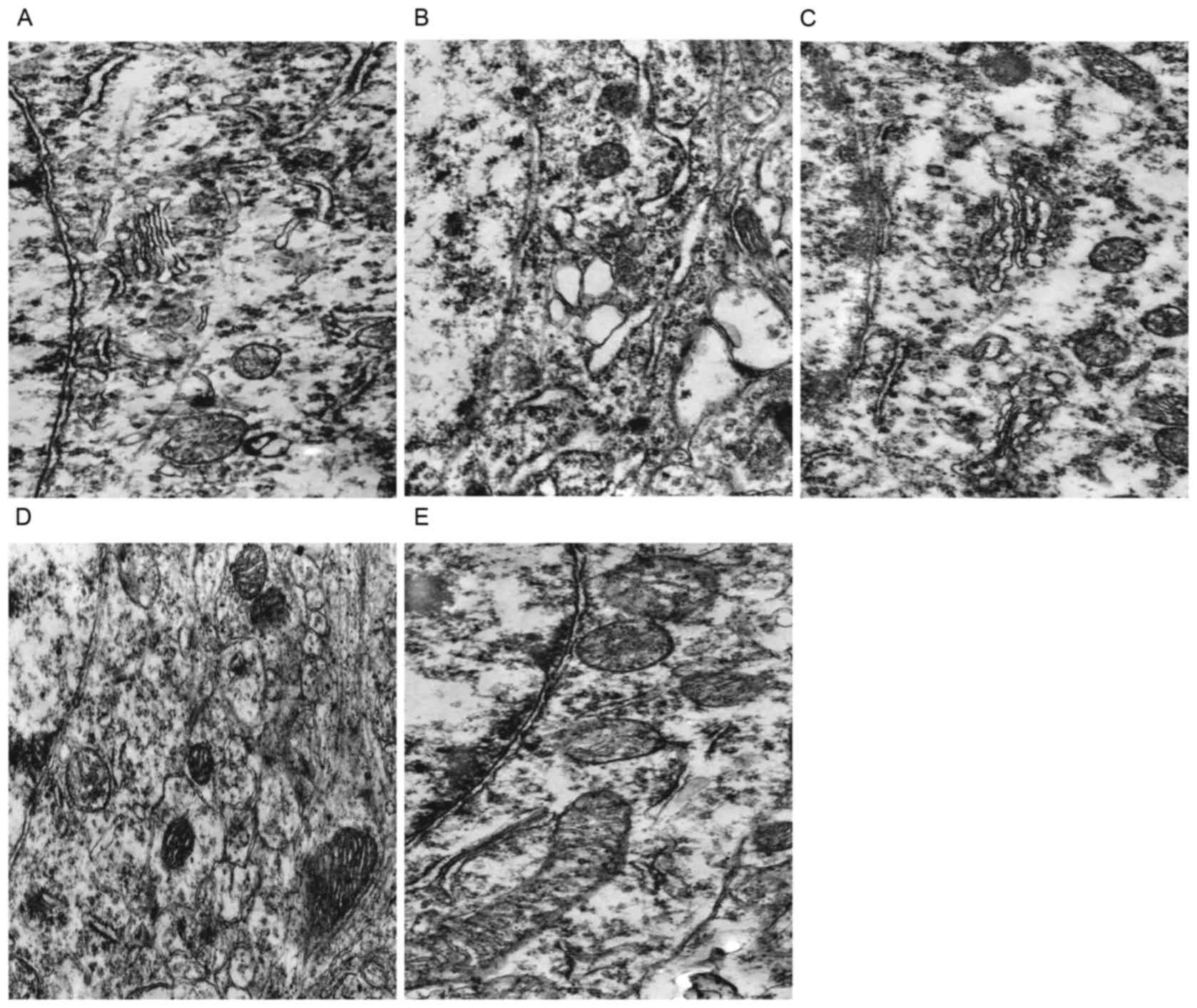
TEM observation
Neuronal changes
In the euthyroid rats, the neurons displayed smooth
and intact nuclear membranes, with chromatins evenly distributed.
The mitochondria developed well, with clear internal ridge
structures and the rough endoplasmic reticulum and ribosomes were
rich (Fig. 2A). In the hypothyroid
rats, the neurons displayed a scanty cytoplasm with few organelles,
the mitochondria were characterized by highly fractured and
degenerated cristae and a clear vacuolation, the endoplasmic
reticulum (ER) was greatly dilated (Fig. 2B). The neurons of the DON group and
the T4 group exhibited clear nuclear membranes, while the
organelles were relatively sparse, and a small amount of
mitochondria exhibited the vacuolation, partial rough endoplasmic
reticulum was mildly dilated (Fig. 2C
and D). The morphologies of neurons, mitochondria, rough ER and
ribosomes of the T4 + DON group were similar to the CON group
(Fig. 2E).
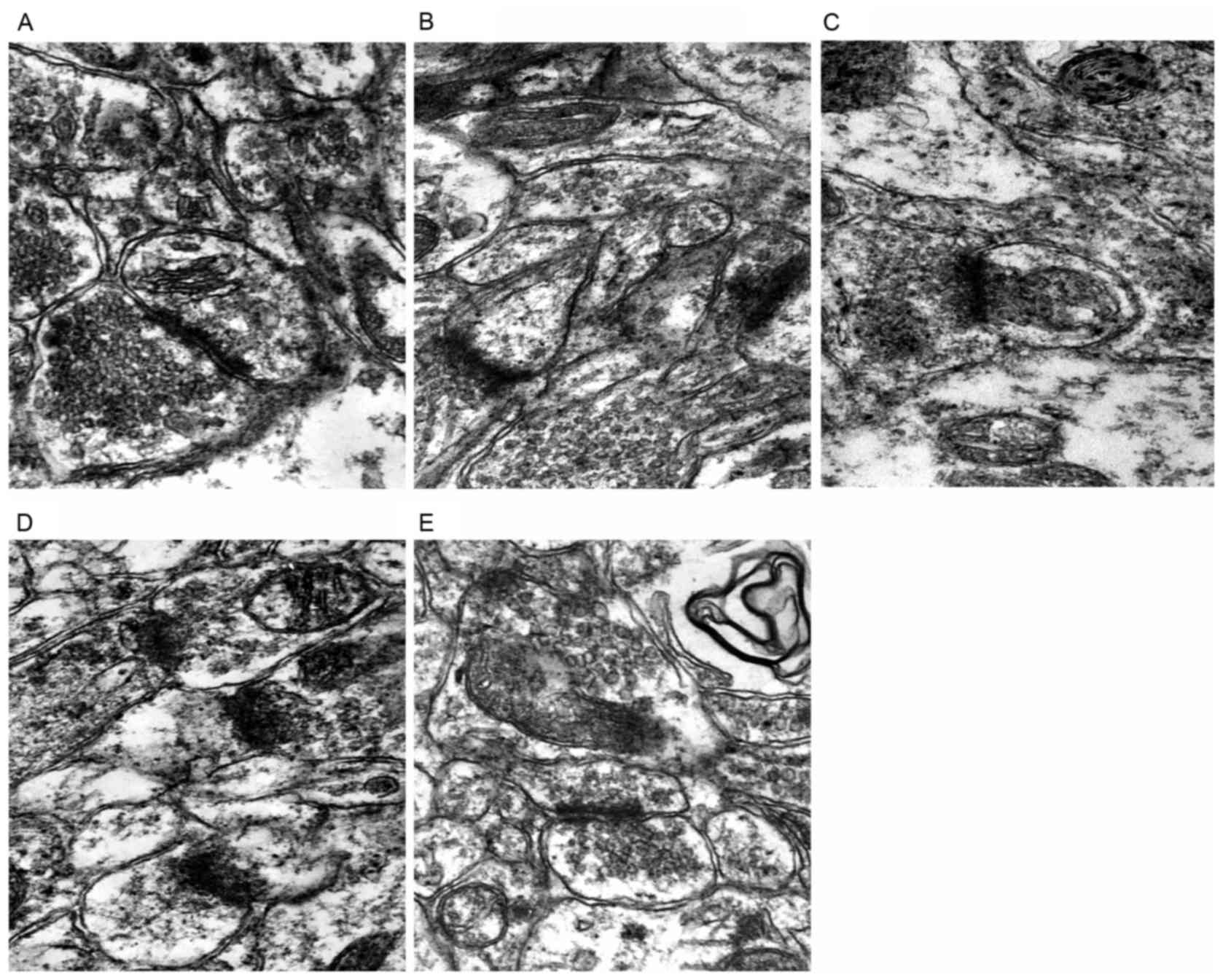
Synaptic changes
The structures of presynaptic membrane, synaptic
cleft and postsynaptic membrane of the CON group were clear, the
specialized belt was obvious, the synaptic vesicles were rich, and
exhibited clear and dense-core vesicles (Fig. 3A). While the presynaptic membrane
and the postsynaptic membrane of the Hypo group were fused, the
synaptic vesicles were significantly reduced, and almost no
clear-type synaptic vesicles could be seen (Fig. 3B). The presynaptic membrane and the
postsynaptic membrane of the DON group and the T4 group were vague,
the synaptic cleft was clear, while the number of clear-type
synaptic vesicles was reduced (Fig. 3C
and D). The structures of three synaptic layers of the T4 + DON
group were relatively clear, the synaptic vesicles were relatively
rich, close to the situations of the CON group (Fig. 3E).
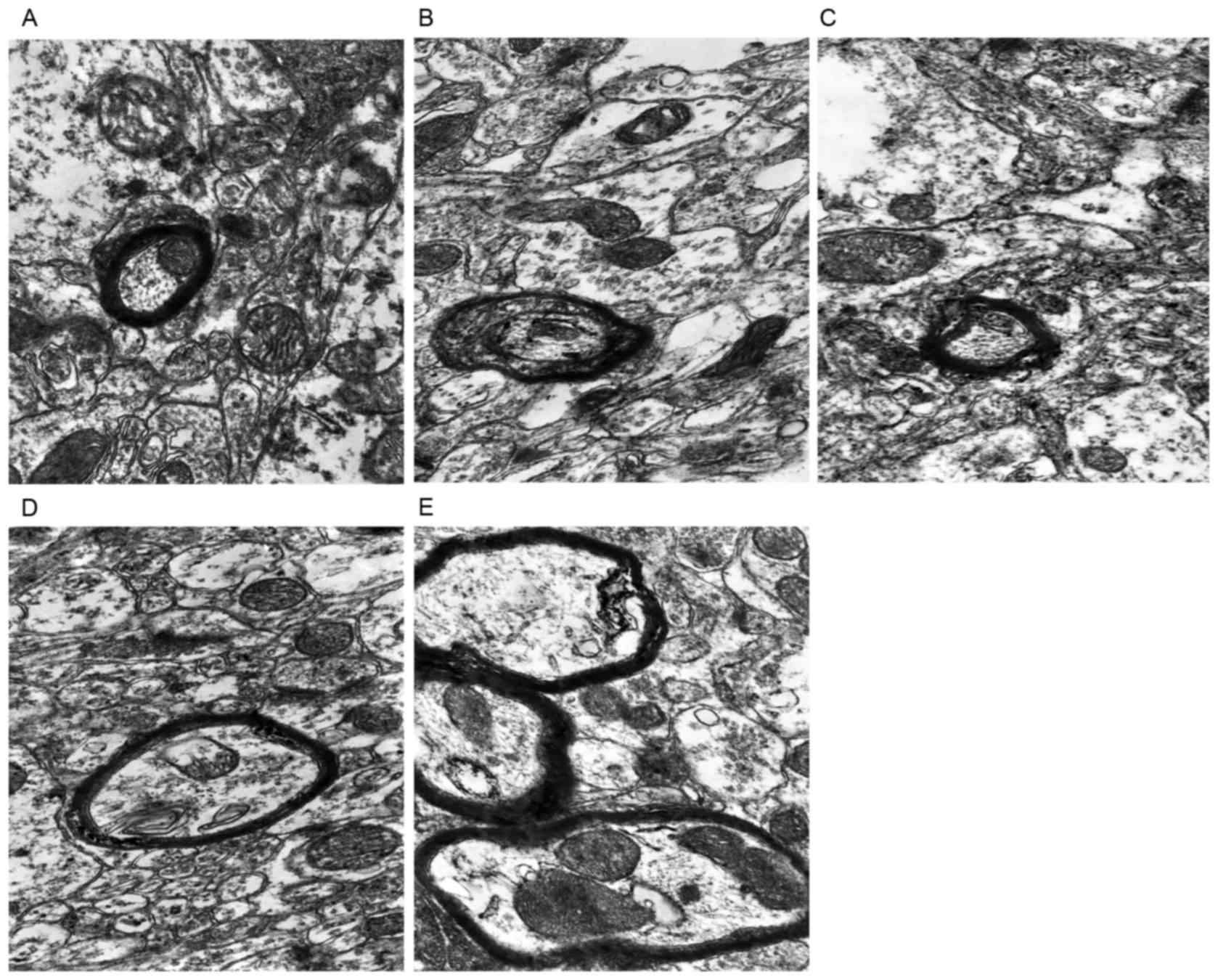
Myelin sheath changes
The structures of the prefrontal myelin sheath in
the rats of CON group were complete, exhibited dense lamination,
normal thickness and smooth edges (Fig. 4A). While the morphological changes
of myelin sheath in the hypothroid rats showed delamination and an
incompact structure, with thinner thickness and irregular outline
(Fig. 4B). Following DON or T4
treatment, there was mild disruption of myelin sheath
microstructures in the rats (Fig. 4C
and D). The morphologies of myelin sheath of the T4 + DON group
were similar to the situations of the CON group (Fig. 4E).
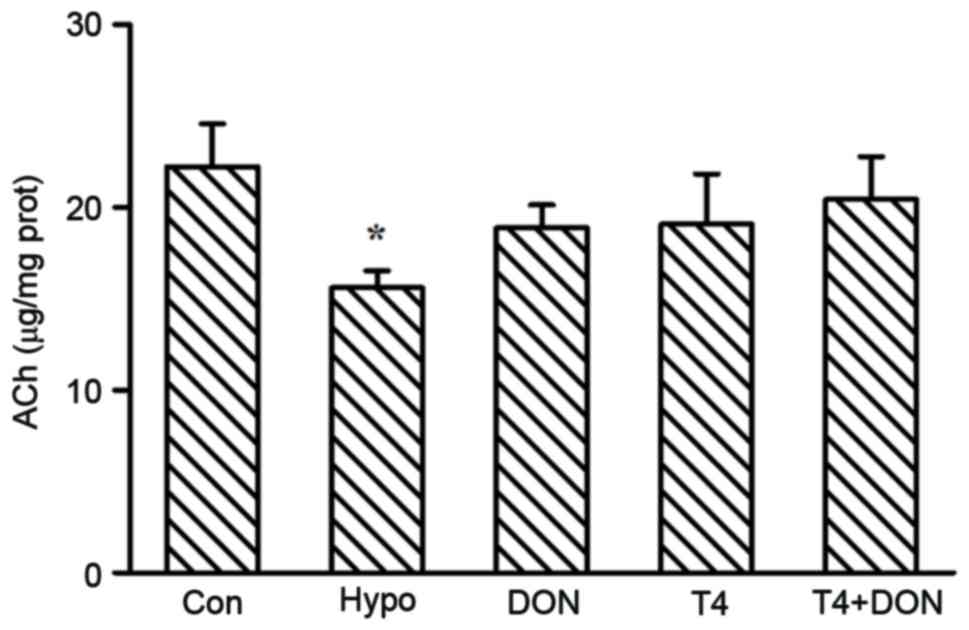
Content of ACh in the rat PFC
The ACh content in the PFC is illustrated in
Fig. 5. The results suggested that
experimentally induced hypothyroidism decreased neurotransmitter
ACh content by 29.8% in the PFC of adult rats, as compared with the
rats in the CON group (P=0.030). The content of ACh could be
restored to control levels (P=0.237, 0.291 and 0.524, respectively)
by administration of DON, T4 or T4 + DON.
AChE activity in the rat PFC
Fig. 6 demonstrates
the activity of AChE in the PFC among the groups. Compared with the
rats in the CON group, the AChE activity in both the Hypo and DON
groups was significantly decreased by 27.8 and 37.3% (P=0.031 and
0.010 respectively), and it was restored to control level by T4
treatment (P=0.144).
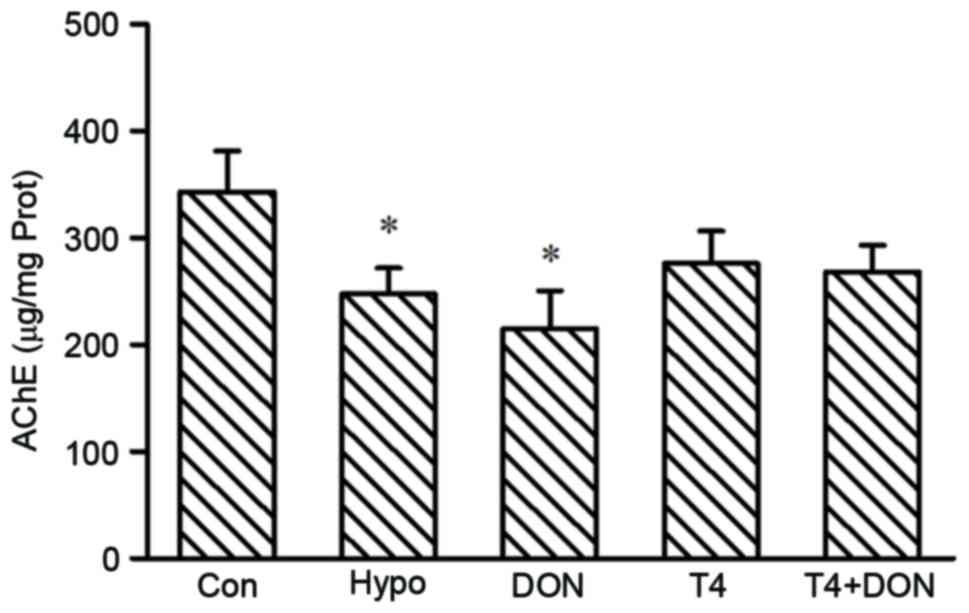 | Figure 6.The AChE activity in the PFC of rats
from CON, Hypo, DON, T4 and T4 + DON groups (n=10), homogenates
were extracted from the PFC of each rat. Data are presented as
means ± standard error of the mean of three independent
experiments. *P<0.05 vs. CON. CON, control; Hypo, hypothyroid;
T4, thyroxine; DON, donepezil; AChE, acetylcholinesterase; Prot,
prefrontal protein. |
Protein levels of syt-1 and SNAP-25 in the rat
PFC
Fig. 7 presents the
relative levels of syt-1 and SNAP-25 in the PFC of all rats. The
results revealed that the protein expressions of syt-1 and SNAP-25
were severely affected by the deficiency of TH. Compared with the
CON group, the prefrontal syt-1 protein expression of the Hypo
Group was significantly increased by 47.8% (P=0.002), and those of
the DON group and the T4 group were increased by 57.4 and 36.0%
(P<0.001 and P=0.016), respectively, while that of the T4 + DON
group presented no difference with the CON group (P=0.211; Fig. 7A and C). The expression of SNAP-25
protein of the Hypo group was significantly increased than the CON
group by 112% (P<0.001), and only increased by 95.6 and 93.3%
following the DON and T4 treatment (both P<0.001), respectively,
following the T4 + DON treatment, the expression of SNAP-25 protein
was restored to the normal level (P=0.070; Fig. 7B and D).
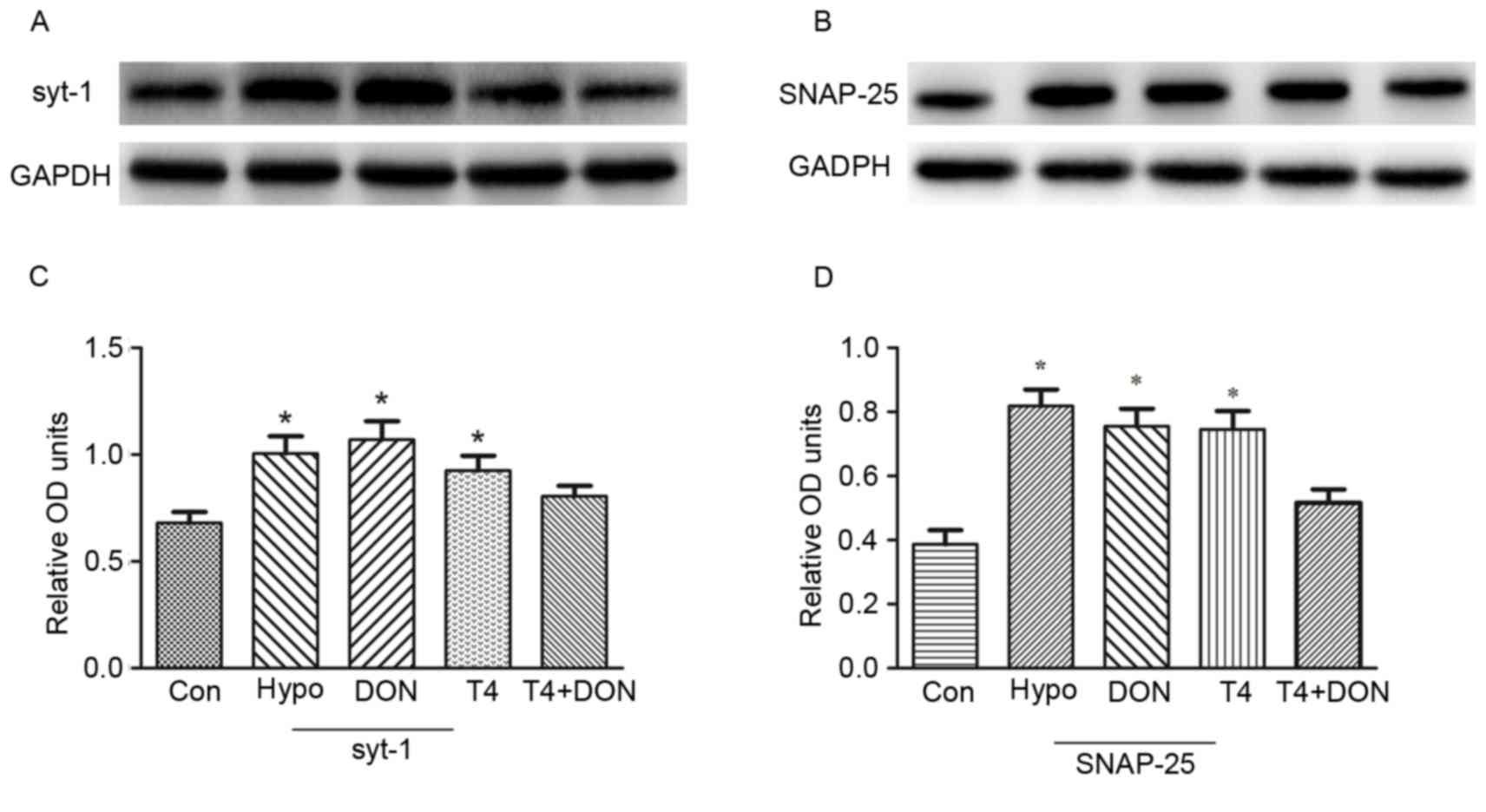 | Figure 7.T4 and DON regulated the expression
of syt-1 and SNAP-25 protein in the PFC of adult hypothyroid rats.
(A and B) Western blot analysis of the expressions of syt-1 and
SNAP-25 protein in the PFC of CON, Hypo, DON, T4 and T4 + DON
groups (C and D). Quantification analysis of the relative protein
levels in each group. The data are presented as the means ±
standard error of the mean (n=10). *P<0.05 vs. CON. CON,
control; Hypo, hypothyroid; DON, donepezil; T4, thyroxine; syt-1,
synaptotagmin-1; SNAP-25, synaptosomal-associated protein of 25
kDa; PFC, prefrontal cortex; OD, optical density. |
Discussion
In the present study, TEM analysis revealed that
adult-onset hypothyroidism induced marked ultrastructural changes,
including the neurons, the synapses and the myelin sheath in the
PFC. The neurons displayed a scanty cytoplasm with few organelles,
the mitochondria were characterized by highly fractured and
degenerated cristae and a clear vacuolation, the endoplasmic
reticulum was greatly dilated. In addition, the synapses exhibited
specialized-belt fusion, the structures became unclear, and the
synaptic vesicles were reduced. The morphological changes of the
myelin sheath demonstrated delamination and an incompact structure,
with reduced thickness and irregular outline. These morphological
alterations caused by hypothyroidism have been described in other
tissues, such as the hippocampus and cuneate nucleus (26–28).
The morphological integrity of the neurons and the efficacy of
synaptic transmission of the PFC are of primary importance for the
role played by this region in various forms of learning and memory.
The complete synaptic and myelin sheath morphology was the
structural basis towards the information transferring among the
neurons, thus the destruction of the above structures may cause the
dysfunction of synaptic transmission by neurotransmitters.
It is well established that the cholinergic system
is involved in higher brain functions such as learning and memory
(29,30). In the present study, the authors
demonstrated that experimentally induced hypothyroidism decreased
ACh content, as well as AChE activity, in the PFC of adult rats. A
significant decrement of ACh levels has also been identified in the
hippocampus and spinal cords of adult-onset hypothyroidism
(14,31). The AChE activity was also indicated
to be decreased in the frontal cortex, the striatum, the cerebellum
and entire brain of hypothyroid rats (10,11,32,33).
Previous research by Kundu et al (34) suggested a central T3 homeostasis
that starts between the 1st and the 2nd day, and terminates between
the days 18 and 20. In the present study, the ACh content and AChE
activity was measured on day 42 following the adult-onset
hypothyroidism in the rat PFC. Thus, the decline in ACh content and
AChE activity following 42 days of PTU-induced hypothyroidism could
be correlated with total disruption of the central homeostatic
mechanism leading to the alteration in the neuronal functional
state, primarily due to metabolic reasons. On the other hand, these
changes may reflect a reduced number of cholinergic synapses in the
PFC, since the PFC has been reported to be diffusely innervated by
the cholinergic fibers ascending from the basal forebrain (35). However, AChE patterns still remain
ambiguous as reports on both unchanged enzyme activity in
thyroidectomized and increased activity in PTU-induced hypothyroid
rats are available (9,36).
Western blot analysis of the synaptic proteins in
the PFC revealed that both syt-1 and SNAP-25 were significantly
increased in the hypothyroid adult rats, compared with that of the
control rats. Previous studies have demonstrated that the
expression of SNAP-25 is upregulated in the dorsal and ventral
hippocampus of hypothyroid mice with mild cognitive impairment
(37). In addition, it was
reported that in rats with thyroid resection, the pituitary SNAP-25
protein expression was increased (38). The increment in the expression of
syt-1 was also observed in the hippocampus of neonatal hypothyroid
rats (39). The regulation
mechanism of the increased synaptic proteins is unknown. It is well
established that these two synaptic proteins are necessary during
the neurotransmitter releasing. In adult hypothyroidism, the
decreased amount of the neurotransmitter ACh within the synaptic
cleft may lead to an upregulation of syt-1 and SNAP-25 expression
to rescue the vesicle exocytosis. However, in a previous study of
the authors' (12), there is
inconsistency with the information regarding the effect of
adult-onset hypothyroidism on the expression of syt-1, which
suggested the level of syt-1 protein was downregulated in the PFC
of hypothyroid rats (three months old rats were enrolled). It is
likely that, among other reasons, PTU-induced hypothyroid rats at
different ages have different expression pattern of the examined
synaptic protein, but the exact mechanism is still elusive.
The T4 replacement therapy was the standard solution
towards the hypothyroidism treatment, which result in attenuating
the alterations induced by hypothyroidism. This experiment
indicated that T4 administration for 2 weeks resulted in
amelioration of adult-onset hypothyroidism-induced morphological
changes in the PFC. A previous study by Ruiz-Marcos et al
(40) clearly demonstrated that
adult hypothyroidism induced by surgical thyroidectomy (T),
performed at 40 or at 120 days of age, affects the pyramidal cell
morphology of the visual cortex and treatment with T4 could reverse
the changes. Previous studies (41,42)
also indicated that treatment of the T rats performed at 10 days of
age with T4 starting at 12 days of age (2 days following T)
prevented the morphological alterations in the visual cortex, as
assessed in 40-day-old animals. In 60-day-old animals, the recovery
was not complete, though treatment with T4 starting at 12 or 15
days of age had an ameliorating effect on the pyramids. However, in
another work of the pyramidal cortical cell morphology studied by
multivariate analysis, treatment with T4 started at 12 days of age
did not prevent the changes due to T performed at 10 days of age
(43). In the present work, the
authors observed a clear-cut ameliorating effect of T4
administration on adult-onset hypothyroidism-induced morphological
changes in the PFC. Detailed quantitative data on the effects of
adult hypothyroidism and T4 substitution therapy upon the
prefrontal morphological parameters is of great interest for the
future studies. In addition, treatment of the adult hypothyroid
rats with T4 was revealed to replenish the decreased ACh levels and
AChE activity in the PFC to control values. The reversible changes
of the ACh concentration and AChE activity by T4 were also observed
in other brain regions, such as the hippocampus of the adult
hypothyroid rats (14). The
results above indicated that ACh and AChE may be under direct
thyroid hormone control. Indeed, previous studies from tissue
culture experiments revealed that as T3 levels approach the level
of total TH in the blood, the activities of acetylcholine
transferase, the enzyme responsible for the synthesis of ACh, and
AChE were markedly enhanced (44,45).
Regretfully, the present results demonstrated that
T4 monotherapy for 2 weeks does not restore the expression levels
of syt-1 and SNAP-25, which is consistent with a previous work by
the author (14). However, the
exact mechanism underlying this remained elusive. It may be that
either the changes in the synaptic proteins are irreversible or
that the restoration could be achieved only by much longer
treatment or much larger dose of T4 treatment. However, a previous
study indicated that even T4 treatment for six weeks failed to
return the decreased PKCγ level back to normal (46). In a previous study, the authors
demonstrated that, when given large-dose T4 shock therapy to the
adult hypothyroid rats, the hippocampal syt-1 and SNAP-25 proteins
could restore to the normal levels, but hyperthyroidism could be
induced at the same time (13).
Alternatively, despite achieving a biochemically euthyroid state,
some patients with hypothyroidism continue to complain of
significant fatigue, weight issues, and diminished neurocognitive
function, perceived to be due to inadequately treated
hypothyroidism (16). Thus, a
therapy combining the administration of T4 and T3 was recommended
by some practitioners and investigators for patients with
hypothyroidism. However, multiple studies have identified no
significant differences in various quality-of-life measures (for
example: Depression, fatigue, cognitive function) with use of the
combination of T4 and T3 compared to T4 alone (15). The findings above indicated that it
is still necessary to discover new alternative therapeutic methods
for treatment of hypothyroidism. DON, as a AChEI, has demonstrated
efficacy in improving cognitive function and providing
neuroprotective effects (23).
Previously, the authors first explored the
efficiency of T4 and DON on hippocampal ACh content, AChE activity
as well as expression changes of several synaptic proteins, such as
syt-1, SNAP-25, munc-18 and syntaxin-1 induced by adult-onset
hypothyroidism, the authors' results demonstrated that T4 and DON
administered in combination resulted in more effective restoration
levels of ACh and synaptic proteins than either alone (14,19).
In the present work, the authors observed that, following the T4 +
DON treatment, the morphological alterations, including the
neurons, the synapses and the myelin sheath in the PFC were similar
to that of the CON group. In addition, the expression levels of
ACh, syt-1and SNAP-25 were closer to the levels of the control
group, which is consistent with previous findings. These results
suggested that DON treatment resulted in amelioration of
impairments induced by hypothyroidism, although the exact
mechanisms underlying the regulation remain largely
uncharacterized. DON, as an AChEI, has been previously used to
compensate for ACh depletion in the AD brain. In the current study,
the decreased levels of ACh induced by hypothyroidism were restored
by DON treatment for 2 weeks. Similar results were observed in
studies revealing that other AChEIs, including galantamine and
physostigmine increase ACh levels (47,48).
Therefore, it is probable that DON increases ACh levels through the
well-established mechanism of preventing the enzymatic degradation
of ACh, thus prolonging its availability. Some clinical and
experimental studies, however, have suggested that DON also provide
neuroprotection. Of note, following the single DON treatment, the
morphological alterations, including the neurons, the synapses and
the myelin sheath in the PFC of the hypothyroid rats recovered to
some extent. Previous studies have reported that the
neuroprotective effects of DON prevent the alterations of the
neuronal dendrite morphology in the PFC caused by aging (49), protect neurons from ischemic
damage, glutamate-induced neurotoxicity and amyloid β neurotoxicity
(50). Furthermore, it has been
demonstrated that DON treatment can preserve presynaptic protein
expression in the hippocampus and spinal cord in a tauopathy mouse
model (23). As indicated in
previous studies, neuroprotective effects of DON may be mediated by
many mechanisms, including an increased expression of AChRs,
activation of the nAChR/PI3K pathway (51–53)
and the σ1 receptor/PLC/PKC pathway (54).
Taken together, the present findings demonstrate
that hypothyroidism could cause pathological damages in the
prefrontal ultrastructures, induce alterations of prefrontal ACh
level and AChE activity, as well as the expression changes of syt-1
and SNAP-25 level, in adult rats. Combined administration of T4 and
DON induce plastic changes in the PFC, different from that of T4
monotherapy, and suggest that the DON treatment may facilitate the
recovery of the synaptic protein impairments induced by
hypothyroidism.
Acknowledgments
The authors would like to thank the Department of
Toxicology of Anhui Medical University (Anhui, China) was greatly
appreciated for the technical assistance. The present study was
financially supported by the National Natural Science Foundation of
China (grant no. 81272152).
References
|
1
|
Dugbartey AT: Neurocognitive aspects of
hypothyroidism. Arch Intern Med. 158:1413–1418. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu DF, Wang ZX, Zhang DR, Pan ZL, He S,
Hu XP, Chen XC and Zhou JN: fMRI revealed neural substrate for
reversible working memory dysfunction in subclinical
hypothyroidism. Brain. 129:2923–2930. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jessell TM and Kandel ER: Synaptic
transmission: A bidirectional and self-modifiable form of cell-cell
communication. Cell. 72:(Suppl). S1–S30. 1993. View Article : Google Scholar
|
|
4
|
Zhou Q, Lai Y, Bacaj T, Zhao M, Lyubimov
AY, Uervirojnangkoorn M, Zeldin OB, Brewster AS, Sauter NK, Cohen
AE, et al: Architecture of the synaptotagmin-SNARE machinery for
neuronal exocytosis. Nature. 525:62–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehta PP, Battenberg E and Wilson MC:
SNAP-25 and synaptotagmin involvement in the final
Ca(2+)-dependent triggering of neurotransmitter
exocytosis. Proc Natl Acad Sci USA. 93:10471–10476. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Wit H, Walter AM, Milosevic I,
Gulyás-Kovács A, Riedel D, Sørensen JB and Verhage M:
Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25
acceptor complexes. Cell. 138:935–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sudhof TC: The synaptic vesicle cycle.
Annu Rev Neurosci. 27:509–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chapman ER: How does synaptotagmin trigger
neurotransmitter release? Annu Rev Biochem. 77:615–641. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salvati S, Attorri L, Campeggi LM,
Olivieri A, Sorcini M, Fortuna S and Pintor A: Effect of
propylthiouracil-induced hypothyroidism on cerebral cortex of young
and aged rats: Lipid composition of synaptosomes, muscarinic
receptor sites, and acetylcholinesterase activity. Neurochem Res.
19:1181–1186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carageorgiou H, Pantos C, Zarros A,
Mourouzis I, Varonos D, Cokkinos D and Tsakiris S: Changes in
antioxidant status, protein concentration, acetylcholinesterase,
(Na+, K+)-, and Mg2+- ATPase
activities in the brain of hyper- and hypothyroid adult rats. Metab
Brain Dis. 20:129–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carageorgiou H, Pantos C, Zarros A,
Stolakis V, Mourouzis I, Cokkinos D and Tsakiris S: Changes in
acetylcholinesterase, Na+, K+-ATPase, and
Mg2+-ATPase activities in the frontal cortex and the
hippocampus of hyper- and hypothyroid adult rats. Metabolism.
56:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang HY, Sun CP, Jia XM, Gui L, Zhu DF and
Ma WQ: Effect of thyroxine on SNARE complex and synaptotagmin-1
expression in the prefrontal cortex of rats with adult-onset
hypothyroidism. J Endocrinol Invest. 35:312–316. 2012.PubMed/NCBI
|
|
13
|
Liu CL, Xu YX, Zhan Y, Hu HL, Jia XM, Chen
GH and Zhu DF: Effect of thyroxine on synaptotagmin 1 and SNAP-25
expression in dorsal hippocampus of adult-onset hypothyroid rats. J
Endocrinol Invest. 34:280–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang F, Zeng X, Zhu Y, Ning D, Liu J, Liu
C, Jia X and Zhu D: Effects of thyroxine and donepezil on
hippocampal acetylcholine content, acetylcholinesterase activity,
synaptotagmin-1 and SNAP-25 expression in hypothyroid adult rats.
Mol Med Rep. 11:775–782. 2015.PubMed/NCBI
|
|
15
|
No authors listed: Drugs for
hypothyroidism. Med Lett Drugs Ther. 57:147–150. 2015.PubMed/NCBI
|
|
16
|
Wekking EM, Appelhof BC, Fliers E, Schene
AH, Huyser J, Tijssen JG and Wiersinga WM: Cognitive functioning
and well-being in euthyroid patients on thyroxine replacement
therapy for primary hypothyroidism. Eur J Endocrinol. 153:747–753.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samuels MH, Schuff KG, Carlson NE, Carello
P and Janowsky JS: Health status, psychological symptoms, mood, and
cognition in L-thyroxine-treated hypothyroid subjects. Thyroid.
17:249–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saravanan P, Chau WF, Roberts N, Vedhara
K, Greenwood R and Dayan CM: Psychological well-being in patients
on ‘adequate’ doses of l-thyroxine: Results of a large, controlled
community-based questionnaire study. Clin Endocrinol (Oxf).
57:577–585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang N, Cai Y, Wang F, Zeng X, Jia X, Tao
F and Zhu D: Effects of thyroxin and donepezil on hippocampal
acetylcholine content and syntaxin-1 and munc-18 expression in
adult rats with hypothyroidism. Exp Ther Med. 7:529–536.
2014.PubMed/NCBI
|
|
20
|
Madeira MD, Sousa N, Lima-Andrade MT,
Calheiros F, Cadete-Leite A and Paula-Barbosa MM: Selective
vulnerability of the hippocampal pyramidal neurons to
hypothyroidism in male and female rats. J Comp Neurol. 322:501–518.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madeira MD, Cadete-Leite A, Andrade JP and
Paula-Barbosa MM: Effects of hypothyroidism upon the granular layer
of the dentate gyrus in male and female adult rats: A morphometric
study. J Comp Neurol. 314:171–186. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pepeu G and Giovannini MG: Cholinesterase
inhibitors and beyond. Curr Alzheimer Res. 6:86–96. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshiyama Y, Kojima A, Ishikawa C and Arai
K: Anti-inflammatory action of donepezil ameliorates tau pathology,
synaptic loss and neurodegeneration in a tauopathy mouse model. J
Alzheimers Dis. 22:295–306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riepe MW: Cholinergic treatment: What are
the early neuropathological targets? Eur J Neurol. 12:(Suppl 3).
S3–S9. 2005. View Article : Google Scholar
|
|
25
|
Gerges NZ, Stringer JL and Alkadhi KA:
Combination of hypothyroidism and stress abolishes early LTP in the
CA1 but not dentate gyrus of hippocampus of adult rats. Brain Res.
922:250–260. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cortes C, Eugenin E, Aliaga E, Carreño LJ,
Bueno SM, Gonzalez PA, Gayol S, Naranjo D, Noches V, Marassi MP, et
al: Hypothyroidism in the adult rat causes incremental changes in
brain-derived neurotrophic factor, neuronal and astrocyte
apoptosis, gliosis, and deterioration of postsynaptic density.
Thyroid. 22:951–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Madeira MD and Paula-Barbosa MM:
Reorganization of mossy fiber synapses in male and female
hypothyroid rats: A stereological study. J Comp Neurol.
337:334–352. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
David S and Nathaniel EJ: Neuronal changes
induced by neonatal hypothyroidism: An ultrastructural study. Am J
Anat. 167:381–394. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gold PE: Acetylcholine modulation of
neural systems involved in learning and memory. Neurobiol Learn
Mem. 80:194–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartus RT, Dean RL III, Beer B and Lippa
AS: The cholinergic hypothesis of geriatric memory dysfunction.
Science. 217:408–414. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Molinengo L, Cassone MC and Oggero L:
Action of hypo- and hyperthyroidism on the postmortal decay of
acetylcholine in the rat spinal cord. Neuroendocrinology. 42:28–31.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Virgili M, Saverino O, Vaccari M, Barnabei
O and Contestabile A: Temporal, regional and cellular selectivity
of neonatal alteration of the thyroid state on neurochemical
maturation in the rat. Exp Brain Res. 83:555–561. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carageorgiou H, Pantos C, Zarros A,
Stolakis V, Mourouzis I, Cokkinos D and Tsakiris S: Effects of
hyper- and hypothyroidism on acetylcholinesterase,
(Na(+), K (+))- and Mg (2+)-ATPase
activities of adult rat hypothalamus and cerebellum. Metab Brain
Dis. 22:31–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kundu S, Pramanik M, Roy S, De J, Biswas A
and Ray AK: Maintenance of brain thyroid hormone level during
peripheral hypothyroid condition in adult rat. Life Sci.
79:1450–1455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Semba K: Phylogenetic and ontogenetic
aspects of the basal forebrain cholinergic neurons and their
innervation of the cerebral cortex. Prog Brain Res. 145:3–43.
2004.PubMed/NCBI
|
|
36
|
Owasoyo JO, Egbunike GN and Iramain CA:
The influence of thyroid gland and thyroxine on the
acetylcholinesterase activity of rat brain and adenohypophysis.
Endokrinologie. 77:242–246. 1981.PubMed/NCBI
|
|
37
|
Cao L, Jiang W, Wang F, Yang QG, Wang C,
Chen YP and Chen GH: The reduced serum free triiodothyronine and
increased dorsal hippocampal SNAP-25 and Munc18-1 had existed in
middle-aged CD-1 mice with mild spatial cognitive impairment. Brain
Res. 1540:9–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quintanar JL and Salinas E: Effect of
hypothyroidism on synaptosomal-associated protein of 25 kDa and
syntaxin-1 expression in adenohypophyses of rat. J Endocrinol
Invest. 25:754–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vara H, Martínez B, Santos A and Colino A:
Thyroid hormone regulates neurotransmitter release in neonatal rat
hippocampus. Neuroscience. 110:19–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ruiz-Marcos A, Sánchez-Toscano F, del
Escobar Rey F and de Morreale Escobar G: Reversible morphological
alterations of cortical neurons in juvenile and adult
hypothyroidism in the rat. Brain Res. 185:91–102. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ruiz-Marcos A, Salas J, Sanchez-Toscano F,
del Escobar Rey F and de Morreale Escobar G: Effect of neonatal and
adult-onset hypothyroidism on pyramidal cells of the rat auditory
cortex. Brain Res. 285:205–213. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ruiz-Marcos A, Sanchez-Toscano F, del
Escobar Rey F and de Morreale Escobar G: Severe hypothyroidism and
the maturation of the rat cerebral cortex. Brain Res. 162:315–329.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ipina SL, Ruiz-Marcos A, del Escobar Rey F
and de Morreale Escobar G: Pyramidal cortical cell morphology
studied by multivariate analysis: Effects of neonatal
thyroidectomy, ageing and thyroxine-substitution therapy. Brain
Res. 465:219–229. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Honegger P and Lenoir D: Triodothyronine
enhancement of neuronal differentiation in aggregating fetal rat
brain cells cultured in a chemically defined medium. Brain Res.
199:425–434. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akuzawa K and Wakabayashi K: A serum-free
culture of the neurons in the septal, preoptic and hypothalamic
region. Endocrinol Jpn. 32:163–173. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alzoubi KH, Gerges NZ and Alkadhi KA:
Levothyroxin restores hypothyroidism-induced impairment of LTP of
hippocampal CA1: Electrophysiological and molecular studies. Exp
Neurol. 195:330–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Saiyed M and Riker WK: Cholinergic and
anticholinergic drug effects on survival during hypoxia:
Significant gender differences. J Pharmacol Exp Ther.
264:1146–1153. 1993.PubMed/NCBI
|
|
48
|
Dimitrova DS and Getova-Spassova DP:
Effects of galantamine and donepezil on active and passive
avoidance tests in rats with induced hypoxia. J Pharmacol Sci.
101:199–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alcantara-Gonzalez F, Juarez I, Solis O,
Martinez-Tellez I, Camacho-Abrego I, Masliah E, Mena R and Flores
G: Enhanced dendritic spine number of neurons of the prefrontal
cortex, hippocampus and nucleus accumbens in old rats after chronic
donepezil administration. Synapse. 64:786–793. 2010.PubMed/NCBI
|
|
50
|
Akasofu S, Kimura M, Kosasa T, Sawada K
and Ogura H: Study of neuroprotection of donepezil, a therapy for
Alzheimer's disease. Chem Biol Interact. 175:222–226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Akaike A, Takada-Takatori Y, Kume T and
Izumi Y: Mechanisms of neuroprotective effects of nicotine and
acetylcholinesterase inhibitors: Role of alpha4 and alpha7
receptors in neuroprotection. J Mol Neurosci. 40:211–216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shen H, Kihara T, Hongo H, Wu X, Kem WR,
Shimohama S, Akaike A, Niidome T and Sugimoto H: Neuroprotection by
donepezil against glutamate excitotoxicity involves stimulation of
alpha7 nicotinic receptors and internalization of NMDA receptors.
Br J Pharmacol. 161:127–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Takada-Takatori Y, Kume T, Izumi Y, Ohgi
Y, Niidome T, Fujii T, Sugimoto H and Akaike A: Roles of nicotinic
receptors in acetylcholinesterase inhibitor-induced neuroprotection
and nicotinic receptor up-regulation. Biol Pharm Bull. 32:318–324.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Meunier J, Ieni J and Maurice T: The
anti-amnesic and neuroprotective effects of donepezil against
amyloid beta25-35 peptide-induced toxicity in mice involve an
interaction with the sigma1 receptor. Br J Pharmacol. 149:998–1012.
2006. View Article : Google Scholar : PubMed/NCBI
|