Introduction
Articular cartilage is a type of hyaline cartilage
that provides a unique low-friction and weight-bearing surface in
diarthrodial joints. Owing to its aneural and avascular
characteristics, articular cartilage may be subject to trauma
without causing pain or other symptoms in the early stages, which
may subsequently become defects with serious symptoms and a poor
regenerative capacity (1).
Therefore, patients with cartilage defects frequently require
surgical interventions to fix defects, relieve pain and reduce the
effects of other symptoms. The current available surgical
interventions for serious cartilage defects include reparative
methods, such as microfracture and drilling, and reconstructive
methods, such as autologous or allogeneic osteochondral grafts,
autologous chondrocyte implantation, cell-seeded scaffolds and
acellular scaffolds (2). Although
these methods may be successful in certain aspects, they exhibit a
common limitation: The generation of fibrocartilage (2,3).
Fibrocartilage may be observed in the filling of microfractures or
implantations, around autografts or allografts, and within
scaffolds; however, almost all regenerative cartilage may be
affected. In contrast with fibrocartilage, articular hyaline
cartilage contains primarily type II collagen with proteoglycan,
rather than type I collagen, and it is therefore able to resist
more compressive loads and be more durable (4). Owing to its vulnerability,
fibrocartilage is considered to be the ‘Achilles’ heel’ of
regenerative cartilage.
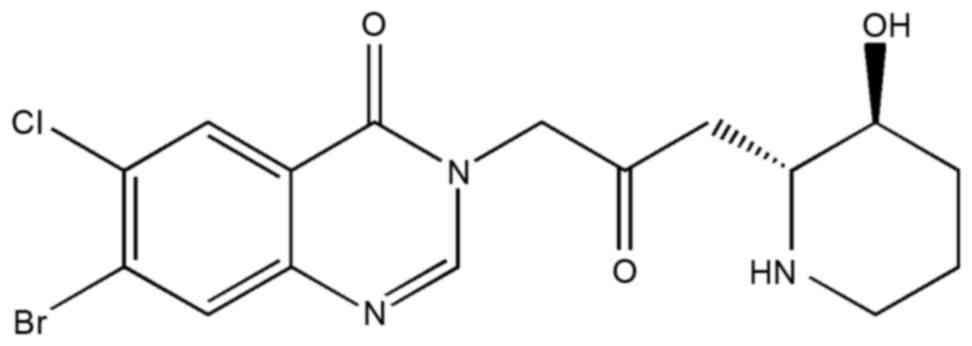
Halofuginone (HF;
7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]-4(3H)-quinazolinone;
Fig. 1) is an analogue of
febrifugine, a type of alkaloid isolated from a plant used in
traditional Chinese medicine, Dichroa febrifuga (5,6). In
previous studies, various pharmacological effects of HF have been
observed in a number of diseases, including malaria, cancer, and
fibrosis-associated and autoimmune diseases (5,7–9). The
present study focused on the antifibrotic properties of HF.
Fibrosis may affect numerous organs and involves multiple signaling
pathways. Among these, the transforming growth factor (TGF)-β
pathway has been the most well studied in in vitro and in
vivo experiments. According to a previous study, HF treatment
may reduce the expression of collagen type I, α1 chain (COL1A1)
gene and prevent type I collagen synthesis by inhibiting the
phosphorylation of mothers against decapentaplegic homolog
(Smad)2/3 in the TGF-β pathway without influencing other types of
collagen, including type II collagen (10). Additionally, HF has been approved
by the Food and Drug Administration of the USA for the treatment of
scleroderma, an autoimmune fibrotic disorder (5).
Similar to fibrosis, the generation of
fibrocartilage also occurs through a tissue repair process,
although it is of relatively poor function and quality. Therefore,
the present study hypothesized that the antifibrotic capacity of HF
may decrease type I collagen synthesis in fibrocartilage and
improve the quality and function of regenerative cartilage, which,
to the best of our knowledge, has not been previously reported. The
present study aimed to elucidate the cause of, and a potential
solution to, the problem of fibrocartilage by investigating the
effect of TGF-β1 and HF on rat chondrocytes.
Materials and methods
Materials
The present study was approved by the ethics
committee of the General Hospital of Shenyang Military Region
(Shenyang, China). Sprague-Dawley rats (total, 20; female, 10;
male, 10; age, 2 weeks; weight, 25–30 g) were provided by the
Animal Center of Nanjing Medical University (Nanjing, China). The
animals were housed in a polycarbonate cage in a temperature and
humidity-controlled (23±1°C; 53±2%) room and maintained on a 12/12
h light/dark cycle with free access to food and water. Dulbecco's
modified Eagle's medium (DMEM-low glucose), fetal bovine serum
(FBS), PBS and trypsin were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Collagenase II and HF were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
Cell Counting Kit-8 (CCK-8) kit was acquired from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Recombinant human TGF-β1 was
obtained from PeproTech, Inc. (Rocky Hill, NJ, USA). Primers for
GAPDH (cat. no. RQP049537), COL1A1 (cat. no. RQP054226), collagen
type II, α1 chain (COL2A1; cat. no. RQP049248), TGF-β1 (cat. no.
RQP050181), Smad2 (cat. no. RQP049947), Smad3 (cat. no. RQP049401),
and Smad7 (cat. no. RQP050884) were provided by GeneCopoeia, Inc.
(Rockville, MD, USA). The RNA Extraction kit, PrimeScript RT Master
Mix and SYBR Premix Ex Taq were acquired from Takara Bio, Inc.
(Otsu, Japan). Anti-collagen I (cat. no. 6308), anti-collagen II
(cat. no. 34712) antibodies, Alexa Fluor® 488-conjugated
anti-mouse immunoglobulin (Ig)G (cat. no. 150113) and Alexa
Fluor® 488-conjugated anti-rabbit IgG (cat. no. 150077)
were purchased from Abcam (Cambridge, UK); anti-phosphorylated
(p)-Smad2/3 (cat. no. 8828), anti-Smad2/3 (cat. no. 8685),
horseradish peroxidase (HRP)-linked anti-rabbit IgG (cat. no. 7074)
and HRP-linked anti-mouse IgG (cat. no. 7076) were supplied by Cell
Signaling Technology, Inc. (Danvers, MA, USA); anti-Smad7 antibody
(cat. no. 365846) was supplied by Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA); and HRP-conjugated anti-GAPDH antibody was
provided by KangChen Bio-tech, Inc. (Shanghai, China). The
Bicinchoninic Acid (BCA) protein assay and the Enhanced
Chemiluminescence (ECL) kits were purchased from Thermo Fisher
Scientific, Inc.
Cell culture
Articular hyaline cartilage was isolated from the
knees of two-week-old rats under sterile conditions and sliced into
pieces (1 mm3) for subsequent digestion. The cartilage
was first incubated with 0.25% trypsin for 30 min at 37°C, followed
by 0.2% collagenase II for 4 h at 37°C. The digested samples were
purified using a cell strainer and cultured in DMEM-low glucose
with 10% FBS in a Heraeus BB 5060 incubator (Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. Chondrocytes were
passaged by trypsinization when cells reached >90% confluence.
When passaged to the second generation, chondrocytes were seeded
onto slides in 6-well or 96-well plates for the following
experiments.
Experimental design
The experiments consisted of three parts. In the
first part, second-generation cells were seeded in 6-well plates
(5×106 cells/well) with gradually increasing
concentrations of TGF-β1 (0, 0.1, 1 and 10 ng/ml). In the second
set of experiments, cells were seeded in 96-well plates
(5×103 cells/well) with gradually increasing
concentrations of HF (0, 1, 3, 10, 30, 100, 300, 1,000, 3,000 and
10,000 ng/ml) for 24 h, and subsequently the concentrations (15, 30
and 60 ng/ml) which exhibited no side effects on proliferation for
24 h was cultured with the cells for 24, 48 and 72 h; these cells
were analyzed by CCK-8 assays as described below. Subsequent to the
above screenings, 10 ng/ml TGF-β1, 30 ng/ml HF as a low-dose (safe
dose) and 100 ng/ml HF as high-dose (overdose) were used for the
further experiments. Cells were seeded in 6-well plates, divided
into 6 groups and treated with HF and/or TGF-β1 as follows: i)
Control, which did not receive HF or TGF-β1 treatment; ii) low-dose
HF; iii) high-dose HF; iv) TGF-β1; v) TGF-β1 with low-dose HF; and
vi) TGF-β1 with high-dose HF. Each experiment was incubated at 37°C
and repeated at least three times.
CCK-8 assay
The cytotoxicity of HF was determined by CCK-8
assay, according to the manufacturer's protocol. Cells
(5×103 cells/well) were seeded in a 96-well plate and
treated with HF as aforementioned. CCK-8 solution (10 µl) was added
and the plates were incubated for 3 h at 37°C; following
incubation, the absorbance was determined using a microplate reader
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 450 nm. Cell
viability was calculated as the ratio of the absorbance at 450 nm
of the treatment groups vs. the control group, using SkanIt
software version 2.4.2 (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using an RNA Extraction kit
by adding 350 µl Buffer RL per well of the 6-well plates, and the
concentration and purity was measured using a spectrophotometer
(Nanodrop 2000; Thermo Fisher Scientific, Inc.). RNA with a 260/280
ratio of 1.8–2.0 was reverse transcribed into cDNA using the
PrimeScript RT Master Mix. qPCR analysis was performed in a 10-µl
mixture using a LightCycler 480 System (Roche Diagnostics, Basel,
Switzerland). Briefly, 1 µl cDNA was mixed with 1 µl specific
primers, 4 µl water and 5 µl SYBR Premix Ex Taq and amplified under
the following cycling conditions: 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec, 60°C for 20 sec, and extension at 72°C
for 10 min. The gene expression of COL1A1, COL2A1, TGFB1, Smad2,
Smad3 and Smad7 was normalized to that of GAPDH using the
2−ΔΔCq method, as previously described (11). Each experiment was repeated at
least three times.
Western blot analysis
Total protein was extracted by adding 50 µl
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) to each well with 1%
phenylmethylsulfonyl fluoride, and the BCA assay was used to
determine the protein concentration. Protein samples (30 µg) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes. Following blocking with 5% skimmed milk in TBS
+ Tween-20 for 1 h at room temperature, membranes were incubated
with primary antibodies (anti-collagen I antibody, 1:1,000;
anti-collagen II antibody, 1:5,000; anti-Smad2/3 antibody, 1:1,000;
anti-p-Smad2/3 antibody, 1:1,000; anti-GAPDH antibody, 1:4,000) at
4°C overnight and with secondary antibodies (HRP-linked anti-mouse
IgG, 1:5,000; HRP-linked anti-rabbit IgG, 1:5,000) at room
temperature for 1 h. Blots were visualized using an ECL kit and a
gel imaging system (ChemiDoc XRS+ system; Bio-Rad Laboratories,
Inc., Hercules, CA, USA); protein bands were normalized to GAPDH
and densitometric analysis was performed using Image Lab 5.0
Software (Bio-Rad Laboratories, Inc.). Each experiment was repeated
at least three times.
Immunofluorescence assay
The cells on the slides in the 6-well plates were
cultured for 3 days and fixed with 4% paraformaldehyde for 30 min
at room temperature. Following washing with PBS and cell membrane
breaking with Triton X-100 (Wuhan Goodbio Technology Co., Ltd.,
Wuhan, China), the cells were incubated with primary antibodies
(anti-collagen I antibody, 1:1,000; anti-collagen II antibody,
1:100) at 4°C overnight and subsequently incubated with secondary
antibodies (Alexa Fluor® 488-conjugated anti-mouse IgG,
1:500; Alexa Fluor® 488-conjugated anti-rabbit IgG,
1:500) at room temperature for 50 min. The cells were
counterstained with DAPI for 10 min in the dark at the room
temperature. Images were captured using a fluorescent microscope
(Nikon Corporation, Inc., Tokyo, Japan) with image capture software
(CapturePro 2.8; Jenoptik AG, Jena, Germany).
Statistical analysis
Statistical analyses were performed using SPSS
software, version 20 (IBM Corp., Armonk, NY, USA), and results are
presented as the mean ± standard error of the mean. One-way and
multi-way analysis of variance with Student-Newman-Keuls post hoc
tests were used to determine the statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TGF-β1 induces type I collagen
expression in chondrocytes
TGF-β1 is a known stimulant of fibrosis in various
tissues, and the present study aimed to investigate whether TGF-β1
is able to induce the production of type I collagen in chondrocytes
in vitro. Second passage chondrocytes were treated a range
of concentrations of TGF-β1 (0, 0.1, 1 and 10 ng/ml) for 3 days.
Gross examination of cell morphology of the four treatment groups
revealed that the extracellular matrix of the chondrocytes was
altered from subovate to spindle, which indicated the formation of
fibrocytes as the concentration of TGF-β1 increased; a marked
difference was observed at 10 ng/ml compared with the other
treatment groups (Fig. 2A).
RT-qPCR and western blot analyses demonstrated that type I and type
II collagen mRNAs and proteins were produced in the
second-generation chondrocytes during monolayer expansion, and
expression increased following treatment with TGF-β1 (Fig. 2B and C, respectively). However, a
difference was observed between the two types of collagen
expressions: A significant increase was noted for type I collagen
at 10 ng/ml, whereas type II collagen also exhibited a significant
increased at 1 ng/ml. Therefore, it was decided to use 10 ng/ml
TGF-β1 to increase type I collagen in the extracellular matrix of
chondrocytes for the experiment discussed below.
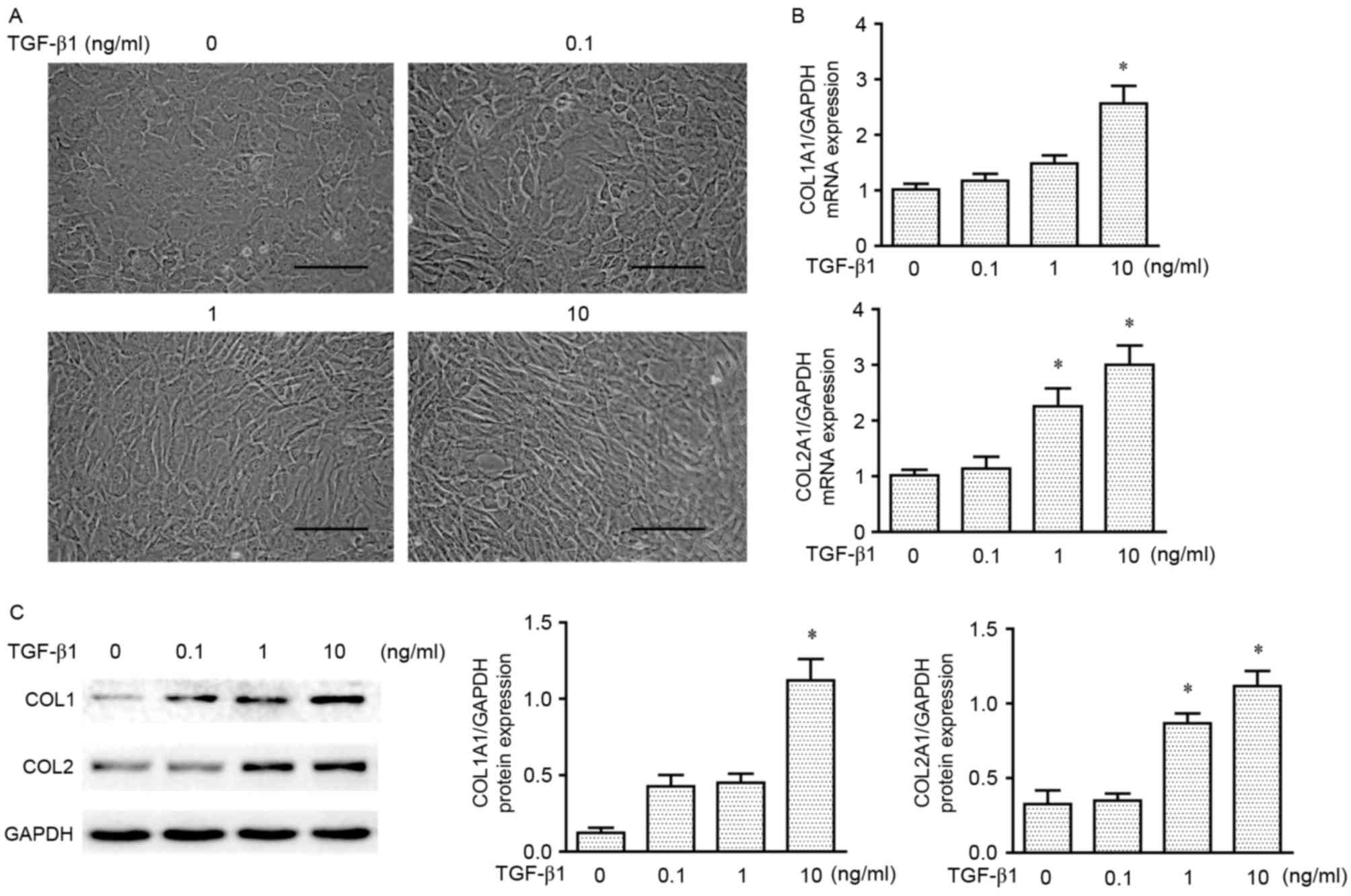 | Figure 2.TGF-β1-induced expression of type I
and type II collagen in chondrocytes. (A) Gross morphology of
chondrocytes treated with various concentrations of TGF-β1 (0, 0.1,
1 and 10 ng/ml) for 3 days. Scale bar, 100 µm. (B) mRNA expression
levels of COL1A1 and COL2A1 (normalized to GAPDH) in chondrocytes
treated with different concentrations of TGF-β1 (0, 0.1, 1 and 10
ng/ml) for 3 days. (C) Representative western blot analysis and
densitometric analysis of COL1 and COL2 in chondrocytes treated
with gradual concentrations of TGF-β1 (0, 0.1, 1 and 10 ng/ml) for
3 days. Data are presented as the mean ± standard error of the
mean; n=3; *P<0.05 vs. 0 ng/ml. COL1, type I collagen; COL2,
type II collagen; COL1A1, collagen type I, α1 chain; COL2A1,
collagen type II, α1 chain; TGF-β1, transforming growth
factor-β1. |
Cytotoxicity of HF in
chondrocytes
To assess the cytotoxicity of HF treatments, the
CCK-8 assay was used to determine a concentration that resulted in
minimal toxicity to chondrocytes. Cells were treated with 10
gradually increasing concentrations of HF (0, 1, 3, 10, 30, 100,
300, 1,000, 3,000 and 10,000 ng/ml) for 24 h. The results
demonstrated that cell viability dropped markedly from >90 to
40% between 30 and 1,000 ng/ml (Fig.
3A). It was additionally observed that the cytotoxicity was
significant at concentrations ≥100 ng/ml HF, compared with the
control group. Based on these results, 4 concentrations of HF (0,
15, 30 and 60 ng/ml) were selected to determine whether the cell
viability was affected by the duration of treatment. Cells were
treated with HF for 24, 48 and 72 h, and the cell viabilities were
analyzed every 24 h. The results demonstrated that viability was
significantly reduced in cells treated with 60 ng/ml for 72 h,
whereas no significant effects were noted in cells incubated with
either 15 or 30 ng/ml (Fig. 3B).
Therefore, 30 ng/ml HF was determined to be a safe concentration;
30 ng/ml as a low-dose (safe dose) and 100 ng/ml as high-dose
(overdose) were used for further cell function tests.
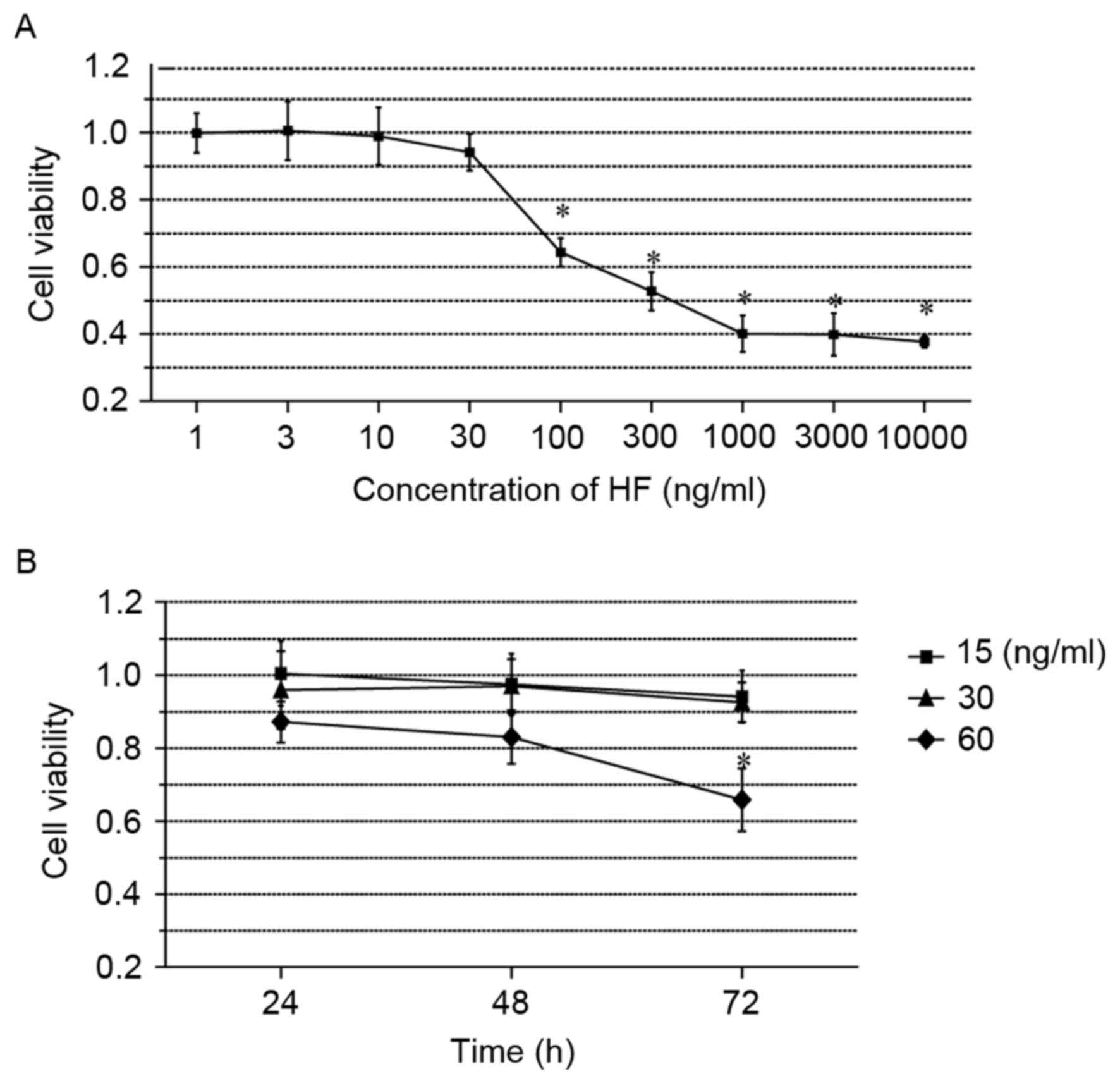 | Figure 3.HF is nontoxic at concentrations ≤30
ng/ml. (A) Dose-dependent cytotoxicity analysis of HF. Chondrocytes
were treated with 10 gradually increasing concentrations of HF (0,
1, 3, 10, 30, 100, 300, 1,000, 3,000 and 10,000 ng/ml) for 24 h.
(B) Time-dependent cytotoxicity analysis of HF. Chondrocytes were
treated with 4 concentrations of HF (0, 15, 30 and 60 ng/ml) for
24, 48, 72 h. Data are presented as the mean ± standard error of
the mean; n=6; *P<0.05 vs. control. HF, halofuginone. |
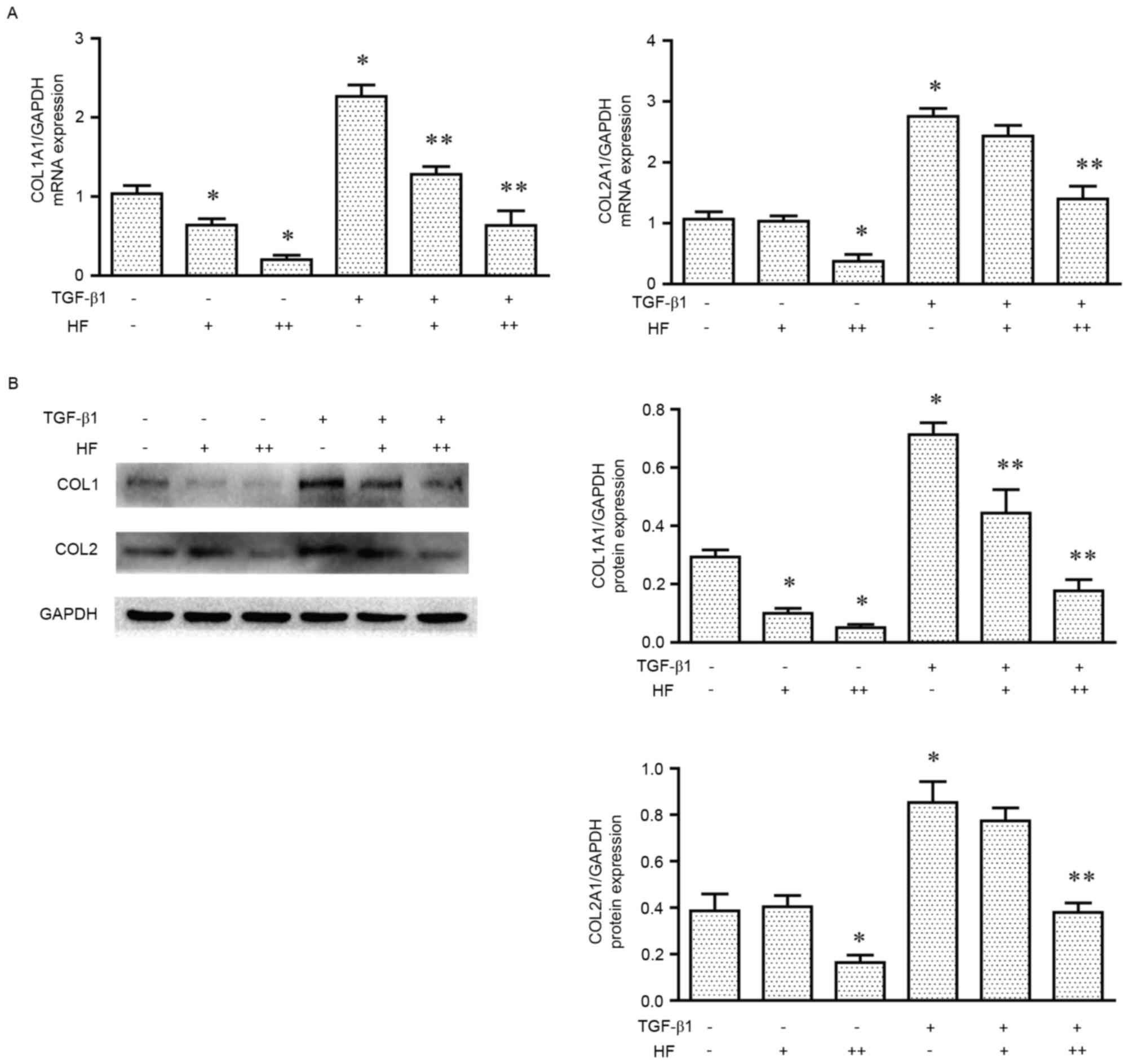
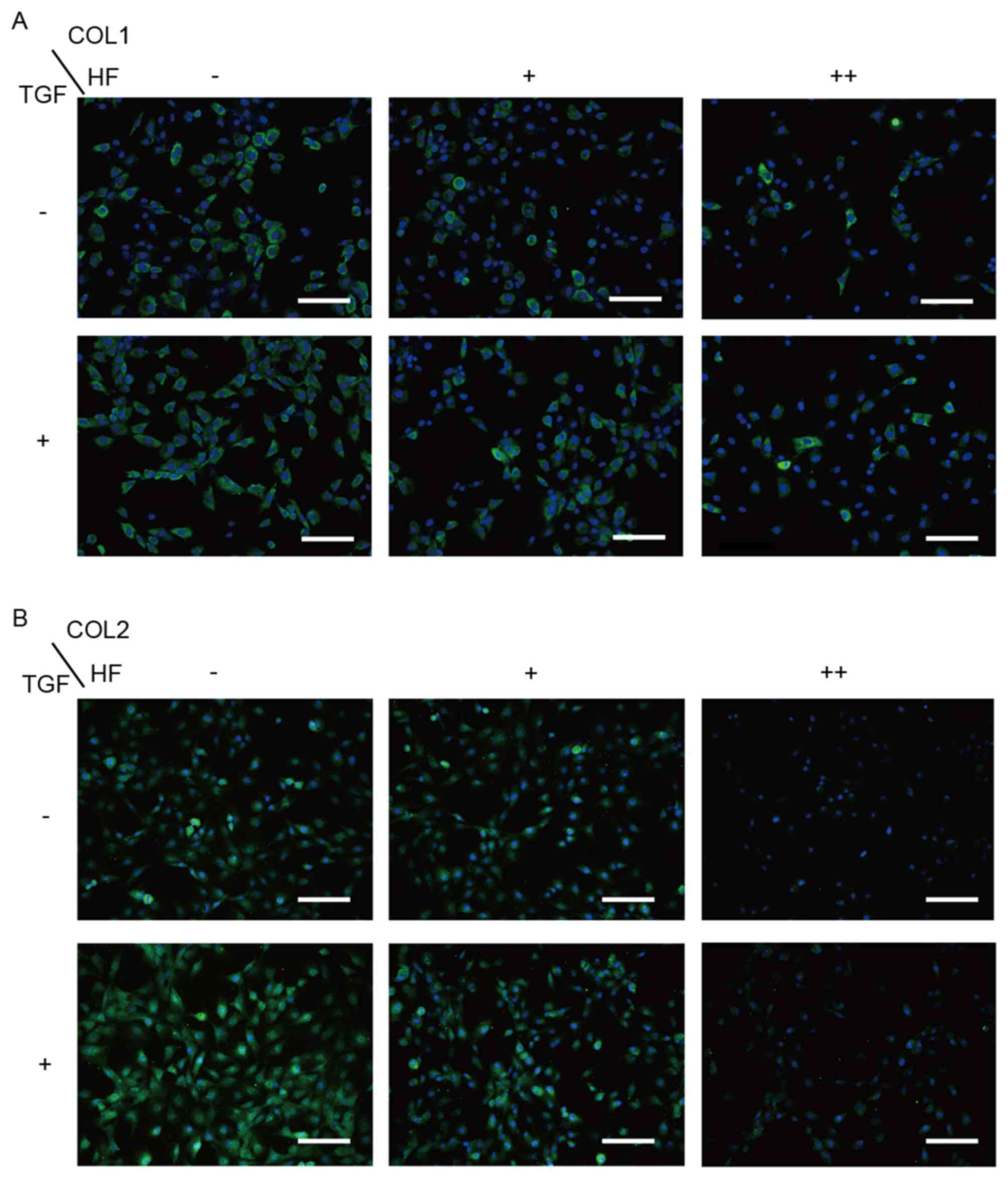
Low-dose HF only suppresses type I
collagen expression in chondrocytes
To determine whether HF was able to decrease type I
collagen synthesis without decreasing type II collagen synthesis,
cells were treated with different concentrations of HF (0, 30 and
100 ng/ml) with or without TGF-β1 (10 ng/ml) co-treatment. In the
RT-qPCR analysis (Fig. 4A), type I
collagen was markedly inhibited with 30 ng/ml HF, whereas type I
and type II collagen were inhibited at a concentration of 100 ng/ml
HF with or without TGF-β1. Western blot analysis (Fig. 4B) demonstrated a similar
concentration-dependent effect on the chondrocytes with or without
TGF-β1. In the immunofluorescence assay (Fig. 5A and B), the cells were observed to
exhibit a markedly decreased expression of type I collagen at
concentrations of 30 and 100 ng/ml HF, while type II collagen only
decreased at 100 ng/ml HF.
HF acts via Smad2/3 and Smad7 in the
TGF-β pathway in chondrocytes
As TGF-β1 was revealed to increase the expression of
type I and type II collagen, whereas their expression levels were
decreased with HF treatment, the expression levels of TGF-β pathway
proteins were analyzed in chondrocytes, as were observed in cells
of other fibrotic tissues and organs (12). The present study examined the mRNA
expression levels of TGFB1, Smad2, Smad3 and Smad7 in the six
treatment groups by RT-qPCR (Fig.
6A). No significant differences were identified for TGFB1,
Smad2 or Smad3 mRNA expression; however, Smad7 mRNA expression
levels were significantly higher in cells treated with high doses
of HF, either with or without TGF-β1 co-treatment. Western blot
analysis of p-Smad2/3 and Smad7 protein expression demonstrated
that HF treatment was able to inhibit the phosphorylation of
Smad2/3 and increase the expression of Smad7, in cells treated with
or without TGF-β1 (Fig. 6B).
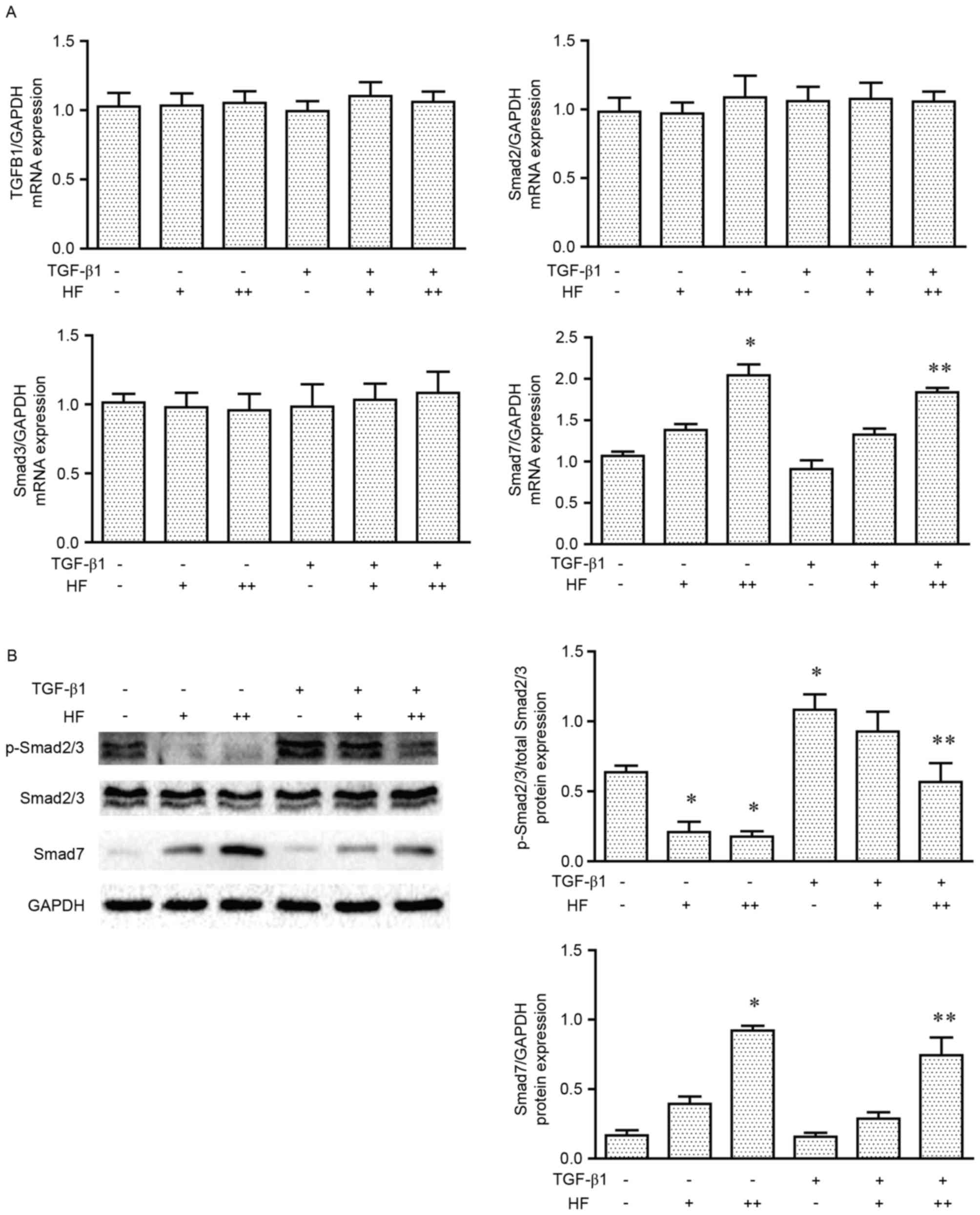 | Figure 6.HF acts via Smad2/3 and Smad7 in the
TGF-β1 signaling pathway. (A) The mRNA levels of TGFB1, Smad2,
Smad3 and Smad7 (compared with GAPDH) of chondrocytes treated with
HF and TGF-β1. (B) Representative western blot analysis and
quantification of p-Smad2/3, Smad2/3 and Smad7 expression in
chondrocytes treated with HF and TGF-β1. HF (−), 0 ng/ml HF; HF
(+), 30 ng/ml HF; HF (++), 100 ng/ml HF; TGF-β1 (−), 0 ng/ml
TGF-β1; TGF-β1 (+), 10 ng/ml TGF-β1. Data are presented as the mean
± standard error of the mean. n=3. *P<0.05 vs. control.
**P<0.05 vs. TGF-β1 (+) HF (−). HF, halofuginone; Smad, mothers
against decapentaplegic homolog; TGF-β1, transforming growth
factor-β1; TGFB1, TGF-β1; p, phosphorylated. |
Discussion
Full-thickness and large area defects of articular
cartilage are unable to repair themselves and require surgical
intervention to regenerate (3).
The durability of regenerative cartilage depends on the
histological structure. Hyaline cartilage with type II collagen is
resistant to loading compression, whereas fibrocartilage with type
I collagen is more resistant to tension than hyaline cartilage,
although it is vulnerable to compression in the joints (Table I) (13). A large proportion of regenerative
cartilage exists in the form of fibrocartilage, which cannot
withstand impact to the same degree as natural hyaline cartilage
(2,14). Therefore, the part of regenerative
cartilage that is fibrocartilage is frequently the earliest site of
failure.
 | Table I.Composition and loading capacity of
fibrocartilage and hyaline cartilage. |
Table I.
Composition and loading capacity of
fibrocartilage and hyaline cartilage.
|
| Composition |
|---|
|
|
|
|---|
| Cartilage type | Type I collagen | Type II collagen |
|---|
| Fibrocartilage | +++ | + |
| Hyaline
cartilage | − | +++ |
|
|
| Loading capacity |
|
|
|
| Cartilage type | Compression | Tension |
|
| Fibrocartilage | + | ++ |
| Hyaline
cartilage | +++ | + |
TGF-β is a central factor in fibrosis, which
promotes the expression of proteins of the extracellular matrix in
various fibrotic conditions through the TGF-β pathway. During the
process of cartilage regeneration, TGF-β is produced and recruited;
however, this recruitment may contradictorily both enhance
cartilage repair and stimulate tissue fibrosis (15,16).
High levels of TGF-β have been demonstrated to induce
osteoarthritis (17). In a
previous study, TGF-β1 was able to dedifferentiate chondrocytes and
cause them to lose their phenotypic characteristics, which led to
the production of more type I collagen compared with type II
collagen (18); this process
additionally occurs in monolayer expansion and 3D regenerative
cartilage in vivo (19).
Therefore, the present study used second-generation
monolayer-cultured chondrocytes, with or without high levels of
TGF-β1 treatment, to mimic the phenotypic alterations of
fibrocartilage. In the second-generation monolayer expansion
chondrocytes, type I collagen was increased in the group treated
with TGF-β1 compared with the untreated cells.
HF was previously identified to be an antifibrotic
agent in the 1990s (20). HF may
prevent the increase in collagen synthesis and promote the
resolution of established fibrosis (5). In addition, HF exhibited no apparent
effects on the collagen in non-fibrotic tissue. In injured rat
carotid arteries, HF was demonstrated to decrease the synthesis of
type I collagen and not type III collagen (21). In liver cirrhosis, chronic
graft-versus-host disease and scleroderma models, HF has been
observed to inhibit type I collagen gene expression and synthesis
without affecting the synthesis of type II or type III collagen
(10). In an osteoarthritis model,
HF was able to attenuate articular cartilage degeneration and
subchondral bone deterioration by decreasing type X collagen and
increasing type II collagen synthesis (22). Owing to its selective inhibition of
collagen, HF was used to treat fibrocartilage in the present study.
A previous study indicated that, during the inhibition of type I
collagen, HF inhibited the phosphorylation of Smad2/3, an important
element of the TGF-β signaling pathway, and increased Smad7
expression, which acts as an inhibitor of the TGF-β pathway
(23). The results of the present
study indicated that a low-dose of HF was able to inhibit the TGF-β
pathway to decrease the synthesis of type I collagen. Notably, the
TGF-β pathway has additionally been reported to be important for
the synthesis of type II collagen (24), which indicated that the selective
inhibition of HF is complex. There are two possible explanations
for this observed effect. The first explanation is that HF may act
in a dose-dependent manner. In the present study, only type I
collagen was decreased at the low-dose (30 ng/ml) HF treatment,
whereas both type I and type II collagen expressions were decreased
at the high dose (100 ng/ml). This may indicate that the regulation
of normally-expressed genes (type II collagen) may differ from that
of abnormally-expressed genes (type I collagen) induced by the
fibrogenic stimulus, which is usually an aggressive and rapid
process (5). Compared with the
native type II collagen, type I collagen may be more sensitive to
HF. The second possible explanation is that TGF-β may exert its
biological effects by non-Smad pathways. For example, type II
collagen synthesis may be stimulated by TGF-β through the
TGFR2-Rac-α serine/threonine protein kinase-serine/threonine
protein kinase mTOR signaling (25).
In the present study, it was observed that HF was
able to inhibit the synthesis of type I collagen, without
influencing type II collagen synthesis, at low concentrations. In
clinical settings, HF may be used to reduce the proportion of type
I collagen in fibrocartilage at the mid-early stage of regeneration
and turn fibrocartilage into hyaline-like cartilage, which is more
similar to the native hyaline cartilage. Treatment with HF may
provide an alternative solution to the problem of fibrocartilage in
cartilage regeneration. HF may also be used in the improvement of
seed cells in monolayer expansion for the tissue engineering of
cartilage.
To the best of our knowledge, the present study
presents the first use of HF to treat the issues associated with
type I collage in chondrocytes, although certain limitations
remain. Owing to the complicated procedures of surgical cartilage
regenerative interventions, the present study did not test the
effect of HF in animal experiments. In addition, chondrocytes in
monolayer expansion may not completely mimic the phenotypic
alterations of fibrocartilage in vivo. Therefore, the
antifibrotic effects of HF in the application of cartilage
regeneration require further investigation.
Acknowledgements
The present study was supported by the Doctoral
Research Initiation Funds of Liaoning Province (grant no.
201601401).
References
|
1
|
Poole AR, Kojima T, Yasuda T, Mwale F,
Kobayashi M and Laverty S: Composition and structure of articular
cartilage: A template for tissue repair. Clin Orthop Relat Res.
(391 Suppl). S26–S33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oussedik S, Tsitskaris K and Parker D:
Treatment of articular cartilage lesions of the knee by
microfracture or autologous chondrocyte implantation: A systematic
review. Arthroscopy. 31:732–744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Zhu T and Fan W: Osteochondral
autograft transplantation or autologous chondrocyte implantation
for large cartilage defects of the knee: A meta-analysis. Cell
Tissue Bank. 17:59–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benya PD, Padilla SR and Nimni ME:
Independent regulation of collagen types by chondrocytes during the
loss of differentiated function in culture. Cell. 15:1313–1321.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pines M and Spector I: Halofuginone-the
multifaceted molecule. Molecules. 20:573–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLaughlin NP, Evans P and Pines M: The
chemistry and biology of febrifugine and halofuginone. Bioorg Med
Chem. 22:1993–2004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang J, Zhang B, Shen RW, Liu JB, Gao MH,
Li Y, Li YY and Zhang W: Preventive effect of halofuginone on
concanavalin A-induced liver fibrosis. PLoS One. 8:e822322013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park MK, Park JS, Park EM, Lim MA, Kim SM,
Lee DG, Baek SY, Yang EJ, Woo JW, Lee J, et al: Halofuginone
ameliorates autoimmune arthritis in mice by regulating the balance
between Th17 and Treg cells and inhibiting osteoclastogenesis.
Arthritis Rheumatol. 66:1195–1207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Derbyshire ER, Mazitschek R and Clardy J:
Characterization of Plasmodium liver stage inhibition by
halofuginone. ChemMedChem. 7:844–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gnainsky Y, Kushnirsky Z, Bilu G, Hagai Y,
Genina O, Volpin H, Bruck R, Spira G, Nagler A, Kawada N, et al:
Gene expression during chemically induced liver fibrosis: Effect of
halofuginone on TGF-beta signaling. Cell Tissue Res. 328:153–166.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson EF, Huang CW, Ewel JM, Chang AA and
Yuan C: Halofuginone down-regulates Smad3 expression and inhibits
the TGFbeta-induced expression of fibrotic markers in human corneal
fibroblasts. Mol Vis. 18:479–487. 2012.PubMed/NCBI
|
|
13
|
Freemont AJ and Hoyland J: Lineage
plasticity and cell biology of fibrocartilage and hyaline
cartilage: Its significance in cartilage repair and replacement.
Eur J Radiol. 57:32–36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bentley G, Biant LC, Carrington RW, Akmal
M, Goldberg A, Williams AM, Skinner JA and Pringle J: A
prospective, randomised comparison of autologous chondrocyte
implantation versus mosaicplasty for osteochondral defects in the
knee. J Bone Joint Surg Br. 85:223–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fortier LA, Barker JU, Strauss EJ,
McCarrel TM and Cole BJ: The role of growth factors in cartilage
repair. Clin Orthop Relat Res. 469:2706–2715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauge C, Girard N, Lhuissier E, Bazille C
and Boumediene K: Regulation and role of TGFβ signaling pathway in
aging and osteoarthritis joints. Aging Dis. 5:394–405.
2013.PubMed/NCBI
|
|
17
|
Zhen G, Wen C, Jia X, Li Y, Crane JL,
Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al: Inhibition
of TGF-β signaling in mesenchymal stem cells of subchondral bone
attenuates osteoarthritis. Nat Med. 19:704–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galera P, Rédini F, Vivien D, Bonaventure
J, Penfornis H, Loyau G and Pujol JP: Effect of transforming growth
factor-beta 1 (TGF-beta 1) on matrix synthesis by monolayer
cultures of rabbit articular chondrocytes during the
dedifferentiation process. Exp Cell Res. 200:379–392. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Darling EM and Athanasiou KA: Rapid
phenotypic changes in passaged articular chondrocyte
subpopulations. J Orthop Res. 23:425–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pines M and Nagler A: Halofuginone: A
novel antifibrotic therapy. Gen Pharmacol. 30:445–450. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo LW, Wang B, Goel SA, Little C,
Takayama T, Shi XD, Roenneburg D, DiRenzo D and Kent KC:
Halofuginone stimulates adaptive remodeling and preserves
re-endothelialization in balloon-injured rat carotid arteries. Circ
Cardiovasc Interv. 7:594–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui Z, Crane J, Xie H, Jin X, Zhen G, Li
C, Xie L, Wang L, Bian Q, Qiu T, et al: Halofuginone attenuates
osteoarthritis by inhibition of TGF-β activity and H-type vessel
formation in subchondral bone. Ann Rheum Dis. 75:1714–1721. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flanders KC: Smad3 as a mediator of the
fibrotic response. Int J Exp Pathol. 85:47–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tekari A, Luginbuehl R, Hofstetter W and
Egli RJ: Transforming growth factor beta signaling is essential for
the autonomous formation of cartilage-like tissue by expanded
chondrocytes. PLoS One. 10:e01208572015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li C, Wang Q and Wang JF: Transforming
growth factor-β (TGF-β) induces the expression of
chondrogenesis-related genes through TGF-β receptor II
(TGFRII)-AKT-mTOR signaling in primary cultured mouse
precartilaginous stem cells. Biochem Biophys Res Commun.
450:646–651. 2014. View Article : Google Scholar : PubMed/NCBI
|