Introduction
Breast cancer has one of the highest incidences in
females worldwide, and it is the primary cause of mortality in
female patients with cancer (1).
At present, various breast cancer treatment methods have
limitations, for example; surgery does not preclude hematopoietic
or lymphatic dissemination leading to distant metastasis (2). A major advancement in the treatment
of breast cancer has been targeted therapy (3). Therefore, research focusing on the
molecular mechanisms of the development and metastasis of breast
cancer is required.
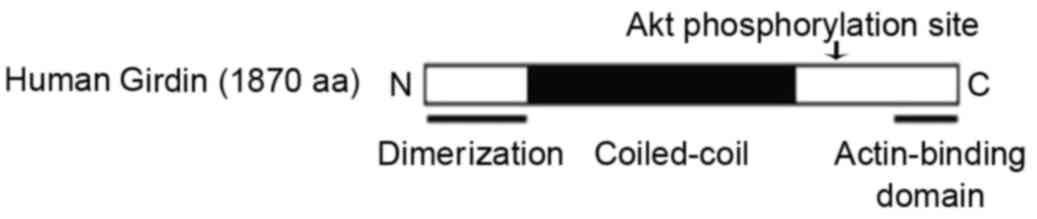
Girdin is a novel actin-binding protein [a
structural schematic which was first published by Jiang et
al (4) in 2008, is depicted in
Fig. 1], that induces cell
migration and angiogenesis (5).
Cell migration is a physiological activity of cells; however, is
also involved in the pathological processes of cancer invasion and
metastasis (6). A previous study
has demonstrated that Girdin promotes DNA synthesis in tumor cells
and inhibits their apoptosis (5).
Girdin has also been demonstrated to induce migration and invasion
of endothelial cells and thus promote angiogenesis (7). However, a systematic study of the
role of Girdin in distinct subtypes of breast cancer has not been
reported to date. Therefore, three cell lines, representing
different subtypes of breast cancer, were selected for use in the
present study: The epithelial MCF-7; the ductal T47D; and the
metastatic MDA-MB-231 breast cancer cell lines. The present study
aimed to investigate the role of Girdin on cell proliferation,
migration and angiogenesis in the various subtypes of breast cancer
cells, and potentially provide insights on novel therapeutic
targets for breast cancer.
Materials and methods
Cell culture
MCF-7, T47D and MDA-MB-231 cells were purchased from
the American Type Culture Collection (Manassas, VA, USA). The
present study was performed in accordance with the Experimental
Guidelines of Harbin Medical University (Harbin, China) and ethical
approval was obtained from Harbin Medical University. MCF-7 cells
were cultured in RPMI 1640 medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS)
(Sigma-Aldrich; Merck KGaA). T47D and MDA-MB-231 cells were
cultured in Dulbecco's Modified Eagle's Medium (DMEM;
Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS. All cells
were cultured with 100 U/ml penicillin and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an
incubator with 5% CO2 at 37°C.
Small interfering RNA (siRNA)
transfection
Girdin siRNA (sc-94984) and non-targeting negative
control siRNA (SIC002) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and GenePharma Co., Ltd.
(Shanghai, China) respectively. Transfection was performed as
described previously (8). Cells
were seeded into 6-well plates at a density of 1×105
cells/well and grown to 60–80% confluency prior to transfection.
siRNAs were transfected into cells using siRNA Transfection Reagent
(Santa Cruz Biotechnology, Inc.), according to the manufacturer's
protocol. Cells were incubated for a further 48 h following
transfection and subsequently used for experiments.
Reverse
transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR)
Total RNA was extracted by TRIzol (Sigma-Aldrich;
Merck KGaA) and relative mRNA was normalized to 18S ribosomal RNA.
The following primers (Hokkaido System Science Co. Ltd, Sapporo,
Japan) were used: Girdin, forward 5′-CCAGGCATGAAGCGAACA-3′ and
reverse 5′-CGAGCATCCGAAAGCAAAT-3′; vascular endothelial growth
factor (VEGF), forward 5′-TTGCCTTGCTGCTCTACCTC-3′ and reverse
5′-AAATGCTTTCTCCGCTCTGA-3′; and 18S, forward
5′-GTAACCCGTTGAACCCCATT-3′ and reverse 5′-CCATCCAATCGGTAGTAGCG-3′.
Reverse transcription was performed using a Transcriptor First
Strand cDNA Synthesis kit (Roche Applied Science, Madison, WI,
USA). A total of 200 ng RNA was used as input for the RT reaction,
and 2 µl input cDNA from the RT product was used for the sqPCR. PCR
was performed using SYBR Premix Ex Taq II (Takara Bio, Inc., Otsu,
Japan) and the ABI 7300 Fast real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were: Holding stage 95°C for 30 sec (1 cycle) and
cycling stage 95°C for 3 sec and 60°C for 31 sec (40 cycles).
Ethidium bromide (Sigma-Aidrich; Merck KGaA) was used as the
agarose gel visualization reagent and ImageJ software (version
1.38e; National Institutes of Health, Bethesda, MD, USA) was used
for the band quantification.
MTT Assay
Cell viability was determined by a colorimetric MTT
assay according to the method described previously (9). Absorbance at 550 nm was measured
using a MTP-800 microplate reader (Corona Electric, Ibaraki,
Japan). Absorbance at 690 nm was also measured as a control, to
compensate for interfering absorbance from potential cell debris or
the microtiter plate. The % of viable cells was calculated as
follows: (Optical density (OD) of treated sample/OD of untreated
control) ×100.
Migration assay
The migration assay was performed using 48-well
migration transwell chambers with polycarbonate membranes
(Sigma-Aldrich; Merck KGaA), according to the method described
previously (10). To prepare the
migration chambers, the upper wells were coated with 0.01% collagen
and incubated for 30 min at 37°C. Then, the cells (5×104
cells/well) were seeded on the upper chamber of the transwells in
RPMI 1640 medium (for MCF-7 cells) or DMEM (for T47D and MDA-MB-231
cells). DMEM with 10% fetal calf serum was added to the lower wells
of the chambers as a chemoattractant. Following incubation at 37°C
for 24 h, the cells that had migrated to the lower surface of the
filters were fixed with 4% paraformaldehyde in PBS for 10 min at
room temperature and stained with crystal violet. Cell migration
was defined as the number of cells that had migrated to the lower
filter surface. Four non-overlapping fields per filter were
selected and the migrated cells were counted under a microscope
(Olympus Corporation, Tokyo, Japan) at a magnification of ×100. The
average number of the cells from four fields was presented as the
results of the migration assay.
Western blot analysis
MCF-7, T47D and MDA-MB-231 cells were lysed by lysis
buffer (1 M Tris-HCl, pH 7.4; 1 M NaCl; 20% Triton X-100; 10% SDS;
and, 0.5 M EDTA; Sigma-Aldrich Merck KGaA). The protein
concentration was determined by Gene Spec III from Hitachi Genetic
Systems (MiraiBio, Alameda, CA, USA). Electrophoresis was performed
using a vertical slab 12% SDS-PAGE, as described previously
(11). A total of 20 µg of protein
was loaded per gel lane. Protein transfer to a membrane
(Immobilon®-P, Merck KGaA) was performed
electrophoretically, as described previously (12) with certain modifications, using a
Semi Dry Electroblotter (Sartorius AG, Goettingen, Germany) for 90
min, with an electric current of 15 V. The membrane was treated
with Block Ace™ (4%; Bio-Rad, Hercules, CA, USA) for 30 min at
22°C. Primary antibody incubations were performed using rabbit
immunoglobulin (Ig) G antibodies against phosphatidyl inositol
3-kinase (PI3K; SAB5500162; 1:100; Sigma-Aldrich; Merck KGaA) and
RAC-α serine/threonine-protein kinase (Akt; SAB4500797; 1:1,000;
Sigma-Aldrich; Merck KGaA) in PBS containing 0.03% Tween-20 (PBST)
for 1 h at 22°C. Following washing in PBST, the secondary antibody
incubation was performed using horseradish peroxidase-conjugated
anti-rabbit goat IgG (A0545; 20 ng/ml; Sigma-Aldrich; Merck KGaA)
for 30 min at 22°C. Following washing in PBST, the enhanced
chemiluminescence (ECL) reaction was performed on the membranes
using the ECL Plus Western Blotting Detection System (GE Healthcare
Life Sciences, Shanghai, China). ImageJ (version 1.38e; National
Institutes of Health) was used for the quantification of western
blots.
Statistical analysis
The paired Student's t-test was used to analyze the
data. Analyses were conducted using SPSS software (version 17.0;
SPSS, Inc., Chicago, IL, USA) and data were expressed as the mean ±
standard deviation. Each experiment was repeated at least three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Girdin knockdown decreases viability
in breast cancer cells
To investigate the effect of Girdin knockdown on the
viability of breast cancer cells, MCF-7, T47D and MDA-MB-231 cells
were seeded onto 6-well plates, grown to 60–80% confluency, and
then transfected with either a Girdin-specific siRNA or a
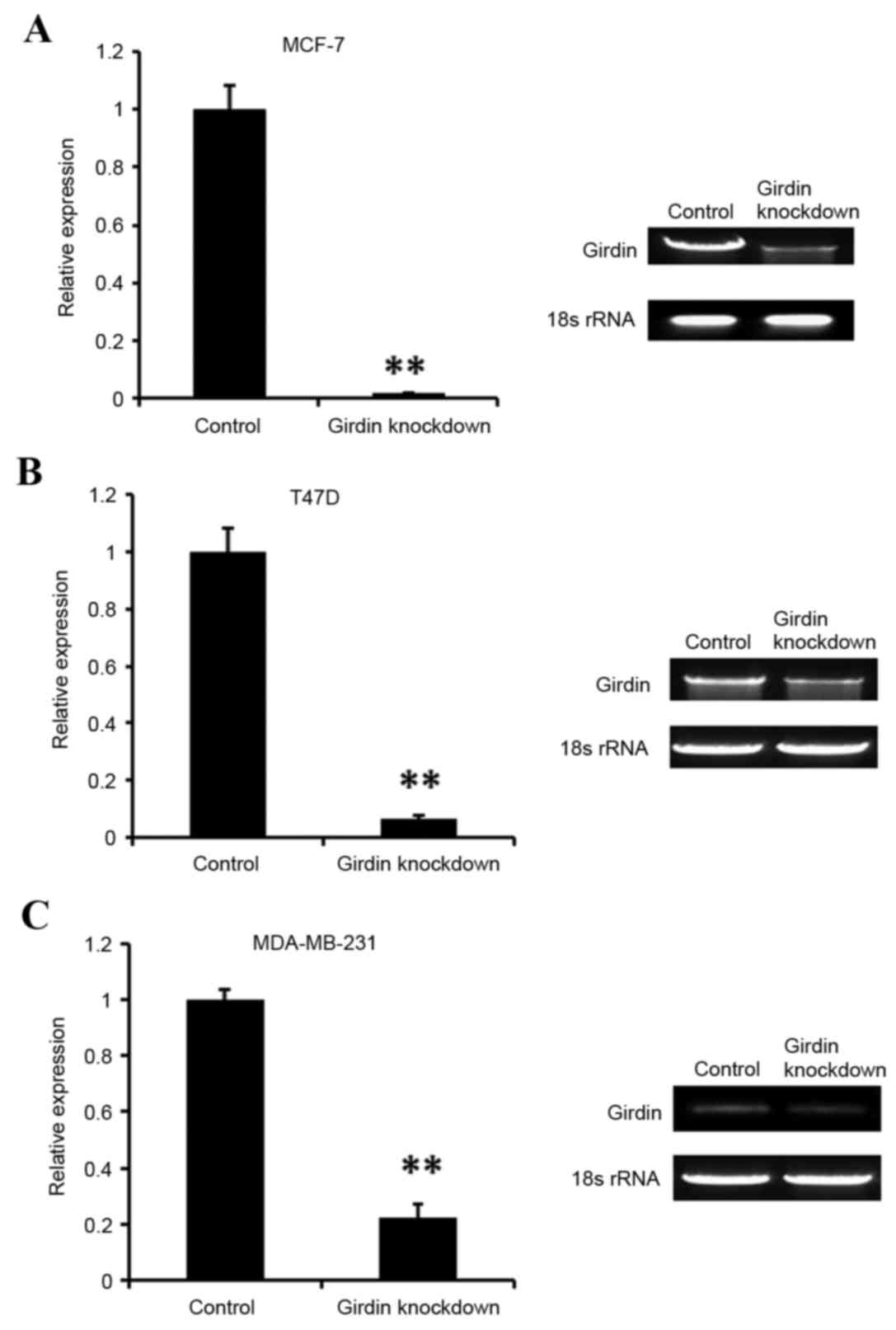
non-specific negative control siRNA. Following 48 h, the efficiency
of Girdin siRNA knockdown was evaluated using RT-sqPCR (Fig. 2). The results demonstrated that
Girdin was efficiently silenced in all three cell lines tested,
compared with the control siRNA-transfected cells (Fig. 2). Cell viability was then
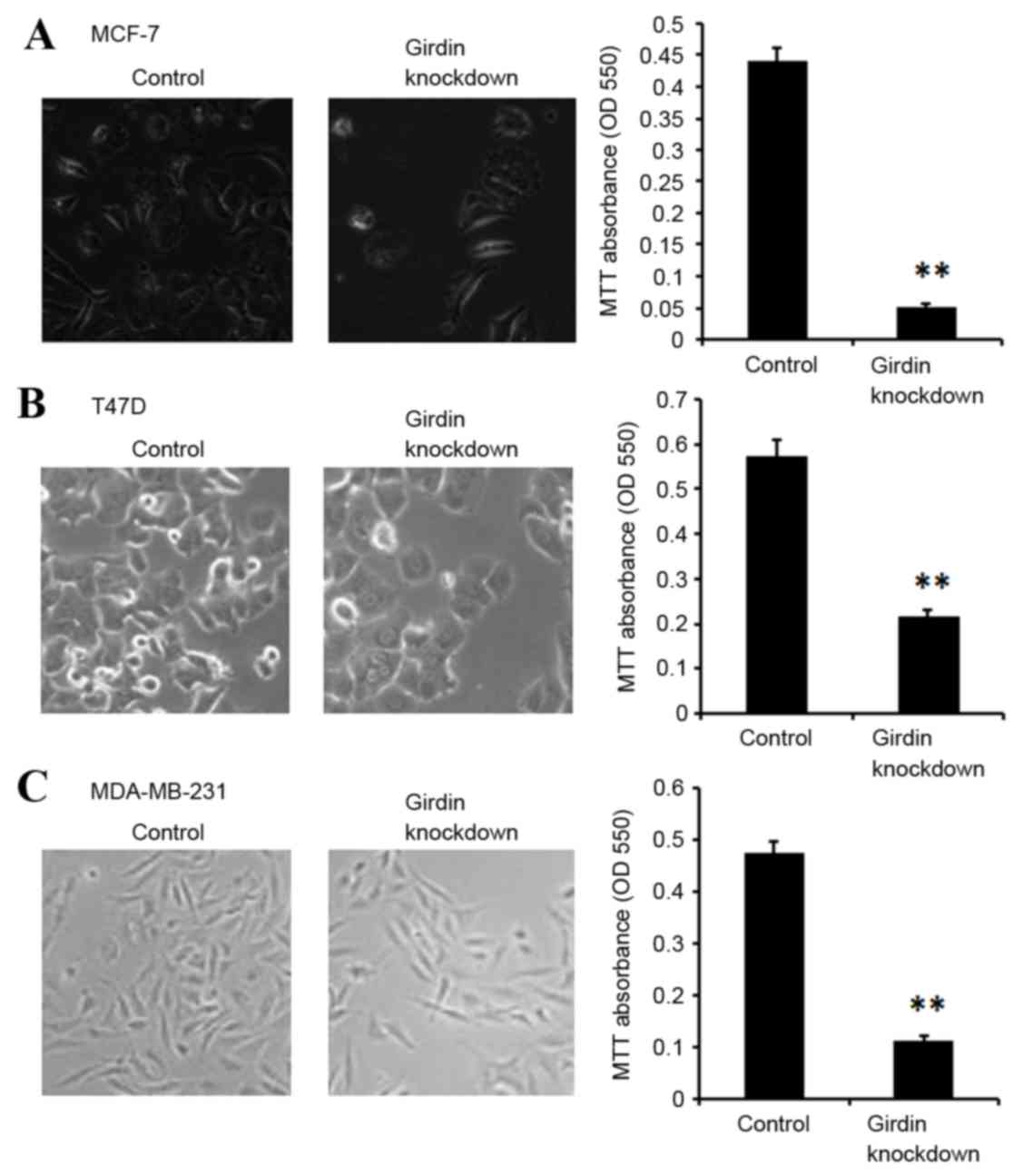
determined by MTT assay. MCF-7, T47D and MDA-MB-231 cells
demonstrated significantly suppressed viability following Girdin
knockdown compared with control siRNA-transfected cells (P<0.01;
Fig. 3).
Girdin knockdown suppresses migration
in breast cancer cells
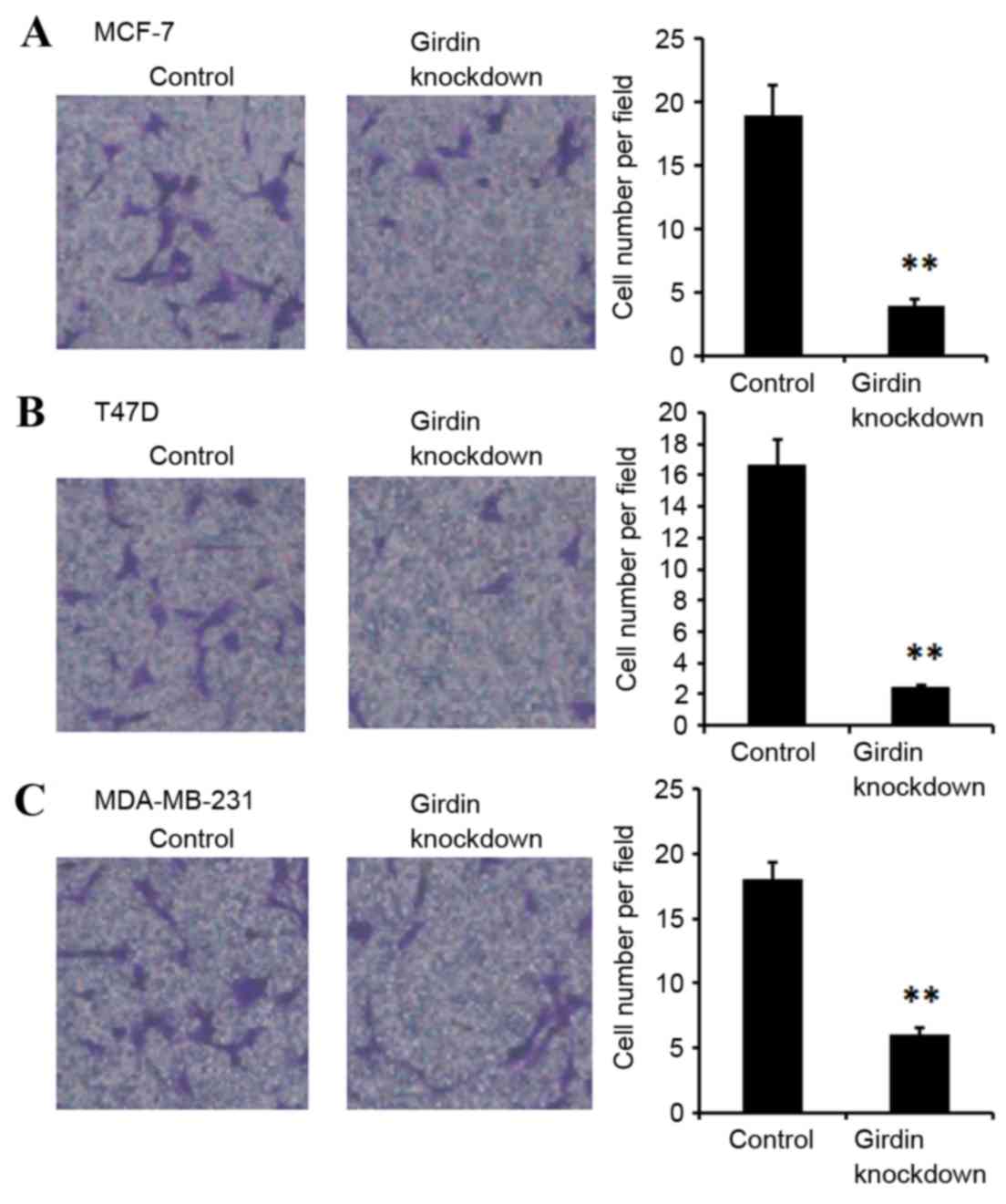
The migration abilities of MCF-7, T47D and
MDA-MB-231 were examined following Girdin knockdown using a
transwell chamber migration assay. Girdin deficient MCF-7, T47D and
MDA-MB-231 cells exhibited significantly suppressed migration
compared with the control siRNA-transfected cells (P<0.01;
Fig. 4).
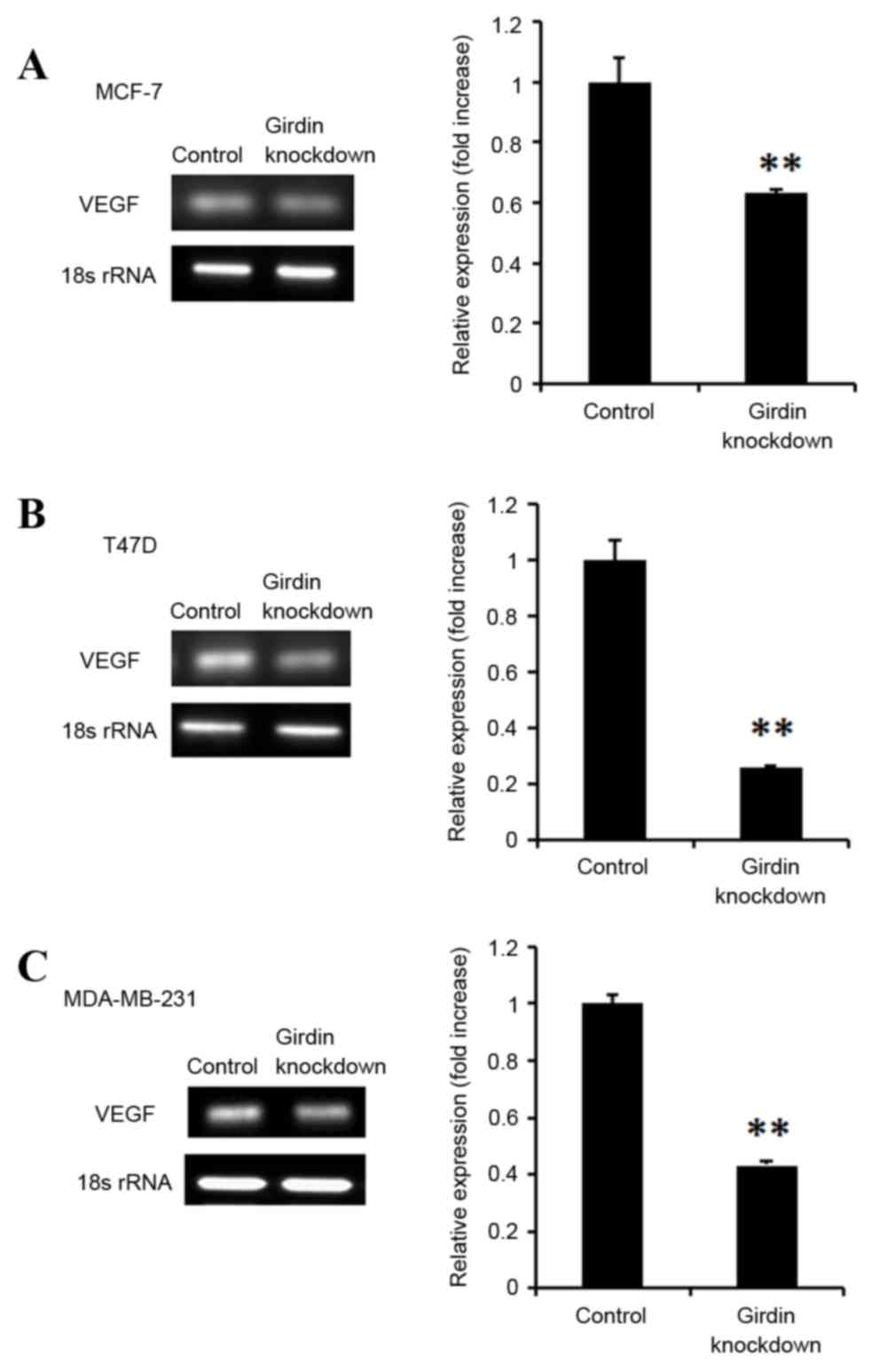
Girdin knockdown suppresses VEGF
expression in breast cancer cells
Breast cancer cells frequently express VEGF, an
endothelial growth and chemotactic agent that promotes
angiogenesis, and subsequently cancer metastasis. The effect of
Girdin knockdown was therefore investigated on VEGF expression in
breast cancer cells lines. Girdin deficient MCF-7, T47D and
MDA-MB-231 cells exhibited significantly decreased VEGF mRNA
expression levels, compared with control siRNA-transfected cells
(P<0.01; Fig. 5).
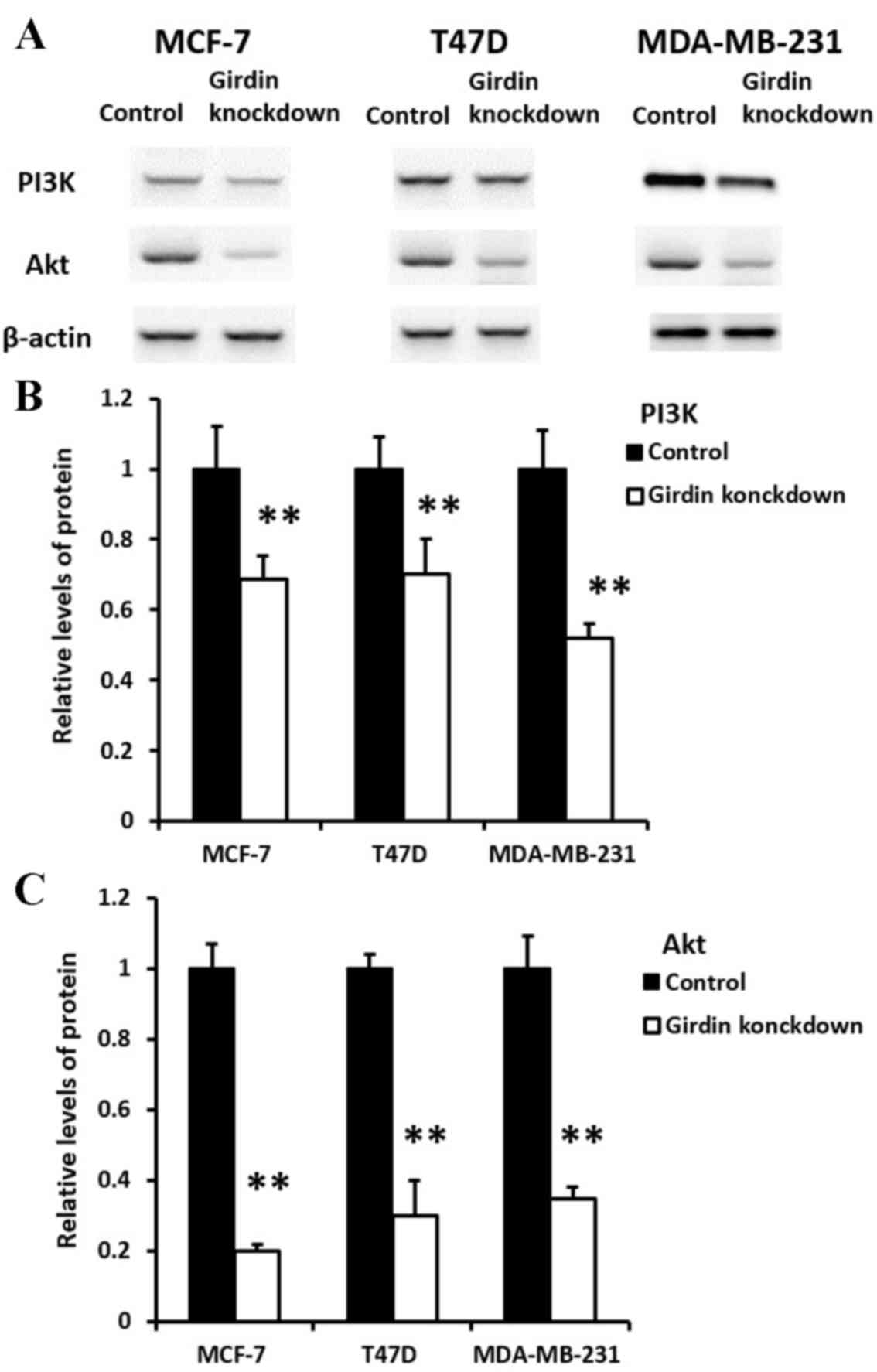
Effect of Girdin knockdown on PI3K and
Akt expression in breast cancer cells
The protein expression levels of PI3K and Akt in
Girdin deficient MCF-7, T47D and MDA-MB-231 cells were measured by
western blot analysis. Expression of PI3K and Akt proteins was
significantly downregulated in MCF-7, T47D and MDA-MB-231 cells
following Girdin knockdown, compared with the control
siRNA-transfected cells. (P<0.01; Fig. 6).
Discussion
To the best of our knowledge, the present study
demonstrated for the first time the effect of Girdin silencing on
different subtypes of breast cancer. Breast cancer has one of the
highest incidences in females worldwide, and it is the primary
cause of mortality in female patients with cancer (1). Breast cancer has long been a leading
cause of mortality in women of both developed and developing
countries (13). Breast cancer is
a heterogeneous disease (14).
There are multiple methods of breast cancer classification. Based
on tissue typing, breast cancers are divided to breast epithelial
cancer, breast ductal carcinoma and metastatic breast cancer
(15). Therefore, in the present
study, three representative cell lines were selected: MCF-7, T47D
and MDA-MB-231 cells, for epithelial, ductal and metastatic breast
cancer respectively. Great advances in the treatment of breast
cancer have come from targeted therapy (3), thus studies investigating the
molecular mechanisms of breast cancer pathogenesis and metastasis
are crucial for the identification of new targets.
Girdin, first discovered by Japanese scholars in
2005 (5), is a novel actin binding
protein that induces cell migration and angiogenesis (16). Girdin maintains the structure of
actin (17), and it induces
invasion and metastasis of tumor cells and angiogenesis (4,7). The
present study is consistent with these previous reports, as it
demonstrated that Girdin deficiency suppressed viability and
migration of different subtypes of breast cancer cells.
A previous study demonstrated that Girdin is
important in vessel formation (18). VEGF is the most important
pathogenic factor in vaso-proliferative disorders (19). Girdin promotes migration of
VEGF-dependent endothelial cells, formation of tubular structures
and remodeling of the micrangium after birth (5,7). In
the present study, VEGF mRNA expression was demonstrated to be
significantly decreased in Girdin deficient MCF-7, T47D and
MDA-MB-231 cells compared with the control siRNA-transfected cells,
suggesting that Girdin may be important in angiogenesis of breast
tumors as well.
PI3K phosphorylates phosphatidylinositol lipids in
response to various growth factors (20). The PI3K/Akt signaling pathway is
important in modulating cell growth, cell survival and cytoskeletal
rearrangement (21). Since cancer
cell proliferation and migration is regulated by the PI3K/Akt
signaling pathway (22,23), the present study aimed to further
investigate the association between Girdin and PI3K/Akt. PI3K and
Akt protein expression levels were significantly decreased in
Girdin deficient MCF-7, T47D and MDA-MB-231 cells compared with the
control siRNA-transfected cells. The present data demonstrated that
Girdin knockdown suppressed viability, migration and angiogenesis
in distinct subtypes of breast cancer, potentially via the PI3K/Akt
signaling pathway.
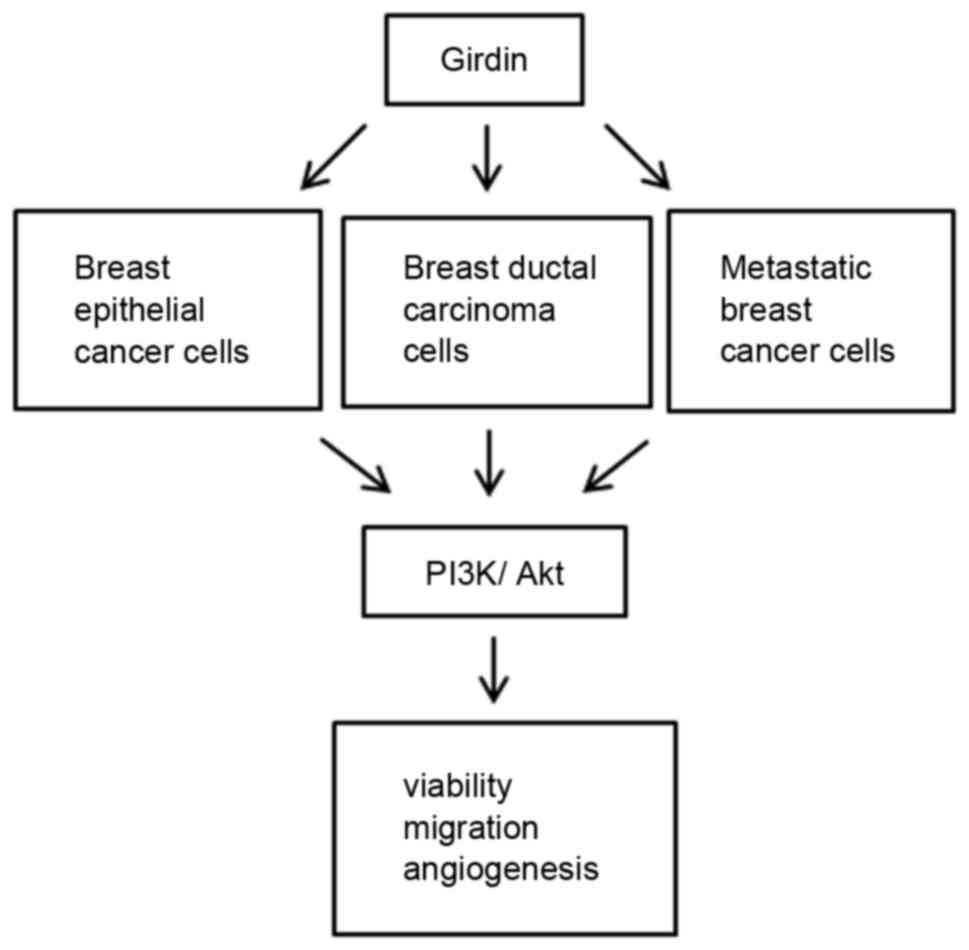
According to the model described in Fig. 7, Girdin knockdown suppressed cell
viability, migration and angiogenesis in different subtypes of
breast cancers, including breast epithelial, breast ductal and
metastatic breast cancer, by downregulating expression of PI3K and
Akt. A previous study has indicated that Girdin is important in
combining with G protein (24).
Although the present study provides evidence that Girdin may be
important in regulating cancer cell viability, migration and
angiogenesis, the underlying mechanism remains unclear and requires
further investigation in the future. In conclusion, the present
study suggested that Girdin may serve as a potential novel target
for the development of novel clinical treatments for breast
cancer.
Acknowledgements
The present work was supported by the Heilongjiang
Provincial Health and Family Planning Commission (grant no.
2014-358).
References
|
1
|
Sun YW, Chen KY, Kwon CH and Chen KM:
CK0403, a 9-aminoacridine, is a potent anti-cancer agent in human
breast cancer cells. Mol Med Rep. 13:933–938. 2016.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang P, Enomoto A, Jijiwa M, Kato T,
Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y and Takahashi M:
An actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Enomoto A, Murakami H, Asai N, Morone N,
Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K and
Takahashi M: Akt/PKB regulates actin organization and cell motility
via Girdin/APE. Dev Cell. 9:389–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enomoto A, Ping J and Takahashi M: Girdin,
a novel actin-binding protein and its family of proteins possess
versatile functions in the Akt and Wnt signaling pathways. Ann NY
Acad Sci. 1086:169–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitamura T, Asai N, Enomoto A, Maeda K,
Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, Kondo T, et al:
Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate
Girdin. Nat Cell Biol. 10:329–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao K, Lu C, Han S, Zou Q, Li J, Xie D, He
S, Yu L, Zhou J, Peng X and Cao P: Expression of Girdin in primary
hepatocellular carcinoma and its effect on cell proliferation and
invasion. Int J Clin Exp Pathol. 8:551–559. 2015.PubMed/NCBI
|
|
9
|
Yuan Z, Feng W, Hong J, Zheng Q, Shuai J
and Ge Y: p38MAPK and ERK promote nitric oxide production in
cultured human retinal pigmented epithelial cells induced by high
concentration glucose. Nitric Oxide. 20:9–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Shu T, Liang Y, Gu W, Wang C, Song
X, Fan C and Wang W: GDC-0152 attenuates the malignant progression
of osteosarcoma promoted by ANGPTL2 via PI3K/AKT but not p38MAPK
signaling pathway. Int J Oncol. 46:1651–1658. 2015.PubMed/NCBI
|
|
11
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyhse-Andersen J: Electroblotting of
multiple gels: A simple apparatus without buffer tank for rapid
transfer of proteins from polyacrylamide to nitrocellulose. J
Biochem Biophys Methods. 10:203–209. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lan T, Wang L, Xu Q, Liu W, Jin H, Mao W
and Wang X and Wang X: Growth inhibitory effect of Cucurbitacin E
on breast cancer cells. Int J Clin Exp Pathol. 6:1799–1805.
2013.PubMed/NCBI
|
|
14
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim G, Ouzounova M, Quraishi AA, Davis A,
Tawakkol N, Clouthier SG, Malik F, Paulson AK, D'Angelo RC, Korkaya
S, et al: SOCS3-mediated regulation of inflammatory cytokines in
PTEN and p53 inactivated triple negative breast cancer model.
Oncogene. 34:671–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng L, Enomoto A, Ishida-Takagishi M,
Asai N and Takahashi M: Girding for migratory cues: Roles of the
Akt substrate Girdin in cancer progression and angiogenesis. Cancer
Sci. 101:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Natsume A, Kato T, Kinjo S, Enomoto A,
Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, Miyata T, et al:
Girdin maintains the stemness of glioblastoma stem cells. Oncogene.
31:2715–2724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito T, Komeima K, Yasuma T, Enomoto A,
Asai N, Asai M, Iwase S, Takahashi M and Terasaki H: Girdin and its
phosphorylation dynamically regulate neonatal vascular development
and pathological neovascularization in the retina. Am J Pathol.
182:586–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Zhang X, Lu H, Matsukura M, Zhao
J and Shinohara M: Silencing heme oxygenase-1 gene expression in
retinal pigment epithelial cells inhibits proliferation, migration
and tube formation of cocultured endothelial cells. Biochem Biophys
Res Commun. 434:492–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Sicience. 296:1655–1657. 2002. View Article : Google Scholar
|
|
21
|
Vivanco I and Sawyers CL: The
phosphatidylonositol 3-kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gallia GL, Tyler BM, Hann CL, Siu IM,
Giranda VL, Vescovi AL, Brem H and Riggins GJ: Inhibition of Akt
inhibits growth of glioblastoma and glioblastoma stem-like cells.
Mol Cancer Ther. 8:386–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woodgett JR: Recent advances in the
protein kinase B signaling pathway. Curr Opin Cell Biol.
17:150–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Le-Niculescu H, Niesman I, Fischer T,
DeVries L and Farquhar MG: Identification and characterization of
GIV, a novel Galpha i/s-interacting protein found on COPI,
endoplasmic reticulum-Golgi transport vesicles. J Biol Chem.
280:22012–22020. 2005. View Article : Google Scholar : PubMed/NCBI
|