Introduction
Ulcerative colitis (UC) is a chronic condition
characterized by continuous inflammation of the colonic mucosa and
submucosa, and it is associated with an increased risk of colon,
rectal and bowel cancer. A previous study reported that the
worldwide incidence of UC was 1.2–20.3 cases per 100,000
individuals per year, with a prevalence of 7.6–246.0 cases per
100,000 individuals per year (1).
UC is one of two conditions referred to as inflammatory bowel
disease, the other condition being Crohn's disease.
Several animal models have been developed to
investigate the pathogenesis of UC in detail. UC can be readily
induced in animal models either chemically or by bacterial
infection. The majority of animal models of UC involve mice;
however, UC has previously also been induced in other animals,
including zebrafish, Drosophila and pigs (2). The administration of dextran sulfate
sodium, intrarectal administration of oxalozone or acetic acid, or
infection with Salmonella typhimurium or Escherichia
coli have all been successfully used to induce UC-like symptoms
and immune responses in animal models. The interleukin (IL)-7 gene
is a candidate risk gene associated with UC. T-cell receptor α
chain (TCRα)-knockout mice have been reported to spontaneously
develop chronic colitis, which was mediated by a Th2-type immune
response closely resembling human UC, with an inflammatory pattern
restricted primarily to the colonic mucosa. Thus, genetic
approaches, including the knockout of IL-7 and TCRα genes have also
been used to induce chronic colitis in animal models (3).
There is substantial evidence suggesting that
UC-associated colon cancer is responsible for 10–15% of
UC-associated mortality (4).
However, how the chronic inflammation of UC develops into cancer
remains to be elucidated.
Toll-like receptor (TLR) and innate immune processes
have been shown to be involved in the pathogenesis of
colitis-associated tumorigenesis (5). The expression of TLR4 has been found
to be increased in colitis-associated tumorigenesis and is
associated with the degree of dysplasia (2). IL-1 receptor-associated kinase (IRAK)
and MyD88 are key factors involved in TLR4-associated signal
transduction. IRAK-1 and IRAK-4 are the active members of the IRAK
family, whereas IRAK-2 and IRAK-3, also known as IRAK-M, are the
inactive members of this family. The expression of IRAK-3 is
localized to the monocyte and macrophage populations only, and it
prevents the formation of a complex containing IRAK-1 and IRAK-4.
The other members of the IRAK family are ubiquitous. Studies have
reported increased inflammation in IRAK-3−/− cells and
mice, suggesting that IRAK-3 is a negative regulator in TLR
pathways, whereas the lack of IRAK-3 is known to be associated with
inflammation and tumorigenesis (6–8).
Specifically, IRAK-3 mediates TLR7-induced MEKK3-dependent second
wave activation of nuclear factor-κB (NF-κB) to produce inhibitory
molecules as negative feedback in the pathway, and exerts an
inhibitory effect on the translational control of cytokines and
chemokines (9,10). Previous studies have also
demonstrated that chronic inflammation is frequently accompanied by
methylation in the development of UC, which occurs prior to
dysplasia (11). Although the
exact mechanism of methylation and its association with
tumorigenesis remains to be elucidated, these findings indicate
that the early detection of methylation may serve to predict and
consequently prevent cancer.
Therefore, the present study aimed to determine the
role of IRAK-3 and changes in its methylation levels in a mouse
model of chemically induced colitis-associated cancer, in order to
examine the potential of IRAK-3 as a therapeutic target.
Materials and methods
Animals
Male ICR mice (5 weeks old) weighing 25–33 g were
purchased from the Shanghai Laboratory Animal Research Center
(Shanghai, China; certificate no. SCXK2013-0016). Mice were
maintained under standard conditions with a temperature of 24±1°C
and 12-h light/dark cycle, and were fed standard laboratory chow
and tap water ad libitum. The experiment was performed
following 3 days of acclimatization. All procedures were performed
in strict accordance with the legislation of the P.R China. The
study was approved by the animal ethics committee of the Second
Affiliated Hospital, School of Medicine, Zhejiang University
(Hangzhou, China).
Development of the mouse model
The ICR mice were randomly allocated into four
groups (n=8 mice/group). The mice in the control group were
injected intraperitoneally with 200 µl physiological saline,
whereas the mice in the DMH and DMH + DSS groups were injected
intraperitoneally with 15 mg/kg 1,2-dimethyl hydrazine (DMH)
dissolved in physiological saline (pH 6.5–7.0). After 1 week, the
mice in the DSS and DMH + DSS groups were provided with 2% dextran
sodium sulfate (DSS) dissolved in tap water, which was provided
ad libitum for 7 days, whereas the mice in the control and
DMH groups were provided with tap water ad libitum. The body
weights, levels of activity and clinical signs of the mice were
monitored every week for a period of 20 weeks. At weeks 4, 9, 13
and 20, one mouse from each group was sacrificed, and tissue
samples were collected for histopathological examination. At week
20, the levels of IRAK3 were analyzed using immunohistochemistry,
western blot analysis, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis and
methylation-specific PCR (MSP) analysis.
Histological examination
For histological examination, one mouse from each
group was sacrificed at weeks 4, 9, 13 and 20. The abdominal cavity
of the mouse was opened, and the colon was isolated and generally
scored. A 1-cm specimen was then cut at 3 cm from the anal verge,
and placed in 40 g/l formalin at room temperature for 48 h. The
specimens were embedded in paraffin and then cut into 4–7-µm-thick
sections. The sections were stained with 2 g/l hematoxylin for 5
min and 0.5% eosin for 2–3 min at room temperature and examined
under a light microscope.
Immunohistochemical analysis of
IRAK-3
The immunohistochemical staining was performed using
a standard avidin-biotin complex technique at week 20. The fresh
frozen (4-µm-thick) sections of mice colon were mounted onto glass
slides, fixed in 100% acetone for 10 min at 4°C, air-dried at room
temperature for 10 min and rehydrated in PBS. Following blocking of
endogenous peroxidase activity for 20 min in 3% hydrogen peroxide
at room temperature, the slides were incubated for 10–15 min at
room temperature with normal goat serum (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) for blocking. The slides
were then incubated with anti-IRAK3 antibodies (cat no. ab238;
1:1,500; Abcam, Cambridge, MA, USA) at 4°C overnight. The slides
were then kept for 20–30 min at room temperature, washed in PBS and
then incubated for 10–15 min at room temperature with a goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(cat no. ab6721; 1:1,000; Abcam). Following washing with PBS, the
slides were incubated with streptavidin-biotin complex (cat no.
ab7403; 1:15,000; Abcam) for 30 min at room temperature, then
washed in PBS 3 times and stained with diaminobenzidine (cat no.
ab103723; Abcam) for 20 min at room temperature. Finally, the
sections were rinsed in distilled water, counterstained with 2 g/l
hematoxylin for 1 min at room temperature, washed in running tap
water and mounted with mounting media. The slides were then
observed under a light microscope (Olympus, Tokyo, Japan) and
images were captured. Immunohistochemical data were quantified
using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
RT-qPCR
The colon tissues were snap-frozen in liquid
nitrogen, and stored at −80°C until further analysis. Colon tissues
were homogenized in diethyl pyrocarbonate-treated water, ice-cold
PBS, 70% ethanol and isopropyl alcohol, and total RNA was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA was
reverse-transcribed into cDNA using RevertAid First Strand cDNA
Synthesis (Thermo Fisher Scientific, Inc.). qPCR was performed on
cDNA using a Toyobo Revertra Ace qRCR RT kit (Toyobo Co., Ltd.,
Osaka, Japan) and SYBR Green Real-Time PCR Master Mix (Thermo
Fisher Scientific, Inc.). The qPCR reaction consisted of 25.0 µl
SYBR Green Real-Time PCR Master Mix, 5.0 µl cDNA, 16.0 µl
nuclease-free water, and 4.0 µl of primer pairs, all in a total
volume of 50 µl. The thermocycling conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec, at 60°C for 30 sec and at 72°C for 30 sec. The
mRNA expression levels were normalized to GAPDH, which was used as
the endogenous control. The following primer sequences were used:
IRAK-3, forward 5′-TTGGTCCTGGGCACAGAAA-3′, reverse
5′-AATAGCTCGACGATGTCCCAT-3′; and GAPDH, forward
5′-GGTATCGTGGAAGGACTCATGAC-3′ and reverse
5′-ATGCCAGTGAGCTTCCCGTTCCCGTTCAGC-3′. Target gene expression was
quantified according to the comparative Cq method (12). Experiments were performed in
triplicate.
Western blot analysis
Total proteins were extracted from the colon samples
obtained at week 20 using ice-cold radioimmunoprecipitation assay
lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM sodium
chloride, 1 mM EDTA (pH 8.0), 1% (v/v) TritonX-100, 0.1% (m/v) SDS
and 1 mM phenylmethylsulfonyl fluoride. Protein concentration was
determined using a Bradford assay. Equal amounts of extracted
protein samples (20 µl) were separated by 10% SDS-PAGE and
transferred onto a polyvinylidene difluoride membrane. The membrane
was blocked with 5% nonfat dried milk in PBS containing 0.1%
Tween-20 for 1 h at room temperature and then incubated with an
anti-IRAK-3 (cat no. ab238; 1:5,000; Abcam) antibody at 4°C
overnight, followed by incubation for 2 h at room temperature with
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibodies [cat no. 70-GAR007; 1:10,000; Multisciences (Lianke)
Biotech Co., Ltd., Hangzhou, China]. The protein bands were
visualized and documented using the ChampGel™6000 image acquisition
and analysis system (Beijing Sage Creation Science Co., Ltd.,
Beijing, China). Blots were semi-quantified using ImageJ software
version 2.1.4.7 (National Institutes of Health, Bethesda, MD,
USA).
Bisulfite modification and MSP
analysis
Genomic DNA was isolated from colon tissues using
the TIANamp Genomic DNA kit (Tiangen Biotech Co., Ltd., Beijing,
China). Briefly, 1 µg genomic DNA was bisulfite-modified using an
EZ DNA Methylation-Lightning™ kit (Zymo Research Corporation,
Irvine, CA, USA) according to the manufacturer's protocol. The MSP
was performed at 94°C for 5 min, followed by 35 cycles at 94°C for
30 sec, 55°C for 30 sec and 72°C for 30 sec. The final extension
step was performed at 72°C for 10 min. The PCR reaction consisted
of 1.5 µl 10X Taq Buffer, 1.0 µl dNTP (20 mM), 0.5 µl Taq DNA
polymerase (Takara Bio, Inc., Otsu, Japan), 1.0 µl cDNA, 20.0 µl
nuclease-free water and 1.0 µl primer pairs, in a total volume of
25 µl. The following MSP primer sequences were used: IRAK-3
unmethylated, forward 5′-AAGTAATTATGGATTGAAGTTTTGA-3′ and reverse
5′-CAAACAAAAACAACCTAAAACATA-3′, IRAK-3 methylated, forward
5′-AAGTAATTATGGATTGAAGTTTCGA-3′ and reverse
5′-CAAACAAAAACAACCTAAAACGTA-3′.
Statistical analysis
Data are expressed as the mean ± standard deviation
for each group. An independent t-test was used to evaluate
differences between two treatment groups. All data were processed
and analyzed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
General condition and pathological
evaluations
At weeks 9 and 13 following the start of the
experiment, colitis was induced in the mice by injection with 15
mg/kg DMH (DMH and DMH + DSS groups) or by provision of drinking
water containing 2% DSS for 7 days (DSS and DMH + DSS groups). At
week 20, the mice in the DMH + DSS group had lost body weight and
developed canalicular adenoma or adenocarcinoma (Fig. 1).
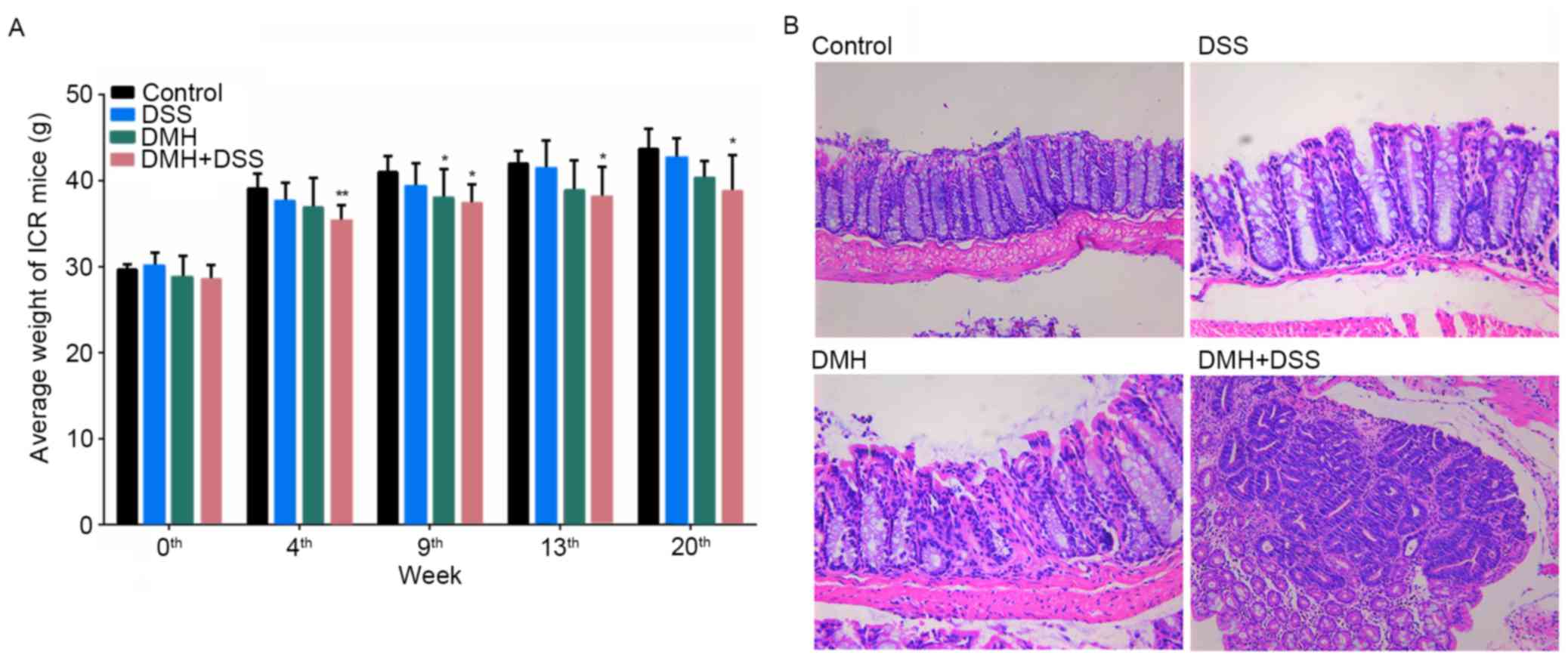 | Figure 1.Comparison of body weights and
histopathological analysis. (A) Graph of body weights during the
experimental period. *P<0.05 and **P<0.01, vs. control group.
(B) Histopathological analyses at week 20. Mice in the DMH + DSS
group developed canalicular adenoma or adenocarcinoma, and mice in
the DSS and DMH groups exhibited increased colitis at week 20.
Control, injected with physiological saline; DSS, provided with tap
water containing DSS; DMH, injected with DMH; DMH + DSS, injected
with 15 mg/Kg DMH and tap water containing DSS. Magnification,
×100. DSS, dextran sodium sulfate; DMH, 1,2-dimethyl hydrazine. |
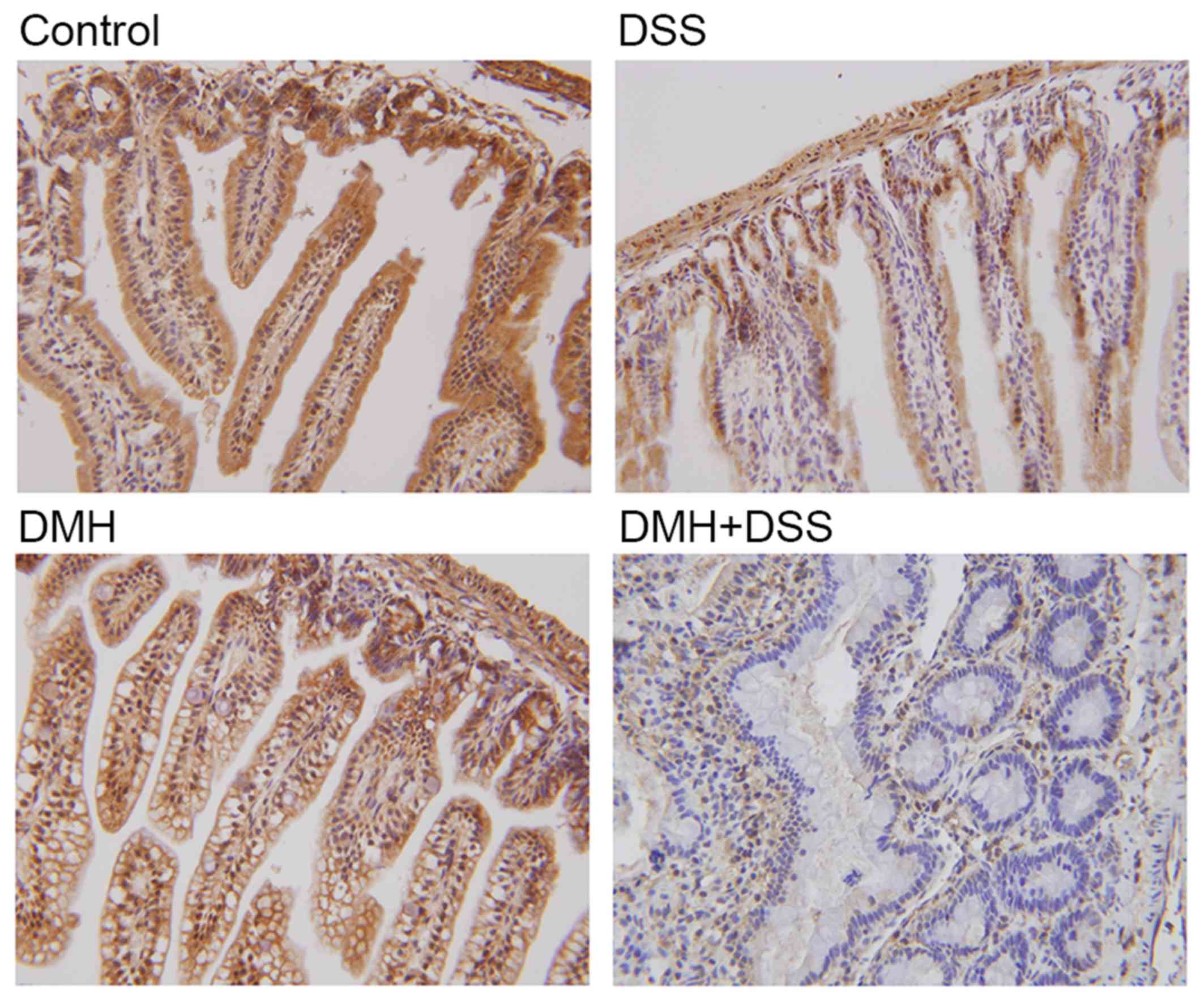
Immunohistochemical localization of
IRAK-3 in the colon
Positive cytoplasmic and nuclear staining for IRAK-3
was found in all groups, and this positive staining was highest in
the mice without DMH or DSS (Fig.
2). By contrast, positive staining for IRAK-3 was minimal in
the colitis-associated cancer model. The quantitative results of
the immunohistochemistry also showed that the expression of IRAK-3
in the colon was decreased significantly in the mice with induced
colitis, particularly in the mice with tumorigenesis (P=0.000;
Table I). Taken together, the
results of the immunohistochemistry indicated that IRAK-3 was
downregulated in the colon cells of DSS- or DMH-induced colitis,
particularly in the colitis-associated cancer model.
 | Table I.Quantitative results of
immunohistochemistry. |
Table I.
Quantitative results of
immunohistochemistry.
| Factor | Control | DSS | DMH | DMH+DSS |
|---|
| Area (pixels) | 675,801 | 309,303 | 605,832 | 57,288 |
| IOD | 168,967.94 | 63,390.00 | 138,182.08 | 9,852.63 |
| Intensity | 0.25003 | 0.20494 | 0.22809 | 0.17198 |
| P-value |
| 0.007 | 0.040 | 0.000 |
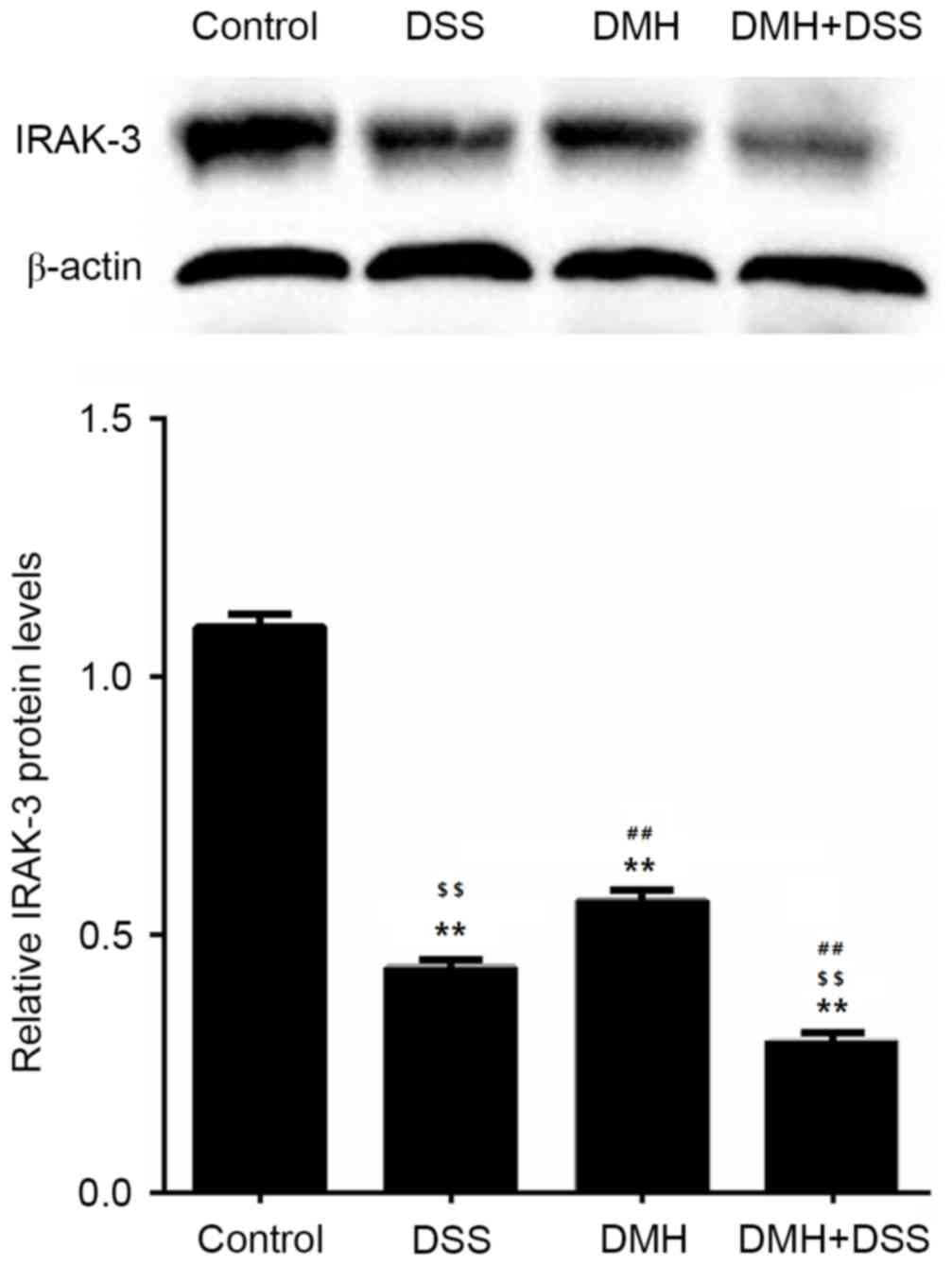
Expression levels of IRAK-3 and gene
methylation in the colon
The gene expression of IRAK-3 in the colon
was significantly lower in mice in the DMH + DSS group with
colitis-associated tumorigenesis (P<0.001; Fig. 3). The results of the western blot
analysis also revealed that the protein levels of IRAK-3 in the
colon were decreased in mice with induced colitis, particularly in
mice with tumorigenesis (Fig. 4),
which was in line with the results of the mRNA levels of IRAK-3.
Methylation of the IRAK-3 gene was observed in the DSS, DMH and DMH
+ DSS groups. However, the methylation levels were higher in the
mice in the DMH + DSS group, compared with those in the other two
groups (Fig. 5).
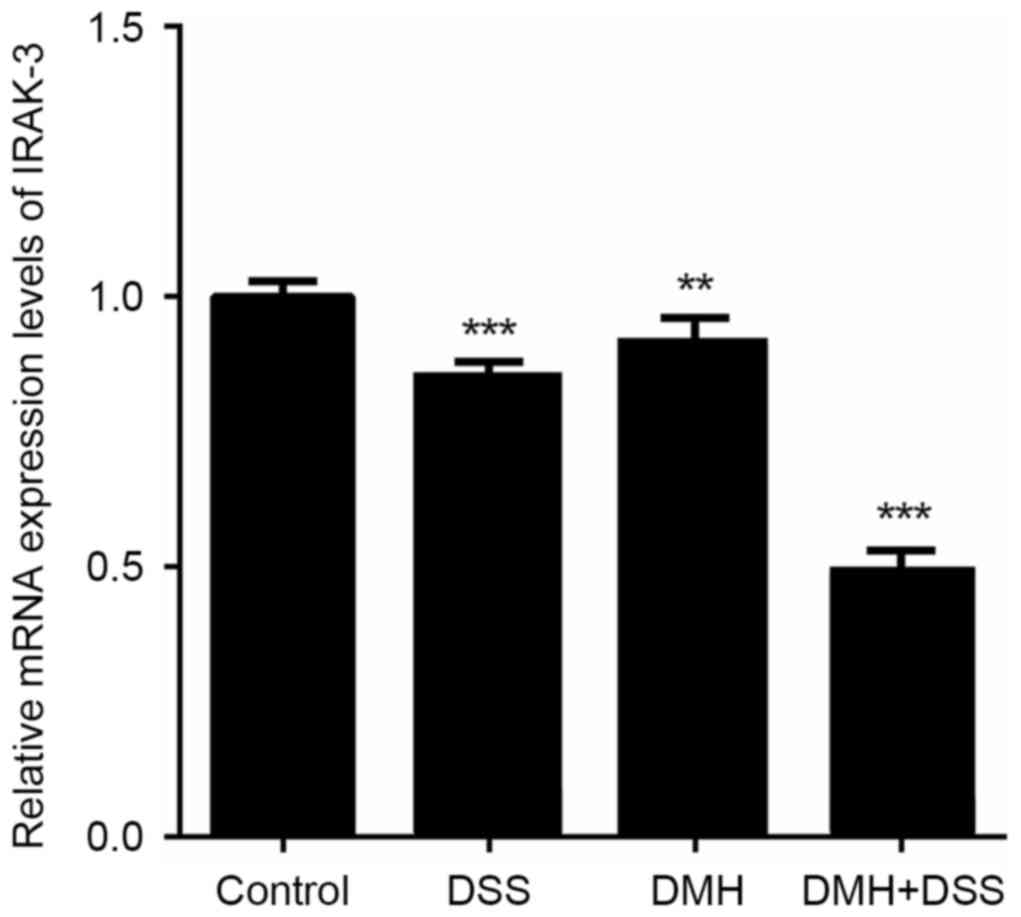 | Figure 3.Reverse transcription-quantitative
polymerase chain reaction analysis of IRAK-3. mRNA expression of
IRAK-3 was significantly lower in the DMH + DSS group, compared
with that in the other groups. **P<0.01 and ***P<0.001, vs.
control group. Control, injected with physiological saline; DSS,
provided with tap water containing DSS; DMH, injected with DMH; DMH
+ DSS, injected with 15 mg/Kg DMH and tap water containing DSS.
IRAK-3, interleukin-1 receptor-associated kinase-3; DSS, dextran
sodium sulfate; DMH, 1,2-dimethyl hydrazine. |
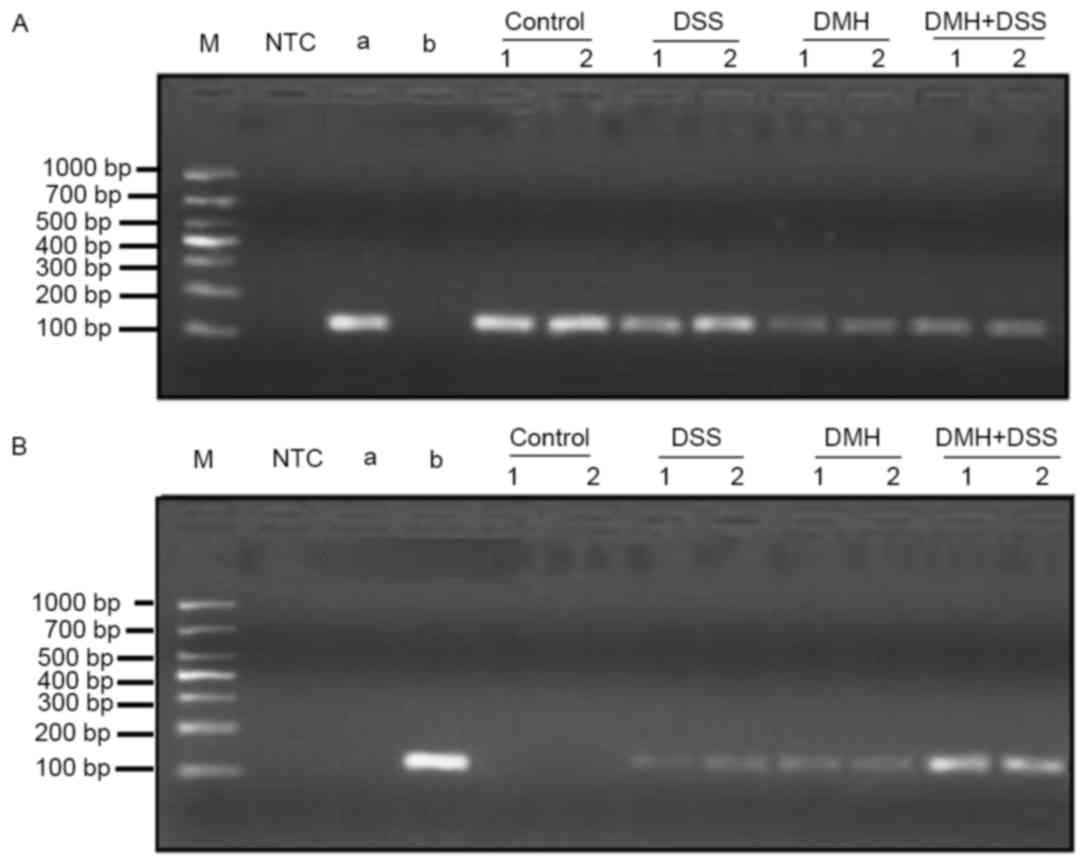 | Figure 5.Gene methylation of IRAK-3. Gene
methylation of IRAK-3 at week 20 was determined using
methylation-specific polymerase chain reaction analysis. (A)
Determination of gene expression levels of IRAK-3 in all the groups
prior to treatment (unmethylated). (B) Determination of IRAK-3 gene
methylation levels at week 20. Samples were examined in duplicate.
NTC, no template control; a, negative reference material; b,
methylation reference material; control, injected with
physiological saline; DSS, provided with DSS dissolved in tap water
for drinking; DMH, injected with DMH; DMH + DSS: mice were injected
with 15 mg/Kg DMH and provided with DSS dissolved in tap water for
drinking. IRAK03, interleukin-1 receptor-associated kinase-3; DSS,
dextran sodium sulfate; DMH, 1,2-dimethyl hydrazine. |
Discussion
Patients with UC are at an increased risk of
colorectal cancer (4). TLR and
innate immune processes are involved in the pathogenesis of
UC-associated tumorigenesis. As the role of IRAK-3 as a negative
regulator in TLR pathways is well known, the present study
investigated the importance of IRAK-3 in a chemically-induced model
of colitis-associated tumorigenesis in mice. Colonic epithelial
cells and microbes are important components of the gut barrier.
Damage to colonic epithelial cells can induce inflammation and, if
the inflammation is aggravated, it increases the risk of cancer
(11). DMH and DSS are widely used
to induce colitis in murine models (13,14).
Therefore, these two agents were used in the present study to
establish a murine UC model.
The mice in the present study were administered with
physiological saline, DMH, DSS, or DMH + DSS, and the expression
levels of IRAK-3 were analyzed. IRAK-3 was expressed in all groups,
and the positive staining for IRAK-3 was highest in the mice
without DMH or DSS (Fig. 2).
IRAK-3 generally acts as a negative regulator of the activation of
NF-κB, IRAK-4/IRAK-1 and IRAK-4/IRAK-2 in TLR and IL-1R signaling,
and favors immunosuppression (6–8,15).
Jain et al (7) demonstrated
that types of cancer with reduced levels of IRAK-3, but elevated
levels of IRAK-1, IRAK-2, and/or IRAK-4, showed increased IRAK-4
signaling and consequently elevated levels of inflammatory
molecules. The absence of IRAK-3 may further sustain IRAK-4
signaling and perpetuate a chronically inflamed tumor environment;
chronic inflammation is a hallmark of tumorigenesis and tumor
progression (16). Several studies
have supported the conclusion that the negative regulation of
IRAK-4 is important in colon cancer resistance (17). The results of the present study
showed similar results in the colitis mice model. It was found that
colitis and dysplasia specimens showed decreased expression of
IRAK-3. However, a previous study showed that IRAK-3 may promote
cancer progression by modulating macrophage activity (7). Evidence from previous studies using
in vivo mouse models showed that the expression level of
IRAK-3 was higher in infiltrating macrophages, and that the
expression levels of IRAK-3 in the tumor cells of patients with
lung cancer were significant and independent predictors of
mortality rates (18–20). These data suggest that the role of
IRAK-3 remains to be fully elucidated. It may be a regulator
between tumor cells and macrophages, which may prevent or promote
tumorigenesis, particularly in UC. Further investigations are
warranted to examine the role of IRAK-3, and its methylated form,
in UC and colon cancer.
During the course of chronic inflammation, various
genes undergo methylation, including, E-cadherin (21) and hyperplastic polyposis protein 1
(HPP1) (22). CpG island
hypermethylation has also been reported to occur in relation to
tumorigenesis or aging. A study on nonneoplastic gastric mucosa
reported that chronic inflammation is closely associated with
increased methylation (23,24).
In a previous study, the methylation of E-cadherin was found in 93%
of dysplasia specimens; HPP1 was considered to be associated with
the adenoma (polyp) cancer path, and methylation was found in 50%
of colitis-associated cancer cases and 40% of dysplasia cases. The
degree of methylation is connected with the level of inflammation
(25). Ullman and Itzkowitz
(11) suggested that DNA
methylation occurs prior to dysplasia. These findings indicate that
methylation may be a potential target for clinical intervention.
The present study further investigated whether IRAK-3 is
methylated, and determined the significance of this change. The
results showed that IRAK-3 was methylated in the cancer
chemically-induced colitis groups, supporting the hypothesis that
the expression and methylation of IRAK-3 may be involved in UC and
subsequent tumorigenesis. However, to examine the effect of IRAK-3
on tumorigenesis, experiments involving the knock down of the
IRAK-3 gene or siRNA are required.
The present study had a number of limitations. T
cells and B cells are not required for the development of
chemically-induced colitis; therefore, the mouse models may not be
an accurate reflection of human colitis. In addition, intestinal
bacteria are important in the development of colitis. Further
investigations should take these factors into account when
establishing animal models of colitis.
In conclusion, the present study demonstrated the
expression of IRAK-3 and gene methylation of IRAK-3 in the colons
of mice with chemically-induced UC. The results revealed that DSS
and DMH induced the downregulation of IRAK-3 and methylation of a
region of IRAK-3, increased inflammation in the colon, and
increased the risk of tumorigenesis. Together, these findings
suggested that IRAK-3 gene methylation may be a predictive factor
in the transition from colitis to cancer, and IRAK-3 may be a
potential therapeutic target for colitis and tumorigenesis.
Acknowledgements
The present study was supported by the Science and
Technology Project of Zhejiang Province, China (grant no.
2012C37105) and the Natural Science Foundation of Zhejiang
Province, China (grant no. LY15H160040).
References
|
1
|
Danese S and Fiocchi C: Ulcerative
colitis. N Engl J Med. 365:1713–1725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Low D, Nguyen DD and Mizoguchi E: Animal
models of ulcerative colitis and their application in drug
research. Drug Des Devel Ther. 7:1341–1357. 2013.PubMed/NCBI
|
|
3
|
Mombaerts P, Mizoguchi E, Grusby MJ,
Glimcher LH, Bhan AK and Tonegawa S: Spontaneous development of
inflammatory bowel disease in T cell receptor mutant mice. Cell.
75:274–282. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: A meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukata M, Shang L, Santaolalla R,
Sotolongo J, Pastorini C, España C, Ungaro R, Harpaz N, Cooper HS,
Elson G, et al: Constitutive activation of epithelial TLR4 augments
inflammatory responses to mucosal injury and drives
colitis-associated tumorigenesis. Inflamm Bowel Dis. 17:1464–1473.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biswas A, Wilmanski J, Forsman H, Hrncir
T, Hao L, Tlaskalova-Hogenova H and Kobayashi KS: Negative
regulation of Toll-like receptor signaling plays an essential role
in homeostasis of the intestine. Eur J Immunol. 41:182–194. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jain A, Kaczanowska S and Davila E: IL-1
receptor-associated kinase signaling and its role in inflammation,
cancer progression, and therapy resistance. Front Immunol.
5:5532014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berglund M, Melgar S, Kobayashi KS,
Flavell RA, Hörnquist EH and Hultgren OH: IL-1 receptor-associated
kinase M downregulates DSS-induced colitis. Inflamm Bowel Dis.
16:1778–1786. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou H, Yu M, Fukuda K, Im J, Yao P, Cui
W, Bulek K, Zepp J, Wan Y, Kim TW, et al: IRAK-M mediates Toll-like
receptor/IL-1R-induced NFκB activation and cytokine production.
EMBO J. 32:583–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi K, Hernandez LD, Galán JE,
Janeway CA Jr, Medzhitov R and Flavell RA: IRAK-M is a negative
regulator of Toll-like receptor signaling. Cell. 110:191–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka T, Kohno H, Suzuki R, Yamada Y,
Sugie S and Mori H: A novel inflammation-related mouse colon
carcinogenesis model induced by azoxymethane and dextran sodium
sulfate. Cancer Sci. 94:965–973. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Saxena A, Li N, Sun J, Gupta A,
Lee DW, Tian Q, Dobaczewski M and Frangogiannis NG: Endogenous
IRAK-M attenuates postinfarction remodeling through effects on
macrophages and fibroblasts. Arterioscler Thromb Vasc Biol.
32:2598–2608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klimesova K, Kverka M, Zakostelska Z,
Hudcovic T, Hrncir T, Stepankova R, Rossmann P, Ridl J, Kostovcik
M, Mrazek J, et al: Altered gut microbiota promotes
colitis-associated cancer in IL-1 receptor-associated kinase
M-deficient mice. Inflamm Bowel Dis. 19:1266–1277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
del Fresno C, Otero K, Gómez-García L,
González-León MC, Soler-Ranger L, Fuentes-Prior P, Escoll P, Baos
R, Caveda L, García F, et al: Tumor cells deactivate human
monocytes by up-regulating IL-1 receptor associated kinase-M
expression via CD44 and TLR4. J Immunol. 174:3032–3040. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soares-Schanoski A, Jurado T, Córdoba R,
Siliceo M, Fresno CD, Gómez-Piña V, Toledano V, Vallejo-Cremades
MT, Alfonso-Iñiguez S, Carballo-Palos A, et al: Impaired antigen
presentation and potent phagocytic activity identifying
tumor-tolerant human monocytes. Biochem Biophys Res Commun.
423:331–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Standiford TJ, Kuick R, Bhan U, Chen J,
Newstead M and Keshamouni VG: TGF-β-induced IRAK-M expression in
tumor-associated macrophages regulates lung tumor growth. Oncogene.
30:2475–2484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wheeler JM, Kim HC, Efstathiou JA, Ilyas
M, Mortensen NJ and Bodmer WF: Hypermethylation of the promoter
region of the E-cadherin gene (CDH1) in sporadic and ulcerative
colitis associated colorectal cancer. Gut. 48:367–371. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato F, Shibata D, Harpaz N, Xu Y, Yin J,
Mori Y, Wang S, Olaru A, Deacu E, Selaru FM, et al: Aberrant
methylation of the HPP1 gene in ulcerative colitis-associated
colorectal carcinoma. Cancer Res. 62:6820–6822. 2002.PubMed/NCBI
|
|
23
|
Kang GH, Lee HJ, Hwang KS, Lee S, Kim JH
and Kim JS: Aberrant CpG island hypermethylation of chronic
gastritis, in relation to aging, gender, intestinal metaplasia, and
chronic inflammation. Am J Pathol. 163:1551–1556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang TJ, Kim DI, Shin YM, Chang HK and
Yang CH: p16(INK4a) Promoter hypermethylation of non-tumorous
tissue adjacent to gastric cancer is correlated with glandular
atrophy and chronic inflammation. Int J Cancer. 93:629–634. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito S, Kato J, Hiraoka S, Horii J,
Suzuki H, Higashi R, Kaji E, Kondo Y and Yamamoto K: DNA
methylation of colon mucosa in ulcerative colitis patients:
Correlation with inflammatory status. Inflamm Bowel Dis.
17:1955–1965. 2011. View Article : Google Scholar : PubMed/NCBI
|