Introduction
Chromosomal abnormalities account for ~6% of
infertility in men, and the prevalence increases to 15% among men
with azoospermia (1). Such
structural Y chromosome abnormalities, including Y-autosomal or Y-X
translocations, inversions, rings, isochromosomes, and dicentrics
are frequently associated with male infertility (2). Among these structural abnormalities,
iso(Y) or idic(Y) chromosomes are the most common. Iso(Y) carries
one centromere and duplication of the short or long arm, and the
idic(Y) consists of two identical arms that are positioned as
mirror images to one another, with an axis of symmetry lying
between two centromeres (3). The
two types of chromosome are often unstable during cell
division.
Patients with iso(Y) or idic(Y) may develop mosaic
karyotypes with variable phenotypes, such as spermatogenic failure,
sexual infantilism, hypospadias, ambiguous genitalia, and a normal
male phenotype (4). The variation
of clinical phenotypes depends on the structure of the abnormal Y
chromosome, the breakpoints, and the types of mosaics, which
consist of a 45,X associated with another cell line that contains a
structurally abnormal Y chromosome (5).
As a result, idic(Y) are structurally dependent on
their breakpoints. Recently, Kalantari et al (6) delineated the association between the
idic(Yq) (breakpoint Yq11.2) and male infertility. The primary
cause of their azoospermia has been demonstrated to be idic(Y) with
a breakpoint in the long arm of the Y chromosome, which may lead to
deletion or rearrangement of critical azoospermia factor (AZF)
regions (7). In the present study,
a rare case of a 45,X/46,X,i(Yq)/46,X,idic(Yq) karyotype identified
in an azoospermic man without an AZF microdeletion is presented. In
addition, the complex sex chromosome mosaicism is evaluated, to
attempt to explain the occurrence of the derivative Y chromosome
and propose how these patients may receive genetic counseling.
Case report
Patient
A 36-year-old man was referred to Center for
Reproductive Medicine, First Hospital, Changchun, China due to
primary infertility for 6 years. He was generally well developed
except for short stature and no malformations were observed. The
patient was 162 cm tall and weighed 52 kg. His father was
33-years-old when the patient was born and his mother was
32-years-old. A detailed history could not identify any infertility
risk factors and there were no documented exposures to
chemotherapy, radiation, smoking or excessive alcohol intake. The
family history was uneventful. Physical examination revealed a
normal penis and pubic hair. The left and right testicular volumes
were 8 ml each. Initial clinical assessment included a repeat semen
analysis and hormones confirming azoospermia and normal hormone
levels with the exception of elevated estradiol (Table I). Cytology revealed neither
spermatozoa nor germ cells.
 | Table I.Semen analysis and hormonal level
results. |
Table I.
Semen analysis and hormonal level
results.
| Variable | Result | Normal range |
|---|
| Semen volume | 1.2 ml | 1.5–5.5 ml |
| Sperm count | 0 | >20
million/ml |
| Prolactin | 0.47 nmol/l | 0.18–0.69 nmol/l |
| Follicle stimulating
hormone | 5.8 U/l | 1.5–12.4 U/l |
| Luteinizing
hormone | 7.9 U/l | 1.7–8.6 U/l |
| Estradiol | 159.75 pmol/l | 27.96–155.92
pmol/l |
| Testosterone | 18.5 nmol/l | 9.9–27.8 nmol/l |
The patient's wife presented with a normal
gynecological status, normal hormone levels and a 46,XX karyotype.
His parents did not exhibit any chromosomal abnormalities.
Appropriate voluntary written consent was obtained
from the patient for the study, which was approved by the Chinese
Association of Humanitarianism and Ethics.
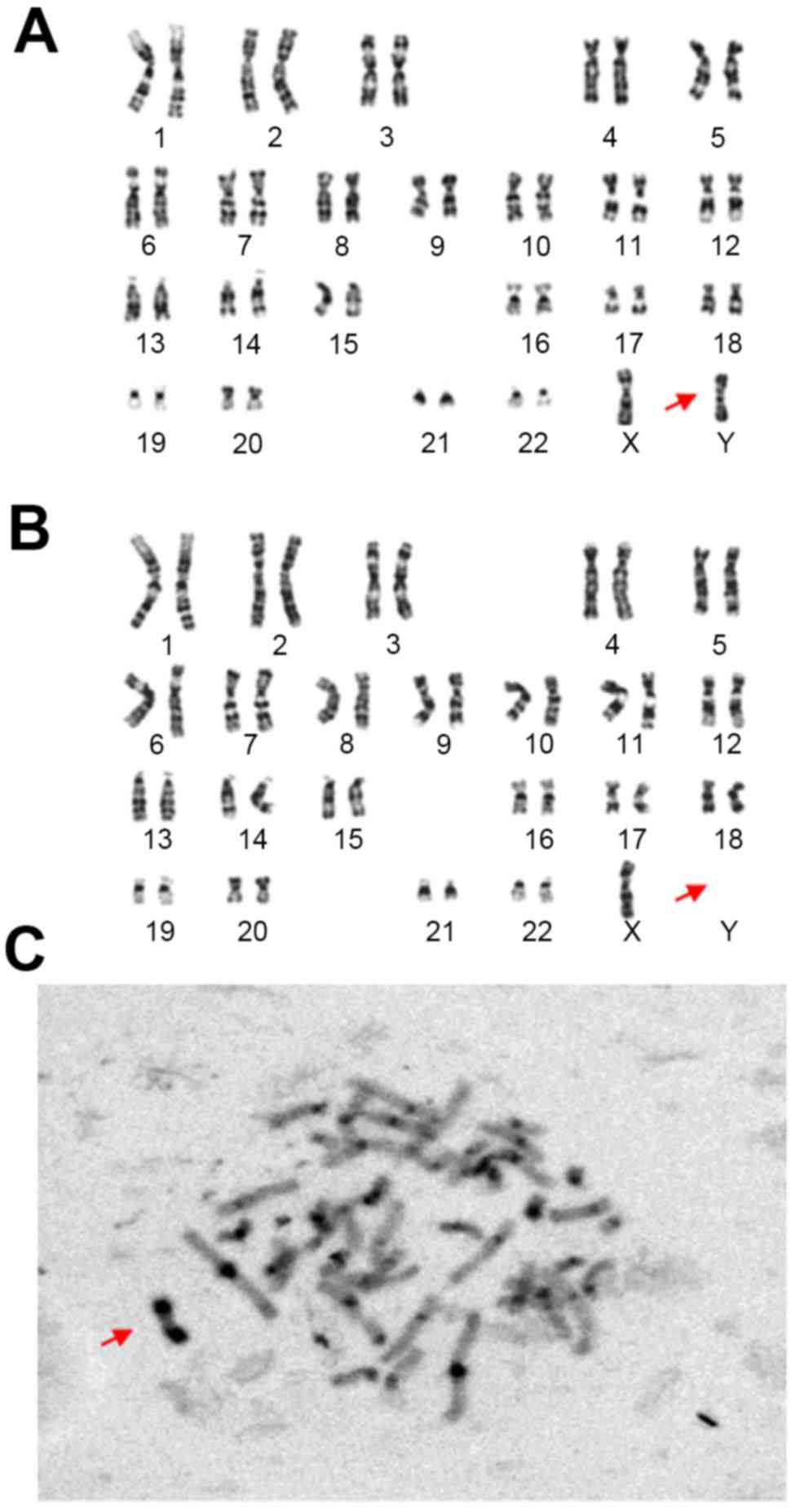
A G-banding karyogram of the proband revealed a
mosaic 45, X/46, X, der(Y), although the exact breakpoint of the Y
chromosome was unclear (Fig. 1A and
B). G-banding demonstrated a 45, X, cell line (13/60 cells) and
a 46, X, der(Y) cell line (47/60 cells). The result of C-banding
suggested der(Y) characterized by the presence of a distal Yq
pseudoautosomal region (Fig.
1C).
Cytogenetic and fluorescent in situ
hybridization (FISH) analyses
Peripheral blood lymphocytes were cultured in
lymphocyte culture medium (Yishengjun; Baidi Biotechnology,
Guangzhou, China) at 37°C for 72 h, followed by 50 µg/ml colchicine
treatment (Yishengjun; Baidi Biotechnology) 1 h before culture
termination to arrest mitoses. The lymphocytes were hypotonically
treated in 0.075 M KCl and fixed in methanol:acetic acid (3:1),
then G-banding and C-banding were performed (8).
FISH was performed using centromeric probes for
chromosomes 18, X and Y (CSP18-Spectrum blue, CSPX-Spectrum green
and CSPY-Spectrum red; Beijing GP Medical Technologies, Beijing,
China). Detailed experimental procedures were performed as
described by Luo et al (9).
FISH was also performed on metaphase chromosome spreads using
dual-color probe combinations for chromosome X and SRY (GLP
SRY Spectrum Red and CEP X Spectrum Green; Beijing Cyto Test
Biotechnology, Beijing, China).
Of the plates, 20% had 2 blue signals, 1 green
signal and no red signal (Fig.
2A); 42% gave 2 blue signals, 1 green signal and 1 red signal
(Fig. 2B) and 38% exhibited 2 blue
signals, 1 green signal and 2 red signals (Fig. 2C). This confirmed that the
uncertain karyotype of der(Y) contained a idic(Yq) and i(Yq). The
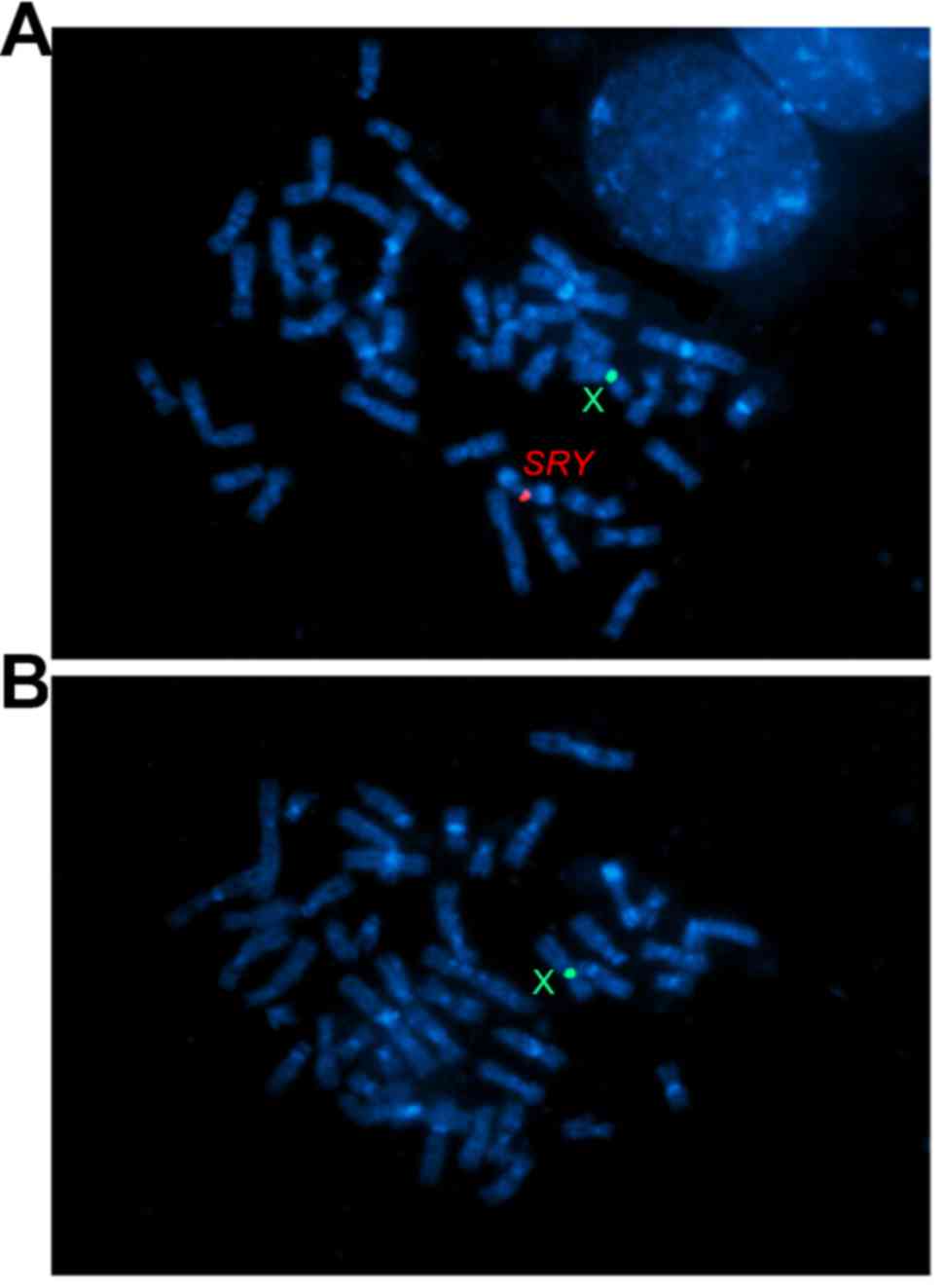
metaphase FISH showed the idic(Yq) with one SRY signal
(Fig. 3A) and the i(Yq) with no
SRY signals (Fig. 3B). The
idic(Yq) was shown to have breakpoints distal to the SRY
locus, likely in the distal Yp pseudoautosomal region. Thus, the
karyotype of the patient was described as 45,X[20]/46,X,
i(Yq)[42]/46,X,idic(Y)(qter→p11.32::p11.32→qter) [38].
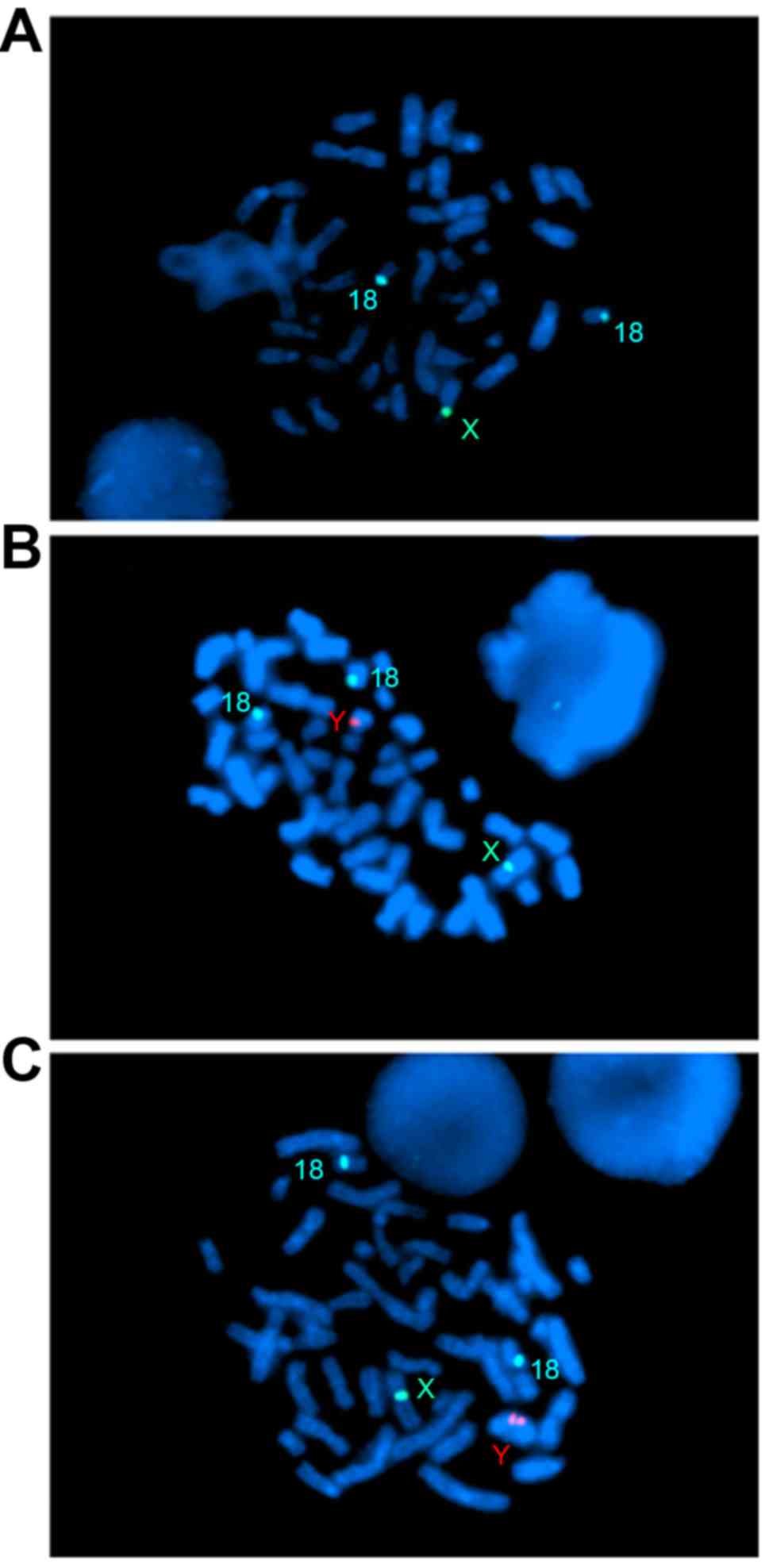 | Figure 2.Fluorescent in situ
hybridization using centromeric probes for chromosomes 18 (blue), X
(green) and Y (red) on metaphase nuclei. (A) Two blue and one green
signal represent the 45, X cell line. (B) Two blue, one green
signal and one red signal represent the 46, X, i(Yq). (C) Two blue,
one green signal and two red signal represent the 46, X, idic(Yq).
Magnification, ×1,000. |
Molecular analysis
To evaluate the sex determining region (SRY)
and AZF zones of the Y chromosome, a set of nine Y
chromosome-specific sequence-tagged sites (STSs) was used (10). The nine STS markers were: sY84 and
sY86 for AZFa; sY127, sY134 and sY143 for AZFb; and sY152, sY157,
sY254, and sY255 for AZFc.
According to the procedure of Al-Achkar et al
(11), mutation screening was
performed using direct DNA sequence analysis. The whole coding
sequence of the SRY gene was amplified by polymerase chain
reaction (PCR) using the primers previously described (12).
No microdeletions were detected in the AZF region of
the Y chromosome in this infertile man. To identify a potential
mutation, the SRY specific PCR fragment was analyzed by DNA
sequencing using a healthy male as a control. No mutation was
exhibited by the patient (data not shown).
Next generation sequencing (NGS)
Whole genome sequencing (WGS) by NGS technology was
performed on an Ion torrent PGM (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) platform according to the standard protocol
(https://ioncommunity.thermofisher.com/community/protocols-home).
Genomic DNA from the peripheral blood of the patient was sheared
into fragments (250–300 bp) using an Ion Shear Plus Reagents kit
(Thermo Fisher Scientific, Inc.). Ion Torrent Barcoded Libraries
were created using an Ion Plus Fragment Library kit (Thermo Fisher
Scientific, Inc.) and an Ion PGM Template OT2 200 kit (Thermo
Fisher Scientific, Inc.) was used for template amplification and
enrichment of the target sequence. Ion Sphere Particles (ISPs) were
recovered and template-positive ISPs were enriched using an Ion
OneTouch ES (Thermo Fisher Scientific, Inc.). Sequencing was
performed using an Ion PGM Sequencing 200 kit v2 (Thermo Fisher
Scientific, Inc.) on a ‘318’ sequencing chip for a total of 500
nucleotide flows, yielding average read lengths of 220–230 bp. The
DNA sample of the patient was pooled and labeled on the ‘318’ chip.
The average whole genomic sequence depth was ~0.02x, and the
average read number was ~500 K. The primary sequencing BAM data
were submitted to the Celloud cloud server (http://www.celloud.org/), which was offered by a
third-party company (JBRH, China), in order to analyze the
chromosomal copy number variants. Data analysis was performed
according to a previous study (13).
No copy number variation of AZF or SRY was
detected through NGS technology (data not shown). These results
demonstrate that WGS could not applied to the detection of certain
mosaic chomosomal abnormalities. For the proportion of mosaicism
cell lines of 45,X/46,X,i(Yq)/46,X,idic(Yq), karyotype analysis and
FISH were recognized as the standard detection methods in the
current case report.
Testicular cytology
A fine-needle aspiration biopsy was performed under
local anesthesia in the pole of the patient's right testis. The
retrieved samples were washed three times in phosphate-buffered
saline, spread onto glass slides and air-dried. The specimens were
then fixed in 95% alcohol and stained with hematoxylin and eosin.
The cells were examined under high magnification using a 40x light
microscope and the spermatogenic status was classified according to
the Meng system (14).
Cytological analysis of a testicular biopsy specimen
demonstrated complete maturation arrest. Neither sperm nor
spermatids were detected. Sperm maturation had stopped in the early
stages of spermatogenesis (data not shown).
Discussion
Increasing cytogenetic and molecular studies in
recent years have demonstrated the association of Y chromosome
abnormalities with male infertility. Correct genetic diagnosis is
essential to ensure proper counseling and avoid unnecessary and
expensive treatments to improve fertility. There are important
ethical consequences regarding patients who are candidates for
assisted reproduction techniques, such as in vitro
fertilization/intra-cytoplasmic sperm injection and preimplantation
genetic diagnosis (15). The
patient, an otherwise healthy 36-year-old man, was referred for
cytogenetic studies due to absolute azoospermia. The results
revealed a mosaic karyotype, 45,X/46,X,i(Yq)/46,X,idic(Yq). A
unique feature of the present case is that three cell lines which
included 45,X, iso(Y) and idic(Y) were identified in the peripheral
blood. The breakpoint occurred at Yp11.32, very close to the
telomere in the idic(Yq) chromosome. The idic(Yq) contains a
duplication of the proximal short arm and entire long arm (q) with
duplicated preservation of AZF regions.
As previously demonstrated (16), the phenotype of patients exhibiting
the idic Y chromosome were highly variable, ranging from
Turner-like females to infertile males, depending on the structure
of the dicentric Y chromosome, the Yp and Yq breakpoints, and the
types of mosaicism. Of the affected subjects, 40.9% were
phenotypical females, 31.8% were phenotypical males and 27.3% had
different degrees of intersexuality. Among aberrant Y chromosomes,
mosaicism of sex chromosomes, which consisted of a 45,X associated
with another cell line, occurs most frequently and represents one
of the main causes of ambiguous genitalia (17). Generally, mosaicism may occur
during meiosis for abnormal X-Y bivalents or as a result of a
postzygotic mitotic error due to structural instability of the Y
chromosome during cell division (18). In the current case, it exerted a
limited effect on the patient's phenotype due to the small
percentage of the 45,X cell line.
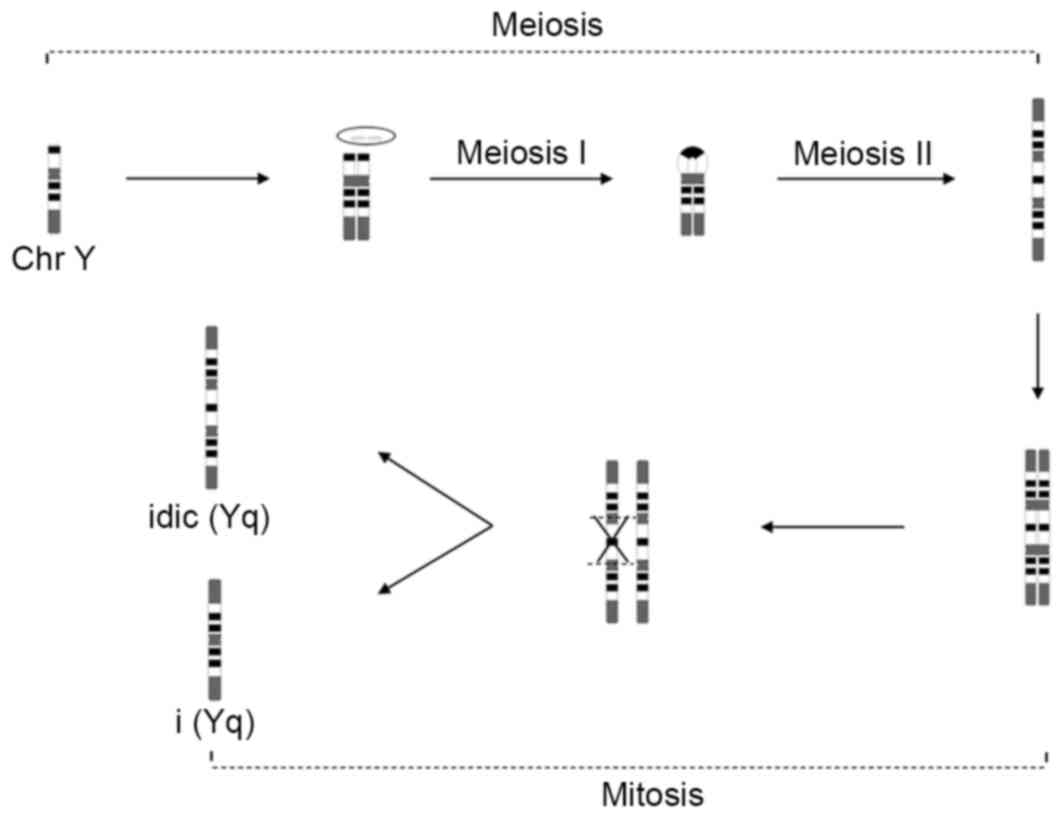
Recently, the mechanisms of iso/idic Y chromosomes
have been proposed to involve activation of intra- and
interchromatid crossover and noncrossover pathways by massive
palindrome-borne mirror-image gene pairs (19). This indicates that the idic(Y)
chromosome resulted from an error during gametogenesis before the
spermatid stage, as two chromatids are required to generate these
rearrangements, or arose from an error in the first zygotic
division. Errors occurring after the first zygotic division would
result in mosaicism. Thus, the present study proposed that the
abnormal idic(Yq) chromosome arose in paternal meiosis I (U type
exchange) and II (nondisjunction), and the i(Yq) chromosome arose
post-zygotically (Fig. 4).
Cases of adult infertile males with idic(Yq) that
have been reported in previous studies are presented in Table II (2,3,20–22).
These patients underwent cytogenetic study due to absolutely male
infertility, a majority of them with azoospermia. Patients with
idic(Yq) and male infertility were selected for this literature
review. A possible reason for spermatogenic failure is the unstable
iso/idic Y chromosome, caused by an inactivated centromere, which
interferes with X-Y bivalent formation and chromosomal separation
during mitosis and leads to a breakdown in spermatogenesis
(23). The other possible reason
is the presence of extra copies of AZF regions in our patient,
which may be a contributing factor for spermatogenic failure,
irrespective of AZF deletions. Kalantari et al (6) indicated that 45,X cells cannot
commence meiosis and induce sperm production. However, without a
high percentage of 45,X, mosaicism of three cell lines may be the
third cause for infertility (24).
 | Table II.Review of adult infertile male with
isodicentric Yq (breakpoint in Yp). |
Table II.
Review of adult infertile male with
isodicentric Yq (breakpoint in Yp).
| Author, date | Karyotype | Age, years | Semen analysis | Height cm | L/R testis volume,
ml | Azoospermia factor
microdeletion | SRY | FSH | LH | T | Testicular
histology | (Refs.) |
|---|
| Present study |
45,X[20]/46,X,i(Yq)[42]/46, X,idic(Y)
(qter→p11.32::p11. 32→qter) [38] | 36 | Azoospermia | 162 | 8/8 | No deletion | + | N | N | N | Maturation arrest of
the primary spermatocyte | n/a |
| Geng et al,
2014 | 45,X[32]/46, X,
der(Y) t(Y; Y)(p11; ?) [18] | 30 | Severe
oligospermia | 151 | 8/8 | No deletion | + | N | N | N | NP | (20) |
| Lehmann et al,
2012 | Mos
45,X[9]/46,X,mar.ish mar(DYZ3+)[3]/46,X,idic(Y) (p11.3).ish
idic(Y)(p11.3) (DYZ3++, SRY++)[33]/47, XY + idic(Y)(p11.3)
.ish idic(Y)(p11.3)(DYZ3++, SRY++)[2]/48, X, + idic
(Y)(p11.3)x2.ish idic(Y) (p11.3)(DYZ3++, SRY++)
[2]/46,XY[1] | 28 | Azoospermia | NP | Small/small | No deletion | + | N | N | N | Maturation arrest
of the primary spermatocyte | (3) |
| Codina-Pascual
et al, 2004 | 46,XY/46,X,idic(Y)
(qter→p11.32::p11.32→qter) | 37 | Azoospermia | NP | 5/5 | NP | + | ↑ | NP | NP | NP | (2) |
| Haaf, et al,
1990 | 45,X/46,X,idic(Yq)
(Yqter→cen→Yp 11.3:: Yp 11.3→cen→Yqter.) | 26 | Azoospermia | NP | Small/small | NP | NP | ↑ | ↑ | N | NP | (21) |
| Micic et al,
1990 (Case 1) |
45,X[33]/46,X,idic(Yq)[17] | 34 | Azoospermia | 165 | Normal/normal | NP | NP | ↑ | ↑ | N | Maturation arrest
of the primary spermatocyte | (22) |
Notably, all the patients were of short stature with
an mean height of 162 cm, which is significantly lower than the
mean height of men (25). The
presence of a 45,X cell line, deletion of the putative growth
controlling gene locus and short stature homeobox-containing gene,
which have been mapped to the pseudoautosomal regions of Xp and Yp,
or a combined effect may result in a short stature (26,27).
In study report by Guevarra et al (28), a 17-year-old with 45,X/46,X,idic(Y)
karyotype was treated with recombinant growth hormone and has
achieved a near final adult height, emphasizing that further
investigation that includes the karyotype is required in male
patients with unexplained short stature.
Molecular-based approaches to investigate mosaicism
are difficult, particularly when three cell lines exist: One cell
line does not contain Y, one cell line contains a normal Y and one
cell line contains an idic Y (29). FISH-based technology is considered
to be more useful in evaluating mosaic genotypes. In the current
case report, no copy number variation of AZF or SRY was
detected using NGS technology. These results demonstrate that WGS
could not be applied to the detection of certain mosaic chomosomal
abnormalities. For the proportion of mosaicism in cell lines
45,X/46,X,i(Yq)/46,X,idic(Yq), karyotype analysis and FISH were
recognized as the standard detection methods in the present case
report.
In conclusion, to the best of our knowledge, this is
the first attempt to detail the clinical characterization and
molecular-cytogenetic studies of patients with complex mosaic
karyotype 45,X/46,X,i(Yq)/46,X,idic(Yq). The major symptoms of the
infertile man include small testes, azoospermia, elevated estradiol
levels and short status. Azoospermia had not been caused by AZF
microdeletions, although there may be other reasons, such as the
abnormal structure of the Y chromosome and mosaicism of the three
cell lines, 45,X/46,X,i(Yq)/46,X,idic(Yq). The results of the
current study highlight that routine karyotype analysis and
FISH-based technology are more useful in detecting mosaic
chromosomal abnormality, predicting the clinical features of the
patient during genetic counseling and planning artificial
reproductive technologies.
Acknowledgements
The authors would like to thank all the staff of the
Genetics Laboratory and Andrology Laboratory, Center for
Reproductive Medicine, First Hospital, Changchun, China for their
excellent work. The present study was supported by the National
Natural Science Fund (grant no. 81471515).
References
|
1
|
Song SH, Chiba K, Ramasamy R and Lamb DJ:
Recent advances in the genetics of testicular failure. Asian J
Androl. 18:350–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Codina-Pascual M, Oliver-Bonet M, Navarro
J, Starke H, Liehr T, Gutiérrez-Mateo C, Sánchez-García JF, Arango
O, Egozcue J and Benet J: FISH characterization of a dicentric Yq
(p11.32) isochromosome in an azoospermic male. Am J Med Genet.
127A:302–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehmann KJ, Kovac JR, Xu J and Fischer MA:
Isodicentric Yq mosaicism presenting as infertility and maturation
arrest without altered SRY and AZF regions. J Assist Reprod Genet.
29:939–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee BY, Kim SY, Park JY, Choi EY, Kim DJ,
Kim JW, Ryu HM, Cho YH, Park SY and Seo JT: Unusual maternal
uniparental isodisomic × chromosome mosaicism with asymmetric y
chromosomal rearrangement. Cytogenet Genome Res. 142:79–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuck-Muller CM, Chen H, Martínez JE, Shen
CC, Li S, Kusyk C, Kusyk C, Batista DA, Bhatnagar YM, Dowling E and
Wertelecki W: Isodicentric Y chromosome: Cytogenetic, molecular and
clinical studies and review of the literature. Hum Genet.
96:119–129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalantari H, Asia S, Totonchi M,
Vazirinasab H, Mansouri Z, Moradi S Zarei, Haratian K, Gourabi H
and Meybodi A Mohseni: Delineating the association between
isodicentric chromosome Y and infertility: A retrospective study.
Fertil Steril. 101:1091–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogt PH, Edelmann A, Hirschmann P and
Köhler MR: The azoospermia factor (AZF) of the human Y chromosome
in Yq11: Function and analysis in spermatogenesis. Reprod Fertil
Dev. 7:685–693. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong Y, Du RC, Jiang YT, Wu J, Li LL and
Liu RZ: Impact of chromosomal translocations on male infertility,
semen quality, testicular volume and reproductive hormone levels. J
Int Med Res. 40:2274–2283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo B, Li W, Deng CH, Zheng FF, Sun XZ,
Wang DH and Dai YP: Utility of fluorescence in situ hybridization
in the diagnosis of upper urinary tract urothelial carcinoma.
Cancer Genet Cytogenet. 189:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Totonchi M, Meybodi A Mohseni, Boroujeni P
Borjian, Gilani M Sedighi, Almadani N and Gourabi H: Clinical data
for 185 infertile Iranian men with Y-chromosome microdeletion. J
Assist Reprod Genet. 29:847–853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Achkar W, Moassass F, Al-Halabi B and
Al-Ablog A: Mutations of the Connexin 26 gene in families with
non-syndromic hearing loss. Mol Med Rep. 4:331–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Wang XR, Liu MG, Wang Q and Liu
JY: Genetic analysis of a family with 46,XY ‘female’ associated
with infertility. Yi Chuan Xue Bao. 33:19–25. 2006.PubMed/NCBI
|
|
13
|
Hou Y, Fan W, Yan L, Li R, Lian Y, Huang
J, Li J, Xu L, Tang F, Xie XS and Qiao J: Genome analyses of single
human oocytes. Cell. 155:1492–1506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng MV, Cha I, Ljung BM and Turek PJ:
Relationship between classic histological pattern and sperm
findings on fine needle aspiration map in infertile men. Hum
Reprod. 15:1973–1977. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foresta C, Moro E and Ferlin A: Y
chromosome microdeletions and alterations of spermatogenesis.
Endocr Rev. 22:226–239. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu LF: Phenotype/karyotype correlations
of Y chromosome aneuploidy with emphasis on structural aberrations
in postnatally diagnosed cases. Am J Med Genet. 53:108–140. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oliveira RM, Verreschi IT, Lipay MV, Eça
LP, Guedes AD and Bianco B: Y chromosome in Turner syndrome: Review
of the literature. Sao Paulo Med J. 127:373–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonelli A, Marcucci L, Elli R, Tanzi N,
Paoli D, Radicioni A, Lombardo F, Lenzi A and Gandini L: Semen
quality in men with Y chromosome aberrations. Int J Androl.
34:453–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lange J, Skaletsky H, van Daalen SK, Embry
SL, Korver CM, Brown LG, Oates RD, Silber S, Repping S and Page DC:
Isodicentric Y chromosomes and sex disorders as byproducts of
homologous recombination that maintains palindromes. Cell.
138:855–869. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geng Q, Luo FW, Wu WQ, Xu ZY, Wang L, Wang
Q and Xie JS: Cytogenetic and molecular analysis of idic(Yp) in 1
infertile man and 1 prenatal fetus. Zhonghua Nan Ke Xue.
19:642–646. 2013.(In Chinese). PubMed/NCBI
|
|
21
|
Haaf T and Schmid M: Y isochromosome
associated with a mosaic karyotype and inactivation of the
centromere. Hum Genet. 85:486–490. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mićić M, Mićić S, Babić M and Diklić V:
Phenotype of two males with abnormal Y chromosomes. Clin Genet.
37:321–326. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Odorisio T, Rodriguez TA, Evans EP, Clarke
AR and Burgoyne PS: The meiotic checkpoint monitoring synapsis
eliminates spermatocytes via p53-independent apoptosis. Nat Genet.
18:257–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Layman LC, Tho SP, Clark AD, Kulharya A
and McDonough PG: Phenotypic spectrum of 45,X/46,XY males with a
ring Y chromosome and bilaterally descended testes. Fertil Steril.
91:791–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang XG, Li YP, Ma GS, Hu XQ, Wang JZ, Cui
ZH, Wang ZH, Yu WT, Yang ZX and Zhai FY: Study on weight and height
of the Chinese people and the differences between 1992 and 2002.
Zhonghua Liu Xing Bing Xue Za Zhi. 26:489–493. 2005.(In Chinese).
PubMed/NCBI
|
|
26
|
Lin YH, Lin YM, Lin YH, Chuang L, Wu SY
and Kou PL: Ring (Y) in two azoospermic men. Am J Med Genet A.
128A:209–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao E, Weiss B, Fukami M, Rump A, Niesler
B, Mertz A, Muroya K, Binder G, Kirsch S, Winkelmann M, et al:
Pseudoautosomal deletions encompassing a novel homeobox gene cause
growth failure in idiopathic short stature and Turner syndrome. Nat
Genet. 16:54–63. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guevarra FM, Nimkarn S, New MI and Lin-Su
K: Long-term growth hormone therapy in an adolescent boy with
45,X/46,XidicY(p11). J Pediatr. 155:752–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu HH, Lee TH, Chen CD, Yeh KT and Chen M:
Delineation of an isodicentric Y chromosome in a mosiac
45,X/46,X,idic(Y)(qter-p11.3::p11.3-qter) fetus bySRY sequencing,
G-banding, FISH, SKY and study of distribution in different
tissues. J Formos Med Assoc. 106:403–410. 2007. View Article : Google Scholar : PubMed/NCBI
|