Introduction
Tumor necrosis factor-alpha (TNF-α) is a cytokine
with complicated bioactivity. It is also a therapeutic target of
autoantigens (1,2). In addition to killing tumor cells
in vitro and in vivo, TNF-α may also induce
inflammation, defend viral infection, regulate immunity, and
promote cell proliferation and activation (3–5).
When TNF-α is overexpressed, it leads to various diseases, such as
inflammatory bowel disease (3–5).
These damages may be mitigated after neutralizing excess TNF-α
using exogenous antibodies or soluble receptors. A monoclonal
antibody against TNF-α, infliximab, is the most commonly used
exogenous antibody that has been approved for the treatment of
Crohn's disease. Clinical trials demonstrated that infliximab also
has a positive role in the treatment of ulcerative colitis (UC)
(6,7). However, using synthetic infliximab is
a passive immunotherapy associated with many inherent defects, such
as short duration and easy-to-induce hypersensitivity. Therefore,
it would be a major breakthrough for the treatment of UC if the
vaccine induced natural anti-TNF-α antibodies in vivo via
active immunization.
The biological characteristics of TNF-α have no
significant species specificity. The amino acids of murine TNF-α
(mTNF-α) are highly homologous to those of human TNF-α with a
similar tertiary structure (8).
Its precursor has 235 amino acid residues containing a signal
peptide with 79 amino acid residues. Based on the primary structure
of TNF-α, bioinformatics was used to predict mTNF-α B-cell
epitopes. Multiple antigenic polypeptides (MAPs) were synthesized
using an eight-branch design (9).
Interleukin-1β (IL-1β) peptide 163–171, as a promiscuous helper
epitope peptide (T-helper epitope), enhances immunity and humoral
immune response of other antigen peptides (10). With combined immunization of
animals, it induced the production of polyclonal antibodies
(9,10). A potential B-cell epitope peptide
was obtained by indirect enzyme-linked immunosorbent assay (ELISA),
western blotting and immunohistochemistry identification of the
polyclonal antibody titer. The aim of the present study was to
provide an initial experimental basis for active immunotherapy
research for mTNF-α targeting UC in mice.
Materials and methods
Materials
The mTNF-α sequence was obtained from GenBank
(https://www.ncbi.nlm.nih.gov/genbank/) as follows:
MST ESM IRD VEL AEE ALP QKM GGF QNS RRC LCL SLFS FLL VAG ATT LFC
LLN FGV IGP QRD EKF PNG LPL ISS MAQ TLT LRS SSQ NSS DKP VAHV VAN
HQV EEQ LEW LSQ RAN ALL ANG MDL KDN QLV VPA DGL YLV YSQ VLF KGQ GCP
DYV LLT HTV SRF AIS YQE KVN LLS AVK SPC PKD TPE GAE LKP WYE PIY LGG
VFQ LEK GDQ LSA EVN LPK YLD FAE SGQ VYF GVI AL.
The following reagents and antibodies were
purchased: Commercial recombinant mTNF-α protein (eBioscience;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), commercial
polyclonal rabbit anti-mouse full-length TNF-α protein antibody
(cat. no. 17590-1-AP). ProteinTech Group, Inc., Chicago, IL, USA),
Freund's complete adjuvant (Sigma-Aldrich; Merck, KGaA, Darmstadt,
Germany), incomplete adjuvant (Sigma-Aldrich; Merck KGaA),
enzyme-labeled goat anti-mouse IgG-horseradish peroxidase II (cat.
no. EK1003). Wuhan Boster Biological Technology, Ltd., Wuhan,
China), wstern blotting chemiluminescence kit (Shanghai Kangcheng
Sheng Biological Engineering Company, Shanghai, China) and protein
marker (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Mouse H22
hepatoma cells were obtained from Huazhong University of Science
and Technology (Wuhan, China). A total of 80 female BALB/c mice
(age, 4–5 weeks; weight, 18–25 g) were purchased from Shanghai
Laboratory Animal Center (Shanghai, China; certificate no.
SCXK20080115). The mice were placed in a plexiglass feeding box and
kept on the clean shelf laminar flow. Each feeder box contained 3–4
mice under the following conditions: Constant temperature, 25–27°C;
humidity, 45–50%; fresh air; high-level dust sterilization; and
specific pathogen-free environment under a 12-h light/dark cycle.
The animals had free access to sterilized water and food. The
animal studies were approved by the ethics committee of Zhejiang
Medical University (approval no. X1002623; Hangzhou, China). Live
animal surgery was performed using 0.3% sodium pentobarbital (30
mg/kg). Upon completion of the experiment, the experimental animals
were sacrificed by cervical dislocation.
B-cell epitope prediction
DNASTAR software version 7.1 (DNASTAR, Inc.,
Madison, WI, USA) and BcePred software (http://www.imtech.res.in/raghava/bcepred/) were used
to analyze 235 amino acid sequences of mTNF-α to evaluate the
physical and chemical properties of the encoded protein, such as
hydrophilicity, accessibility, and plasticity, which was used for
assessing the mTNF-α molecule antigenicity in the highly
hydrophilic region. The two analyses were combined, and the
overlapping result area was selected as the candidate mTNF-α B-cell
epitope.
MAP synthesis
The MAP of the B-cell epitope peptide was
synthesized by the Chinese Peptide Company Ltd. (Hangzhou, China).
Based on the selected amino acid peptide and lysine core matrix, an
eight-branch polypeptide was designed. Synthesis was performed on a
peptide synthesizer using the solid-phase method, where amino acid
peptides were linked with the 8 amino terminals of the 4 lysine
located at the end of the MAP core structure. According to the
amino acid sequence of the peptide, they were sequentially
connected one by one from the carboxyl terminal to the amino
terminal. Synthesized polypeptides purified by high-pressure liquid
chromatography had a purity of >97%.
Binding force between MAP peptide and
whole-protein mTNF-α antibodies
A 96-well ELISA plate was coated with 100 µl of
different MAPs or recombinant TNF-α protein at a concentration of
10 mg/l and maintained overnight at 4°C. After blocking in 1%
bovine serum albumin (BSA), a rabbit anti-mouse full-length TNF-α
antibody (1:4,000; 100 µl/well; cat. no. 17590-1-AP; ProteinTech
Group, Inc.) was added, with normal BALB/c mouse serum (1:4,000)
serving as a control. The results were expressed as the mean of
three experiments. The absorbance of the plates were measured at a
wavelength of 490 nm using an Imark Micoplate Absorbance Reader
13550 (Bio-RAD, Hercules, California, USA).
Material grouping
Eighty male BALB/c mice (age, 3–4 weeks; weight,
18–25 g), were randomly divided into four groups as follows: MAP1
group (amino acids 54–65), MAP2 group (amino acids 78–92), mTNF-α
group, and phosphate-buffered saline (PBS) control group, with 20
mice in each group.
Immunization
The MAP1 and MAP2 groups were treated with
eight-branch MAP immunogen. The mice were injected four times at
2-week intervals. The mice were first immunized with Freund's
complete adjuvant (Sigma-Aldrich; Merck KGaA) and boosted with
incomplete Freund's adjuvant. The quantity of MAP used for the
first immunization and boosting was 0.2 mg per mouse. The mTNF-α
control group was immunized with commercial recombinant mTNF-α
protein at a dose of 20 µg per mice. An equal quantity of T helper
(TH) linear peptide was mixed, dissolved in 0.5 ml PBS, and
emulsified with 0.5 ml Freund's adjuvant. Multi-point injections
were performed intradermally on the backs of the mice. The PBS
control group was also injected in the same way with 0.5 ml PBS
emulsified with an equal volume of Freund's complete adjuvant.
Specimen collection and antibody
detection
Retro-orbital blood (0.5 ml per mouse) was collected
prior to the first immunization and 2 weeks after each
immunization. Blood was collected a total of five times, and the
mice were sacrificed by cervical dislocation. Serum was centrifuged
for 4 min at 1,000 × g at 4°C and serum antibody titers were
measured by standard indirect ELISA. The 96-well ELISA plate was
coated with 10 mg/l MAP1, MAP2, mTNF-α protein, or TH full peptide,
100 µl/well, and maintained overnight at 4°C. After blocking with
1% BSA, diluted test animal antiserum (dilutions ranging from
1:10,000 to 1:150,000) was used as the primary antibody for
measurement, with pre-immune serum serving as a negative control.
The results were presented as an A value (the mean of duplicated
wells) and the experiments were repeated three times. The positive
standard was >2.1, which was determined by the A value of the
measured sample/negative control.
Protein electrophoresis and western
blotting
H22 cells (1×109), which express mTNF-α
stably and efficiently, were centrifugally washed. Pro-cooling
suspended buffer solution (1 ml) was added to the cell pellet for
dissociation. The cells were vortexed for 5 min and centrifuged for
5 min at 10,000 × g at 4°C. The supernatant was discarded, and
total protein was extracted with an equal volume of 2X sodium
dodecyl sulfate. A total of 20 mg protein was analyzed by SDS
polyacrylamide gel electrophoresis, then it was transferred to a
polyvinylidene difluoride membrane at 400 mA for 90 min, and a
commercial mTNF-α protein served as a control and a mouse
pre-immune serum served as a negative control.
Glyceraldehyde-3-phosphate dehydrogenase was selected as an
internal reference. Chemiluminescent western blot detection was
performed according to the manufacturer's instructions. The
antibodies used for western blotting were either commercially
available antibodies against full-length mTNF-α (cat. no.
17590-1-AP; ProteinTech Group, Inc.) (1:2,000) or mouse antiserum
against MAP or mTNF-α (1:3,000 dilution). The specificity of the
protein detected by the antiserum was determined by the molecular
weight of the bands. BandScan version 5.0 software (Glyko Inc.,
Novato, CA, USA) was used to compare the relative gray scale of the
bands.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Quantitative data were presented
as means ± standard deviation. Pluralities of samples were compared
using single factor analysis. The Student-Newman-Keuls post-test
method was used for comparing variances between groups and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Prediction of B-cell epitope and
peptide synthesis
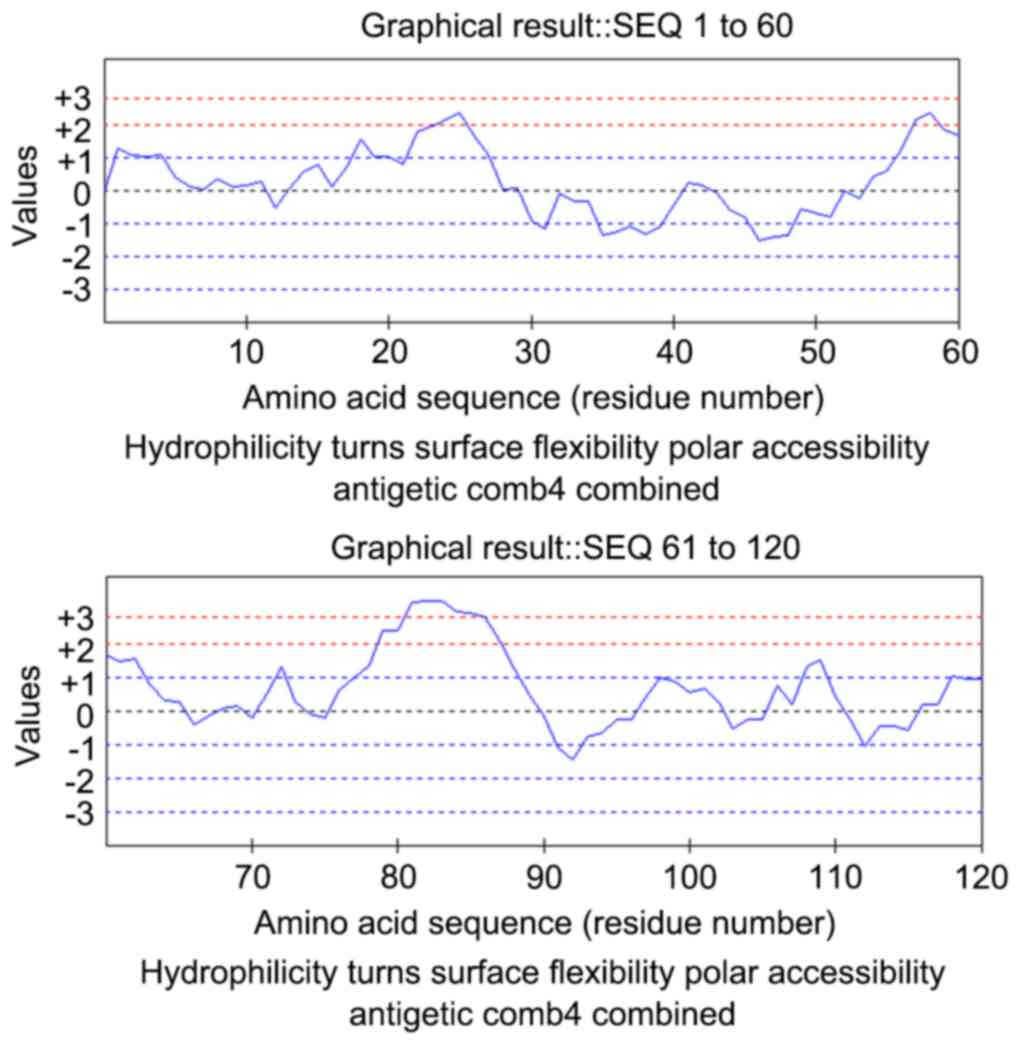
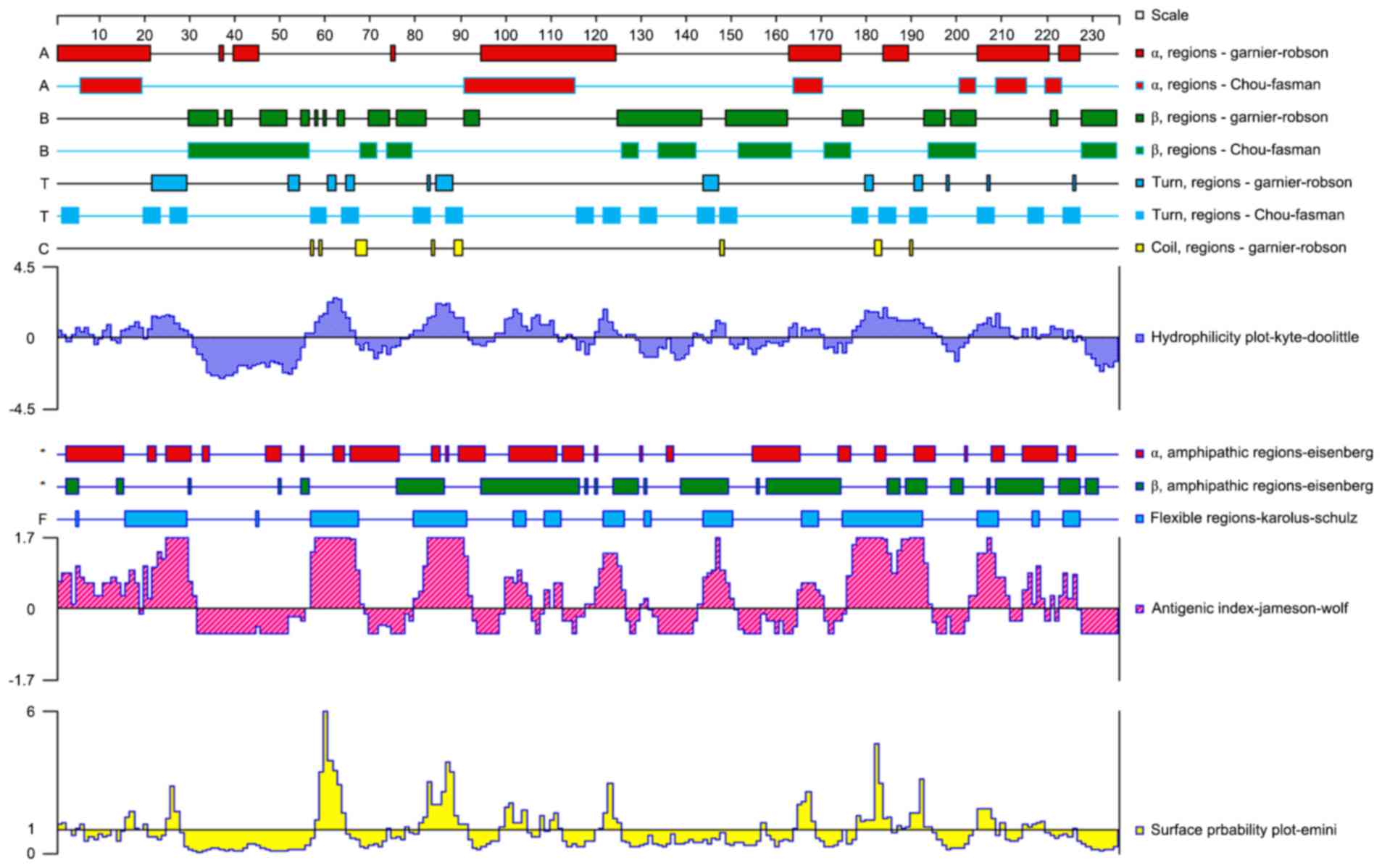
The predictions of B-cell epitopes using BcePred and
DNAStar are presented in Figs. 1
and 2, respectively. The results
of the two methods were almost identical. Amino acids 54–65
(GVIGPQRDEKFP) and 78–92 (LTLRSSSQNSSDKPV) exhibited good
hydrophilicity, accessibility and plasticity, and were located in
protein extended structures or with no coil in the secondary
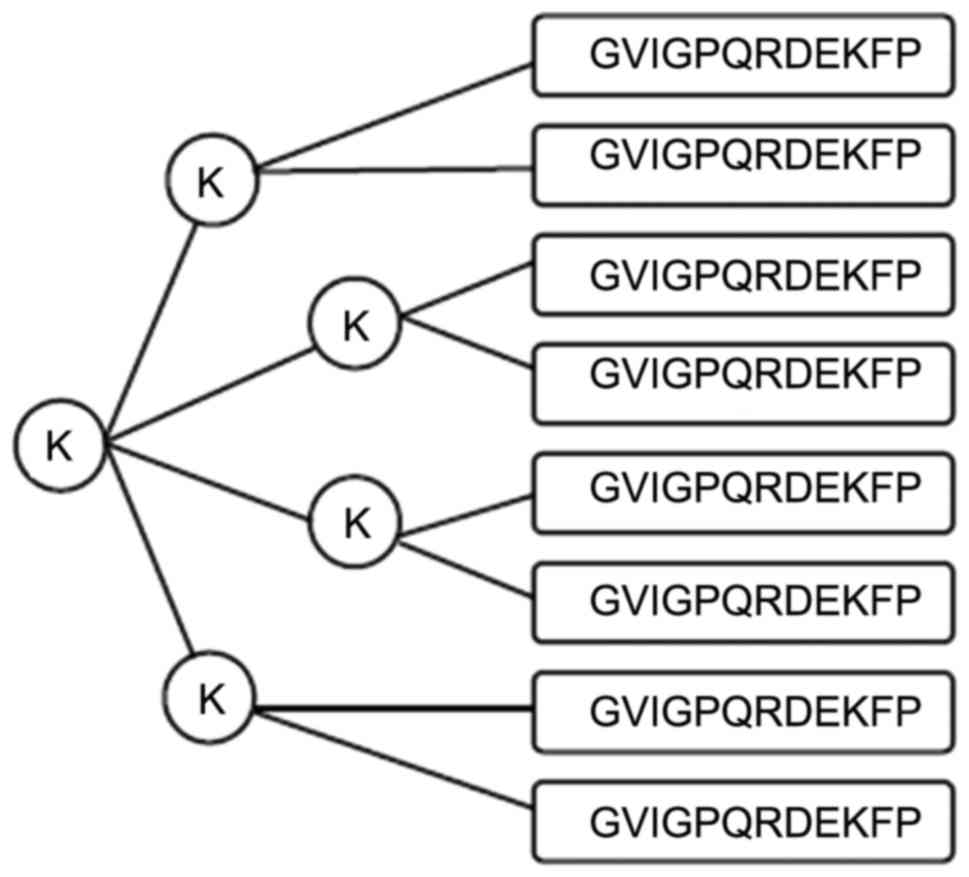
structure. They were presumed to be B-cell epitopes. Therefore, two
eight-branch MAPs (Fig. 3) were
synthesized based on the predicted amino acid sequences (Table I).
 | Table I.B cell epitopes of mTNF-α were
predicted using BcePred (http://www.imtech.res.in/raghava/bcepred/) and DNAStar
software (DNASTAR, Inc., Madison, WI, USA). |
Table I.
B cell epitopes of mTNF-α were
predicted using BcePred (http://www.imtech.res.in/raghava/bcepred/) and DNAStar
software (DNASTAR, Inc., Madison, WI, USA).
| MAP | Amino acid | Peptide | Software |
|---|
| MAP1 | 54–65 | GVIGPQ | BcePred, |
|
|
| RDEKFP | DNAStar |
| MAP2 | 78–92 | LTLRSSSQ | BcePred, |
|
|
| NSSDKPV | DNAStar |
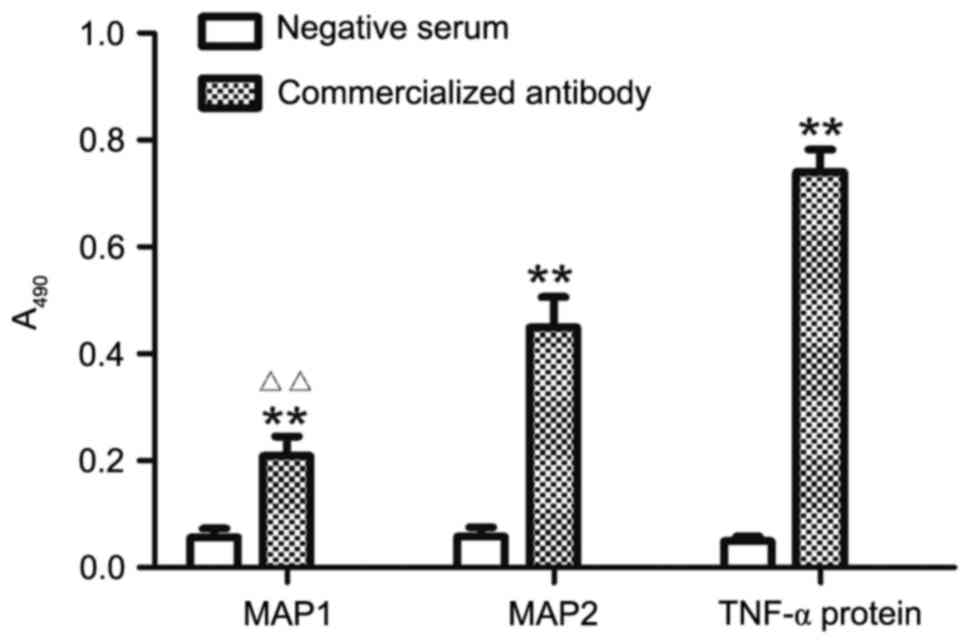
Binding affinity of synthesized MAPs
and mTNF-α full-length protein to anti-mTNF-α antibody
The binding affinity of synthesized MAP and mTNF-α
full-length protein to anti-mTNF-α antibody was measured by
indirect ELISA. On the basis of absorbance at a wavelength of 490
nm, MAP1 and MAP2 exhibited rather high affinity to the
commercialized antibody. The binding of MAP2 and mTNF-α full-length
protein to the antibody was stronger than that of MAP1 (Fig. 4).
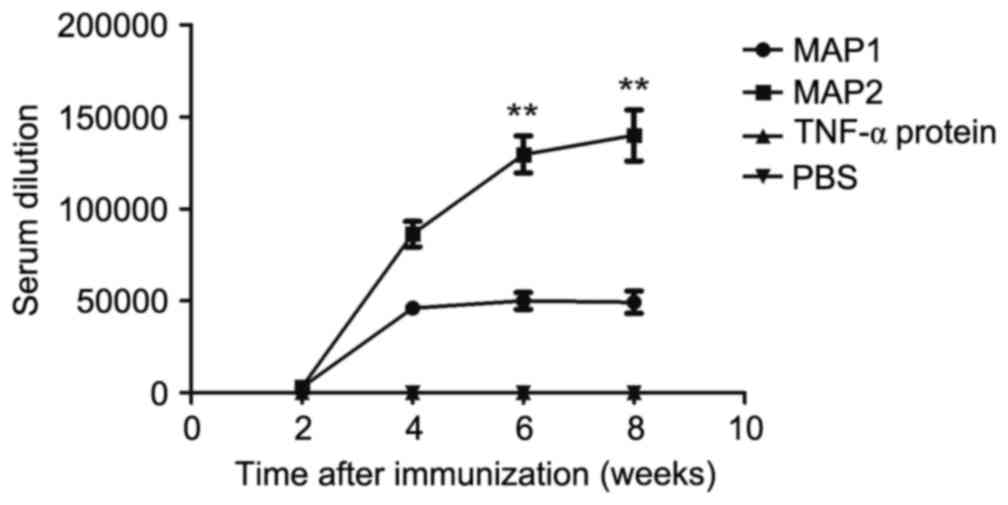
Dynamic changes in mouse antibody
titer
Specific antibodies were detected 2 weeks after the
first immunization. The MAP1 antibody titer reached a plateau after
4 weeks with the mean highest measured antibody titer of 1:50,000.
The MAP2 antibody titer reached the mean highest antibody titer of
1:130,000 6 weeks after immunization and peaked at 8 weeks. The
mean highest antibody titer for MAP2 was 1:140,000. No antibodies
were generated in the mTNF-α and PBS control groups (Fig. 5). Furthermore, no reactions between
TH and the two MAP antiserums, or cross-reactions between the two
serums and two MAPs were observed.
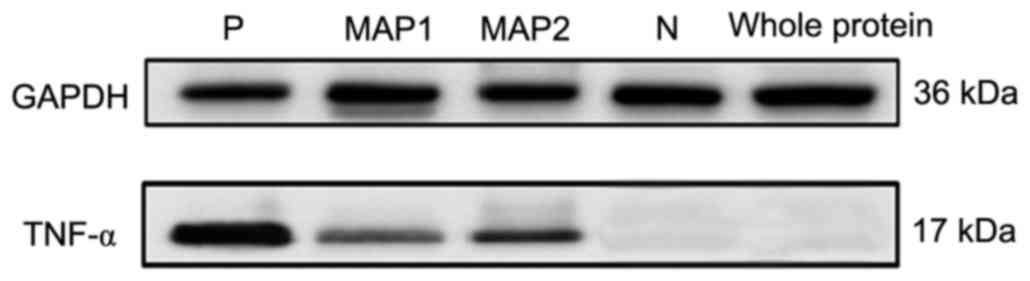
Protein electrophoresis and western
blotting
Total protein from H22 cells was used to evaluate
the MAP1 antiserum, MAP2 antiserum and commercial mTNF-α antiserum.
A commercial rabbit anti-mouse TNF-α antibody served as a positive
control and a mouse pre-immune serum served as a negative control.
Glyceraldehyde-3-phosphate dehydrogenase was selected as an
internal reference. A very clear band was observed in a molecular
weight of ~17 kDa from the commercial antibody. This was also
observed for for MAP1 and MAP2 antiserums. Based upon a commercial
antibody datasheet, 17 kDa indicated the mTNF-α protein. The mTNF-α
recombinant protein antiserum and negative controls did not exhibit
a band (Fig. 6).
Discussion
The prevalence of UC has increased in China in
recent years. Without effective treatment, its persistence and
recurrent attacks severely affect the quality of life. TNF-α has
emerged as a target for the treatment of UC (11,12).
The bioactivity of TNF-α, a human self-antigen, is
complicated. Studies have demonstrated that TNF-α overexpression
co-produces various types of disease, such as inflammatory bowel
disease (3,4,6,7).
Anti-TNF-α monoclonal antibody (infliximab) is the most commonly
used antibody for passive immunotherapy. It neutralizes TNF-α in
vivo and alleviates pathological damage. Infliximab has been
approved for the treatment of Crohn's disease, and its positive
role in UC treatment has been confirmed by clinical studies
(11,12). However, anti-TNF-α monoclonal
antibody treatment has various drawbacks. It is associated with
high medication volume use and high production cost. This type of
treatment also requires long-term repeated use and is prone to
causing hypersensitivity reactions (13,14).
This has given a new direction for developing anti-self molecule
immunotherapies, and active immunization vaccines against human
self-proteins (autologous vaccines) rather than passive acceptance
of monoclonal antibody drugs (15). Autoantigen epitope vaccines present
as a better treatment modality for autoimmune disease. Due to their
low molecular weight and unitary structure, self-antigens reduce
immunogenicity and cannot induce the desired immune responses,
particularly in B-cell epitope-mediated humoral immune responses
(16). To solve this problem,
short peptide vaccine epitope and carrier protein crosslinking
methods were used to improve immunogenicity. However, carrier
proteins are foreign antigen molecules and often induce antibodies
against the carrier protein itself rather than the vaccine
epitopes. In recent years, a design for MAPs has been proposed.
Low-molecular weight and low-immunogenicity lysines are used as the
core matrix coupling monomeric peptides (usually four or eight) to
form a dendritic structure. Such a design mimics the natural
conformational epitopes, but also activates humoral immunity and
induces high-titer and high-affinity antibodies without coupling to
a carrier protein (17).
Therefore, the use of a branched MAP epitope complex designed using
an MAP program may improve the quality of peptide vaccines with the
removal of carrier protein defects.
The present study was based on previous
investigations of a heparanase MAP vaccination strategy (18–22),
with the establishment of a method to induce a high-titer humoral
immune response (23). To achieve
the inhibition of TNF-α, internet software was used to predict the
TNF-α B-cell epitope and an optimized immunization strategy (MAP +
T cell epitope peptide) was used to induce high titers of antibody
in vivo. To validate the immunogenic specificity of the
predicted B-cell epitopes, the corresponding MAP, together with
T-helper epitope for polyclonal antisera, was synthesized and the
BALB/c mice were immunized. ELISA results demonstrated that MAP1
and MAP2 induced TNF-α-specific antibodies in vivo, with a
higher-titer antibody observed in MAP2, which indicated a high
affinity to the commercial full-length antibody. This suggested
that MAP1 and MAP2 were possible epitope peptides, with MAP2
located in amino acids 78–92 and demonstrating a slightly higher
immunogenicity. MAP2 is more likely an immunogen for inducing
highly specific humoral immune response to TNF-α.
To further verify the specificity of the antibody
produced by MAP immunization, total protein was extracted from
mouse hepatoma H22 cells, that were stably expressing mTNF-α, and
reacted with MAP1 antiserum, MAP2 antiserum, and commercial mTNF-α
recombinant protein antiserum. A commercial rabbit anti-mouse
mTNF-α antibody served as positive control, with the corresponding
pre-immune mouse serum serving as a negative control. The results
demonstrated a clear band at a molecular weight of ~17 kDa for MAP1
and MAP2, but no clear bands were observed for mTNF-α recombinant
protein antiserum and the negative control. The western blotting
results demonstrated that mTNF-α, as a self-antigen, could not
induce a specific humoral immune response alone, whereas the MAP
strategy could markedly enhance its B-cell epitope immunogenicity
and induce polyclonal antibodies specifically for TNF-α.
In conclusion, the results of the present study
indicated that amino acids 54–65 and 78–92 of mTNF-α were B-cell
epitopes, with the strongest immunogenicity for amino acids 78–92.
The study provided a theoretical basis for further investigations
into a TNF-α polypeptide antibody and B-cell peptide vaccine.
However, there were some limitations in this study, such as the
optimal dose of MAP was not attentively selected, and the
possibility of inducing autoimmune diseases or hypersensitivity
reactions were not fully investigated. In addition, for TNF-α
immunotherapy, further studies are required to clarify whether the
above-mentioned B-cell epitopes are neutralizing epitopes of TNF-α,
which inhibit TNF-α activity after binding and facilitate with the
treatment of inflammatory bowel disease.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81400682) and
Zhejiang pharmaceutical and health care plans (grant no.
2013KYA014).
References
|
1
|
Park JH and Brentjens RJ: Adoptive
immunotherapy for B-cell malignancies with autologous chimeric
antigen receptor modified tumor targeted T cells. Discov Med.
9:277–288. 2010.PubMed/NCBI
|
|
2
|
Castro FV, Al-Muftah M, Mulryan K, Jiang
HR, Drijfhout JW, Ali S, Rutkowski AJ, Kalaitsidou M, Gilham DE and
Stern PL: Regulation of autologous immunity to the mouse 5T4
oncofoetal antigen: Implications for immunotherapy. Cancer Immunol
Immunother. 61:1005–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopylov U, Ben-Horin S, Zmora O, Eliakim R
and Katz LH: Anti-tumor necrosis factor and postoperative
complications in Crohn's disease: Systematic review and
meta-analysis. Inflamm Bowel Dis. 18:2404–2413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawalec P, Mikrut A, Wiśniewska N and Pilc
A: Tumor necrosis factor-α antibodies (infliximab, adalimumab and
certolizumab) in Crohn's disease: Systematic review and
meta-analysis. Arch Med Sci. 9:765–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marchioni RM and Lichtenstein GR: Tumor
necrosis factor-α inhibitor therapy and fetal risk: A systematic
literature review. World J Gastroenterol. 19:2591–2602. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laharie D, Bourreille A, Branche J, Allez
M, Bouhnik Y, Filippi J, Zerbib F, Savoye G, Nachury M, Moreau J,
et al: Ciclosporin versus infliximab in patients with severe
ulcerative colitis refractory to intravenous steroids: A parallel,
open-label randomised controlled trial. Lancet. 380:1909–1915.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reinisch W, Sandborn WJ, Rutgeerts P,
Feagan BG, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers
WJ, Blank M, Lang Y, et al: Long-term infliximab maintenance
therapy for ulcerative colitis: The ACT-1 and −2 extension studies.
Inflamm Bowel Dis. 18:201–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jun Y, Hong Z and Jie T: The study of
mouse TNF-α functional domain and its neutralizing antibody binding
site. Prog Biochem Biophys. 36:4302009.
|
|
9
|
Amexis G and Young NS: Multiple antigenic
peptides as vaccine platform for the induction of humoral responses
against dengue-2 virus. Viral Immunol. 20:657–663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dechamma HJ, Dighe V, Kumar CA, Singh RP,
Jagadish M and Kumar S: Identification of T-helper and linear B
epitope in the hypervariable region of nucleocapsid protein of PPRV
and its use in the development of specific antibodies to detect
viral antigen. Vet Microbiol. 118:201–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thorlund K, Druyts E, Mills EJ, Fedorak RN
and Marshall JK: Adalimumab versus infliximab for the treatment of
moderate to severe ulcerative colitis in adult patients naïve to
anti-TNF therapy: An indirect treatment comparison meta-analysis. J
Crohns Colitis. 8:571–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fausel R and Afzali A: Biologics in the
management of ulcerative colitis-comparative safety and efficacy of
TNF-α antagonists. Ther Clin Risk Manag. 11:63–73. 2015.PubMed/NCBI
|
|
13
|
Marehbian J, Arrighi HM, Hass S, Tian H
and Sandborn WJ: Adverse events associated with common therapy
regimens for moderate-to-severe Crohn's disease. Am J
Gastroenterol. 104:2524–2533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Can super-antibody drugs be tamed? Nature.
440:855–856. 2006.PubMed/NCBI
|
|
15
|
Rolinski J and Hus I: Breaking
immunotolerance of tumors: A new perspective for dendritic cell
therapy. J Immunotoxicol. 11:311–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park KB, Lim BK, Ye MB, Chung SY and Nam
JH: A peptide vaccine based on a B-cell epitope on the VP1 protein
of enterovirus 70 induces a strong antibody response. Acta Virol.
56:337–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haro I and Gómara MJ: Design of synthetic
peptidic constructs for the vaccine development against viral
infections. Curr Protein Pept Sci. 5:425–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Yang JM, Wang HJ, Ru GQ and Fan
DM: Synthesized multiple antigenic polypeptide vaccine based on
B-cell epitopes of human heparanase could elicit a potent
antimetastatic effect on human hepatocellular carcinoma in vivo.
PLoS One. 8:e529402013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Yang J, Fan D, Tao H, Wang H and
Yu T: Peptide FLNPDVLDI of heparanase is a novel HLA-A2-restricted
CTL epitope and elicits potent immunological antitumor effects
in vitro with an 8-branched design. Oncol Rep. 29:1955–1961.
2013.PubMed/NCBI
|
|
20
|
Zhang J, Fan DM and Yang JM: Immunotherapy
targeting Heparanase-1 may be the the dawn of cancer sufferers. J
Gastroen Hepatol Res. 1:32012.
|
|
21
|
Zhang J, Yang J, Han X, Zhao Z, Du L, Yu T
and Wang H: Overexpression of heparanase multiple antigenic peptide
2 is associated with poor prognosis in gastric cancer: Potential
for therapy. Oncol Lett. 4:178–182. 2012.PubMed/NCBI
|
|
22
|
Zhang J, Yang J, Cai Y, Jin N, Wang H and
Yu T: Multiple antigenic polypeptide composed of heparanase B-cell
epitopes shrinks human hepatocellular carcinoma in mice. Oncol Rep.
33:1248–1256. 2015.PubMed/NCBI
|
|
23
|
Du L, Wang H, Yang J, Gao H, Zhou Y and
Tao H: T-helper epitope peptide improves immunological effects of
the B cell epitopes of human heparanase protein. Chin J Microbiol
Immunol. 869–872. 2008.
|