Introduction
Inflammatory bowel disease (IBD) is the term for
chronic and relapsing-remitting inflammatory disorders of the
gastrointestinal tract, including Crohn's disease and ulcerative
colitis (1,2). While the incidence and prevalence of
IBD are increasing, the etiology and pathogenesis are complex and
remain unclear (3,4). Various factors, including immunity,
heredity, the intestinal environment (defective epithelial barrier
and increased intestinal permeability) and bacterial infection,
appear to be associated with the pathogenesis of IBD, particularly
immunological factors (1,5).
The hypothesis that dysfunction of the immune system
and disproportionality of pro- and anti-inflammatory mediators
serve important roles in the pathogenesis of IBD has been widely
accepted (6,7). Naïve cluster of differentiation
(CD)4+ T cells generally divide into different lineages,
which encompass effector CD4+ T helper (Th) cells and
regulatory CD4+ T (Treg) cells, and vary in cytokine
production and function (8,9).
Apart from classical Th1 and Th2 cells, Th17 lymphocytes are a
novel subpopulation of effector Th cells that have been
demonstrated to be an important pathogenic element in IBD (5). In addition, Th17 cells are
characterized by the production of a large amount of interleukin
(IL)-17A, IL-17F, IL-21, IL-22 and IL-23, and have been observed in
the mucosa and lamina propria of patients with IBD (1,10–12).
By contrast, Treg cells produce a number of anti-inflammatory
cytokines which suppress the activation and effector functions of T
cells. It has been reported that Treg cells inhibit abnormal
inflammatory responses against the commensal flora or dietary
antigens, and are involved in the maintenance of intestinal
homeostasis (1,13). Therefore, the quantity and function
of Treg cells may be associated with the initiation and progression
of IBD (14). A previous study
demonstrated that breakdown of the Th17/Treg balance may promote
inflammation and autoimmune disease, including IBD (15).
IL-17, an important pro-inflammatory cytokine
secreted by Th17, is associated with a number of chronic
inflammatory disorders (15,16).
It has been observed that IL-17 is overexpressed in patients with
IBD (1). Additionally,
fibrinogen-like protein 2 (FGL2) is one of the immunosuppressive
cytokines expressed by Treg cells and has been identified to be an
important effector molecule by which Treg cells exerts their
immunosuppressive effects (17–20).
FGL2 has been demonstrated to inhibit the proliferation of T cells
and the maturation of bone marrow-derived cells. Previous studies
have demonstrated that subsets of Treg cells with increased levels
of FGL2 are more suppressive and that Treg cells from
FGL2−/− mice have been observed exhibit decreased
suppressive activity (17,20,21).
It was previously identified that the expression of FGL2 in
intestinal mucosal biopsies and peripheral blood was increased in
patients with active disease, and decreased in inactive disease.
Additionally, FGL2 levels were significantly positively correlated
with clinical disease activity index, erythrocyte sedimentation
rate and C-reactive protein levels (22). Therefore, it was hypothesized that
the increased levels of FGL2 may be associated with a mass of Treg
cells recruited to regions of inflammation to counter
inappropriately activated effector T cells. In addition, Dothel
et al (2) demonstrated that
acute inflammation in animal models of trinitro-benzene-sulfonic
acid (TNBS)-induced colitis is characterized by a Th1/Th17-oriented
response, with increased levels of tumor necrosis factor (TNF)-α,
IL-1β, IL-12, IL-17, IL-18 and IL-6. It has been additionally
suggested that Crohn's disease is oriented to a Th1 and Th17 immune
response and, in order to mimic the chronic inflammation typical of
Crohn's disease, models have been developed via multiple TNBS
administrations. Therefore, the TNBS-induced model presented in the
present study is a model of the acute phase of Crohn's disease
(2,23).
The present study investigated alterations in FGL2
and IL-17 expression in the TNBS-induced colitis model, in order to
identify whether the balance between Th17 and Treg was disrupted
and whether the imbalance may be involved in the immunopathogenesis
of IBD. Therefore, the purpose of the present study was to
demonstrate variation in FGL2 and IL-17 expression in an animal
model of IBD and to examine their involvement in the
immunopathogenesis of IBD.
Materials and methods
Animals
A total of 22 BALB/c mice of 6–8 weeks of age (male,
~20 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd
[Shanghai, China; license no. SCXK (Shanghai) 2012-0002]. Animals
were housed in standard cages (5 mice/cage) under specific
pathogen-free conditions in the Laboratory Animal Research Center
of Wenzhou Medical University (Wenzhou, China). Mice were given
free access to autoclaved tap water and to a standard diet,
maintained under controlled conditions of light (12-h light/dark
cycle), temperature (22–24°C) and humidity (45–55%). All procedures
were conducted ethically according to the Guide for the Care and
Use of the Administration Committee of Experimental Animals of
Wenzhou Medical University (permit no. wydw2015-0123).
TNBS-induced colitis
Mice were skin sensitized with a mixture of acetone,
olive oil and TNBS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany;
64:16:20 v/v/v), prior to TNBS administration. A total of 7 days
subsequently, mice were weighed and anesthetized by intraperitoneal
injection of ketamine/xylazine solution (80 ml/10 g body weight).
TNBS was dissolved in anhydrous ethanol (50:50 v/v). A total of 100
µl TNBS solution (100 mg/kg body weight) was administered
intrarectally (via 3.5 F catheter) to mice maintained for 60 sec in
a vertical position. The catheter was inserted into the colon 4 cm
proximal to the anus. Control mice received 100 µl 0.9% saline
intrarectally (24). The sample
size in each group was 8.
Clinical and macroscopic analysis of
colitis
The clinical analysis of colitis was performed by
daily monitoring of body weight, diarrhea and hemafecia (25). Loss of body weight was calculated
as the percentage difference relative to initial body weight.
Diarrhea was scored as follows: 0, Normal; 2, loose stools; and 4,
diarrhea that remained adhesive to the anus. Bleeding was scored as
follows: 0, Negative hemoccult test; 2, positive hemoccult test;
and 4, apparent bleeding (23).
Mice were euthanized by cervical dislocation 3 days subsequent to
TNBS administration, when the inflammation was most severe. The
colon was removed and opened longitudinally. The macroscopic damage
was assessed by a blinded observer with the following score system
(5,26): 0, Normal; 1, hyperemia, edema, no
ulcer; 2, hyperemia, edema, small linear ulcers or petechiae; 3,
hyperemia, edema, wide ulcers, necrosis or adhesions; and 4,
hyperemia, edema, megacolon, stenosis or perforation.
Histology
For histological analysis, the colonic fragments
(0.5 cm) were fixed in 4% paraformaldehyde at 4°C for 48 h,
dehydrated, embedded in paraffin, sectioned (4-µm thickness), and
stained with hematoxylin and eosin. The pathological slices were
observed under a light microscope at a magnification of ×200 by two
blinded pathologists and the microscopic damage was scored as
follows (27): 0, No evidence of
inflammation; 1, low level of inflammation with scattered
infiltrating mononuclear cells (1–2 foci); 2, moderate inflammation
with multiple foci; 3, high level of inflammation with increased
vascular density and marked wall thickening; and 4, maximal
severity of inflammation with transmural leukocyte infiltration and
loss of goblet cells.
Immunohistochemistry
The colon specimens were fixed with 4%
paraformaldehyde at 4°C for 48 h, embedded with paraffin, and
sectioned (4-µm thickness) for immunohistochemical staining of
FGL2, IL-17 and TNF-α. Following incubation with xylene and
descending concentrations of ethanol, antigens were retrieved using
citrate buffer for 15 min at 100°C. Endogenous peroxidases were
removed in 3% hydrogen peroxidase for 15 min at room temperature
(RT) followed by 5% goat serum (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 1 h at 37°C for blocking.
Sections were incubated with antibodies [FGL2 (cat. no. ab198029;
1:100), TNF-α (cat. no. ab6671; 1:250) and IL-17 (cat. no. ab79056;
1:300) (all Abcam, Cambridge, UK)] overnight at 4°C, and
subsequently incubated with horseradish peroxidase-conjugated
secondary antibody (PV.6001; 1:100; Zhongshan Golden Bridge
Biotechnology, Beijing, China) for 30 min at 37°C. Antibody
bindings were counterstained with 10% hematoxylin at room
temperature for 2 sec, dehydrated with ascending concentrations of
ethanol, cleared with xylene and mounted. A negative control was
performed according to the same procedure. Images were captured
using a biological imaging microscope at a magnification of ×200 or
×400 (BX53; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Total proteins of colon specimens were extracted in
radioimmunoprecipitation lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd.) and phenylmethylsulfonyl fluoride. The
protein concentration was analyzed by a BCA kit (Tiangen
Biotechnology, Beijing, China). Equal amounts of proteins (40 µg)
were separated using SDS-PAGE on a 12% gel and transferred onto
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). Following blocking in 5% non-fat milk for 90 min at room
temperature, the membranes were incubated overnight at 4°C with
anti-FGL2 (polyclonal; rabbit anti-mouse; ab198029; 1:1,000),
anti-TNF-α (polyclonal; rabbit anti-mouse; ab667; 1:1,000),
anti-IL-17 (polyclonal; rabbit anti-mouse; ab79056; 1:1,000) (all
Abcam) and anti-GAPDH (polyclonal; rabbit anti-mouse; BS60630;
1:5,000; Bioworld Technology, Inc., St. Louis Park, MN, USA), and
washed three times in TBS with Tween-20. The membranes were
subsequently incubated with secondary antibodies conjugated to
horseradish peroxidase (7074; 1:5,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) for 1 h at RT, followed by washing three
times. Immunoreactive bands were visualized using an enhanced
chemiluminescence kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Image Lab software version 4.1 (Bio-Rad Laboratories, Inc.)
was used for densitometric analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the colon tissue and
spleen mononuclear cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. A total of 1 µg total RNA was
reverse-transcribed into cDNA using the RevertAid First Strand cDNA
Synthesis kit (K1622; Thermo Fisher Scientific, Inc.). The mRNA
expression of a number of genes was quantified using SYBR Green
Real-time PCR Master Mix Plus (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on an ABI 7500 Sequence-Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR cycling
conditions were as follows: An initial denaturation and activation
at 95°C for 10 min, followed by 40 amplification cycles of 95°C for
15 sec and 60°C for 60 sec. The primer sequences are listed in
Table I. GAPDH was used as
reference gene. Relative gene expression levels were calculated
using the 2−∆∆Cq method (28).
 | Table I.Primers used for the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for the reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer
sequence |
|---|
| GAPDH | sense:
5′-GGTTGTCTCCTGCGACTTCA-3′ |
|
|
antisense:5′-TGGTCCAGGGTTTCTTACTCC-3′ |
| TNF-α | sense:
5′-ACGGCATGGATCTCAAAGAC-3′ |
|
|
antisense:5′-GTGGGTGAGGAGCACGTAGT-3′ |
| ROR-γt | sense:
5′-GAACCAGAACAGGGTCCAGA-3′ |
|
|
antisense:5′-TCGGAAGGACTTGCAGACAT-3′ |
| Foxp3 | sense:
5′-ACTCGCATGTTCGCCTACTT-3′ |
|
|
antisense:5′-GTCCACACTGCTCCCTTCTC-3′ |
| FGL2 | sense:
5′-ATTAGATGTTGAACTGGCTGTGA-3′ |
|
|
antisense:5′-TGGCAAATCTAACCGTTGTGG-3′ |
| IL-17 | sense:
5′-TCCCTCTGTGATCTGGGAAG-3′ |
|
|
antisense:5′-CTCGACCCTGAAAGTGAAGG-3′ |
ELISA analysis
Peripheral blood from the mice was drawn into
EDTA-anticoagulant tubes, and centrifuged for 15 min (3,000 × g) at
4°C. The plasma was collected and stored at −80°C until tested.
ELISA kits were used to assess the plasma concentrations of FGL2
(BPE10791R; Shanghai Boyun Biotech Co., Ltd., Shanghai China),
IL-17 (BPE10871R; Shanghai Boyun Biotechnology Co., Ltd.) and TNF-α
(BPE10912R; Shanghai Boyun Biotechnology Co., Ltd.), according to
the manufacturer's protocol.
Flow cytometric analysis
The spleen was removed from mice 3 days subsequent
to TNBS administration and prepared into spleen mononuclear cells
The spleen was milled into a homogenate and gradiently centrifuged
for 20 min (716 × g) at room temperature with lymphocyte separation
solution (Tianjin Haoyang Biotechnology Co., Ltd., Tianjin China)
leaving the spleen mononuclear cells in the middle of the
centrifugal layer. For analysis of Th17 cells, cells were
stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml) and
ionomycin (1 µg/ml) in the presence of brefeldin A (10 µg/ml) and
monensin (1.4 µg/ml), at 37°C with 5% CO2 for 4 h. Cells
were washed with PBS and surface-labeled with fluorescein
isothiocyanate-(FITC-) conjugated anti-CD4 (1:200; 85-11-0041-81;
eBioscience, San Diego, CA, USA) for 40 min at 4°C. Following
fixing and permeabilizing using a fixation/permeabilization buffer
(BD Biosciences, San Jose, CA, USA), cells were labeled with
phycoerythrin-(PE-) conjugated anti-IL-17 (1:100; 85-12-7177-81;
eBioscience) for 40 min at 4°C. For analysis of Treg cells, without
PMA and ionomycin stimulation, surface staining was performed with
FITC-conjugated anti-CD4 (1:200; 85-11-0041-81; eBioscience) and
allophycocyanin-conjugated anti-CD25 (1:67; 85-17-0390-82;
eBioscience) for 40 min at 4°C. Subsequently, cells were fixed and
permeabilized, and intracellular staining was performed with
PE-conjugated anti-forkhead box protein 3 (Foxp3; 1:80;
85-12-5773-82; eBioscience) for 40 min at 4°C. Appropriate isotype
controls were used in the experiments. The stained cells were
assessed using a FACSCalibur flow cytometer (BD Biosciences) and
the data were analyzed using FlowJo software (version 7.6.1; Tree
Star, Inc., Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software version 16.0 (SPSS Inc., Chicago, IL, USA). Data were
analyzed using the Student's t-test for two-group comparisons.
Results are presented as the mean ± standard error of the mean and
P<0.05 was considered to indicate a statistically significant
difference.
Ethical considerations
In accordance with the Association for the
Assessment and Accreditation of Laboratory Animal Care
International (Frederick, MD, USA), mice were maintained under
specific pathogen-free conditions and studied according to
protocols approved by the Wenzhou Medical University Animal Care
and Use Committee.
Results
TNBS-induced mice develop severe
clinical manifestations and gross morphological alterations
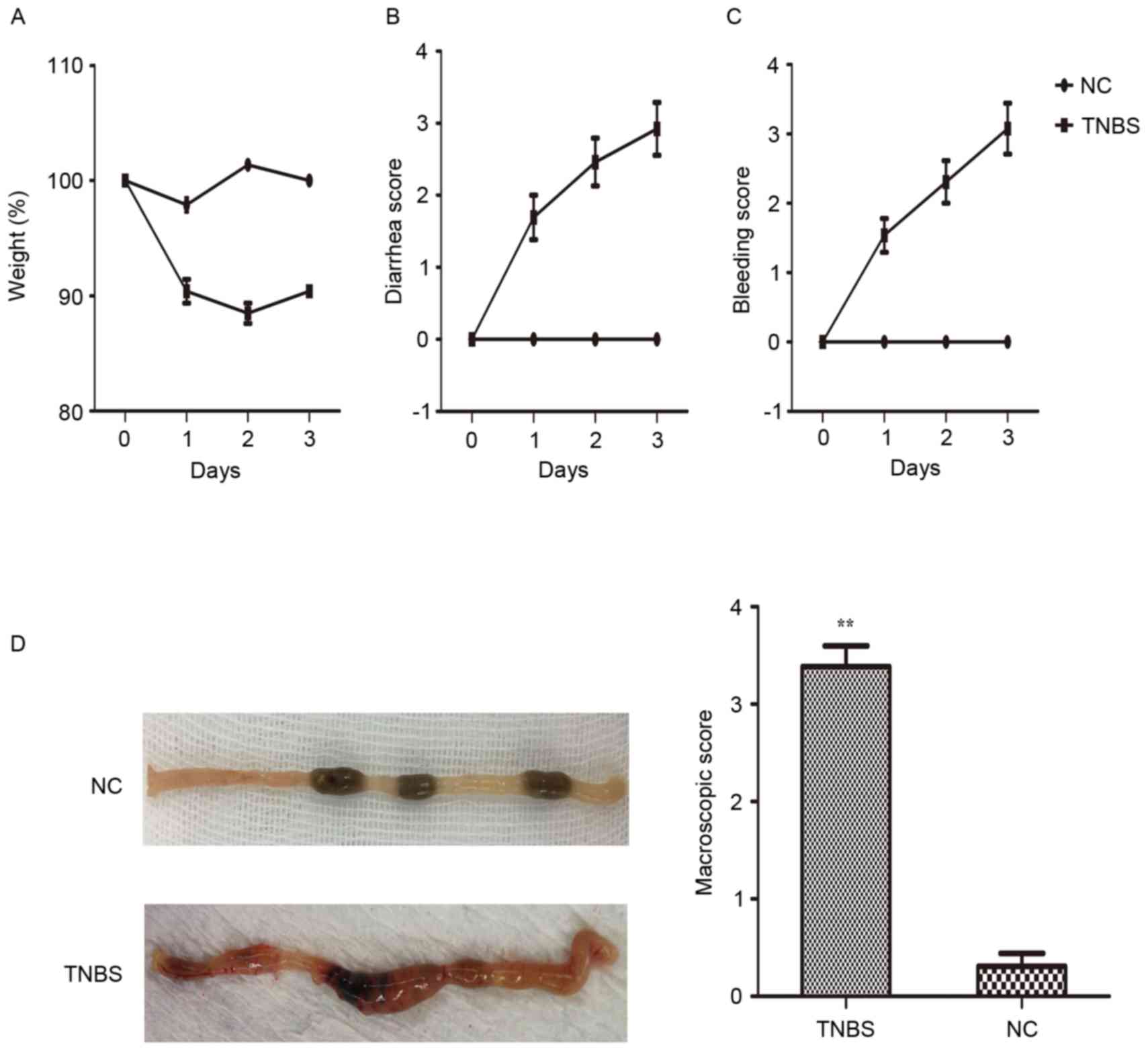
The clinical symptoms and macroscopic scores in
TNBS-induced colitis were evaluated. The mice exhibited a disease
phenotype characterized by body weight loss, diarrhea, defecate
occult blood or hemafecia following TNBS administration (Fig. 1A-C). In addition, 40% mortality was
observed relative to the saline control (P<0.05). Body weight
loss began immediately in the TNBS-induced mice. By contrast, the
control group did not develop colitis or succumb to the treatment.
Subsequently, the colonic samples were observed and scored by a
blinded observer (Fig. 1D).
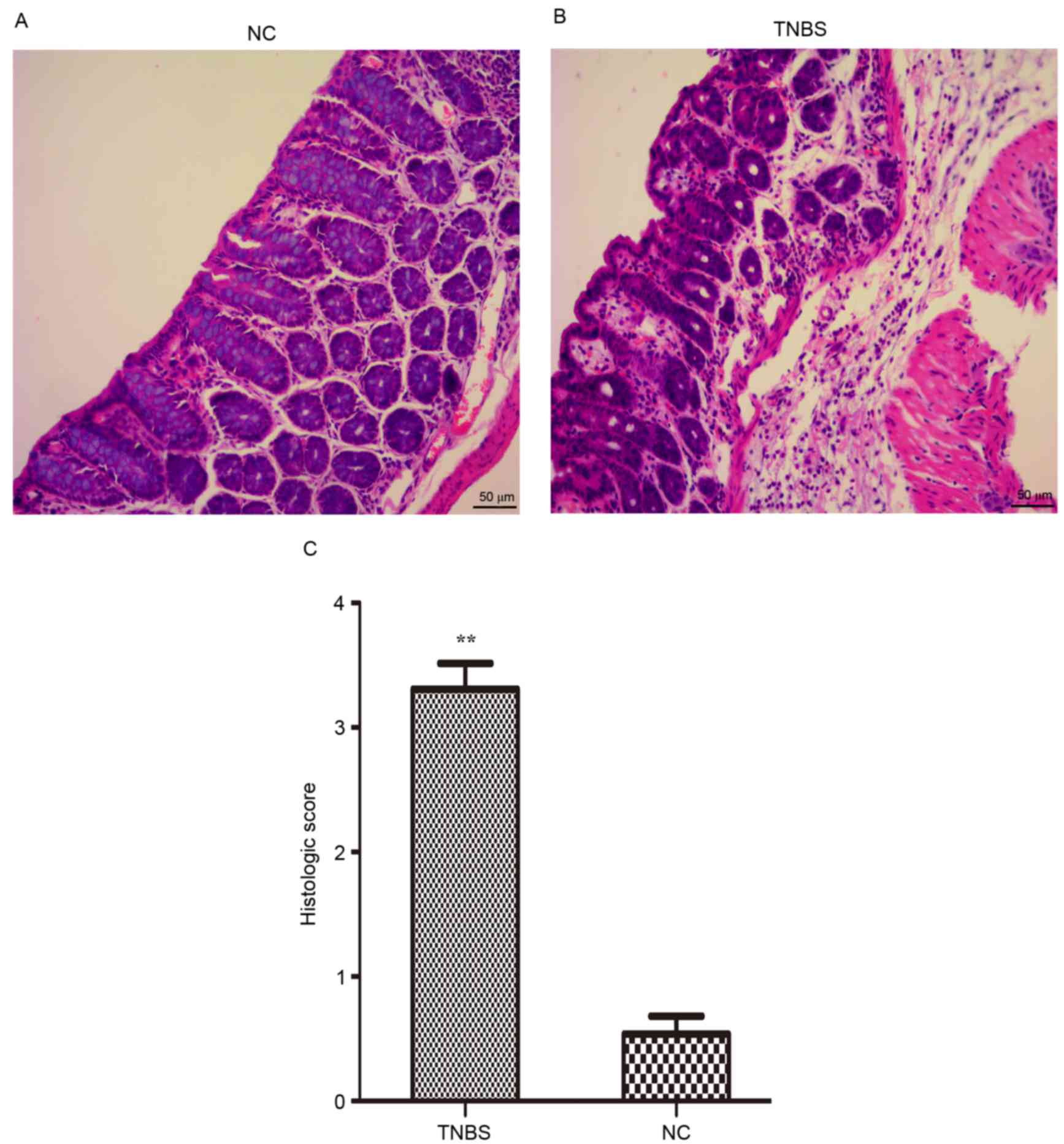
Histological alterations and
inflammation score
In accordance with the macroscopic test, it was
observed that the colon of the IBD model animals exhibited
hyperemia, edema, ulcers, necrosis and adhesion. Histological
examination demonstrated alterations in the TNBS-induced mice,
including a decrease in goblet cells, loss of crypts, damage to
crypts, infiltration by inflammatory cells and extensive
destruction of the mucosal layer (Fig.
2A and B). Histological scoring was performed as described
above. The results of the present study demonstrated that the
histological scores of the TNBS-induced mice were markedly
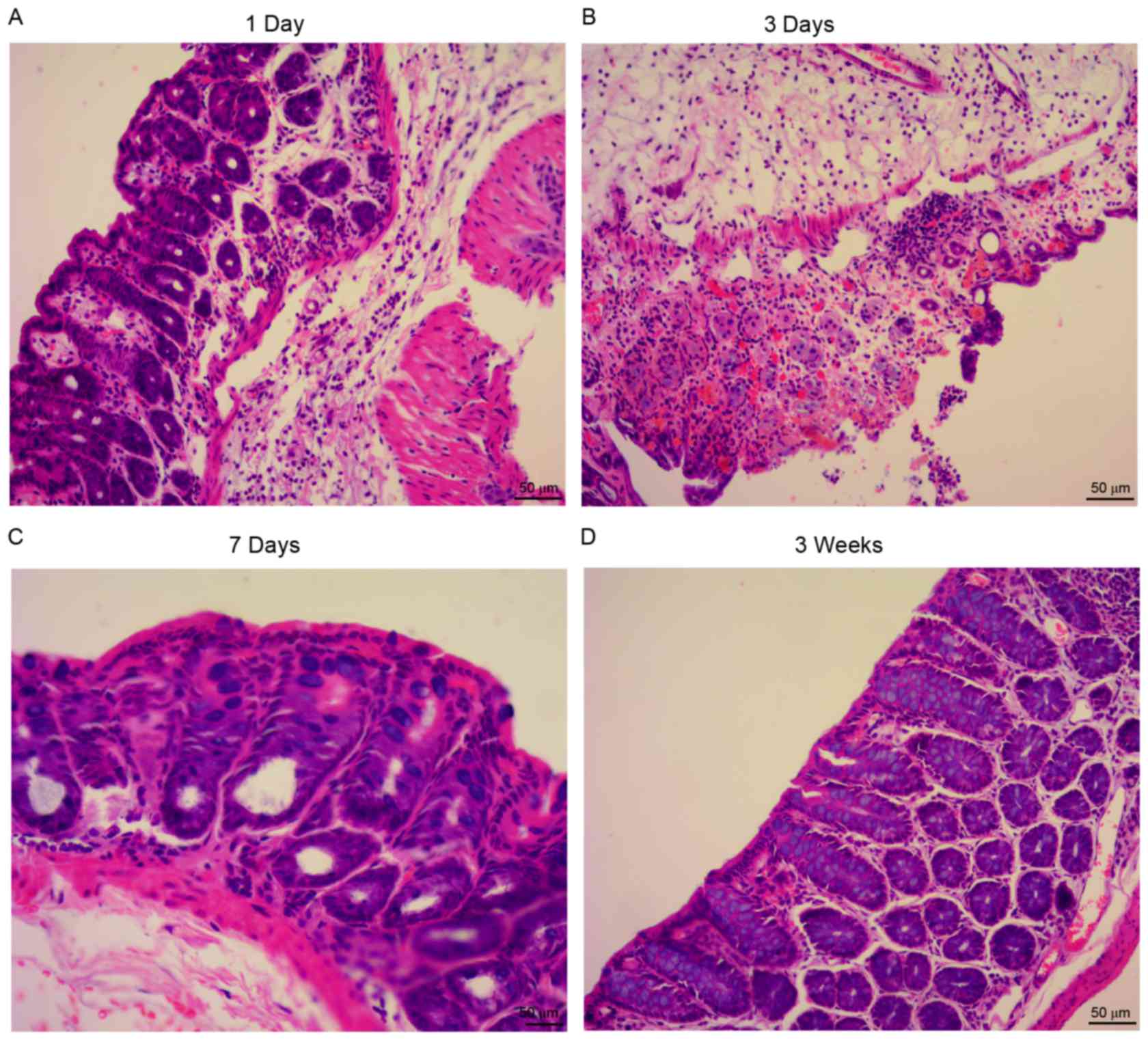
increased, compared with the control group (Fig. 2C). The inflammation was most severe
3 days subsequent to TNBS administration, compared with other time
points (days 1 and 7, and 3 weeks; Fig. 3).
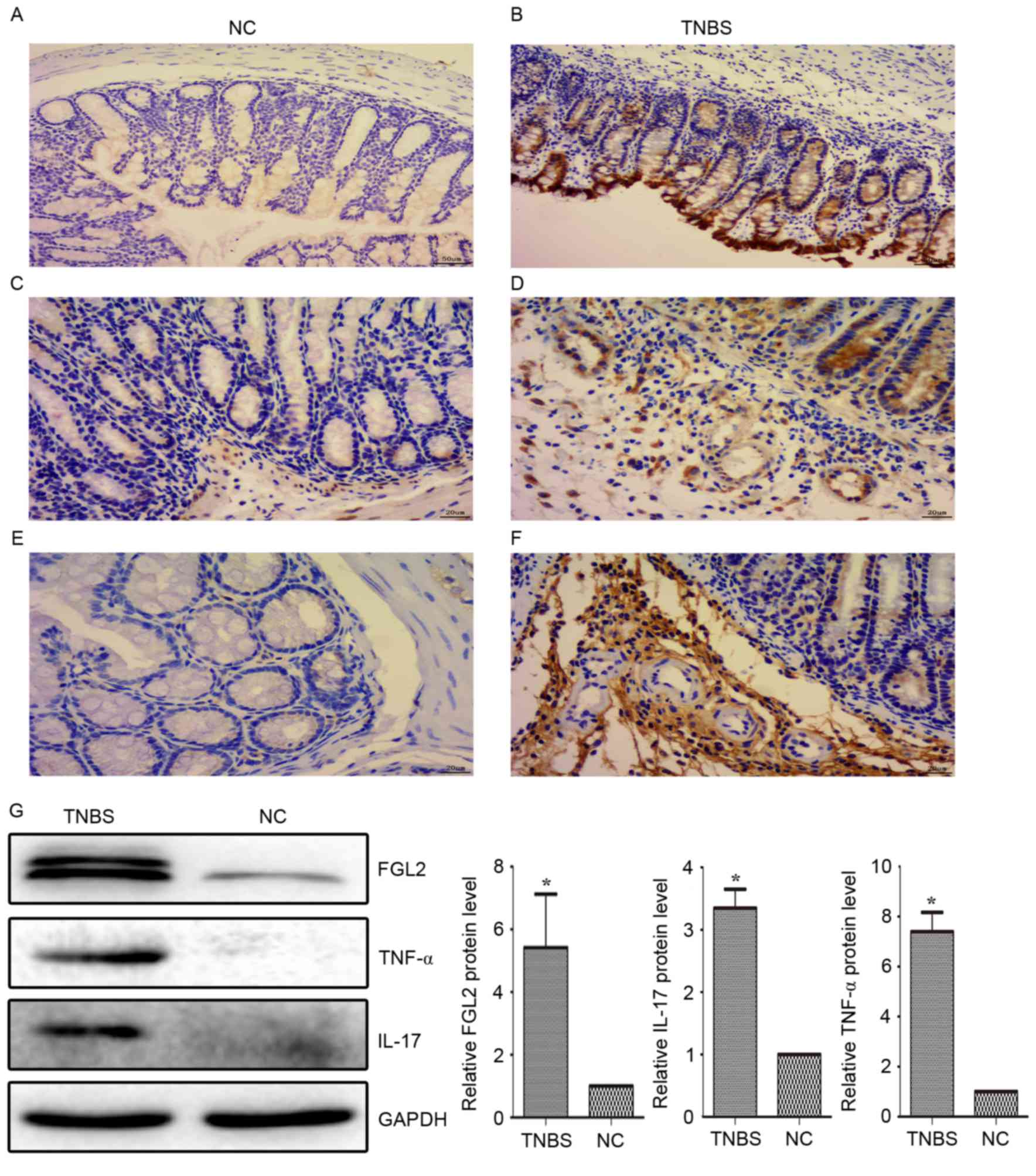
FGL2 expression is increased in the
colon tissue from TNBS-induced mice, accompanied by elevated
pro-inflammatory cytokine expression
Compared with the control group, pro-inflammatory
cytokine expression in the colon tissue, including IL-17 and TNF-α,
was significantly increased on day 3 following TNBS administration.
The expression of FGL2 exhibited the same trend as IL-17 and TNF-α,
which was consistent with a previous study in patients with IBD
(22). The results of the
immunohistochemistry and western blotting of FGL2, IL-17 and TNF-α
in the present study demonstrated upregulated expression in colonic
sections from TNBS-induced colitis mice, compared with the control
group (Fig. 4).
MRNA levels of Th17 and Treg
transcription factors and associated cytokines are modulated in the
colonic tissue of IBD mice
Previous studies have demonstrated a decrease in
Treg cell count and an increase in Th17 (29). Therefore, the present study
investigated the mRNA expression of signature transcription factors
of Th17 and Treg cells, and associated cytokines, in the colonic
tissue. RT-qPCR analysis demonstrated that the mRNA expression of
IL-17, retinoic acid related orphan receptor-γt (ROR-γt), TNF-α,
Foxp3, and FGL2 in the TNBS group were markedly increased compared
with the control group (Fig.
5).
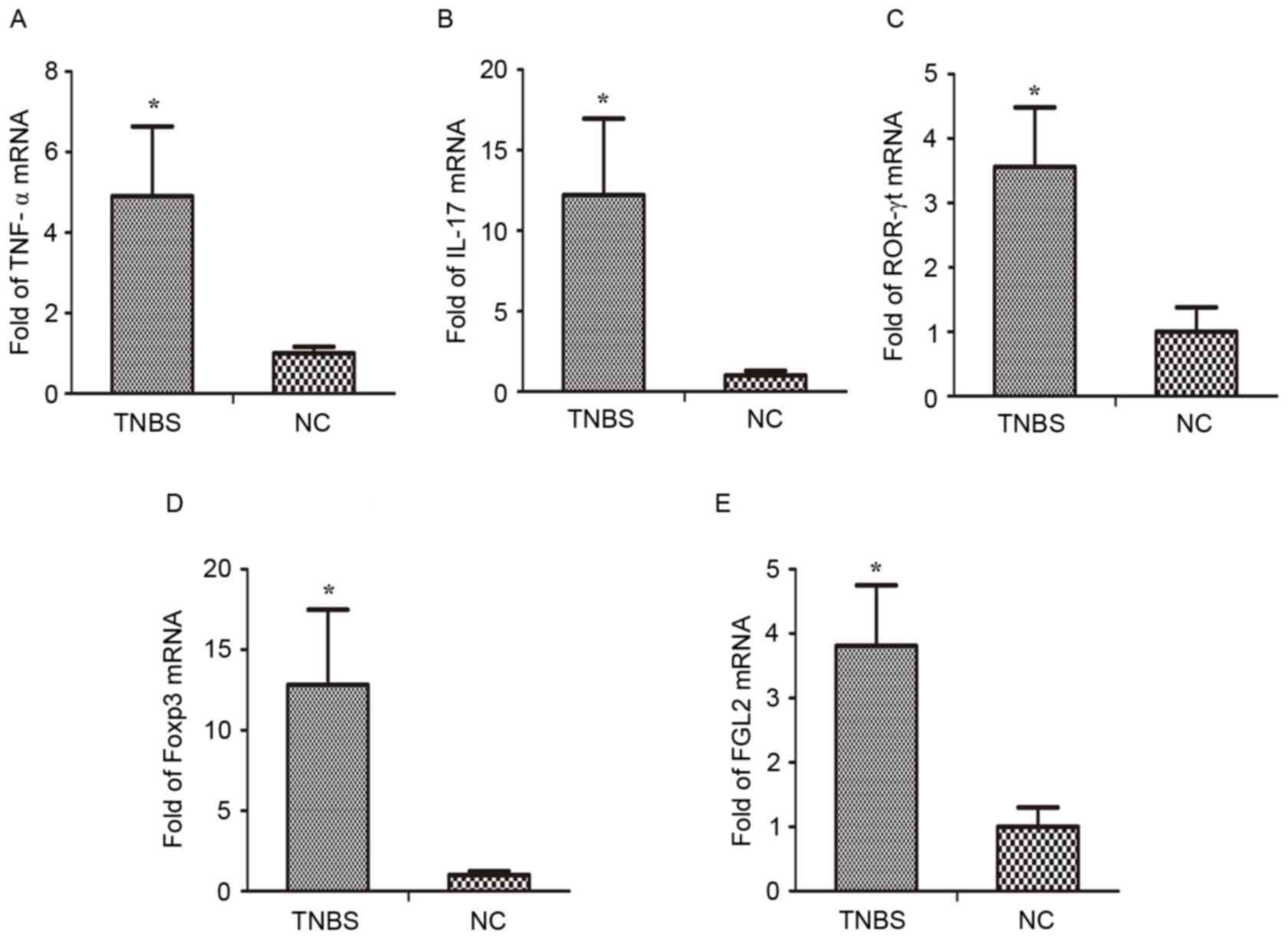 | Figure 5.Altered gene expression in the colon
of TNBS-induced inflammatory bowel disease mice. Reverse
transcription-quantitative polymerase chain reaction analysis was
performed of the mRNA expression of (A) TNF-α, (B) IL-17, (C)
ROR-γt, (D) Foxp3 and (E) FGL2 in colonic tissue. *P<0.05 vs.
NC. n=8 mice/group. TNBS, trinitro-benzene-sulfonic acid; NC,
normal control; TNF-α, tumor necrosis factor-α; FGL2,
fibrinogen-like protein 2; IL-17, interleukin-17; ROR-γt, retinoic
acid related orphan receptor-γt; Foxp3, forkhead box protein 3. |
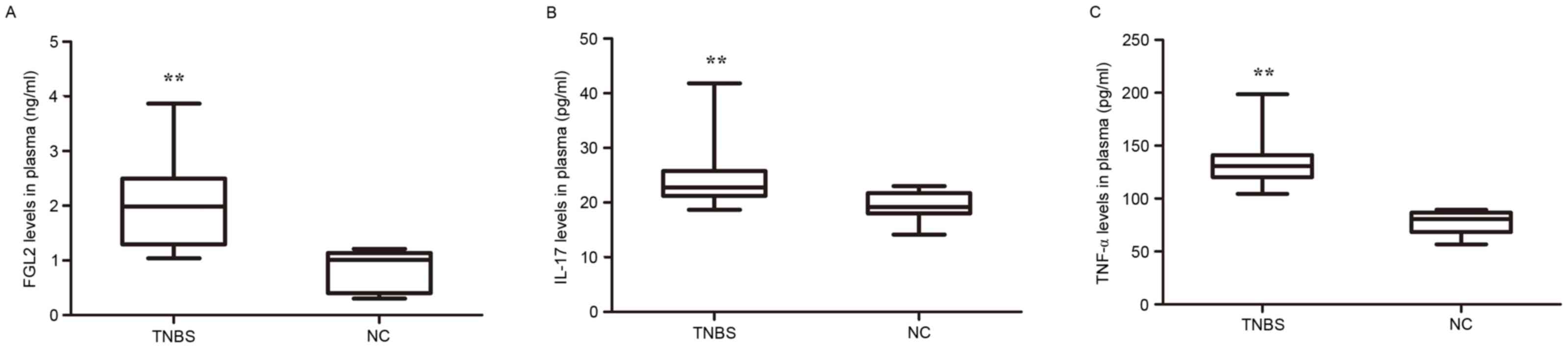
Levels of FGL2 are increased in plasma
from TNBS-induced mice in addition to elevated pro-inflammatory
cytokine expression
In order to determine whether the expression of FGL2
and IL-17 was altered in the peripheral blood of TNBS-induced mice,
the plasma expression of FGL2, IL-17 and TNF-α was analyzed. As
presented in Fig. 6, significantly
increased FGL2 secretion and increased production of IL-17 and
TNF-α in plasma, were detected in the TNBS group compared with the
control group.
Distinct frequencies of splenic
Treg/Th17 cells in TNBS-induced mice
It has been hypothesized that an imbalance of Treg
and Th17 cells may be associated with the pathogenesis of IBD
(29,30). The aim of the present study was to
further investigate the polarization of CD4+ T cells in
spleens from TNBS-induced mice (Fig.
7). Flow cytometric analysis demonstrated that
CD4+CD25+Foxp3+ (Treg) cells were
markedly decreased, while CD4+IL-17+ (Th17)
cells were significantly increased following TNBS challenge
(Fig. 7E). In parallel, the
expression of Th17- and Treg-specific transcription factors and
associated cytokines in the splenic lymphocytes were analyzed using
RT-qPCR. The results of the present study indicated that the mRNA
expression of IL-17 and ROR-γt in the TNBS group was markedly
increased compared with the control group, while Foxp3 and FGL2
mRNA were significantly downregulated (Fig. 7A-D).
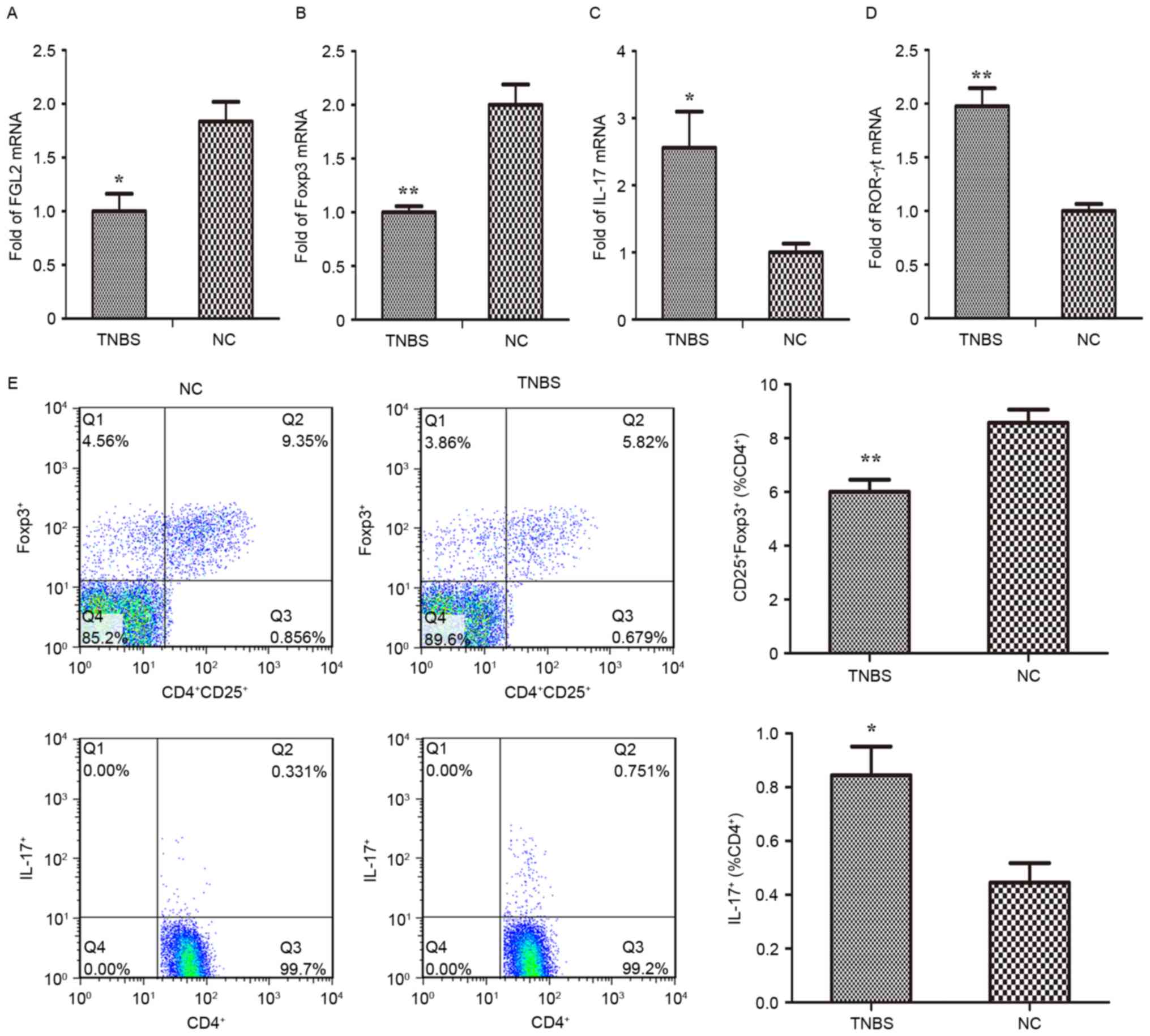 | Figure 7.Analysis of gene expression in
splenic lymphocytes of TNBS-induced inflammatory bowel disease
mice. Reverse transcription-quantitative polymerase chain reaction
analysis was performed to assess the mRNA expression of (A) FGL2,
(B) Foxp3, (C) IL-17 and (D) ROR-γt in splenic lymphocytes. (E)
Flow cytometric analysis of regulatory T and T helper 17 cells was
performed and quantified. *P<0.05, **P<0.001 vs. NC. n=8
mice/group. TNBS, trinitro-benzene-sulfonic acid; NC, normal
control; TNF-α, tumor necrosis factor-α; FGL2, fibrinogen-like
protein 2; IL-17, interleukin-17; ROR-γt, retinoic acid related
orphan receptor-γt; Foxp3, forkhead box protein 3. |
Discussion
The etiopathogenesis of IBD is complex, involving
defects in the mucosal barrier and immune system. In the digestive
system, in the context of IBD, destruction of immune tolerance
results in pathological inflammation. An excessive inflammatory
milieu, and insufficient functioning or quantity of cellular
constituents that downregulate the immune response, may be the
principal causes for the imbalance of the intestinal immune system.
Previous studies have demonstrated that a lack of equilibrium
between Treg and Th17 cells serves a role in inflammation in IBD
(31,32).
Th17 cells and associated pro-inflammatory
mediators, including IL-17, IL-12, IL-23 and IL-26, have been
identified to be novel factors implicated in the pathogenesis of
autoimmune disease. As the receptors for the above cytokines are
expressed by intestinal epithelial cells, Th17 cells exert an
important impact on the development and progression of IBD. Among
several cytokines, IL-17 response is thought to act as the initial
trigger of colitis (11,30,33–35).
While Th17 cells facilitate intestinal inflammation in IBD, Treg
cells exhibit effective anti-inflammatory properties by inhibiting
the cell proliferation and cytokine secretion of Th1 and Th2 cells
(30,32,36).
Previous studies have indicated that Treg cells serve an important
role in the maintenance of mucosal tolerance and are able to cure
experimentally-induced colitis. In addition, the transcription
factor of functional Treg cells, Foxp3, is associated with their
regulatory effect (32,37,38).
Certain studies have demonstrated that important mediators of Treg
cells, including transforming growth factor-β (TGF-β), IL-35 and
IL-10, serve an important role in the anti-inflammatory effect
(39,40); however, a number of studies have
demonstrated that inhibitors of these molecules are unable to block
the inhibitory effect of Treg cells (39,40).
It has been previously demonstrated that FGL2, secreted by T cells,
is involved in the immunomodulatory activity of Treg cells
(39,41). One hypothesis is that FGL2
indirectly inhibits T cell proliferation and immune activity by
suppressing the nuclear factor-κB signaling pathway, to inhibit the
maturity of dendritic cells. FGL2 directly decreases B cell immune
responses through the induction of B cell apoptosis (42). Th17 are able to regulate the
development of Treg cells, and vice versa, to maintain immune
homeostasis and accomplish pathogen clearance (43). TGF-β has been proposed to be the
essential common factor for the proliferation of Th17 and Treg
cells, and not only an anti-inflammatory cytokine produced by Treg
cells. It has been reported that TGF-β promotes the differentiation
of Treg cells by inducing Foxp3 expression, whereas it promotes the
differentiation of Th17 cells with concurrent administration of
IL-6 and TGF-β (43).
In a previous study, the expression of FGL2 was
detected in peripheral blood samples and intestinal tissue from
patients with IBD, and the results demonstrated that the level of
FGL2 was markedly increased in the active period of IBD and
positively correlated to the disease activity index (22). Based on the above previous results,
it may be hypothesized that the immunological activity of FGL2 may
contribute to the pathogenesis of IBD by maintaining the balance
between Treg and Th17 cells. In the present study, the expression
of FGL2 and other cytokines, which are secreted by Treg and Th17
cells, was measured in the TNBS-induced colitis model. The clinical
manifestation, macroscopic scoring and histological examination in
the animal model indicated that the TNBS-induced experimental
animals conformed to the features of IBD. The experimental group
was observed to exhibit an increased expression of TNF-α, which
serves an explicit pathogenic role in IBD. The elevated expression
of FGL2 and IL-17 was observed in the colon tissue of IBD mice
compared with the control group. Similar to the lesion tissue in
the colon, an increase in FGL2 and IL-17 was additionally observed
in the serum of IBD mice. In addition, the proportion of Treg and
Th17 cells was detected in the IBD mice and control group. The
results of the present study exhibited an elevated frequency of
Th17 cells, in addition to a decreased frequency of Treg cells.
This result is consistent with other previous studies (29,33,44).
The results of the present study demonstrated that the mRNA level
of ROR-γt, the transcription factor which guides Th17
differentiation, was increased in the colon tissue and splenic
lymphocytes of IBD mice. However, Foxp3 mRNA was minimally
expressed in splenic lymphocytes, although it was highly expressed
in the colon tissue, suggesting that Treg cells may be recruited to
the intestinal lesion area in order to repress the pro-inflammatory
response (22). Consistent with
Foxp3, increased expression of FGL2 mRNA was demonstrated in the
colon of IBD mice; however, levels in the splenic lymphocytes were
decreased. The alteration in levels of FGL2 was hypothesized to
reflect their accumulation in the inflamed region of the intestinal
tract (22). Therefore,
upregulation of FGL2 in the peripheral blood and intestinal lesions
of IBD mice may counter the pro-inflammatory role of the effecter T
cells, so as to regain balance in the intestinal immune system.
However, the increased level of FGL2 appears to be an insufficient
counter regulation, which fails to inhibit the progression of
IBD.
In conclusion, it was observed that the intestinal
and peripheral expression of FGL2 and IL-17 in TNBS-induced colitis
mice was significantly increased compared with healthy mice, and
that the balance between Th17 and Treg cells was disrupted in the
spleen of the model animals. The present study demonstrated that
FGL2 was associated with the immunopathogenesis of IBD and with
Th17/Treg balance. The present results suggested that the increased
level of Treg-expressed FGL2 may be a potential biomarker for the
assessment of IBD and may lead to a novel therapeutic strategy,
although this hypothesis requires further investigation. Therefore,
future studies will aim to gain an insight into the feasible
mechanisms underlying the immunosuppressive effect of FGL2 in
IBD.
Acknowledgements
The authors of the present study would like to
acknowledge Professor Huiping Zhou of the Department of
Microbiology and Immunology, Virginia Commonwealth University
(Richmond, VA, USA) for assistance with professional
English-language editing. The present study was supported by the
Public Technology Research Project of Zhejiang Province (grant no.
2015C33120) and the Natural Science Foundation of China (grant no.
81570495).
Glossary
Abbreviations
Abbreviations:
|
IBD
|
inflammatory bowel disease
|
|
FGL2
|
fibrinogen-like protein 2
|
|
Treg
|
regulatory cluster of differentiation
4+ T cells
|
|
IL-17
|
interleukin17
|
|
Th17
|
effector cluster of differentiation
4+ T helper 17
|
|
TNBS
|
trinitro-benzene-sulfonic acid
|
|
TNF-α
|
tumor necrosis factor-α
|
|
RT
|
room temperature
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
ROR-γt
|
retinoic acid related orphan
receptor-γt
|
|
Foxp3
|
forkhead box protein 3
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Geremia A, Biancheri P, Allan P, Corazza
GR and Di Sabatino A: Innate and adaptive immunity in inflammatory
bowel disease. Autoimmun Rev. 13:3–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dothel G, Vasina V, Barbara G and De Ponti
F: Animal models of chemically induced intestinal inflammation:
Predictivity and ethical issues. Pharmacol Ther. 139:71–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54.e42; quiz e30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prideaux L, Kamm MA, De Cruz PP, Chan FK
and Ng SC: Inflammatory bowel disease in Asia: A systematic review.
J Gastroenterol Hepatol. 27:1266–1280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou Y, Li WY, Wan Z, Zhao B, He ZW, Wu ZG,
Huang GL, Wang J, Li BB, Lu YJ, et al: Huangqin-tang ameliorates
TNBS-induced colitis by regulating effector and regulatory CD4 (+)
T cells. Biomed Res Int. 2015:1020212015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strober W, Fuss I and Mannon P: The
fundamental basis of inflammatory bowel disease. J Clin Invest.
117:514–521. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matricon J, Barnich N and Ardid D:
Immunopathogenesis of inflammatory bowel disease. Self Nonself.
1:299–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maynard CL and Weaver CT: Intestinal
effector T cells in health and disease. Immunity. 31:389–400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4 T cell populations (*). Annu Rev
Immunol. 28:445–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Almeida Cde S, Andrade-Oliveira V, Câmara
NO, Jacysyn JF and Faquim-Mauro EL: Crotoxin from crotalus durissus
terrificus is able to down-modulate the acute intestinal
inflammation in mice. PLoS One. 10:e01214272015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dambacher J, Beigel F, Zitzmann K, De Toni
EN, Göke B, Diepolder HM, Auernhammer CJ and Brand S: The role of
the novel Th17 cytokine IL-26 in intestinal inflammation. Gut.
58:1207–1217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geremia A and Jewell DP: The IL-23/IL-17
pathway in inflammatory bowel disease. Expert Rev Gastroenterol
Hepatol. 6:223–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valencia X, Stephens G, Goldbach-Mansky R,
Wilson M, Shevach EM and Lipsky PE: TNF downmodulates the function
of human CD4+CD25hi T-regulatory cells. Blood. 108:253–261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cătană CS, Neagoe I Berindan, Cozma V,
Magdaş C, Tăbăran F and Dumitraşcu DL: Contribution of the
IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease.
World J Gastroenterol. 21:5823–5830. 2015.PubMed/NCBI
|
|
16
|
Himmel ME, Hardenberg G, Piccirillo CA,
Steiner TS and Levings MK: The role of T-regulatory cells and
Toll-like receptors in the pathogenesis of human inflammatory bowel
disease. Immunology. 128:145–153. 2008. View Article : Google Scholar
|
|
17
|
Chruscinski A, Sadozai H, Rojas-Luengas V,
Bartczak A, Khattar R, Selzner N and Levy GA: Role of regulatory T
cells (Treg) and the treg effector molecule fibrinogen-like protein
2 in alloimmunity and autoimmunity. Rambam Maimonides Med J.
6:2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bilate AM and Lafaille JJ: Induced
CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev
Immunol. 30:733–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joller N, Lozano E, Burkett PR, Patel B,
Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, et al: Treg cells
expressing the coinhibitory molecule TIGIT selectively inhibit
proinflammatory Th1 and Th17 cell responses. Immunity. 40:569–581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shalev I, Liu H, Koscik C, Bartczak A,
Javadi M, Wong KM, Maknojia A, He W, Liu MF, Diao J, et al:
Targeted deletion of fgl2 leads to impaired regulatory T cell
activity and development of autoimmune glomerulonephritis. J
Immunol. 180:249–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan CW, Kay LS, Khadaroo RG, Chan MW,
Lakatoo S, Young KJ, Zhang L, Gorczynski RM, Cattral M, Rotstein O
and Levy GA: Soluble fibrinogen-like protein 2/fibroleukin exhibits
immunosuppressive properties: Suppressing T cell proliferation and
inhibiting maturation of bone marrow-derived dendritic cells. J
Immunol. 170:4036–4044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong X, Ye X, Chen X, Chen T, Xie S, Li Q,
Lin X and Huang Z: Intestinal and peripheral fibrinogen-like
protein 2 expression in inflammatory bowel disease. Dig Dis Sci.
59:769–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alex P, Zachos NC, Nguyen T, Gonzales L,
Chen TE, Conklin LS, Centola M and Li X: Distinct cytokine patterns
identified from multiplex profiles of murine DSS and TNBS-induced
colitis. Inflamm Bowel Dis. 15:341–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 7:541–546. 2007. View Article : Google Scholar
|
|
25
|
Glauben R, Batra A, Stroh T, Erben U,
Fedke I, Lehr HA, Leoni F, Mascagni P, Dinarello CA, Zeitz M and
Siegmund B: Histone deacetylases: Novel targets for prevention of
colitis-associated cancer in mice. Gut. 57:613–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bell CJ, Gall DG and Wallace JL:
Disruption of colonic electrolyte transport in experimental
colitis. Am J Physiol. 268:G622–G630. 1995.PubMed/NCBI
|
|
27
|
Scheiffele F and Fuss IJ: Induction of
TNBS colitis in mice. Curr Protoc Immunol: Chapter. 15:Unit 15. 19.
2002. View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eastaff-Leung N, Mabarrack N, Barbour A,
Cummins A and Barry S: Foxp3+ regulatory T cells, Th17 effector
cells, and cytokine environment in inflammatory bowel disease. J
Clin Immunol. 30:80–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaudhry A, Rudra D, Treuting P, Samstein
RM, Liang Y, Kas A and Rudensky AY: CD4+ regulatory T cells control
TH17 responses in a Stat3-dependent manner. Science. 326:986–991.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaser A, Zeissig S and Blumberg RS:
Inflammatory bowel disease. Annu Rev Immunol. 28:573–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Veltkamp C, Anstaett M, Wahl K, Möller S,
Gangl S, Bachmann O, Hardtke-Wolenski M, Länger F, Stremmel W,
Manns MP, et al: Apoptosis of regulatory T lymphocytes is increased
in chronic inflammatory bowel disease and reversed by anti-TNFα
treatment. Gut. 60:1345–1353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brand S: Crohn's disease: Th1, Th17 or
both? the change of a paradigm: New immunological and genetic
insights implicate Th17 cells in the pathogenesis of Crohn's
disease. Gut. 58:1152–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kugathasan S, Saubermann LJ, Smith L, Kou
D, Itoh J, Binion DG, Levine AD, Blumberg RS and Fiocchi C: Mucosal
T-cell immunoregulation varies in early and late inflammatory bowel
disease. Gut. 56:1696–1705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson NJ, Boniface K, Chan JR, McKenzie
BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel
F, et al: Development, cytokine profile and function of human
interleukin 17-producing helper T cells. Nat Immunol. 8:950–957.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vignali DA, Collison LW and Workman CJ:
How regulatory T cells work. Nat Rev Immunol. 8:523–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maul J, Loddenkemper C, Mundt P, Berg E,
Giese T, Stallmach A, Zeitz M and Duchmann R: Peripheral and
intestinal regulatory CD4+CD25high T cells in inflammatory bowel
disease. Gastroenterology. 128:1868–1878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shalev I, Wong KM, Foerster K, Zhu Y, Chan
C, Maknojia A, Zhang J, Ma XZ, Yang XC, Gao JF, et al: The novel
CD4+CD25+ regulatory T cell effector molecule fibrinogen-like
protein 2 contributes to the outcome of murine fulminant viral
hepatitis. Hepatology. 49:387–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: Mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Foerster K, Helmy A, Zhu Y, Khattar R,
Adeyi OA, Wong KM, Shalev I, Clark DA, Wong PY, Heathcote EJ, et
al: The novel immunoregulatory molecule FGL2: A potential biomarker
for severity of chronic hepatitis C virus infection. J Hepatol.
53:608–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Shalev I, Manuel J, He W, Leung E,
Crookshank J, Liu MF, Diao J, Cattral M, Clark DA, et al: The
FGL2-FcgammaRIIB pathway: A novel mechanism leading to
immunosuppression. Eur J Immunol. 38:3114–3126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Elshal MF, Aldahlawi AM, Saadah OI and
McCoy JP: Reduced dendritic cells expressing CD200R1 in children
with inflammatory bowel disease: Correlation with Th17 and
regulatory T cells. Int J Mol Sci. 16:28998–29010. 2015. View Article : Google Scholar : PubMed/NCBI
|