Introduction
Age-related macular degeneration (AMD) is a common
cause of irreversible blindness in the elderly in the western
world. It is estimated that 1.47% of the US population (>40
years) are suffering from AMD, and the likelihood of AMD is
expected to increase by 50% (to 2.95 million) by 2020 (1). Although the pathogenic mechanism
underlying the progression of AMD is currently unknown, research
has demonstrated that degenerative and progressive conditions of
retinal pigmented epithelial (RPE) cells are the key pathogenic
mechanisms in AMD (2).
RPE cells serve a critical role in protecting the
outer retina from photo-oxidative stress, maintaining the viability
of photoreceptors and inhibiting retinal edema and
neovascularization (3). Oxidative
stress is considered to be particularly vital in the development of
RPE cell degeneration, dysfunction and apoptosis (4).
NF-E2-related factor 2 (Nrf2) has been demonstrated
to regulate the expression of genes encoding detoxification
enzymes, antioxidant proteins and other stress-response mediators,
including heme oxygenase-1 (HO-1) and NAD(P)H, quinone
oxidoreductase 1 (NQO1) (5). In
vivo and in vitro studies have highlighted the central
role of Nrf2 in protecting RPE cells from a variety of oxidative
challenges. Previous studies have demonstrated that antioxidants
could mitigate oxidative stress in RPE cells by upregulating
Nrf2-regulated phase II enzymes (6,7). A
study demonstrated that RPE cells of aged mice expressed higher
levels of the Nrf2 target genes compared with RPE of younger mice,
especially under unstressed conditions, thereby indicating an
age-associated increase in basal oxidative stress (8). RPE cells of older mice exhibited
impaired induction of the Nrf2 signaling pathway following
oxidative stress, thereby suggesting that the aged RPE cells are
vulnerable to oxidative damage due to impaired Nrf2 signaling
(8). The Nrf2 pathway may serve an
important role in AMD pathogenesis and function as a promising
target for novel pharmacologic or genetic therapeutic
strategies.
Endoplasmic reticulum (ER) stress is considered to
be an early or initial response of cells to stress or damage.
Disturbed ER homeostasis could induce the unfolded protein response
(UPR) accumulation, thereby triggering cell death responses
(9). Growing evidence has
suggested that ER stress serves an important role in retinal
diseases. Libby and Gould (10)
proposed that ER stress could be an important mechanism in the
pathogenesis of AMD. A previous study demonstrated that exposure of
A2E containing ARPE-19 cells to blue light resulted in significant
apoptosis and increased levels of ER stress markers, indicating
that photo-oxidative damage to RPE cells was mediated by the ER
stress-induced intrinsic apoptotic pathway (11). Therefore, therapeutic agents that
inhibit ER stress may protect RPE from dysfunction and apoptosis
during the course of development of AMD.
Quercetin is a ubiquitous flavonoid compound, which
is widely distributed in different fruits and vegetables, including
onions, capers, cranberries, fennel, dark grapes and cocoa.
Experimental data has suggested that quercetin serves as a strong
free radical scavenger and possesses anti-apoptosis, anti-oxidant,
anti-inflammatory and anti-cancer properties (12,13).
Although a previous study demonstrated that precubation with
quercetin could protect RPE cells from
H2O2-induced oxidative damage and attenuate
cellular senescence increase in vitro in a dose-dependent
manner (14), the underlying
mechanism remains unclear. Furthermore, the association between
Nrf2 and quercetin remains to be investigated. The role of
quercetin in promoting the expression of Nrf2 and downstream
signaling molecules and modulating ER stress and apoptosis proteins
remains unclear. To confirm this, the present cultured ARPE-19
cells with quercetin prior to H2O2
stimulation and investigated the underlying molecular
mechanism.
Materials and methods
Materials
The human retinal pigment epithelial cell line
ARPE-19 was obtained from the American Type Culture Collection
(Manassas, VA, USA). Dulbecco's modified Eagle's medium/nutrient
mixture F12 (DMEM/F12) trypsin-EDTA was obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Quercetin,
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), dimethyl
sulfoxide (DMSO) and H2O2 were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). A CellTiter
96® AQueous One Solution Cell Proliferation Assay kit
(MTS) was obtained from Promega Corporation (Madison, WI, USA). The
NE-PER™ Nuclear and Cytoplasmic Extraction reagent was obtained
from Thermo Fisher Scientific, Inc. The antibodies for Nrf2 (cat.
no. ab31163), NQO1 (cat. no. ab80588), HO-1 (cat. no. ab68477) and
Laminin B1 (cat. no. ab109293) were obtained from Abcam (Cambridge,
MA, USA). Antibodies for binding of immunoglobulin protein (Bip;
cat. no. 3177S), CCAAT/enhancer-binding protein homologous protein
(CHOP; cat. no. 5554S), phosphorylated (p) eukaryotic translation
initiation factor 2α (eIF2α; cat. no. 3398), eIF2α (cat. no.
5324S), B-cell lymphoma 2 (Bcl-2; cat. no. 15071S), Bcl-2
X-associated protein (Bax; cat. no. 5023S) and β-actin (cat. no.
12620S), and Alexa Fluor 488-conjugated anti-rabbit secondary
antibody (cat. no. 4412S) were purchased from Cell Signaling
Technology (Beverly, MA, USA). Horseradish peroxidase
(HRP)-conjugated anti-mouse (cat. no. ab6789) and anti-rabbit (cat.
no. ab6721) secondary antibodies were purchased from Abcam. An
enhanced chemiluminescence (ECL) kit and polyvinylidene difluoride
(PVDF) membranes were obtained from EMD Millipore (Billerica, MA,
USA).
In addition, quercetin was freshly dissolved in 100%
DMSO and further diluted with culture medium on the day of
experiment. The final concentration of DMSO in each experiment was
<0.1%. H2O2 was diluted with
double-distilled water to the desired concentration at the
beginning of each experiment.
Cell culture
ARPE-19 cells were cultured in DMEM/F12 supplemented
with 10% fetal bovine serum and 1X penicillin-streptomycin solution
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2/95% air. For monolayer culture, ARPE-19 cells were
seeded at high confluence at a density of 4×105
cells/well, and maintained for 3 days to form a monolayer. The
culture medium was replaced every 48 h. The cells were passaged
once a week, with a split ratio of 1:3.
Cell viability studies
Inhibition of cell proliferation by different
concentrations of H2O2 or quercetin was
measured using an MTS kit according to the manufacturer's protocol.
Briefly, the cells were seeded into 96-well culture plates
(104 cells/well) in 100 µl media, and different
concentrations of H2O2 or quercetin were
added. After treatment, 20 µl MTS solution was added to each well,
and the cells were further incubated in 5% CO2 for 1 h.
The plates were read at 450 nm using a microplate reader (Model
EL800; Omega Bio-Tek, Inc., Norcross, GA, USA). All the experiments
were conducted in triplicate.
Measurement of intracellular reactive
oxygen species (ROS) levels
Production was determined by DCFH-DA staining assay.
Briefly, following treatment, ARPE-19 cells were incubated with 10
µM DCFH-DA at 37°C for 30 min in the dark. The cells were washed
twice with PBS, resuspended, and subjected to flow cytometry with a
CytoFLEX flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) at
excitation and emission wavelengths of 488 nm and 525 nm,
respectively. The results were expressed as fluorescence intensity
of DCF.
Immunofluorescence microscopy
ARPE-19 cells were seeded into 24-well glass slides
(Merck KGaA) at a density of 4×105 per well. After
treatment, the cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde at
room temperature for 20 min. The cells were permeabilized with
0.05% Triton X-100 in PBS for 10 min. After washing with ice-cold
PBS, the cells were blocked with 3% bovine serum albumin (Gibco;
Thermo Fisher Scientific, Inc.) for 15 min, followed by incubation
with primary antibodies against Nrf2 (1:200) overnight at 4°C. The
cells were then washed three times with PBS and incubated for 1 h
with an Alexa Fluor 488-conjugated secondary antibody at room
temperature. 4′-6-diamidino-2-phenylindole (DAPI; 5 mg/ml; Beyotime
Institute of Biotechnology, Nanjing, China) in PBS was used to
stain the nuclei. Fluorescence photographs were acquired by a
fluorescence microscope.
Protein extraction
Following appropriate treatment, cells were
trypsinized and collected by centrifugation at 1,000 × g for 10 min
at room temperature, washed briefly with PBS, resuspended in lysis
buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton
X-100] and supplemented with protease and phosphatase inhibitors
(Thermo Fisher Scientific, Inc.). The suspension was left on ice
for 30 min and then centrifuged at 15,000 × g for 10 min at 4°C.
Cytoplasmic and nuclear extracts were collected using NE-PER™
Nuclear and Cytoplasmic Extraction reagents according to the
manufacturer's protocol. All the protein extracts were stored at
−80°C until use. Protein concentrations were determined using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology).
Western blotting
Equal amounts of extracted protein samples (40 µg)
were separated on Tris-HCl 10% polyacrylamide gels (Ready Gel;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 100 V and
subsequently transferred onto a PVDF membrane. After blocking with
5% instant non-fat dry milk for 1 h, the membranes were incubated
with the following primary antibodies at 4°C overnight: Anti-Nrf2
(1:2,000), anti-NQO1 (1:5,000), anti-HO-1 (1:5,000), anti-Bip
(1:1,000), anti-Chop (1:1,000), anti-p-eIF2α (1:1,000), anti-eIF2α
(1:1,000), anti-Bcl-2 (1:1,000), anti-Bax (1:1,000), anti-β-actin
(1:1,000) and anti-Laminin B1 (1:2,000), followed by incubation
with the following secondary antibodies for 1 h at room
temperature: Alexa Fluor 488-conjugated anti-rabbit secondary
antibody (1:1,000) and HRP-conjugated anti-mouse and anti-rabbit
secondary antibodies (1:1,000). Protein bands were detected with an
enhanced chemiluminescence (ECL) detection kit (EMD Millipore)
using an ECL detection system (GE Healthcare, Chicago, IL, USA).
Blots were semi-quantified by densitometry using Quantity One
software version 4.62 (Bio-Rad Laboratories, Inc.).
Statistics analysis
Each experiment was repeated at least three times.
Data are expressed as the mean ± standard deviation. Statistical
analyses were performed using unpaired Student's t-test for
two-group data and one-way analysis of variance followed by a post
hoc Bonferroni's multiple comparison test for three groups or more.
Statistical analysis was performed using GraphPad Prism software
version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
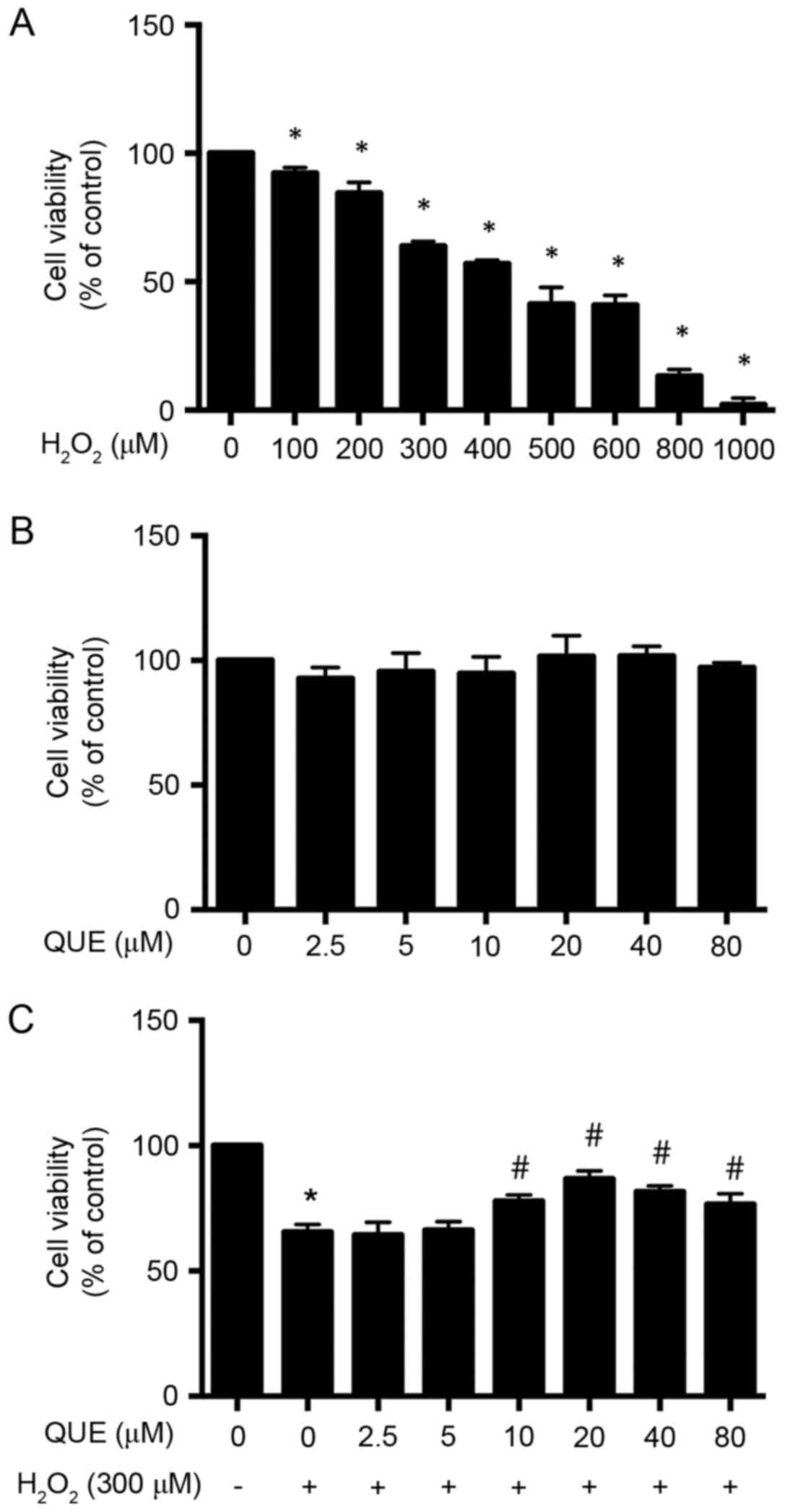
Effects of H2O2
and quercetin on cell viability in ARPE-19 cells
To determine the concentration of
H2O2, ARPE-19 cells were treated with 0–1,000
µM H2O2 for 24 h, and the dose-dependent cell
toxicity of H2O2 was measured by MTS assay.
As presented in Fig. 1A,
H2O2 progressively decreased the viability of
ARPE-19 cells. However, for further studies, 300 µM
H2O2 was used, where the cell viability was
decreased to 63.97±0.99% (P<0.05). In order to evaluate the
potential toxicity quercetin towards ARPE-19 cells, the of cells
were incubated with 0–80 µM quercetin for 24 h, and the cell
viability was tested (Fig. 1B).
Experimental data demonstrated that quercetin treatment alone had
no effect on cell viability; thereby demonstrating that quercetin
at the tested concentrations was safe for cultured ARPE-19
cells.
Effect of quercetin on inhibiting
H2O2-induced toxicity in ARPE-19 cells
To investigate whether quercetin treatment could
protect ARPE-19 cells from H2O2-induced cell
damage, the cells were first incubated in medium containing
different concentrations of quercetin. After 24 h of treatment, the
medium was removed and the cells were exposed to 300 µM
H2O2 for another 24 h. Cell viability was
determined by MTS assay. As presented in Fig. 1C, the cell viability was
significantly decreased to 65.53±1.77% (P<0.05) in cultures
treated with H2O2 alone as compared with
untreated cells. There was no statistically significant difference
in cell viability of incubation with quercetin at low
concentrations of 2.5 µM (64.40±2.89%; P>0.05) and 5 µM
(66.33±1.91%; P>0.05) compared with the
H2O2 group; however, pretreatment of
quercetin at concentrations of 10, 20, 40 and 80 µM protected up to
77.97±1.36% (P<0.05), 86.87±1.80% (P<0.05), 81.67±1.29%
(P<0.05) and 76.67±2.40% (P<0.05) of ARPE-19 cells from
H2O2-induced cell death, compared with
65.53±1.77% in the control group (300 µM
H2O2; Fig.
1C). In this study, 20 µM quercetin was used for all the
experiments.
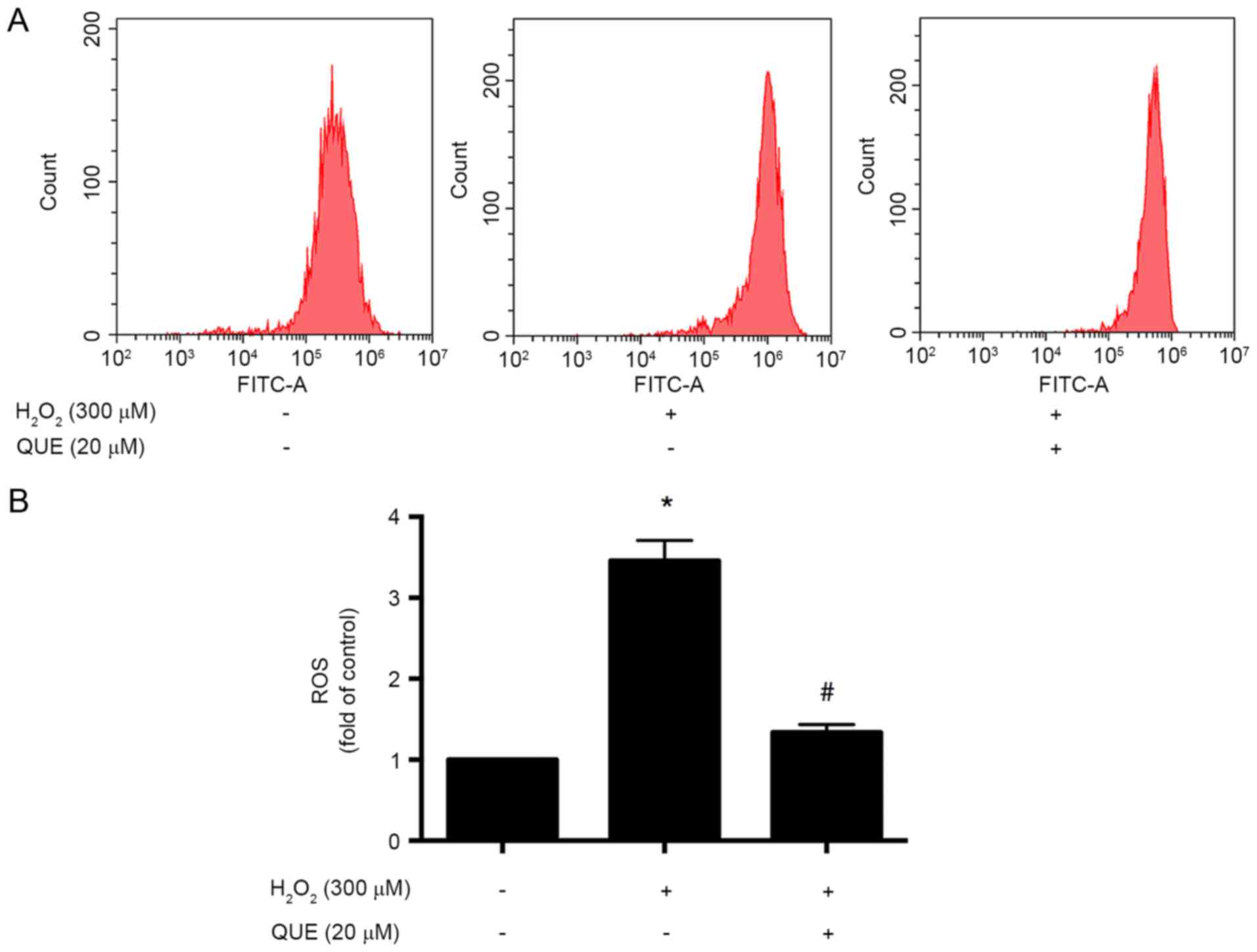
Quercetin pretreatment reduces
H2O2-induced intracellular generation of
ROS
To evaluate the effect of quercetin on
H2O2-induced ROS generation in ARPE-19 cells,
the cells were incubated with 20 µΜ quercetin for 24 h before
exposure to 300 µM H2O2 for another 24 h. It
was observed that treatment of cells with 300 µM
H2O2 resulted in an increase in the
intracellular level of ROS. ROS accumulation was reduced following
pretreatment with quercetin at concentration of 20 µM (Fig. 2).
Effect of quercetin on activation of
Nrf2 and phase II enzymes
It has been well documented that Nrf2 and dependent
genes, NQO1 and HO-1, are important components of the cellular
stress response. To investigate the potential effect of quercetin
on the expression of Nrf2 and NQO1, the cells were incubated with
20 µM quercetin for different time intervals (0–48 h), and the
expression levels of Nrf2 and NQO1 were examined. It was observed
that 20 µM quercetin administration induced protein expression of
Nrf2 and NQO1 in a time-dependent manner (Fig. 3A).
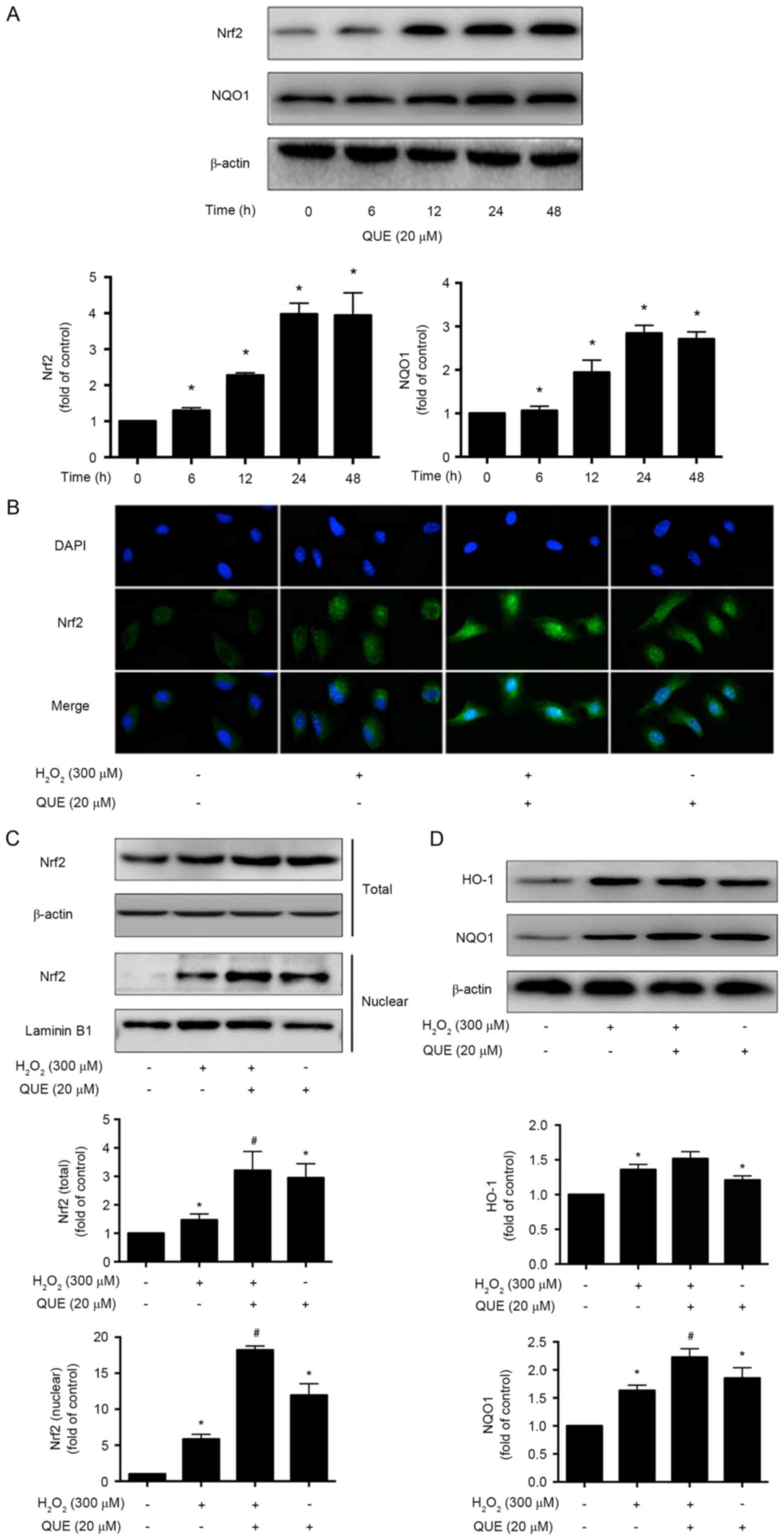 | Figure 3.Effects of quercetin on activation of
Nrf2 and phase II enzymes in ARPE-19 cells. (A) Western blot images
and quantification of Nrf2 and NQO1 protein expression levels in
cells incubated with 20 µM quercetin for 0, 6, 12, 24 and 48 h. (B)
Representative immunofluorescence images of activation of Nrf2 in
ARPE-19 cells. Magnification, ×400. Western blot images and
quantification of (C) total and nuclear Nrf2, and (D) HO-1 and NQO1
protein expression levels in cells. Data are presented as the mean
± standard deviation. *P<0.05 vs. control, #P<0.05
vs. H2O2-induced cells without pretreatment
with quercetin. QUE, quercetin; Nrf2, NF-E2-related factor 2; NQO1,
NAD(P)H, quinone oxidoreductase 1; HO-1, heme oxygenase-1. |
Furthermore, the location of Nrf2 was examined by
immunofluorescence experiments. It was observed that
H2O2 alone could only slightly enhance the
Nrf2 expression in the cytoplasm; however, quercetin treatment
significantly stimulated the expression and accumulation of Nrf2
and induced the nuclear translocation of Nrf2 in ARPE-19 cells
(Fig. 3B). Western blotting
results also revealed that quercetin supplementation enhanced the
total expression levels of Nrf2 and significantly increased nuclear
levels of Nrf2 in the quercetin pretreatment group, compared with
H2O2 (Fig.
3C). The expression level of NQO1 was increased significantly
as well. HO-1 was also increased, but no significant difference was
detected (Fig. 3D).
Effect of quercetin on ER stress
markers under H2O2 stimulation
To confirm the protective effect of quercetin
against H2O2-induced ER stress in ARPE-19
cells, expression levels of ER stress markers were assessed by
western blotting. The results demonstrated that Bip and CHOP
expression levels were downregulated, with eIF2α phosphorylation
reduction, following quercetin pretreatment, compared with
H2O2 alone. This finding demonstrated that
quercetin could inhibit ER stress in ARPE-19 cells during
H2O2-induced injury (Fig. 4A-D).
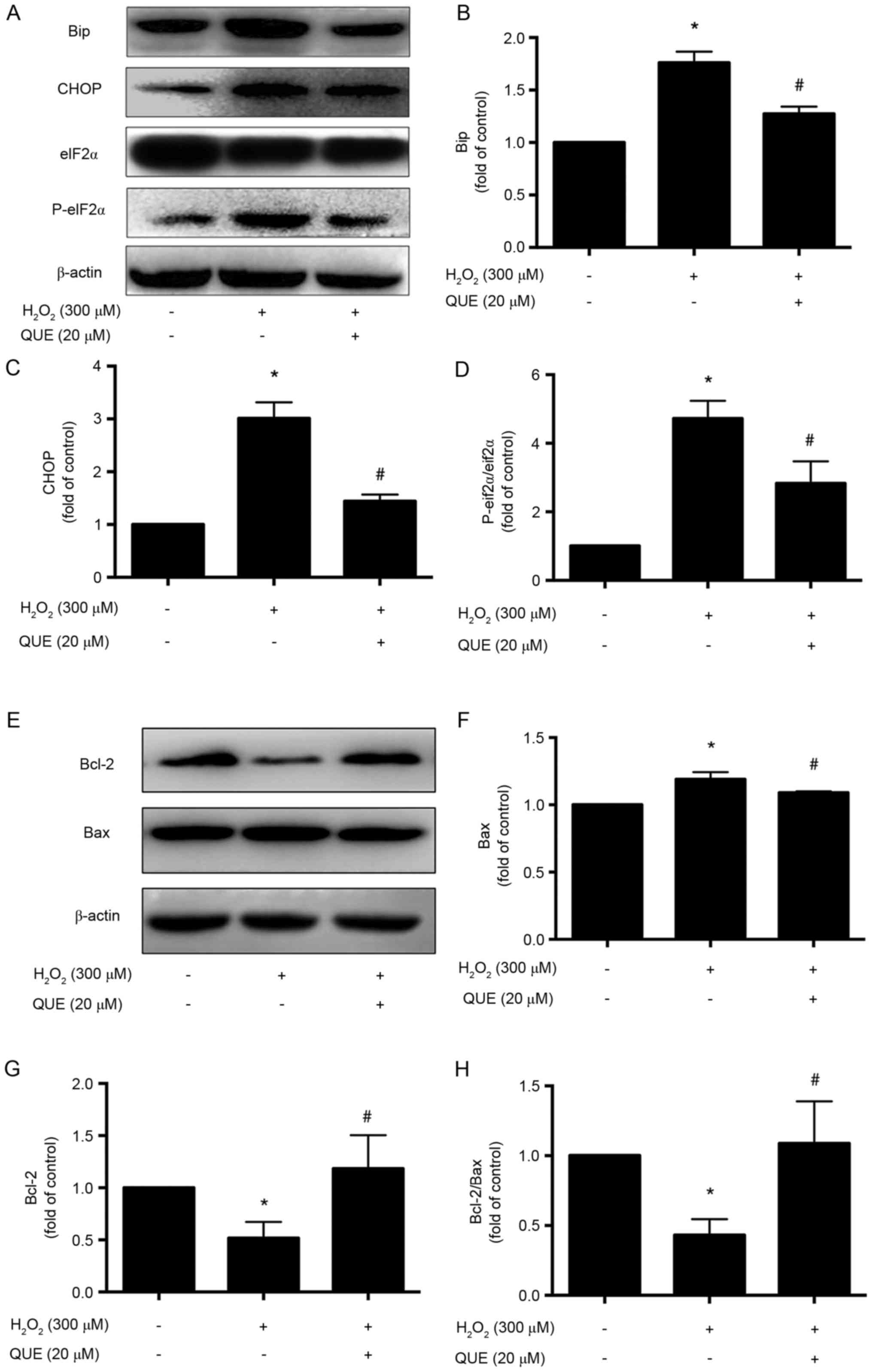 | Figure 4.Effects of quercetin on endoplasmic
reticulum stress markers and apoptosis-associated proteins in
ARPE-19 cells. Cells were incubated with 300 µM
H2O2 with or without 20 µM quercetin
administration. (A) Representative western blot images and
quantification of (B) Bip, (C) CHOP and (D) eIF2α and p-eIF2α
protein expression levels. (E) Representative western blot images
and quantification of (F) Bax, (G) Bcl-2 and (H) the ratio of
Bcl-2/Bax protein expression levels. Data are presented as the mean
± standard deviation. *P<0.05 vs. control, #P<0.05
vs. H2O2-induced cells without pretreatment
with quercetin. QUE, quercetin; Bip, binding of immunoglobulin
protein; CHOP, CCAAT/enhancer-binding protein homologous protein;
p, phosphorylated; eIF2α, eukaryotic translation initiation factor
2α; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 X associated protein. |
Effect of quercetin on
apoptosis-associated proteins in ARPE-19 cells
To further investigate the effect of quercetin on
apoptosis-associated proteins, the expression levels of Bcl-2 and
Bax were evaluated. The results demonstrated that in the
quercetin-pretreated group, the expression level of Bcl-2 was
upregulated, and Bax was downregulated (Fig. 4E-G). The Bcl-2/Bax ratio declined
in H2O2 group, while it was significantly
increased with the administration of quercetin (20 µΜ; Fig. 4H).
Discussion
Quercetin is an important dietary polyphenol, which
is present in several foods. The present study assessed the
cytotoxic effects of quercetin on ARPE-19 cells and observed that
quercetin does not influence the viability of APRE-19 cells. The
results demonstrated that a higher concentration of quercetin
attenuated H2O2-induced cytotoxicity in
ARPE-19 cells, particularly at 20 µΜ concentration.
ROS have been implicated in the etiology of a number
of physiological and pathological conditions, and diseases.
Oxidative stress, produced by excess ROS, has been identified to
serve a critical role in the injury and degeneration of RPE. In the
present study, it was observed that the level of intercellular ROS
were increased significantly after H2O2
treatment, as demonstrated by the conversion of DCFH-DA into DCF,
while pretreatment with quercetin significantly reduced ROS
accumulation, indicating that the cytoprotection conferred by
quercetin was due to its antioxidant effect.
It is well-known that Nrf2 is a ubiquitous
transcription factor, which significantly influences the
maintenance of cellular redox status. During homeostasis, Nrf2 is
sequestered in the cytoplasm by binding to cytoskeleton-binding
Kelch-like ECH-associated protein 1 (Keap1) and degraded through
the ubiquitin-26S proteasome pathway. Under oxidative stress, Nrf2
is uncoupled with Keap1 and is rapidly translocated into the
nucleus, were it heterodimerizes with Maf proteins and binds to the
antioxidant response elements (AREs) in the promoters of its target
genes, such as NQO1 and HO-1 (15). A previous study demonstrated that
quercetin could activate Nrf2 by upregulating the steady-state
level of Nrf2 at the transcriptional level, as well as stabilizing
Nrf2 protein post-transcription in HepG2 cells (16). In keeping with these findings, the
present study provided direct evidence that quercetin alone had the
potential of elevating the expression levels of Nrf2 and NQO1 in a
time-dependent manner. It was confirmed that quercetin treatment
significantly resulted in translocation of Nrf2 to the nucleus, as
detected by immunostaining. In addition, the expression levels of
Nrf2 and its target genes were significantly elevated in cells
following quercetin pretreatment, compared with
H2O2 treatment alone. Therefore, quercetin
exerts anti-oxidative effects on ARPE-19 cells via the Nrf2-ARE
signaling pathway.
There is increasing evidence that induction of ER
stress occurs in exposure to oxidative stress, which serves a
crucial role in the RPE cell dysfunction and death. Oxidative
stress and ER stress are interrelated biological events, and both
participate in RPE apoptosis. Previous studies have demonstrated
that oxidative stress increases the accumulation of ROS in the ER
and subsequently triggers ER stress after
H2O2 stimulation or A2E and blue
light-induced damage (17,18). Huang et al (19) revealed that cigarette smoke extract
(CSE) exposure induced a dose- and time-dependent increase in ER
stress markers, and enhanced ROS, mitochondrial fragmentation and
apoptosis of RPE cells, while the ROS scavenger N-acetylcysteine
reduced the expression of ER stress protein. These findings
suggested a close interaction between oxidative and ER stress in
CSE-induced apoptosis (19). In
the present study, it was observed that H2O2
increased the level of intracellular ROS and triggered ER stress
markers expression in ARPE-19 cells. However, quercetin could
significantly reduce ROS accumulation, and decrease the expression
levels of ER stress markers Bip and CHOP. As ROS have been
demonstrated to trigger ER stress, it could be hypothesized that
the anti-oxidative activity of quercetin partially contributed to
the inhibitory effect on ER stress. In addition, the results
demonstrated that CHOP upregulation was in consistent with the
increase in phosphorylation of eIF2α, a downstream target of
protein kinase RNA-like endoplasmic reticulum kinase (PERK),
suggesting activation of the PERK-eIF2α-activator of transcription
factor 4 (ATF4)-CHOP branch of ER stress pathways. In response to
stressors, PERK phosphorylates eIF2α to repress translation of
proteins. eIF2α phosphorylation preferentially upregulates the
translation of ATF4, which then activates expression of its
downstream target genes (20).
Thus, pretreatment with quercetin may re-establish ER homoeostasis.
Hayakawa et al (21)
demonstrated that quercetin could reduce eIF2α phosphorylation and
ATF4 expression via damaged-inducible gene 34 induction in the
brain. However, the ROS-independent effects of quercetin on
alleviating ER stress require further investigation.
Bcl-2 and Bax, belonging to the Bcl-2 family, are
associated with physiological and pathological apoptosis. Bcl-2
mediates cell survival through sequestration of BH3-only proteins,
which are necessary for Bax-mediated mitochondrial permeabilization
and apoptosis (22). The
anti-apoptotic effects of quercetin have been reported in several
cells, including neural, cardiomyoblast and endothelial cells
(23,24). The results of the present study
have demonstrated that quercetin pretreatment reduced the
production of Bax and promoted the generation of Bcl-2, thereby
indicating that quercetin might protect ARPE-19 cells by activating
pro-survival proteins and inhibiting pro-apoptotic proteins. ER
stress can regulate a number of apoptosis-associated proteins that
localize on the mitochondrial membrane, particularly the members of
the Bcl-2 family. CHOP has been defined as a pivotal mediator of
cell death signaling in ER stress, and it also has been suggested
that prolonged activation of CHOP promotes apoptosis by
downregulating the expression of Bcl-2 (25). Quercetin may upregulate Bcl-2
expression partially by suppressing the expression of CHOP.
However, according to previous research, quercetin treatment could
result in cell apoptosis in different kinds of cancer cells.
Quercetin directly binds to the BH3 domain of Bcl-2 and Bcl-xL
proteins, thereby inhibiting their activity and promoting cancer
cell apoptosis (26). Therefore,
quercetin might have contrasting effects under different
conditions.
Xu et al (27) demonstrated that quercetin did not
effectively reduce the contents of ROS in
H2O2-treated ARPE-19 cells, whereas the
phospholipid complex (PC) of quercetin could significantly decrease
their level. Quercetin-PC, but not quercetin, was demonstrated to
upregulate the protein expression levels of HO-1 and NQO1.
Contradictory to this, the result of the present study demonstrated
that 20 uM quercetin could significantly elevate Nrf2 and NQO1 in a
time-dependent manner, ROS accumulation was reduced following
pretreatment with quercetin. Consistent with this, Zhu et al
(28) revealed that quercetin (20
µΜ) significantly blocked UVB irradiation (15
mJ/cm2)-induced intracellular ROS generation (28). Therefore, it was hypothesized that
quercetin could have antioxidant properties in
H2O2-treated ARPE-19 cells at relatively low
concentrations. Kook et al (29) first demonstrated that quercetin was
able to protect RPE cells from oxidative damage and cellular
senescence in vitro in a dose-dependent manner.
In conclusion, the present study confirmed the
antioxidant effect of quercetin in ARPE-19 cells and further
highlighted the possible mechanisms-activation of the Nrf2
signaling pathway, attenuation of ER stress, and the involvement in
regulation of apoptosis. These findings offer some novel
therapeutic strategies and methods for various oxidative
stress-associated retinal degenerative diseases, such as AMD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81300774 and
81200701) and the Natural Science Foundation of Shanghai (grant no.
12ZR1424500).
References
|
1
|
Friedman DS, O'Colmain BJ, Muñoz B, Tomany
SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J, et al:
Eye Diseases Prevalence Research Group: Prevalence of age-related
macular degeneration in the United States. Arch Ophthalmol.
122:564–572. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Lookeren Campagne M, LeCouter J,
Yaspan BL and Ye W: Mechanisms of age-related macular degeneration
and therapeutic opportunities. J Pathol. 232:151–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blasiak J, Petrovski G, Vereb Z, Facskó A
and Kaarniranta K: Oxidative stress, hypoxia and autophagy in the
neovascular processes of age-related macular degeneration. Biomed
Res Int. 2014:7680262014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plafker SM, O'Mealey GB and Szweda LI:
Mechanisms for countering oxidative stress and damage in retinal
pigment epithelium. Int Rev Cell Mol Biol. 298:135–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Q, Pi J, Woods CG and Andersen ME: A
systems biology perspective on Nrf2-mediated antioxidant response.
Toxicol Appl Pharmacol. 244:84–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao X and Talalay P: Induction of phase 2
genes by sulforaphane protects retinal pigment epithelial cells
against photooxidative damage. Proc Natl Acad Sci USA.
101:10446–10451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y,
Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from
oxidative stress via upregulation of Nrf2-regulated phase II
enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666.
2013.PubMed/NCBI
|
|
8
|
Sachdeva MM, Cano M and Handa JT: Nrf2
signaling is impaired in the aging RPE given an oxidative insult.
Exp Eye Res. 119:111–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dandekar A, Mendez R and Zhang K: Cross
talk between ER stress, oxidative stress, and inflammation in
health and disease. Methods Mol Biol. 1292:205–214. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Libby RT and Gould DB: Endoplasmic
reticulum stress as a primary pathogenic mechanism leading to
age-related macular degeneration. Adv Exp Med Biol. 664:403–409.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Z, Sun T, Jiang Y, Wu L, Cai X and
Sun X and Sun X: Photooxidative damage in retinal pigment
epithelial cells via GRP78 and the protective role of grape skin
polyphenols. Food Chem Toxicol. 74:216–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Andrea G: Quercetin: A flavonol with
multifaceted therapeutic applications? Fitoterapia. 106:256–271.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobylinska A and Janas KM:
Health-promoting effect of quercetin in human diet. Postepy Hig Med
Dosw (Online). 69:51–62. 2015.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kook D, Wolf AH, Yu AL, Neubauer AS,
Priglinger SG, Kampik A and Welge-Lüssen UC: The protective effect
of quercetin against oxidative stress in the human RPE in vitro.
Invest Ophthalmol Vis Sci. 49:1712–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanigawa S, Fujii M and Hou DX: Action of
Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free
Radic Biol Med. 42:1690–1703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He S, Yaung J, Kim YH, Barron E, Ryan SJ
and Hinton DR: Endoplasmic reticulum stress induced by oxidative
stress in retinal pigment epithelial cells. Graefes Arch Clin Exp
Ophthalmol. 246:677–683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng J, Chen X and Sun X, Wang F and Sun
X: Expression of endoplasmic reticulum stress markers GRP78 and
CHOP induced by oxidative stress in blue light-mediated damage of
A2E-containing retinal pigment epithelium cells. Ophthalmic Res.
52:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang C, Wang JJ, Ma JH, Jin C, Yu Q and
Zhang SX: Activation of the UPR protects against cigarette
smoke-induced RPE apoptosis through up-regulation of Nrf2. J Biol
Chem. 290:5367–5380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang ZQ, Ma YX, Li MH, Zhan XQ, Zhang X
and Wang MY: 5-Hydroxymethylfurfural protects against ER
stress-induced apoptosis in GalN/TNF-α-injured L02 hepatocytes
through regulating the PERK-eIF2α signaling pathway. Chin J Nat
Med. 13:896–905. 2015.PubMed/NCBI
|
|
21
|
Hayakawa M, Itoh M, Ohta K, Li S, Ueda M,
Wang MX, Nishida E, Islam S, Suzuki C, Ohzawa K, et al: Quercetin
reduces eIF2α phosphorylation by GADD34 induction. Neurobiol Aging.
36:2509–2518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng JH, Follis A Viacava, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–7000. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suematsu N, Hosoda M and Fujimori K:
Protective effects of quercetin against hydrogen peroxide-induced
apoptosis in human neuronal SH-SY5Y cells. Neurosci Lett.
504:223–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang XQ, Yao RQ, Liu X, Huang JJ, Qi DS
and Yang LH: Quercetin protects oligodendrocyte precursor cells
from oxygen/glucose deprivation injury in vitro via the activation
of the PI3K/Akt signaling pathway. Brain Res Bull. 86:277–284.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao
M, Liu D, Qiao L, Li N, Zheng J and Chen D: CHOP mediates
ASPP2-induced autophagic apoptosis in hepatoma cells by releasing
Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2.
Cell Death Dis. 5:e13232014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Primikyri A, Chatziathanasiadou MV, Karali
E, Kostaras E, Mantzaris MD, Hatzimichael E, Shin JS, Chi SW,
Briasoulis E, Kolettas E, et al: Direct binding of Bcl-2 family
proteins by quercetin triggers its pro-apoptotic activity. ACS Chem
Biol. 9:2737–2741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu XR, Yu HT, Yang Y, Hang L, Yang XW and
Ding SH: Quercetin phospholipid complex significantly protects
against oxidative injury in ARPE-19 cells associated with
activation of Nrf2 pathway. Eur J Pharmacol. 770:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu X, Li N, Wang Y, Ding L, Chen H, Yu Y
and Shi X: Protective effects of quercetin on UVB
irradiation-induced cytotoxicity through ROS clearance in
keratinocyte cells. Oncol Rep. 37:209–218. 2017.PubMed/NCBI
|
|
29
|
Kook D, Wolf AH, Yu AL, Neubauer AS,
Priglinger SG, Kampik A and Welge-Lüssen UC: The protective effect
of quercetin against oxidative stress in the human RPE in vitro.
Invest Ophthalmol Vis Sci. 49:1712–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|