Introduction
It is widely accepted that microbiota in the gut
serves a pivotal role in host physiology by interacting with the
immune and neuroendocrine systems in gastrointestinal (GI) tissues
(1–4). In addition, gut hormones, which are
key players in the neuroendocrine system, have various important
functions in secretion, metabolism and motility (5,6).
Therefore, it is hypothesized that gut microbiota may be involved
in the fine-tuning of GI motility through modification of
neuroendocrine signaling; however, this hypothesis has not been
completely tested to date.
Serotonin or 5-hydroxytryptamine (5-HT),
predominantly synthesized by GI enterochromaffin cells (7), acts as a neurotransmitter and/or
local hormone in the enteric nervous system, resulting in
alteration of GI motility (7,8). Gut
microbiota is known to affect the behavior of 5-HT-producing
enterochromaffin cells in the GI mucosa (9), and have been reported to regulate GI
motility by modifying 5-HT expression in the GI tract (10). Previously, evidence that 5-HT
signaling affects the enteric nervous system and the immune system
in the GI wall has been reported (8). 5-HT is important in M2 macrophage
polarization and cytokine production in GI tissues (11). In addition, it has been reported
that macrophages in the muscular layer may act on the enteric
nervous system via the GI wall (2), although the mechanism underlying the
5-HT/muscularis macrophage axis and its effect on GI motility
remains unclear. 5-HT has been extensively studied as a therapeutic
target for functional GI disorders, which involve a significant
disturbance in GI motility (7). In
this regard, it would be meaningful to clarify the role of the
5-HT/muscularis macrophage axis in GI motility in humans. However,
GI muscular layer tissues cannot be collected from humans,
therefore animal experiments are required to approach these issues.
In addition, it would be of interest to determine how gut
microbiota are involved in the 5-HT/muscularis macrophage axis in
GI motility in humans. However, this theme is also difficult to
examine in the human body, and therefore germ-free (GF) animals are
vital to examine the effect of gut microbiota on GI physiology. In
the present study, intestinal microorganisms were transplanted into
GF mice in order to examine how gut microbiota affects the
association between GI motility, and 5-HT expression and M2
macrophage abundance in the GI tract.
Materials and methods
Animal and experimental design
A total of 22 GF (6 or 10 weeks old; male; ICR
strain; weight, 26 to 40 g) and 6 specific pathogen-free (SPF; 7 to
9 weeks old; male; ICR strain; weight, 33 to 42 g) mice were
purchased from CLEA Japan, Inc. (Tokyo, Japan). Fecal suspensions
were freshly prepared from SPF mice by 10-fold dilution of colonic
content with saline, as reported previously (12). GF mice at 6 weeks of age were
orally administered these fecal suspensions to reconstitute the
intestinal flora. Thereafter, the GF mice that had undergone fecal
transplantation (FT) were housed under SPF conditions and
sacrificed 4 weeks following FT. Non-microflora-reconstituted GF
mice at 6 and 10 weeks of age were used as controls. The
experimental protocol was approved by the Animal Use and Care
Committee at Hyogo College of Medicine (Nishinomiya, Japan).
Histopathological evaluation
The GI tissues were removed from experimental mice
and fixed in 10% buffered formalin overnight at room temperature,
sliced perpendicularly to the surface, embedded in paraffin, and
cut into sections 4-µm thick. The sections were deparaffinized in
xylene, rehydrated in a series of ethanol and stained with 100%
hematoxylin (room temperature for 2 min) and 0.1% eosin (room
temperature for 1 min) for histopathological observation under a
light microscope (Olympus CX41; Olympus Corporation, Tokyo,
Japan).
Immunohistochemistry
Immunohistochemical staining for 5-HT and mannose
receptor (MR; a marker of M2-polarized macrophages) was performed
using the Envision Kit (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA), according to the manufacturer's protocol. The
primary antibodies used were: anti-5-HT (1:100,000 dilution; cat.
no. IST-20080; ImmunoStar, Inc., Hudson, WI, USA) and anti-MR
(1:1,000 dilution; cat. no. ab64693; Abcam, Cambridge, UK). As
previously described (13), the
sections were deparaffinized, rehydrated, placed in 1X Dako REAL
Target Retrieval Solution (Dako; Agilent Technologies, Inc.), and
heated in the microwave for 20 min. The sections were subsequently
preincubated with 0.3% H2O2 in methanol for
25 min at room temperature to quench endogenous peroxidase
activity. The sections were incubated with primary antibodies for 1
h at room temperature. The slides were incubated with horseradish
peroxidase-conjugated secondary antibodies (ready-to-use; cat. nos.
K4001 or K4003; Dako; Agilent Technologies, Inc.) at room
temperature for 30 min, visualized by 3,3′-diaminobenzide
tetrahydrochloride with 0.05% H2O2 for 3 min,
and counterstained with Mayer's hematoxylin. Under a light
microscope (Olympus CX41; Olympus Corporation), 5-HT-positive
epithelial cells were counted by eye in a 1,000 µm stretch of the
entire length of well-oriented tissue sections in at least 5
randomly selected fields from the stomach to colon of each mouse,
and the average was calculated. Similarly, the number of
MR-positive cells was evaluated in the lamina propria and muscle
layer throughout the GI tract.
GI transit time (GITT)
GITT was measured as described previously (14). The mice orally received 0.3 ml of
0.5% methylcellulose solution including 6% carmine red (Wako Pure
Chemical Industries, Ltd., Osaka, Japan). Following administration
of the solution, the mice were allowed free access to food and
water ad libitum until the first red fecal pellet appeared.
GITT was determined as the time period between oral gavage and the
appearance of the first red fecal pellet.
Statistical analysis
Statistical analyses were performed using StatView
5.0 software (SAS Institute, Inc., Buckinghamshire, UK). All values
were expressed as the mean ± standard error of the mean.
Differences between two animal groups were analyzed using the
Mann-Whitney U-test. The correlation among GITT, 5-HT expression
and MR expression was assessed by linear regression analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of 5-HT in the GI tract of
FT-treated mice
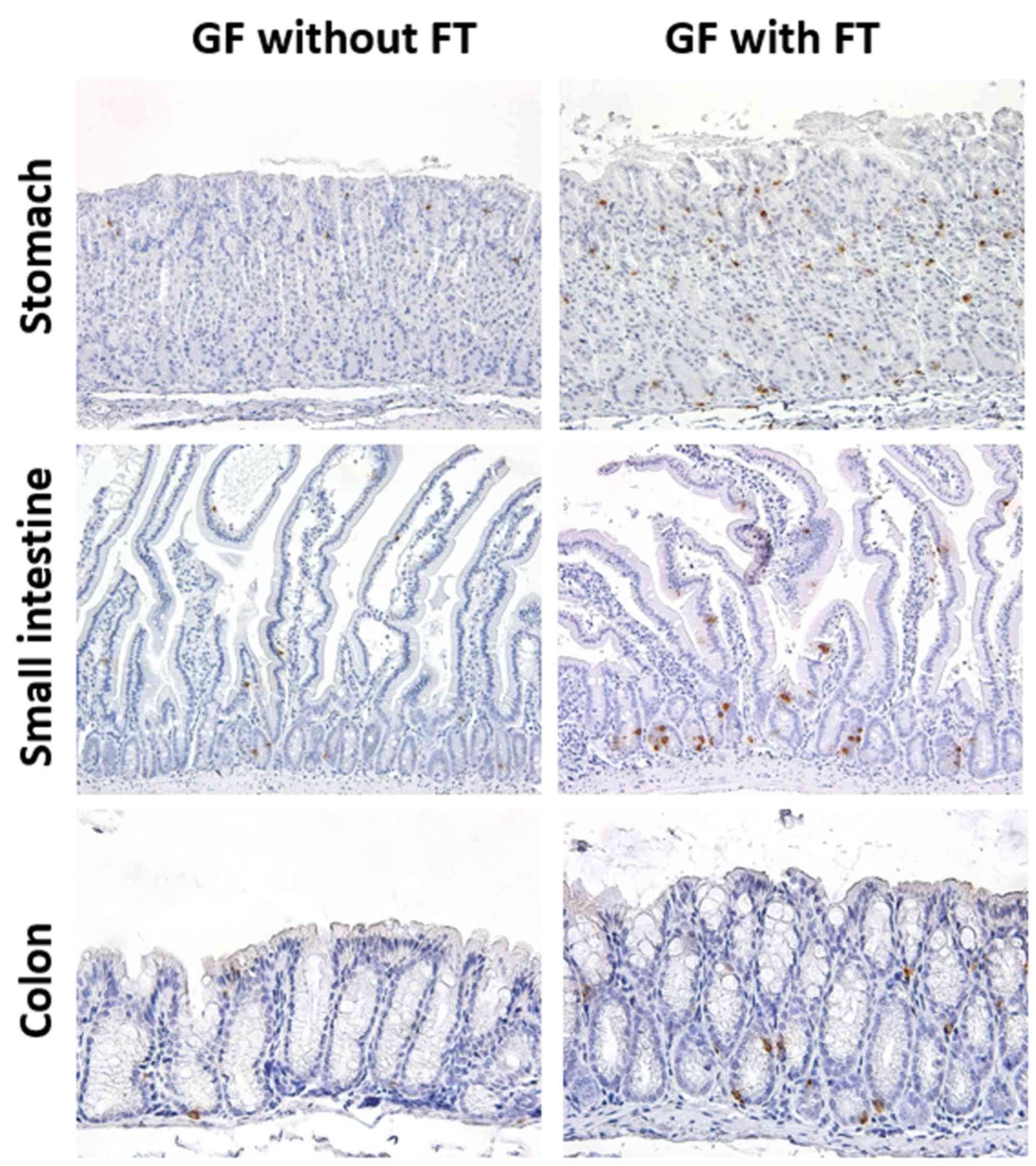
In GF mice, 5-HT-positive cells were observed
throughout the GI epithelium (Fig.
1). The 5-HT immunoreactivity was localized in the cytoplasm of
ovoid or pyramidal epithelial cells throughout the GI mucosa, a
staining pattern that was morphologically compatible with endocrine
cells (Fig. 1). Expression of 5-HT
protein was subsequently evaluated in the GI tissues of 6-week-old
GF mice at 4 weeks post-FT, and age-matched control mice that did
not receive FT (Figs. 1 and
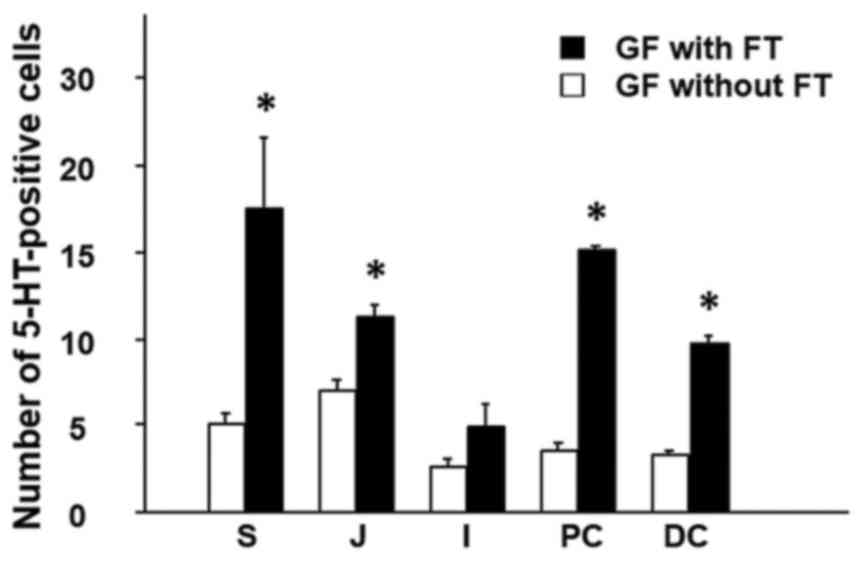
2). The results demonstrated that
the number of 5-HT-positive cells was significantly increased in
the colonic mucosa (proximal and distal colon) of GF mice with FT,
compared with mice without FT (Fig.
2). In addition, the number of 5-HT-positive cells was
significantly increased in not only the gastric mucosa (stomach),
but also the proximal small-intestinal mucosa (jejunum) in mice
with FT compared with mice without FT (Fig. 2).
Expression of MR in the GI tract of
FT-treated mice
MR protein expression, as a marker of M2-polarized
macrophages, was evaluated in GI tissues by immunohistochemistry.
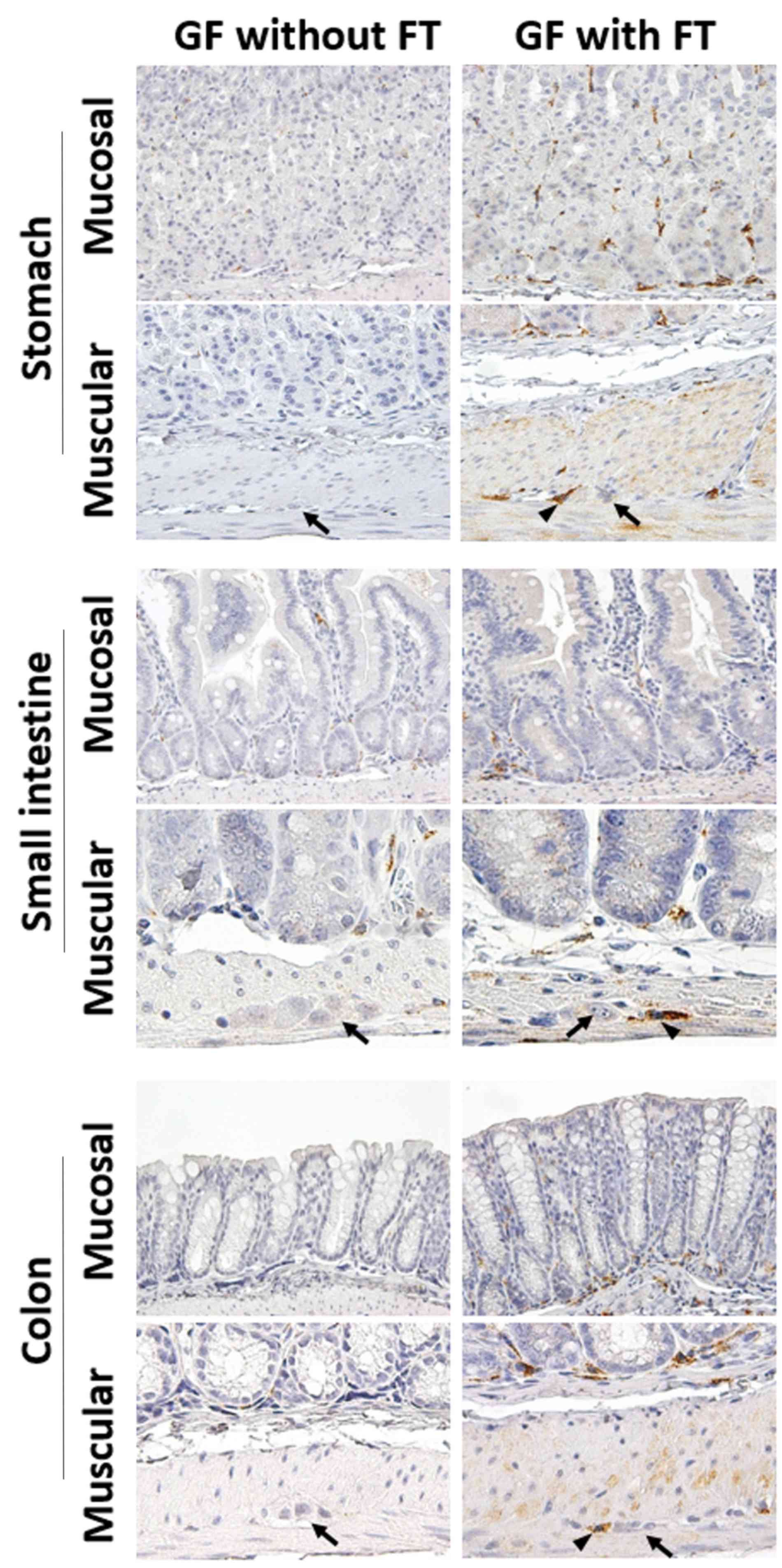
In GF mice, immunoreactivity for MR was detected in immune cells
of, not only the lamina propria (mucosal layer), but also the
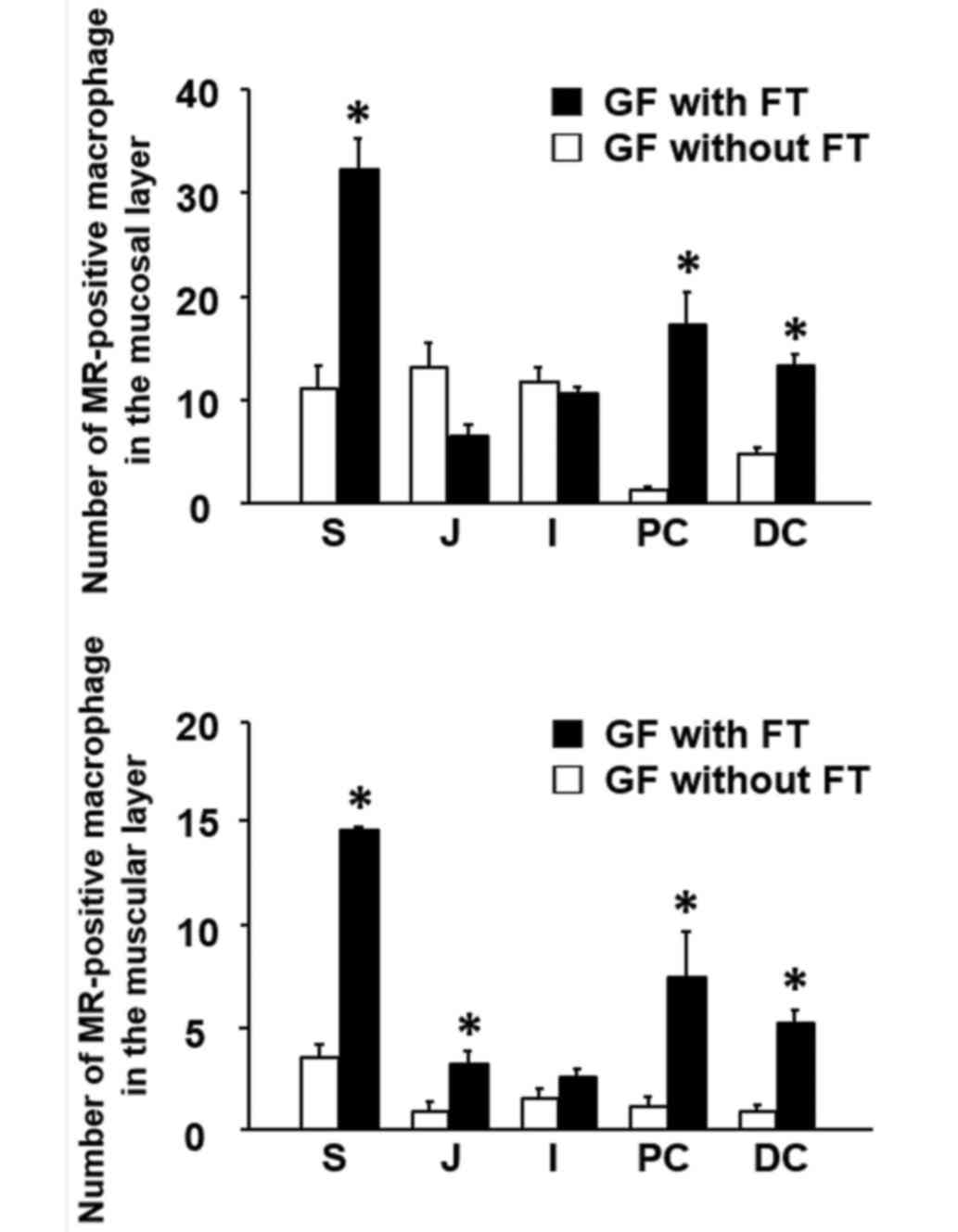
muscular layer throughout the GI tract (Fig. 3). Following FT, the number of
MR-positive cells was significantly increased in the lamina propria
of the stomach and colon in GF mice, compared with mice without FT
(Fig. 4). The number of
MR-positive cells was also significantly increased in the muscular
layer of the upper GI tract (stomach and jejunum) and colon
(proximal and distal) of FT-treated mice, compared with mice
without FT (Fig. 4). Notably,
MR-positive cells were frequently present around the myenteric
neural cells between the circular and longitudinal muscle layers
throughout the GI tract (Fig. 3).
These results suggest that treatment with FT is associated with
increased infiltration of MR-positive cells, presumably M2
macrophages, into both the mucosal and the muscular layers
throughout the GI tract.
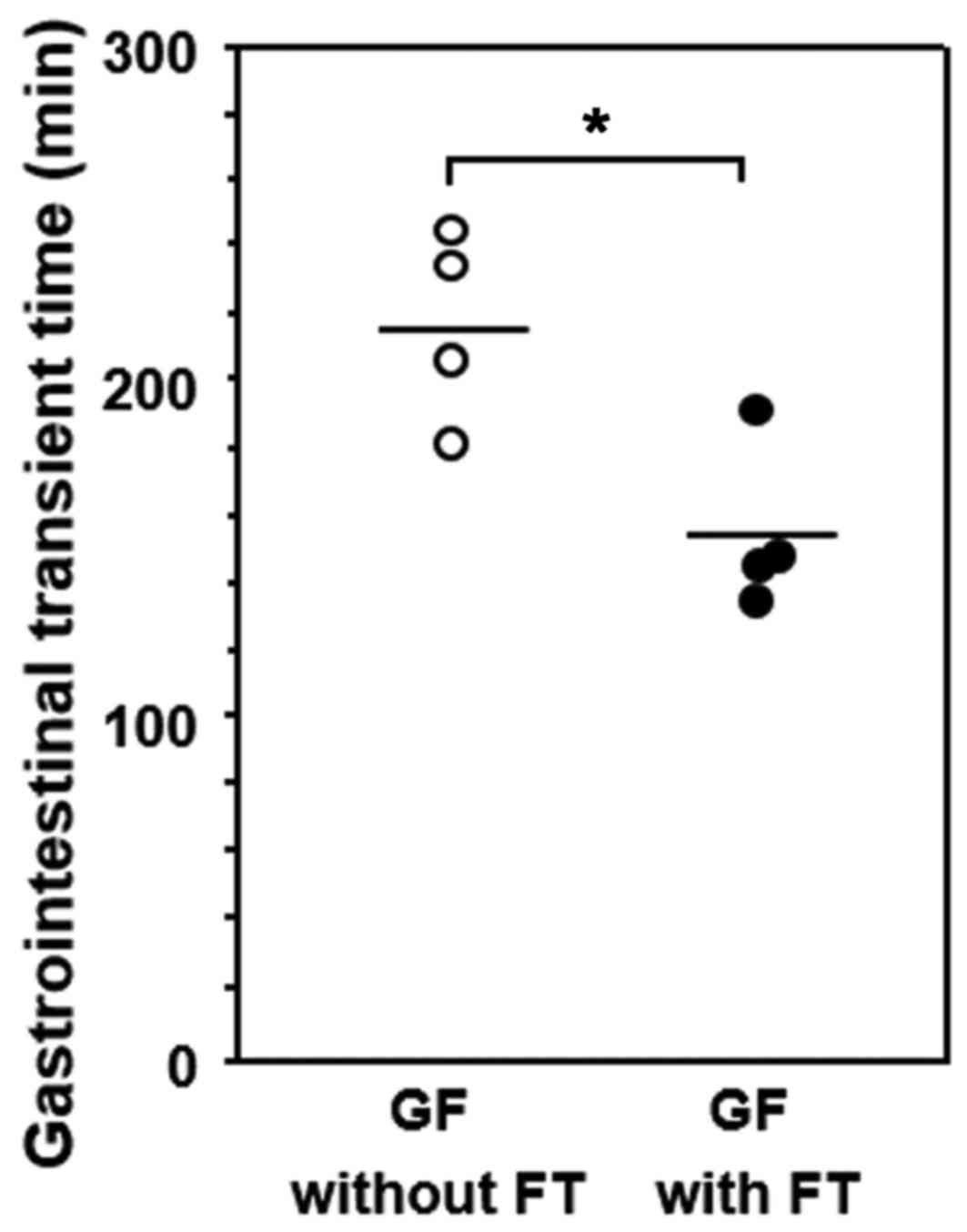
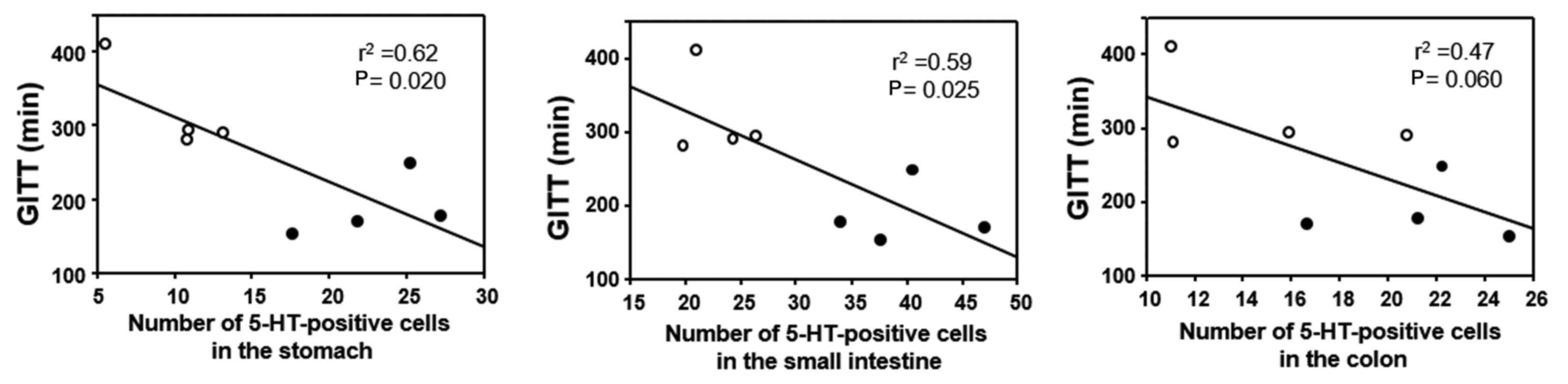
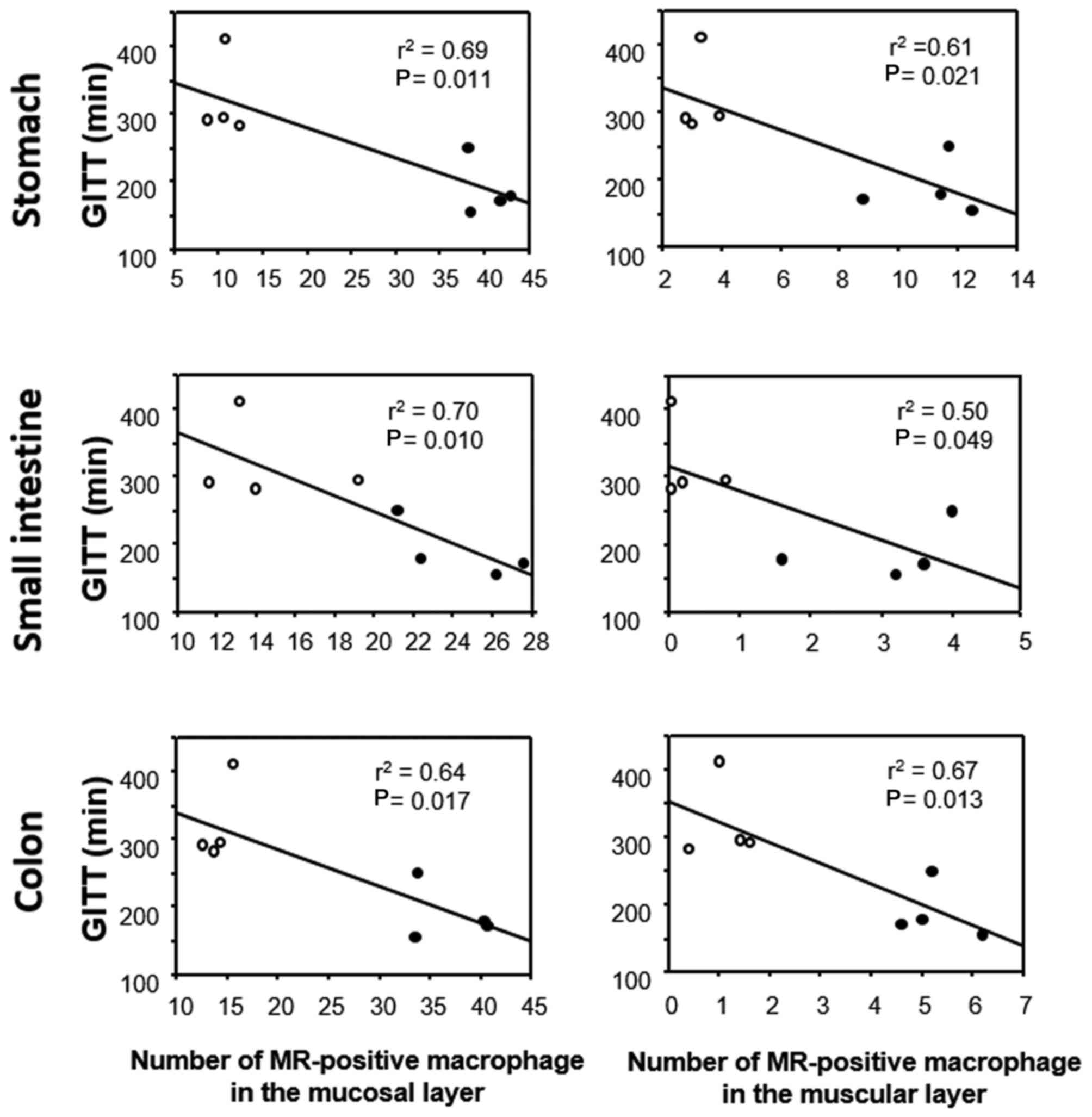
GITT and its association with 5-HT or
MR expression in GF mice
GITT was significantly decreased in GF mice
subjected to FT compared with age-matched GF mice without FT
(Fig. 5). The correlation between
GITT and 5-HT or MR expression was investigated in the experimental
mice (FT-treated and untreated) by linear regression analysis. GITT
was negatively correlated with the number of 5-HT-positive cells in
the gastric and small-intestinal mucosa (Fig. 6), whereas no correlation was
evident for GITT with 5-HT-positive cells in the colonic mucosa
(Fig. 6). GITT was negatively
correlated with the number of MR-positive cells in the lamina
propria and the number of MR-positive cells in the muscular layer
throughout the GI wall (Fig.
7).
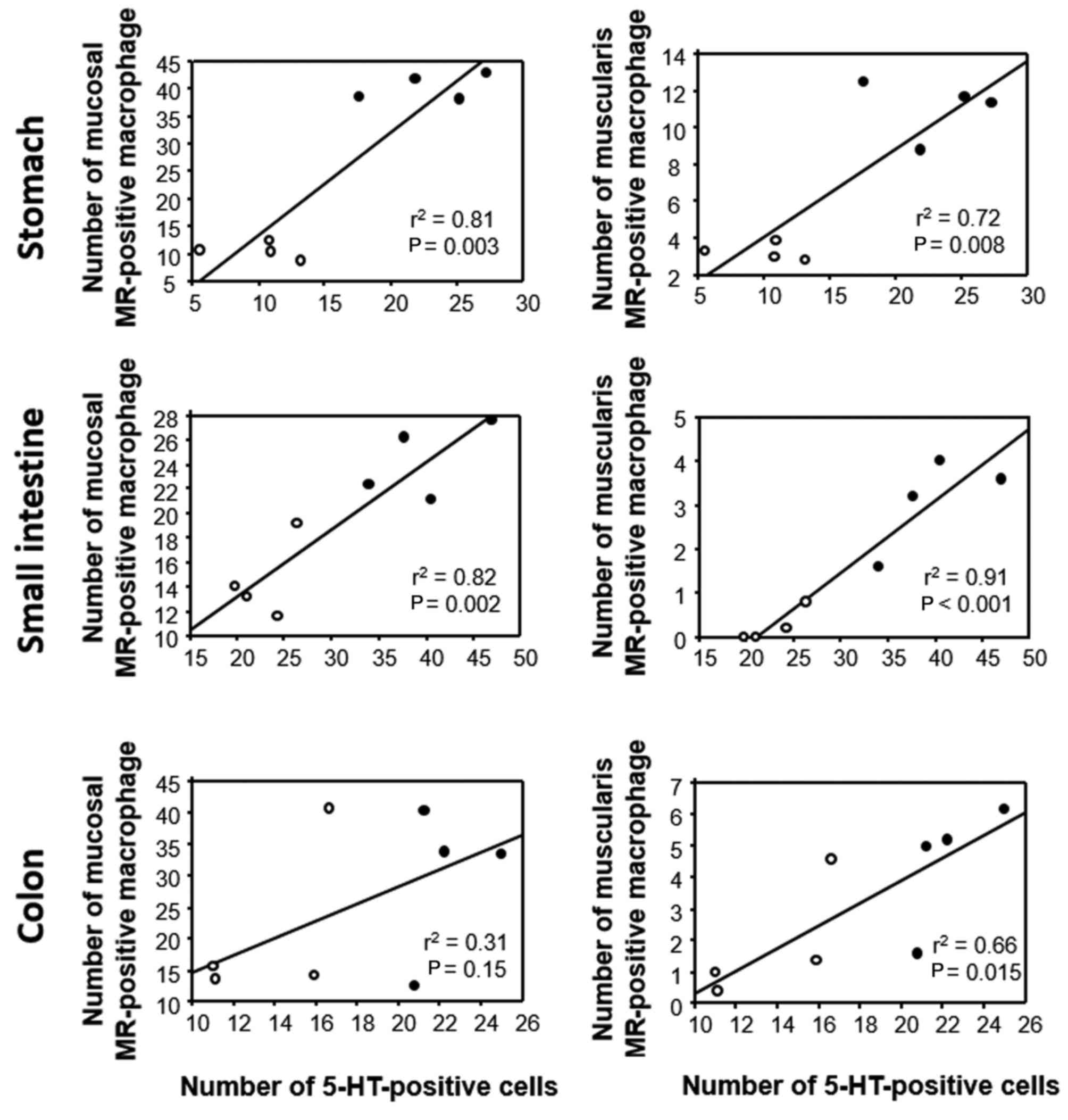
Association between 5-HT and MR
expression in GI tissues of GF mice
The association between 5-HT and MR expression in
the experimental mice (FT-treated and untreated) was also
investigated by linear regression analysis. In the stomach and
small intestine, the number of MR-positive cells in the mucosal
layer was positively correlated with the number of 5-HT-positive
cells, whereas no such correlation was evident in the colon
(Fig. 8). The number of
MR-positive cells in the muscular layer was positively correlated
with the number of 5-HT-positive cells throughout the GI wall
(Fig. 8).
Discussion
In the present study, 5-HT was demonstrated to be
expressed in endocrine cells throughout the GI mucosa in GF mice,
and the number of 5-HT-positive cells was increased in GF mice
following transplantation of gut microbiota, consistent with
previous reports (10). Although
in humans the population of 5-HT-positive endocrine cells is larger
in the upper GI and rectum (15),
no significant differences were observed among the various parts of
the GI tract of the GF mice examined in the present study. However,
the number of 5-HT-positive endocrine cells increased in the upper
GI and colon of FT-treated GF mice compared with untreated mice,
resulting in a distribution of 5-HT-positive cells in the GI tract
that resembled that of humans. This finding suggests that gut
microbiota may be essential in determining the distribution profile
of 5-HT-producing endocrine cells in the GI tract.
The distribution of M2 macrophages was also
investigated by immunostaining for MR (16), and the results revealed that
MR-positive macrophages were present in the lamina propria and the
muscular layer throughout the GI wall. Notably, MR-positive
macrophages were also distributed around myenteric neural cells
between the circular and longitudinal muscle layers of the GI wall,
suggesting that these macrophages may serve a role in the
physiology of the enteric nervous system. The numbers of M2
macrophages in the muscular layer were markedly increased in the
upper GI tract and colon of FT-treated GF mice, suggesting that gut
microbiota may promote infiltration of macrophages into the GI
muscular layer.
Furthermore, the role of gut microbiota in GI
motility was examined and demonstrated to be suppressed in control
untreated GF mice in comparison with mice with gut microbiota,
consistent with previous reports (17,18).
Conversely, this result may suggest that transplantation of gut
microbiota accelerated GI motility in GF mice. To clarify the
mechanism by which gut microbiota may have accelerated GI motility,
the correlation between GITT and 5-HT expression or MR-positive
macrophages in the GI tract was further examined. The results
demonstrated that GITT was negatively correlated with the number of
5-HT-positive cells in the upper GI tract, suggesting that the
increase of 5-HT expression is associated with acceleration of GI
motility. A previous study has also reported that 5-HT promotes
motility of the GI tract (19). In
addition, GITT was negatively correlated with the number of
MR-positive macrophages in the muscular layer of the GI tract.
Recently, Muller et al (20) have reported that musclaris
macrophages are important in GI motility through cross-talk with
enteric nerve cells through bone morphogenetic protein 2. In the
present study, although the specific mediators produced by M2
macrophages were not identified, the numbers of M2 macrophages
following FT were demonstrated to be significantly correlated with
acceleration of GI motility.
Since the movement of the GI tract is orchestrated
by the enteric nervous system (21,22),
the mechanism by which endocrine-produced 5-HT acts on the enteric
nervous system is of great interest. It is believed that the 5-HT
secreted by enterochromaffin cells acts locally on the submucosal
and the myenteric neural cells in a paracrine manner (7,23).
In addition, as 5-HT may also act on immune cells that express its
receptor (8), 5-HT-induced
mediators from the immune cells may affect the enteric nervous
system function (24). Notably,
previous evidence suggests that 5-HT is important in the
polarization of macrophages toward an M2 phenotype (11). In this context, it was notable that
in the present study the number of MR-positive cells in the
muscular layer was positively correlated with the number of
5-HT-positive cells throughout the GI wall. Therefore, it is
speculated that 5-HT may act on the enteric nervous system directly
or indirectly via macrophage stimulation.
In conclusion, the present study has demonstrated
that the numbers of 5-HT-positive endocrine cells and muscularis
MR-positive macrophages are positively correlated in the GI tract,
and are significantly increased in the upper GI and colon of GF
mice following transplantation of gut microbiota, compared with
age-matched untreated mice. Furthermore, GITT was decreased in GF
mice following FT compared with untreated mice, and it was
negatively correlated with the number of 5-HT-positive endocrine
cells and muscularis MR-positive macrophages. The precise mechanism
by which gut microbiota accelerated GI motility remains unknown.
However, the present data suggest that gut microbiota may be
important in inducing increased numbers of 5-HT-producing endocrine
cells and muscularis M2 macrophages in the GI tract, which may act
on the enteric nervous system to modulate GI function.
Acknowledgements
The present work was supported in part by
Grants-in-aid for Scientific Research (grant no. 26460953) from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology. The authors of the present study would like to thank
Miss Mayumi Yamada and Miss Chiyomi Itoh (Hyogo College of
Medicine, Nishinomiya, Japan) for their technical assistance.
Glossary
Abbreviations
Abbreviations:
|
GI
|
gastrointestinal
|
|
5-HT
|
5-hydroxytryptamine
|
|
GF
|
germ-free
|
|
SPF
|
specific pathogen-free
|
|
MR
|
mannose receptor
|
|
FT
|
fecal transplantation
|
|
GITT
|
gastrointestinal transit time
|
References
|
1
|
Furness JB, Rivera LR, Cho HJ, Bravo DM
and Callaghan B: The gut as a sensory organ. Nat Rev Gastroenterol
Hepatol. 10:729–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kabouridis PS and Pachnis V: Emerging
roles of gut microbiota and the immune system in the development of
the enteric nervous system. J Clin Invest. 125:956–964. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Psichas A, Reimann F and Gribble FM: Gut
chemosensing mechanisms. J Clin Invest. 125:908–917. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stasi C, Rosselli M, Bellini M, Laffi G
and Milani S: Altered neuro-endocrine-immune pathways in the
irritable bowel syndrome: The top-down and the bottom-up model. J
Gastroenterol. 47:1177–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drucker DJ: The role of gut hormones in
glucose homeostasis. J Clin Invest. 117:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy KG and Bloom SR: Gut hormones and
the regulation of energy homeostasis. Nature. 444:854–859. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gershon MD and Tack J: The serotonin
signaling system: From basic understanding to drug development for
functional GI disorders. Gastroenterology. 132:397–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baganz NL and Blakely RD: A dialogue
between the immune system and brain, spoken in the language of
serotonin. ACS Chem Neurosci. 4:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uribe A, Alam M, Johansson O, Midtvedt T
and Theodorsson E: Microflora modulates endocrine cells in the
gastrointestinal mucosa of the rat. Gastroenterology.
107:1259–1269. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yano JM, Yu K, Donaldson GP, Shastri GG,
Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK and Hsiao EY:
Indigenous bacteria from the gut microbiota regulate host serotonin
biosynthesis. Cell. 161:264–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de las Casas-Engel M, Domínguez-Soto A,
Sierra-Filardi E, Bragado R, Nieto C, Puig-Kroger A, Samaniego R,
Loza M, Corcuera MT, Gómez-Aguado F, et al: Serotonin skews human
macrophage polarization through HTR2B and HTR7. J Immunol.
190:2301–2310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogawa H, Fukushima K, Sasaki I and Matsuno
S: Identification of genes involved in mucosal defense and
inflammation associated with normal enteric bacteria. Am J Physiol
Gastrointestinal Liver Physiol. 279:G492–G499. 2000.
|
|
13
|
Ogata H, Sekikawa A, Yamagishi H, Ichikawa
K, Tomita S, Imura J, Ito Y, Fujita M, Tsubaki M, Kato H, et al:
GROα promotes invasion of colorectal cancer cells. Oncol Rep.
24:1479–1486. 2010.PubMed/NCBI
|
|
14
|
Welch MG, Margolis KG, Li Z and Gershon
MD: Oxytocin regulates gastrointestinal motility, inflammation,
macromolecular permeability, and mucosal maintenance in mice. Am J
Physiol Gastrointest Liver Physiol. 307:G848–G862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sjölund K, Sandén G, Håkanson R and
Sundler F: Endocrine cells in human intestine: An
immunocytochemical study. Gastroenterology. 85:1120–1130.
1983.PubMed/NCBI
|
|
16
|
Nakanishi Y, Nakatsuji M, Seno H, Ishizu
S, Akitake-Kawano R, Kanda K, Ueo T, Komekado H, Kawada M, Minami M
and Chiba T: COX-2 inhibition alters the phenotype of
tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse
polyps. Carcinogenesis. 32:1333–1339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kashyap PC, Marcobal A, Ursell LK,
Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA,
Higginbottom SK, Million M, et al: Complex interactions among diet,
gastrointestinal transit, and gut microbiota in humanized mice.
Gastroenterology. 144:967–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samuel BS, Shaito A, Motoike T, Rey FE,
Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J,
Yanagisawa M and Gordon JI: Effect of the gut microbiota on host
adiposity are modulated by the short-chain fatty-acid binding G
protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA.
105:16767–16772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanger GJ: 5-Hydroxytryptamine and
functional bowel disorders. Neurogastroenterol Motil. 8:319–331.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muller PA, Koscsó B, Rajani GM, Stevanovic
K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et
al: Crosstalk between muscularis macrophages and enteric neurons
regulates gastrointestinal motility. Cell. 158:300–313. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mayer EA, Tillisch K and Gupta A:
Gut/brain axis and the microbiota. J Clin Invest. 125:926–938.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wood JD: Neuropathophysiology of
functional gastrointestinal disorders. World J Gastroenterol.
13:1313–1332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mawe GM and Hoffman JM: Serotonin
signalling in the gut-functions, dysfunctions and therapeutic
targets. Nat Rev Gastroenterol Hepatol. 10:473–486. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gershon MD: Nerves, reflexes, and the
enteric nervous system: Pathogenesis of the irritable bowel
syndrome. J Clin Gastroenterol. 39:(5 Suppl 3). S184–S193. 2005.
View Article : Google Scholar : PubMed/NCBI
|