Introduction
Stress urinary incontinence (SUI) is defined as the
involuntary loss of urine that occurs when intra-abdominal pressure
exceeds urethral pressure during coughing, sneezing or physical
exertion. SUI is a distressing problem with a profound impact on
health-associated quality of life (1). The etiology of SUI is unclear.
Several studies have proved that its onset is associated with the
response of tissue to overstress, inflammation and
hypoxia-associated injury (2–6).
Aquaporins (AQPs) are a family of transmembrane
proteins that transport water and small solutes, including
glycerol, across cell membranes. AQPs are mediators of
transcellular water flow and serve an important role in maintaining
intra/extracellular fluid homeostasis by facilitating water
transport in response to altering osmotic gradients. AQPs have been
demonstrated to serve a role in skin hydration, cellular
proliferation, migration, immunity, wound healing and vascular
remodeling in multiple organs, and during acute lung injury and
cancer (7–12). Furthermore, a previous study
confirmed that abnormal synthesis and degradation of collagens
during extracellular matrix (ECM) remodeling contributes to SUI, by
altering normal tissue architecture and mechanical properties
(13), and that ECM repair and/or
remodeling-associated proteins serve a role in the development of
SUI.
The authors previously demonstrated that AQP2
expression in the human endometrium varies during the menstrual
cycle (14). The present study
used anterior vaginal wall tissue as this is an important part of
the periurethral support structure (15,16).
The vaginal wall consists of a dense ECM, rich in collagen, that is
maintained and remodeled by fibroblastic cells. Fibroblasts are
involved in normal and pathological soft tissue repair processes
(17). Therefore, the present
study was designed to determine AQP2 location and expression in the
anterior vaginal wall, and investigate the association between AQP2
and the ECM in the pathogenesis of SUI.
Materials and methods
Ethics statement
Ethical approval for the present study was granted
by the Ethics Committee of the School of Medicine of Zhejiang
University (Hangzhou, China). All participants provided written
informed consent to participate in the study, and the ethics
committee approved the consent procedure.
Subjects and sample collection
A total of 12 women aged 47.5±9.2 years, diagnosed
with SUI according to the recommendations of the International
Continence Society (18), who
underwent tension-free vaginal tape surgery at the Department of
Gynecology (Women's Hospital, School of Medicine, Zhejiang
University) between March 2013 and March 2014 were recruited to the
present study. A total of 12 female patients aged 47.6±9.9 in the
Department of Gynecology (Women's Hospital, School of Medicine,
Zhejiang University) without SUI or pelvic organ prolapse (POP) who
were undergoing intravaginal cystectomy for vaginal wall cyst
between March 2013 and March 2014 were recruited as controls. The
study groups were as followings: i) SUI patients were divided into
two groups, the premenopausal group (6 cases) and postmenopausal
group (6 cases); and ii) the control group (12 cases), which were
also divided into a premenopausal group (6 cases) and
postmenopausal group (6 cases). There were no statistically
significant differences in ages, body mass indices and parity among
the groups. Criteria for exclusion from the control group were:
Hormone replacement therapy within the previous 3 months; signs of
urinary infection; estrogen-associated disease (endometriosis,
myoma or functional ovarian tumor); clear clinical evidence of POP
(grade 2 or higher); and urge incontinence. All participants were
diagnosed via a combination of medical history, gynecological
examination, urinary stress test, ultrasonography and urodynamic
examination, including a POP-quantification test.
Biopsy samples of the anterior vaginal wall were
taken 1–2 cm from the uterine cervix, and included mucosa,
submucosa, connective tissue and smooth muscle.
Each sample (24 cases) was divided into two parts;
one part of the tissue was processed for paraffin embedding for
immunohistochemical analysis, and the tissue was fixed in 10%
neutral buffered formalin at 25°C for 12–24 h followed by paraffin
embedding. Another part of the tissue (from each of the 24 cases)
was frozen at −80°C for western blotting, and 3 cases of SUI tissue
(premenopausal, n=2; postmenopausal, n=1) was separated for cell
culture; the tissues were washed with PBS, cut up into pieces and
digested by collagenase for cell culture.
Immunohistochemical analysis of AQP2
expression and localization
The tissue was processed for paraffin embedding.
Sections of 3 µm were prepared and blocked in 3% hydrogen peroxide
[cat. no. GK600711; Gene Tech (Shanghai) Co., Ltd., Shanghai,
China] for 10 min at 25°C. The immunohistochemical analysis of AQP2
expression and localization was performed on the anterior vaginal
wall using an anti-AQP2 antibody (1:400; cat. no. ab15081; Abcam,
Cambridge, UK) incubated for 1 h at 20°C. Anti-immunoglobulin (Ig)
G-horseradish peroxidase (HRP) [cat. no. GK600711.A; Gene Tech
(Shanghai) Co., Ltd.] and 3′-3′-diaminobenzidine kit
immunohistochemical detection reagent [cat. no. GK600711.B; Gene
tech (Shanghai) Co., Ltd.] were used for immunohistochemical
staining according to the manufacturer's protocol. The results of
the immunohistochemistry for AQP2 were examined with a light
microscope (LEICA DM 2000).
Western blotting for AQP2
Total protein from the anterior vaginal walls of
each group was extracted using Radioimmunoprecipitation Assay Lysis
and Extraction Buffer (containing a protease inhibitor cocktail;
Haoji Biological Co., Ltd., Hangzhou, China) and the protein
concentration was determined using a Bicinchoninic Acid Protein
assay kit (Haoji Biological Co., Ltd.). A total 60 µg proteins/lane
were separated by SDS-PAGE on a 10% gel and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% nonfat milk for 12 h at 4°C
(cat. no. 000105; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Protein signals were detected by incubating membranes with
1:500 dilution of anti-AQP2 antibody (cat. no. SC-28629; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-β-actin as an
internal control (cat. no. SC-47778; Santa Cruz Biotechnology,
Inc.) at 22°C for 2 h. The membranes were subsequently incubated
with goat anti-rabbit IgG-HRP (1:500; cat. no. 32430; Thermo Fisher
Scientific, Inc.) antibody and goat anti-mouse IgG-HRP (1:500; cat.
no. 32430; Thermo Fisher Scientific, Inc.) antibody at 22°C for 1
h. Protein signals were visualized using Enhanced Chemiluminescence
Plus™ Western Blotting Reagents (GE Healthcare Life Sciences,
Little Chalfont, UK) and Kodak X-OMAT film (Kodak, Rochester, NY,
USA). Band intensities were quantitated using Quantity one
software, version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell culture and identification
The tissue size was ~0.5×0.5×1.0 cm, with blood and
mucus in the SUI and non-SUI groups. The tissues were washed with
PBS and cut up into pieces. The tissues were subsequently
centrifuged for 5 min at 250 × g at 25°C, followed by successive
digesting with collagenase for 120 min and DNase I for 20 min. The
cells were washed with PBS and cultured in Dulbecco's modified
Eagle's medium (DMEM)/high glucose (cat. no. SH30022.01; HyClone;
GE Healthcare Life Sciences, Logan, UT, USA) containing 20% bovine
serum (cat. no. SH30406.02; HyClone; GE Healthcare Life Sciences).
After 3–5 days of primary culture, fibroblasts were digested with
pancreatin for 2 min and passaged in culture bottles. The third
generation of the cells were identified to be fibroblasts by
morphological and immunohistochemical analysis, according to
previous methods (15). The
fibroblasts in the 3rd-6th generation were taken for experiments
for identifying AQP2 by immunofluorescence. The fibroblasts were
blocked in 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 30 min at 25°C and subsequently incubated
with a 1:50 dilution of the primary anti-AQP2 antibodies at 4°C
overnight. The cells were incubated with a 1:100 dilution of the
secondary antibody goat anti-rabbit IgG-fluorescein isothiocyanate
(cat. no. 65-6111; Thermo Fisher Scientific, Inc.) for 30 min at
25°C. Fibroblasts incubated without the primary antibodies were
used as the control. A portion of the cells were also used for
identifying AQP2 expression by western blotting as described
above.
Small interfering RNA (siRNA)
knockdown and vector-mediated overexpression
Following seeding of 200,000 cells/well in 6-well
plates, cell transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Cells
were harvested subsequent to two days of culture. For knockdown
experiments, 100 pmol/well siRNA targeting the AQP2 gene (target
sequence, AGC TGT CGT CAC TGG CAA ATT) and the siRNA negative
control (cat. no. siN05815122147-1-5; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) were added. For overexpression experiments, AQP2
overexpression vector pcDNA-AQP2 constructed from pcDNA3.1 was
purchased from OriGene Technologies, Inc. (Rockville, MD, USA), and
2 µg/well vector was used for transfection, with pcDNA3.1 as the
control. Negative control cells were untransfected.
ELISA
The fibroblasts in the 3rd-6th generation were
cultured for 24 h with DMEM/high glucose medium containing 20%
bovine serum. The culture medium was collected and collagen I/III,
expression levels were tested using Human Pro-Collagen I alpha I
DuoSet ELISA kit (cat. no. DY6220-0; R&D Systems, Inc.,
Minneapolis, MN, USA5) and Human Pro-Collagen III ELISA kit (cat.
no. E-EL-H0182; Elabscience Biotechnology Co., Ltd., Wuhan,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from fibroblasts using
RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol. Then cDNA was synthesized
using PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.).
qPCR was carried out using SYBR® Premix Ex Taq™ (Takara
Biotechnology, Co., Ltd.) in an Applied Biosystems 7900 Fast
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following primers: AQP2-F, 5′-GGCCCCGACGGACGCTTGT-3′ and AQP2-R,
5′-TGCGCTGGGGGGCCAACTT-3′, as the sense primer and anti-sense
primer, respectively. GAPDH was used as the internal control. The
primers were GAPDH-F, 5′-CCATGACAACTTTGGTATCGTGGAA-3′ and GAPDH-R,
5′-GGCCATCACGCCACAGTTTC-3′. The samples were run under the
following conditions: 95°C for 5 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 25 sec. A total of three replicates were
performed. Data were analyzed using the 2−ΔΔCq method
(19).
Statistical analysis
Statistical analyses were performed using SPSS
statistical software (version 20.0; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference. The results are presented as the mean ± standard
deviation. Fisher's exact test significant difference test was used
to analyze the immunohistochemical analysis, and independent
samples t-tests were used to analyze the AQP2 protein and mRNA
expression, and the collagen I/III protein and mRNA expression.
Results
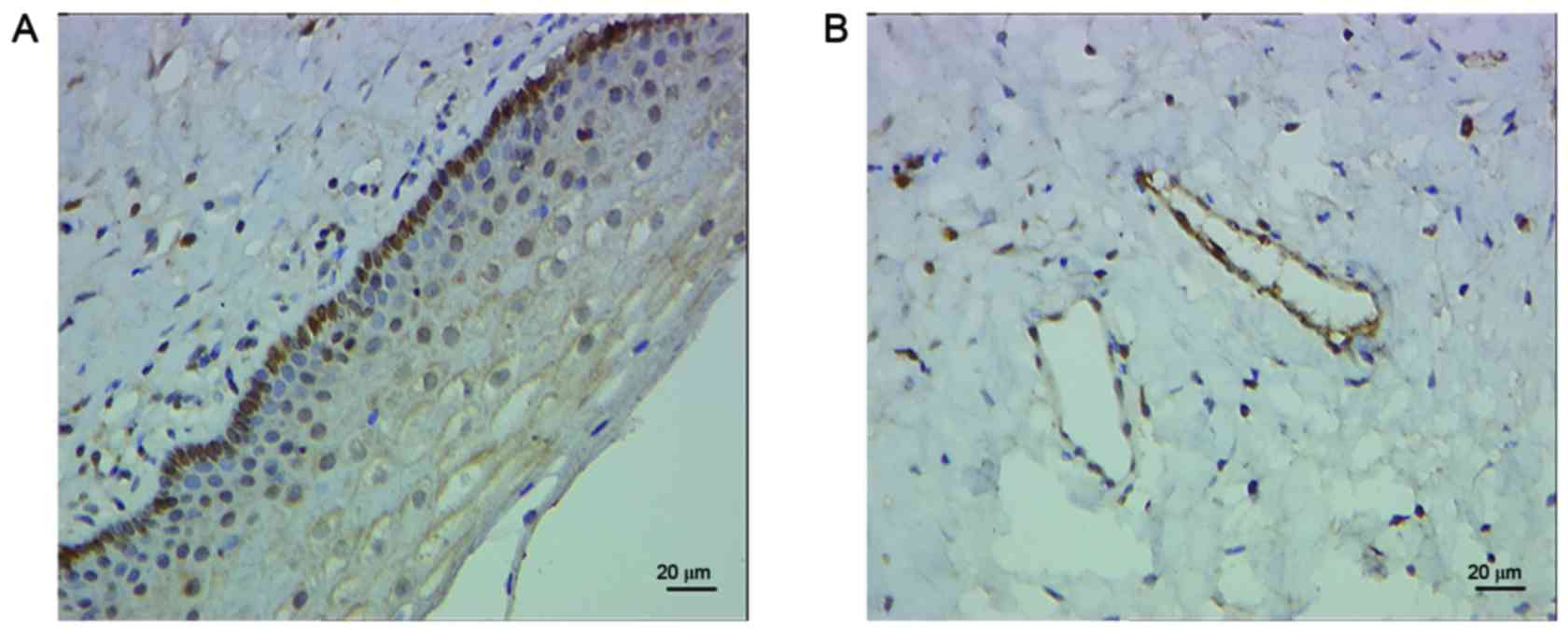
Expression of AQP2 in the anterior
vaginal wall
Expression of AQP2 was detected in the anterior
vaginal wall of patients with and without SUI via
immunohistochemical analysis (Fig.
1).
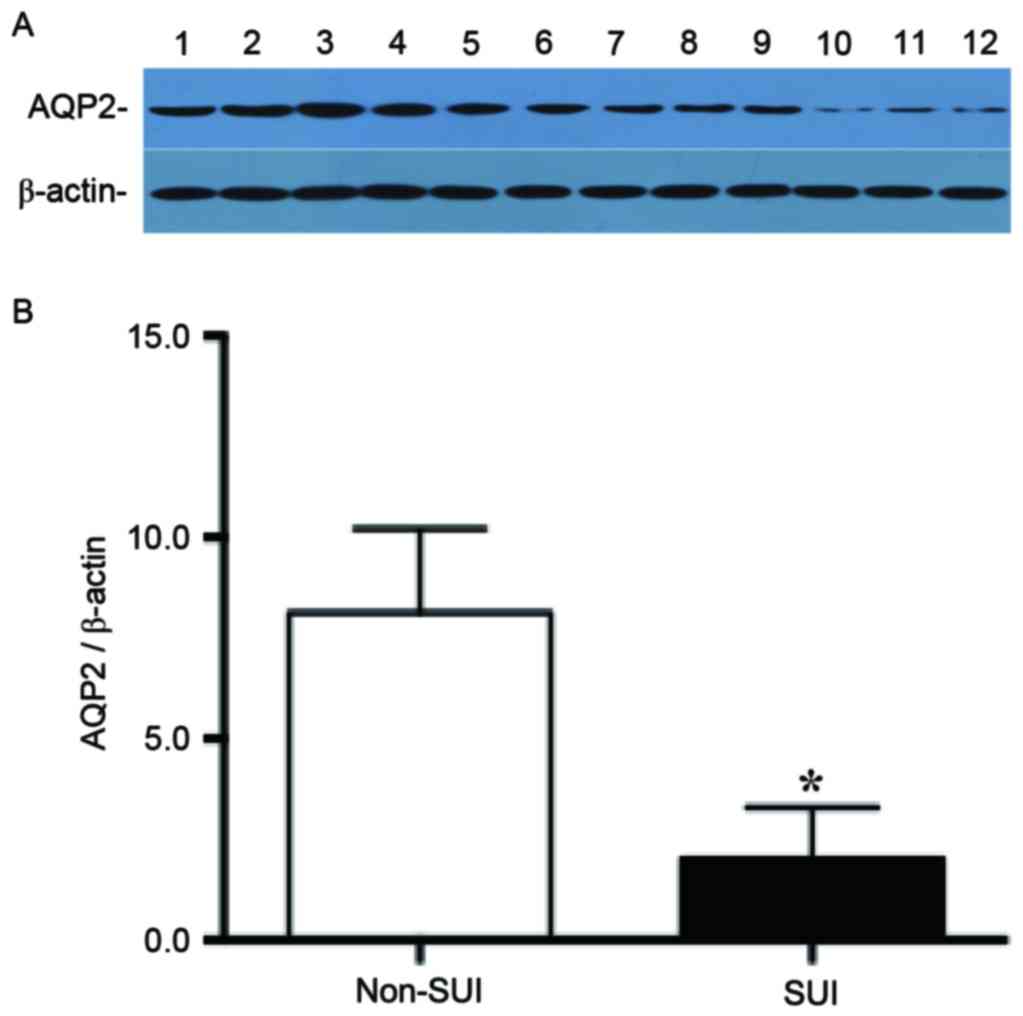
AQP2 expression in the anterior
vaginal wall is decreased in patients with SUI
Expression of AQP2 was detected in the anterior
vaginal wall in patients with and without SUI by western blotting,
and the expression of AQP2 was significantly decreased in patients
with SUI compared with patients without SUI (non-SUI AQP2/β-actin
ratio, 8.13±0.85; SUI AQP2/β-actin ratio, 2.02±0.52; P<0.01;
Fig. 2).
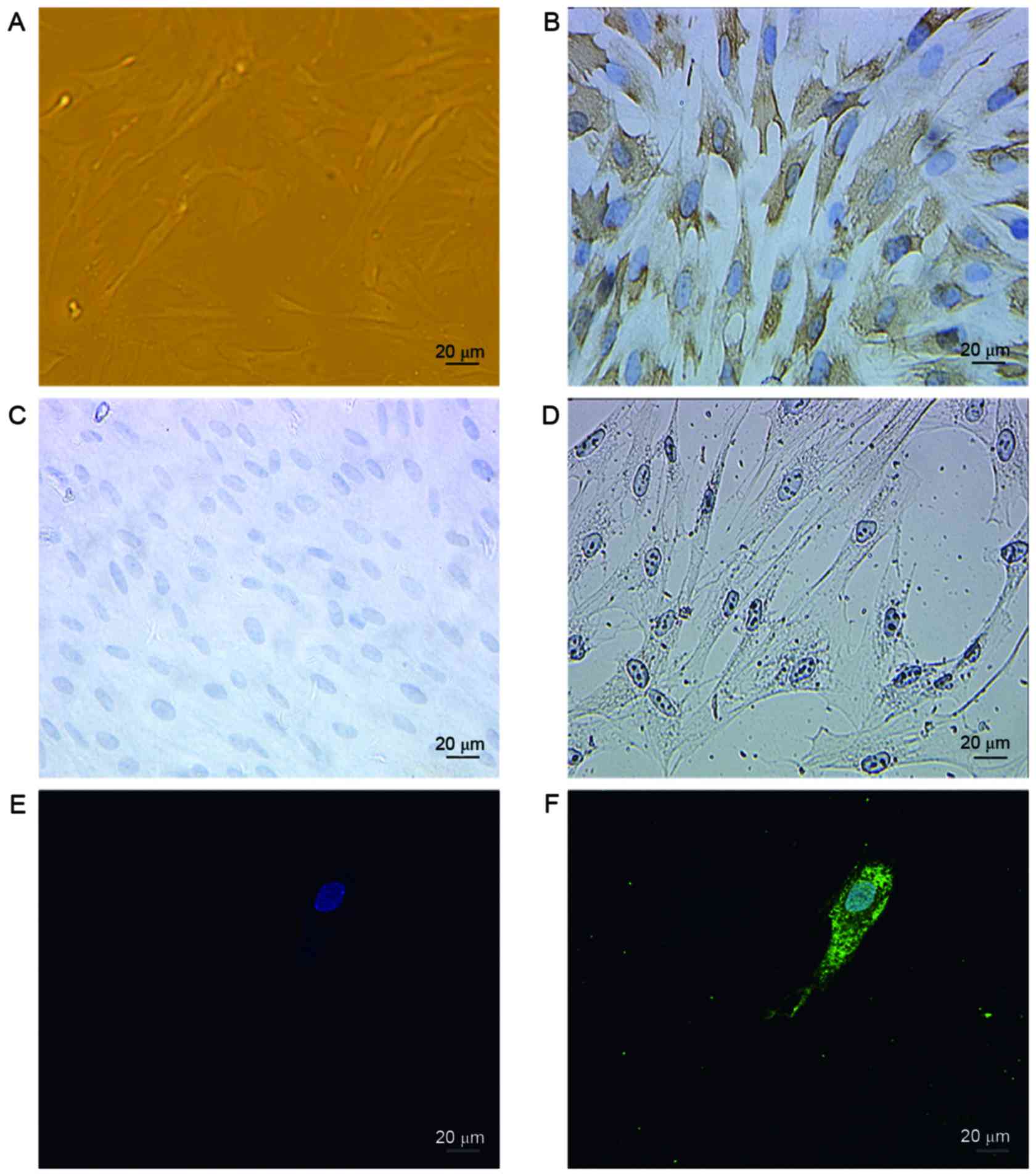
Identification of AQP2 expression in
fibroblasts by immunofluorescence
As immunohistochemical and western blot analysis
demonstrated AQP2 to be expressed in the anterior vaginal wall, it
was confirmed whether AQP2 is expressed in fibroblasts. Fibroblasts
were separated from the anterior vaginal wall, cultured in
vitro and identified by their spindle-shaped morphology
(Fig. 3A). Fibroblasts are widely
distributed in the majority of tissue types, particularly
connective tissues. These cells are of mesenchymal origin and
express vimentin (Fig. 3B), but
not keratin (Fig. 3C) or α-smooth
muscle actin (Fig. 3D) as
described previously (15). In
addition, immunofluorescence was performed to identify the location
and expression of AQP2 in fibroblasts, as shown in Fig. 3E and F. AQP2 stained positively in
the cell membrane and cytoplasm.
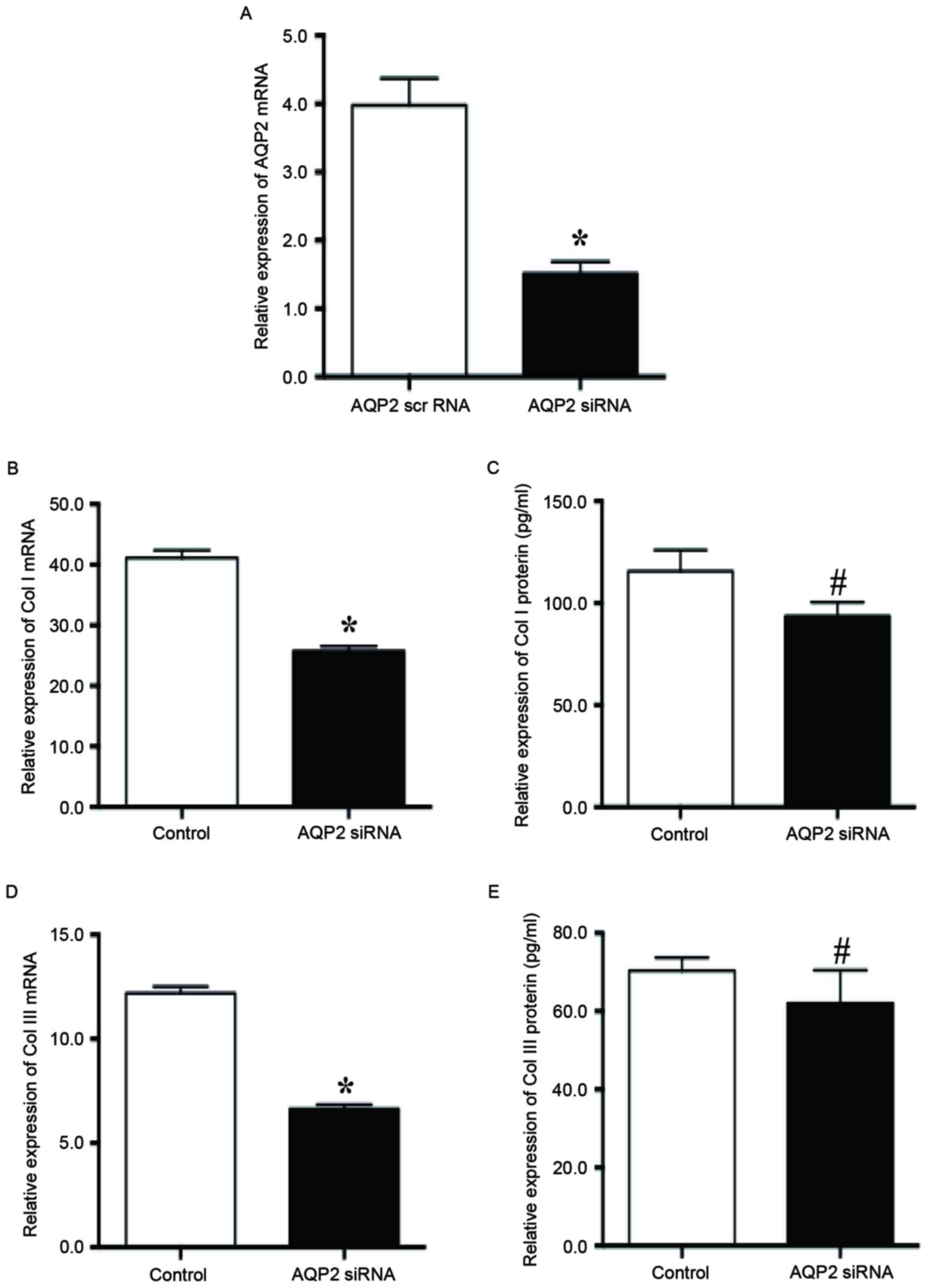
Inhibition of AQP2 downregulated the
secretion of collagen I and III in SUI fibroblasts
In order to determine the role of AQP2 in ECM
secretion in SUI fibroblasts, fibroblasts were transfected with
siRNA targeting AQP2. Compared with the scrambled siRNA, treating
fibroblasts with AQP2 siRNA for 36 h reduced the expression level
of AQP2 mRNA by 67.4% (Fig. 4A).
Inhibition of AQP2 with AQP2 siRNA significantly reduced the
relative expression of collagen I mRNA (25.78±0.73 vs. 41.12±1.28;
P<0.01; Fig. 4B) and protein
(93.66±6.74 vs. 115.68±10.32; P<0.05; Fig. 4C) compared with the scrambled
siRNA. Furthermore, inhibition of AQP2 with AQP2 siRNA
significantly reduced the relative expression of collagen III mRNA
(6.64±0.20 vs. 12.20±0.32; P<0.01; Fig. 4D) and protein (62.01±8.47 vs.
70.36±3.33; P<0.05; Fig. 4E)
compared with the scrambled siRNA. Therefore, inhibition of AQP2
expression may reduce the secretion of collagen I/III in the
anterior vaginal wall-associated fibroblasts in patients with
SUI.
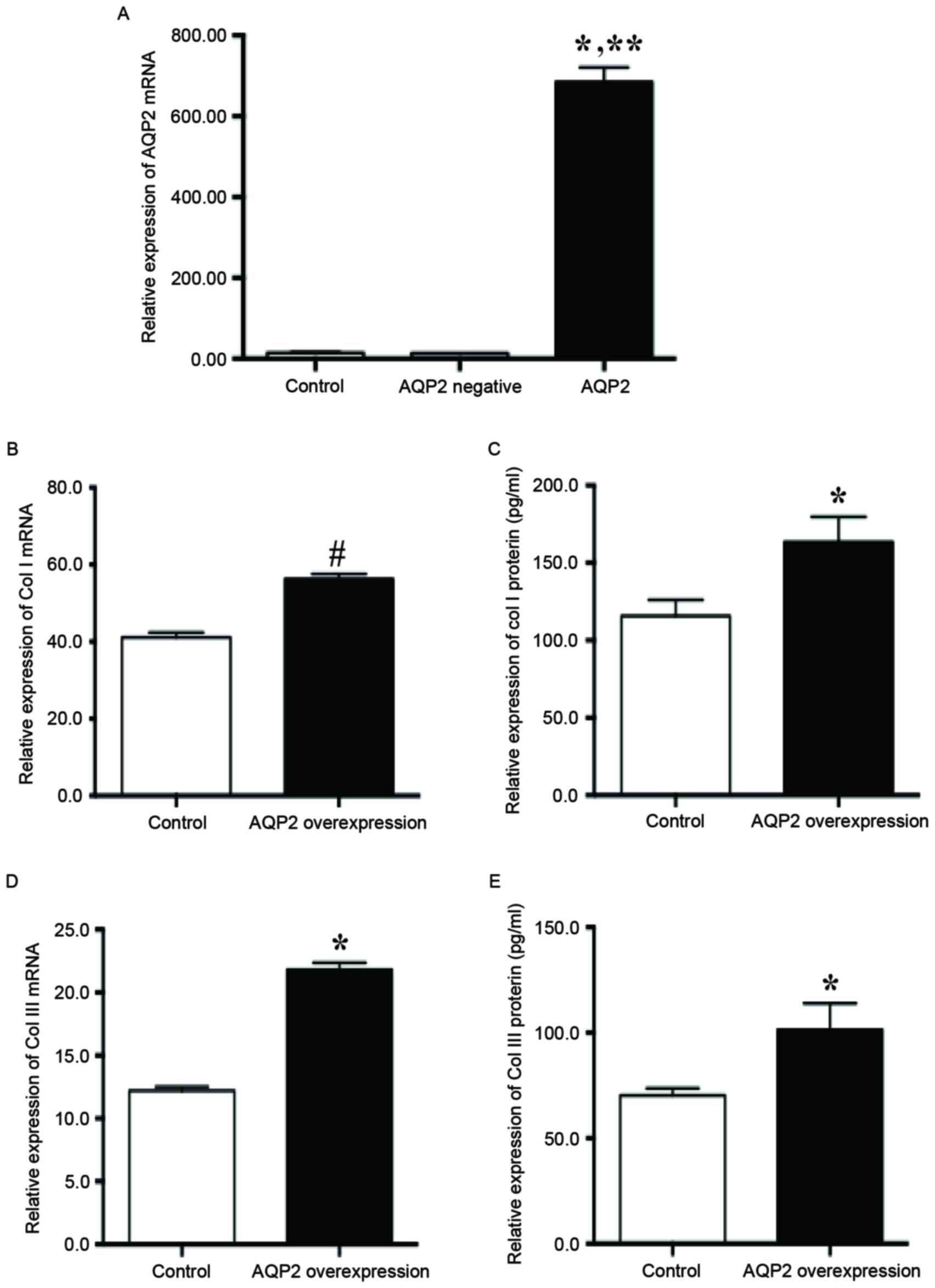
Overexpression of AQP2 enhanced the
secretion of collagen I/III into the ECM by fibroblasts in SUI
In order to confirm that high AQP2 expression serves
an important role in the secretion of ECM proteins by fibroblasts
in SUI, AQP2 was overexpressed in fibroblasts. After 48 h of AQP2
overexpression vector transfection, the AQP2 mRNA level was
significantly increased compared with the control (684.80±36.13 vs.
14.75±1.21; P<0.01; Fig. 5A).
AQP2 overexpression significantly increased the relative expression
of collagen I mRNA (56.32±1.82 vs. 41.12±1.28; P<0.05; Fig. 5B) and protein (163.55±16.19 vs.
115.68±10.32; P<0.01; Fig. 5C)
compared with the control. In addition, AQP2 overexpression
significantly increased the relative expression of collagen III
mRNA (21.80±0.57 vs. 12.20±0.32; P<0.01; Fig. 5D) and protein (101.46±12.42 vs.
70.36±3.33; P<0.01; Fig. 5E)
compared with the control.
Discussion
The results of the present study indicated that AQP2
is expressed in anterior vaginal wall tissue, including
fibroblasts, and that the expression of AQP2 in the anterior
vaginal wall was significantly decreased in patients with SUI
compared with those without SUI. In addition, AQP2 expression was
demonstrated to be associated with collagen I/III metabolism in
fibroblasts of the anterior vaginal wall. Inhibition of AQP2
downregulated collagen I/III secretion in fibroblasts, while
overexpression of AQP2 upregulated it.
Previous studies have confirmed the abnormal
synthesis and degradation of collagens in ECM remodeling
contributes to SUI, by altering normal tissue architecture and
mechanical properties (13);
tissue maintenance and remodeling is performed by fibroblasts,
therefore altered cellular functionality may influence tissue
quality (20). However, the
mechanism of regulation of fibroblastic cells in the ECM is
unclear.
AQPs are widely expressed in human tissue, including
the skin (7), lung (8), vascular (9), and overexpression of AQPs has been
reported in several types of human cancer (10–12).
The roles of AQPs in pelvic support tissues and the pathology of
female SUI remain unknown; however, the present study used anterior
vaginal wall tissue as this is an important part of the
periurethral support structure and has been previously examined
(15,16). In the present study,
immunohistochemical and immunofluorescence analysis revealed that
AQP2 is expressed in the vaginal wall, including the fibroblasts,
and that AQP2 protein expression in the anterior vaginal wall is
decreased in patients with SUI. This indicates that abnormalities
in the AQP2 expression may serve a role in the pathogenesis of
SUI.
In healthy women, the bladder is kept in place by
the connective-tissue layer of the anterior vaginal wall which is a
dense ECM with relatively few cells. The ECM obtains its strength
from fibrillar proteins (collagen I and III), and is produced and
maintained by fibroblastic cells (17,20).
Abnormal collagen metabolism in SUI is well documented (21,22).
Collagen types I and III are important in maintaining tissue
tensile strength and the mechanical stability of pelvic support
tissues. In the present study, knockdown of AQP2 reduced the
relative expression of collagen I/II mRNA and protein, while AQP2
overexpression significantly increased the relative expression of
collagen I/III mRNA and protein, suggesting that AQPs are crucial
for collagen I/III synthesis in the pelvic floor. AQP2 promotes
collagen synthesis in the ECM and inhibits collagen decomposition.
The results suggested that AQP2 may be associated with the
pathogenesis of female SUI through collagen metabolism regulated by
fibroblasts.
In conclusion, female SUI is associated with reduced
levels of AQPs compared with non-SUI controls and inhibition of
AQP2 inhibits collagen I/III synthesis in vaginal wall fibroblasts.
AQP2 regulates the expression level of collagen I/III in the
anterior vaginal wall and fibroblasts. It is possible that
abnormalities of AQP2 expression may be involved in the
pathogenesis of female SUI through ECM metabolism. Furthermore,
larger scale investigations are required to fully elucidate the
influence of AQP2 on ECM metabolism in SUI.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81200429),
Zhejiang Province Natural Science Foundation of China (grant no.
LY16H040006), Zhejiang Provincial Education Committee Research
Foundation (grant no. Y201328819) and Zhejiang Province Health
Family Planning Commission Population Planning and Research Project
(grant no. 2014KYA248).
References
|
1
|
Zhu L, Lang J, Liu C, Han S, Huang J and
Li X: The epidemiological study of women with urinary incontinence
and risk factors for stress urinary incontinence in China.
Menopause. 16:831–836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luginbuehl H, Baeyens JP, Kuhn A, Christen
R, Oberli B, Eichelberger P and Radlinger L: Pelvic floor muscle
reflex activity during coughing-an exploratory and reliability
study. Ann Phys Rehabil Med. 59:302–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chai TC, Richter HE, Moalli P, Keay S,
Biggio J, Zong W, Curto T, Kim HY, Stoddard AM and Kusek JW:
Inflammatory and tissue remodeling urinary biomarkers before and
after mid urethral sling surgery for stress urinary incontinence. J
Urol. 191:703–709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malone L, Schuler C, Leggett RE and Levin
RM: Effect of estrogen and ovariectomy on response of the female
rabbit urinary bladder to two forms of in vitro oxidative stress.
Int Urogynecol J. 25:791–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan SSC, Cheung RYK, Lee LL, Choy RKW and
Chung TKH: A longitudinal follow-up of levator ani muscle avulsion:
Does a second delivery affect it? Ultrasound Obstet Gynecol. Jul
1–2016.(Epub ahead of print).
|
|
6
|
Sumino Y, Yoshikawa S, Mori K, Mimata H
and Yoshimura N: IGF-1 as an important endogenous growth factor for
recovery from impaired urethral continence function in rats with
simulated childbirth injury. J Urol. 195:1927–1935. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sebastian R, Chau E, Fillmore P, Matthews
J, Price LA, Sidhaye V and Milner SM: Epidermal aquaporin-3 is
increased in the cutaneous burn wound. Burns. 41:843–847. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang X, Zhang B, Chen Q, Zhang J, Lei B,
Li B, Wei Y, Zhai R, Liang Z, He S and Tang B: The mechanism
underlying alpinetin-mediated alleviationof pancreatitis-associated
lung injury through upregulating aquaporin-1. Drug Des Devel Ther.
10:841–850. 2016.PubMed/NCBI
|
|
9
|
Iguchi H, Oda M, Yamazaki H and Yokomori
H: Participation of aquaporin-1 in vascular changes and remodeling
in cirrhotic liver. Med Mol Morphol. 46:123–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Wang Z, Xu D, Liu Y and Gao Y:
Aquaporin 3 promotes prostate cancer cell motility and invasion via
extracellular signal-regulated kinase 1/2-mediated matrix
metalloproteinase-3 secretion. Mol Med Rep. 11:2882–2888.
2015.PubMed/NCBI
|
|
11
|
Dorward HS, Du A, Bruhn MA, Wrin J, Pei
JV, Evdokiou A, Price TJ, Yool AJ and Hardingham JE:
Pharmacological blockade of aquaporin-1 water channel by AqB013
restricts migration and invasiveness of colon cancer cells and
prevents endothelial tube formation in vitro. J Exp Clin Cancer
Res. 35:362016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao XC, Zhang WR, Cao WF, Liu BW, Zhang F,
Zhao HM, Meng R, Zhang L, Niu RF, Hao XS, et al: Aquaporin3 is
required for FGF-2-induced migration of human breast cancers. PLoS
One. 8:e567352013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen B and Yeh J: Alterations in
connective tissue metabolism in stress incontinence and prolapse. J
Urol. 186:1768–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He RH, Sheng JZ, Luo Q, Jin F, Wang B,
Qian YL, Zhou CY, Sheng X and Huang HF: Aquaporin-2 expression in
human endometrium correlates with serum ovarian steroid hormones.
Life Sci. 79:423–429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Q, Jin H, Xie Z, Wang M, Chen J and
Zhou Y: The role of the ERK1/2 signalling pathway in the
pathogenesis of female stress urinary incontinence. J Int Med Res.
41:1242–1251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Zhang L, Jin H, Zhou J and Xie Z:
The role of calpain-calpastatin system in the developmentof stress
urinary incontinence. Int Urogynecol J. 21:63–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ruiz-Zapata AM, Kerkhof MH, Ghazanfari S,
Zandieh-Doulabi B, Stoop R, Smit TH and Helder MN: Vaginal
fibroblastic cells from women with pelvic organ prolapse produce
matrices with increased stiffness and collagen content. Sci Rep.
6:229712016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haylen BT, de Ridder D, Freeman RM, Swift
SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK and
Schaer GN: An international urogynecological association
(IUGA)/international continence society (ICS) joint report on the
terminology for female pelvic floor dysfunction. Int Urogynecol J.
21:5–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruiz-Zapata AM, Kerkhof MH,
Zandieh-Doulabi B, Brölmann HA, Smit TH and Helder MN: Functional
characteristics of vaginal fibroblastic cells from premenopausal
women with pelvic organ prolapse. Mol Hum Reprod. 20:1135–1143.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han L, Wang L, Wang Q, Li H and Zang H:
Association between pelvic organ prolapse and stress urinary
incontinence with collagen. Exp Ther Med. 7:1337–1341.
2014.PubMed/NCBI
|
|
22
|
Mangera A, Bullock AJ, Roman S, Chapple CR
and MacNeil S: Comparison of candidate scaffolds for tissue
engineering for stress urinary incontinence and pelvic organ
prolapse repair. BJU Int. 112:674–685. 2013. View Article : Google Scholar : PubMed/NCBI
|