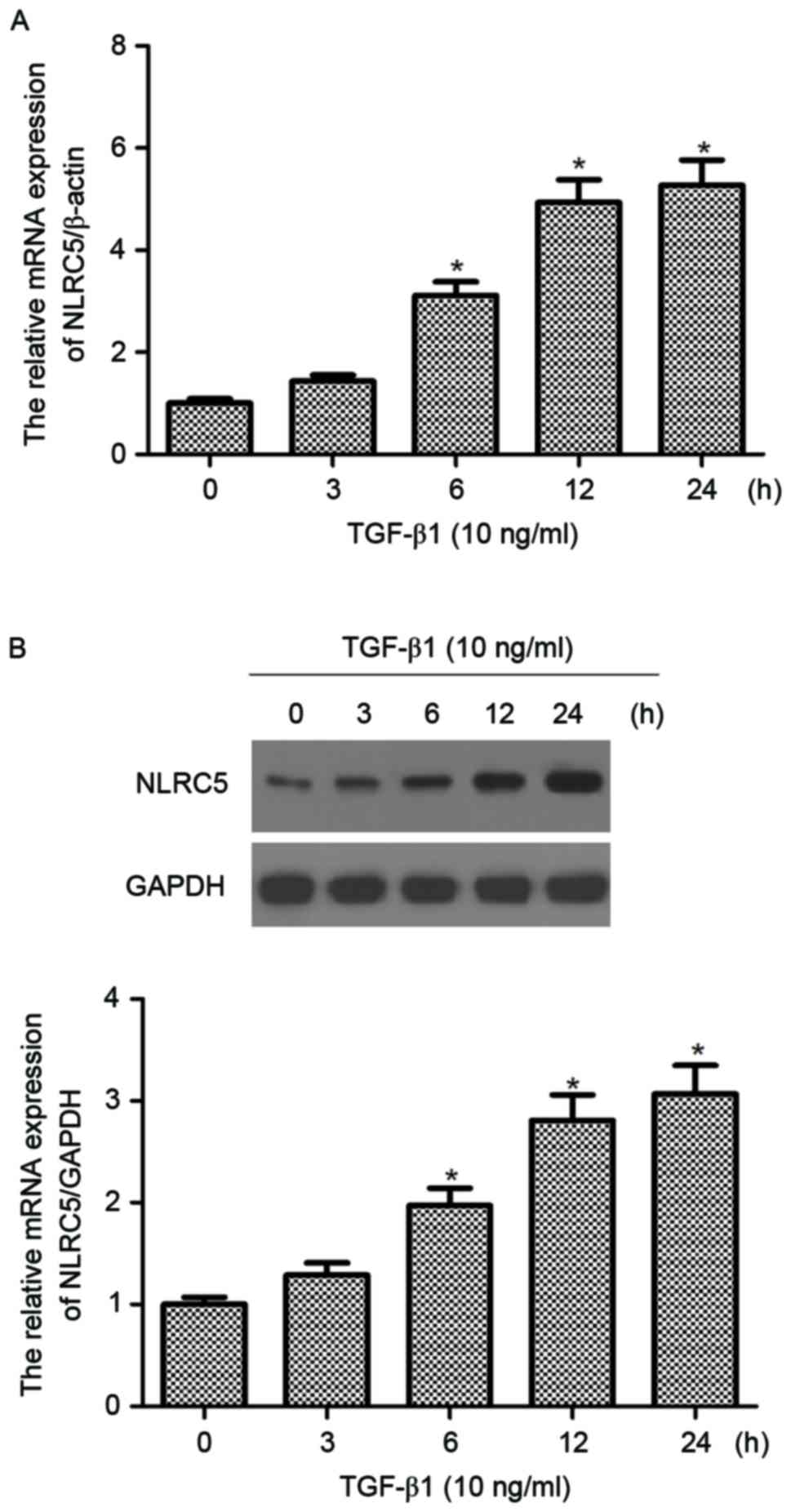
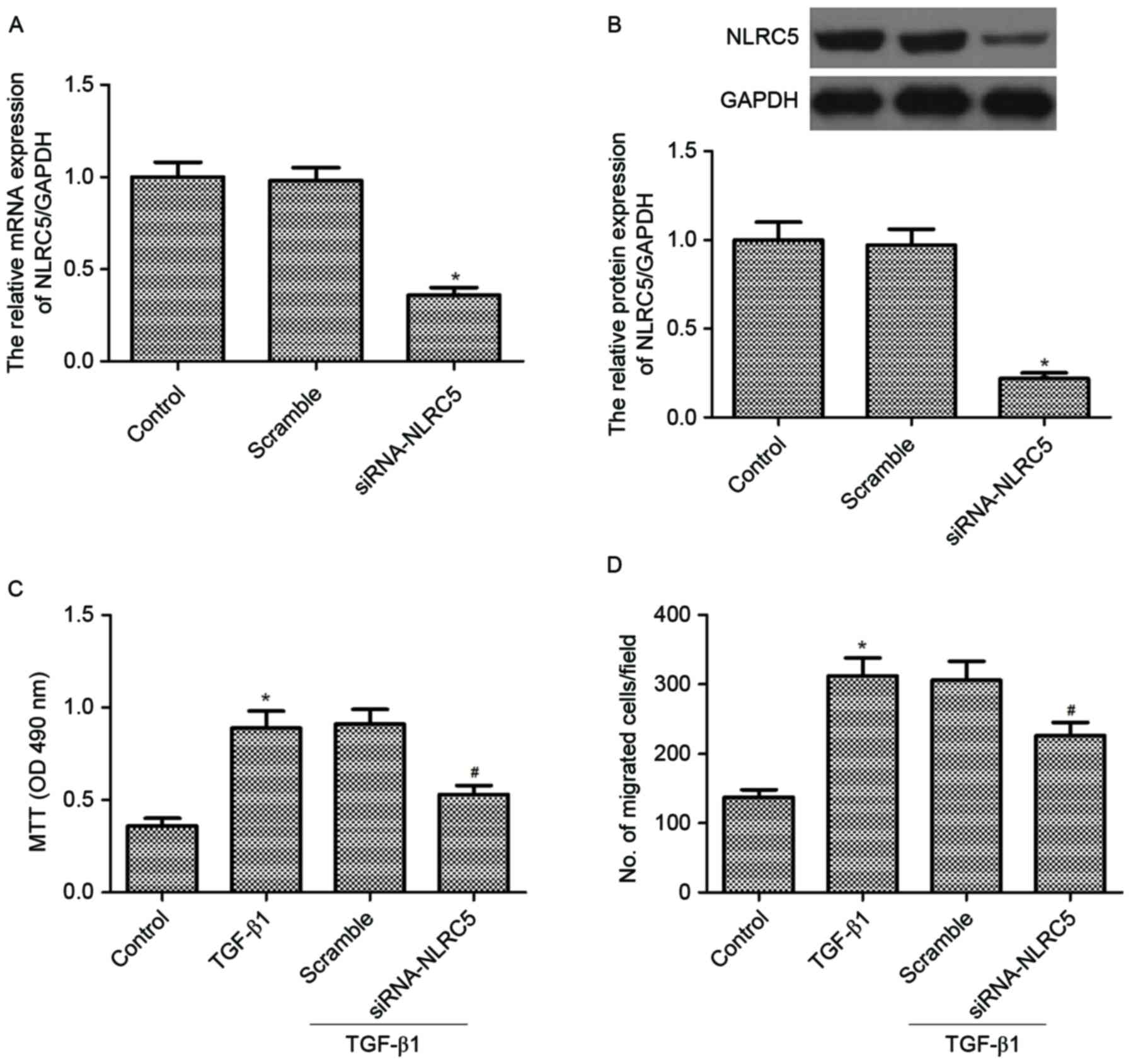
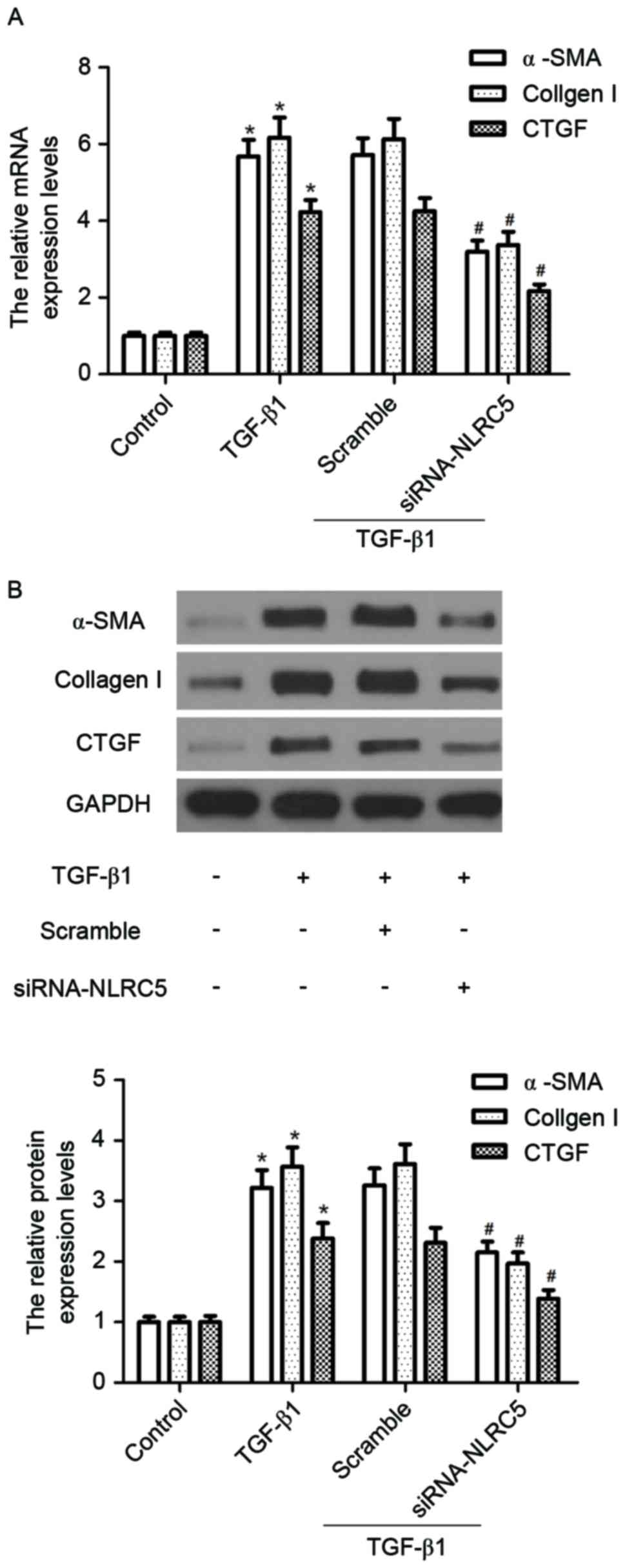
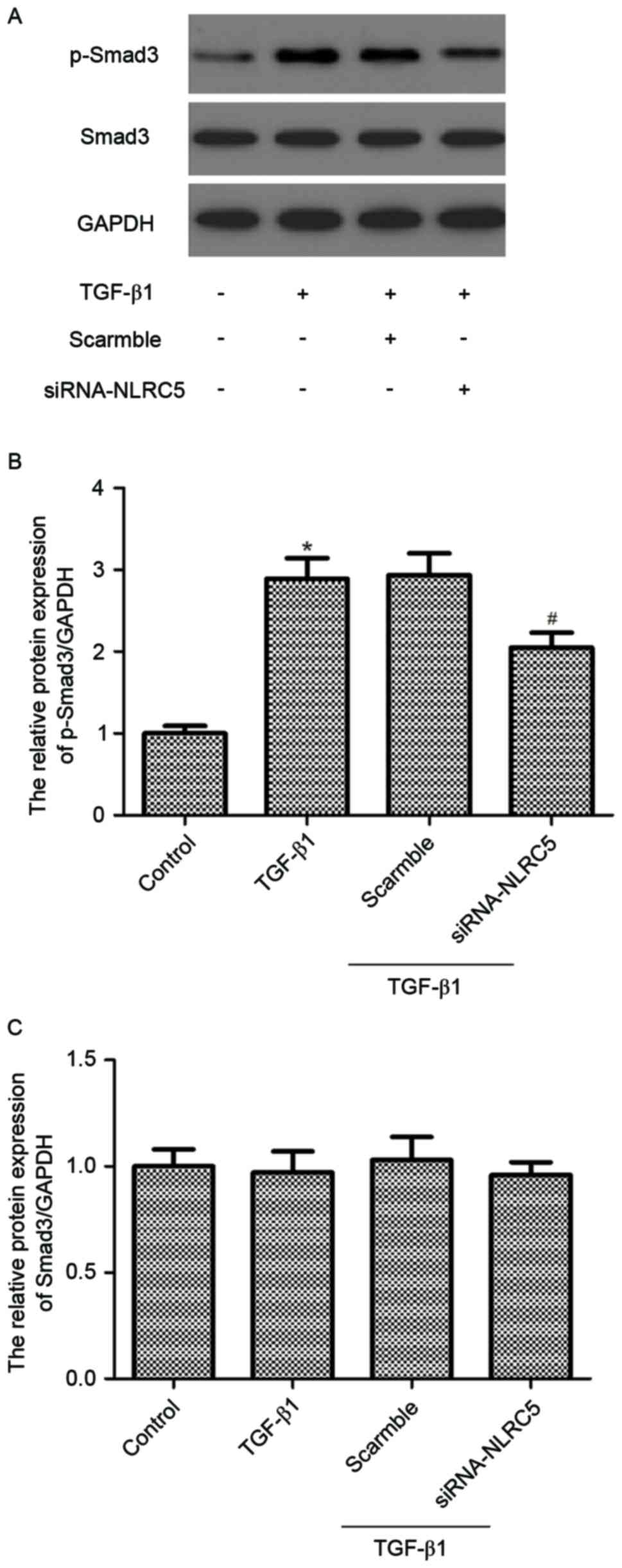
|
1
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burlew BS and Weber KT: Cardiac fibrosis
as a cause of diastolic dysfunction. Herz. 27:92–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leask A: Potential therapeutic targets for
cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF,
partners in fibroblast activation. Circ Res. 106:1675–1680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spinale FG, Coker ML, Bond BR and Zellner
JL: Myocardial matrix degradation and metalloproteinase activation
in the failing heart: A potential therapeutic target. Cardiovasc
Res. 46:225–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brilla CG, Funck RC and Rupp H:
Lisinopril-mediated regression of myocardial fibrosis in patients
with hypertensive heart disease. Circulation. 102:1388–1393. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lijnen PJ, Petrov VV and Fagard RH:
Induction of cardiac fibrosis by transforming growth
factor-beta(1). Mol Genet Metab. 71:418–435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis BK, Roberts RA, Huang MT, Willingham
SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ,
Accavitti-Loper MA, et al: Cutting edge: NLRC5-dependent activation
of the inflammasome. J Immunol 186. 186:1333–1337. 2011. View Article : Google Scholar
|
|
8
|
Kuenzel S, Till A, Winkler M, Häsler R,
Lipinski S, Jung S, Grötzinger J, Fickenscher H, Schreiber S and
Rosenstiel P: The nucleotide-binding oligomerization domain-like
receptor NLRC5 is involved in IFN-dependent antiviral immune
responses. J Immunol. 184:1990–2000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meissner TB, Li A, Biswas A, Lee KH, Liu
YJ, Bayir E, Iliopoulos D, van den Elsen PJ and Kobayashi KS: NLR
family member NLRC5 is a transcriptional regulator of MHC class I
genes. Proc Natl Acad Sci USA. 107:13794–13799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staehli F, Ludigs K, Heinz LX,
Seguín-Estévez Q, Ferrero I, Braun M, Schroder K, Rebsamen M,
Tardivel A, Mattmann C, et al: NLRC5 deficiency selectively impairs
MHC class I-dependent lymphocyte killing by cytotoxic T cells. J
Immunol. 188:3820–3828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu T, Ni MM, Xing-Li, Li XF, Meng XM,
Huang C and Li J: NLRC5 regulates TGF-β1-induced proliferation and
activation of hepatic stellate cells during hepatic fibrosis. Int J
Biochem Cell Biol. 70:92–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM
and Lan HY: miR-29b as a therapeutic agent for angiotensin
II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol
Ther. 22:974–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Nieuwenhoven FA and Turner NA: The
role of cardiac fibroblasts in the transition from inflammation to
fibrosis following myocardial infarction. Vasc Pharmacol.
58:182–188. 2013. View Article : Google Scholar
|
|
16
|
Lijnen P, Petrov V and Fagard R:
Transforming growth factor-beta 1-mediated collagen gel contraction
by cardiac fibroblasts. J Renin Angiotensin Aldosterone Syst.
4:113–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carver W, Nagpal ML, Nachtigal M, Borg TK
and Terracio L: Collagen expression in mechanically stimulated
cardiac fibroblasts. Circ Res. 69:116–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen MM, Lam A, Abraham JA, Schreiner GF
and Joly AH: CTGF expression is induced by TGF- beta in cardiac
fibroblasts and cardiac myocytes: a potential role in heart
fibrosis. J Mol Cell Cardiol. 32:1805–1819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bujak M and Frangogiannis NG: The role of
TGF-beta signaling in myocardial infarction and cardiac remodeling.
Cardiovasc Res. 74:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seeland U, Haeuseler C, Hinrichs R,
Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K and Böhm M:
Myocardial fibrosis in transforming growth factor-beta(1)
(TGF-beta(1)) transgenic mice is associated with inhibition of
interstitial collagenase. Eur J Clin Invest. 32:295–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H,
Shang FJ, Niu XL, He YP and Lu XL: Chymase induces profibrotic
response via transforming growth factor-beta 1/Smad activation in
rat cardiac fibroblasts. Mol Cell Biochem. 310:159–166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Araújo-Jorge TC, Waghabi MC,
Hasslocher-Moreno AM, Xavier SS, Higuchi Mde L, Keramidas M, Bailly
S and Feige JJ: Implication of transforming growth factor-beta1 in
Chagas disease myocardiopathy. J Infect Dis. 186:1823–1828. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shyu KG, Wang BW, Chen WJ, Kuan P and Hung
CR: Mechanism of the inhibitory effect of atorvastatin on endoglin
expression induced by transforming growth factor-beta1 in cultured
cardiac fibroblasts. Eur J Heart Fail. 12:219–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sassoli C, Chellini F, Pini A, Tani A,
Nistri S, Nosi D, Zecchi-Orlandini S, Bani D and Formigli L:
Relaxin prevents cardiac fibroblast-myofibroblast transition via
notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS One.
8:e638962013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leask A: TGFbeta, cardiac fibroblasts, and
the fibrotic response. Cardiovasc Res. 74:207–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bujak M, Ren G, Kweon HJ, Dobaczewski M,
Reddy A, Taffet G, Wang X-F and Frangogiannis NG: Essential role of
Smad3 in infarct healing and in the pathogenesis of cardiac
remodeling. Circulation. 116:2127–2138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dobaczewski M, Bujak M, Li N,
Gonzalez-Quesada C, Mendoza LH, Wang XF and Frangogiannis NG: Smad3
signaling critically regulates fibroblast phenotype and function in
healing myocardial infarction. Circ Res. 107:418–428. 2010.
View Article : Google Scholar : PubMed/NCBI
|