Introduction
Lung cancer, additionally termed lung carcinoma or
pulmonary carcinoma, is the leading cause of cancer-associated
mortality worldwide and is characterized by uncontrolled cell
growth in tissues of the lung. Lung cancer is the most fatal type
of cancer in the USA, responsible for more fatalities than
colorectal, breast and prostate cancers combined. An estimated
158,040 Americans are expected to have succumbed to lung cancer in
2015, accounting for ~27% of all cancer mortality in America
(1–3).
It has previously been demonstrated that ~80–90% of
cases of lung cancer are associated with cigarette smoking
(4). Symptoms of lung cancer
include, however are not limited to, coughing, chest pain and
weight loss. The prognosis for a lung cancer patient is frequently
poor, however outcomes have improved, due to the identification of
certain mutations that maybe targeted for therapy (5). Depending on the stage of the disease,
lung cancer treatment may include surgery, chemotherapy, radiation
therapy or a combination of these.
It is reported that numerous active substances
produced by animals, plants and microorganisms have been employed
in the development of novel drugs to treat cancer (6). Of these identified substances, the
peptide melittin in bee venom has been demonstrated to exhibit
antitumor activity (7). Melittin
is reported to exhibit the antitumor potential, and is isolated
from bee venom, acting via differing mechanisms on the physiology
of cancer cells (8). It is
suggested that the cytotoxicity of melittin in tumor cell lines,
and its action on signaling pathways, lead to the inhibition of
cellular proliferation (9).
Melittin is the principal toxic component in the
venom of the European honey bee (Apis mellifera) and is a
cationic, hemolytic peptide that is small and linear and composed
of 26 amino acid residues. The amino-terminal region of the peptide
is predominantly hydrophobic, whereas the carboxy-terminal region
is hydrophilic. This is due to the presence of a stretch of
positively charged amino acids (10). The sequence of melittin is as
follows:
Gly-Ile-Gly-Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro-Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-Gln
(6). It is reported that melittin
targets a range of cancer cells, including those in leukemia, lung,
renal, liver, bladder and prostate cancers (11). Heinen and da Veiga (6) reported that lytic peptide conjugates
were all highly effective in targeting and destroying disseminated
breast cancer metastases in lymph nodes, bones, lungs and other
organs). In addition, Jeong et al (12) demonstrated that melittin suppresses
epidermal growth factor (EGF)-induced cell motility and invasion by
inhibiting the phosphoinositide 3-kinase/protein kinase
B/mechanistic target of rapamycin (mTOR) signaling pathway in
breast cancer cells. Furthermore, Shin et al (13) reported that melittin may be able to
suppress the expression of hypoxia-inducible factor (HIF)-1α and
vascular endothelial growth factor (VEGF) through inhibiting the
extracellular signal-regulated kinase and mTOR/p70S6K pathways in
cervical carcinoma cells.
Therefore, the aim of the present study was to
determine if melittin has a direct action on processes of non-small
cell lung cancer cells.
Materials and methods
Ethical approval
All animal experiments in the present study were
approved by the Ethics Committee of Tongji University School of
Medicine (Shanghai, China).
Cell culture
The human non-small cell lung cancer cell line,
A549, was purchased from the Shanghai Institute for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% FBS at 37°C in an environment containing 5% CO2.
Cell viability assays
Cells at a density of 1×103 cells/well
were plated in 96-well culture plates and cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
Following a 24 h attachment period, media were replaced with 100 µl
of medium containing different concentrations (0, 0.5, 1, 2 or 4
µg/ml) of melittin (cat no. M4171; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and cultured for 24 h. A total of 10 µl Cell
Counting Kit (CCK)-8 (cat no. CK04-3000T; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well for 4 h
at 37°C in a 5% CO2 incubator, then the absorption value
was tested according to the manufacturer's protocol. The reaction
product was quantified by measuring the absorbance value at 490 nm
using a DU® 800 spectrophotometer (Beckman Coulter,
Inc., Brea, CA, USA).
Flow cytometric analysis of
apoptosis
A549 non-small cell lung cancer cells were seeded
onto 6-well dishes at a density of 2×106 cells/ml.
Following a 24 h period for attachment, the cells were treated with
different concentrations of melittin (0, 0.5, 1 or 2 µg/ml) for 24
h. Cells were treated with 1% paraformaldehyde for 15 min at room
temperature, permeabilized with 10% Triton X-100 (cat no. 93443;
Sigma-Aldrich; Merck KGaA) for 10 min at room temperature, and
stained using the FITC-Annexin V Apoptosis Detection kit I (BD
Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. The proportion of apoptotic cells was
measured using a flow cytometer (BD FACSAria™ III; BD Biosciences)
and analyzed using FlowJo software (version 10; Tree Star, Inc.,
Ashland, OR, USA). All experiments were repeated three times
(14).
Transwell invasion assay
Cell invasion serves a pivotal role in the
progression of cancer. Non-small cell lung cancer cells (100 µl
cell suspension; 1×106 cells/ml) were plated on the
Matrigel-coated upper chamber, and the serum-free media with or
without drugs were added to the upper chamber of the Transwell
insert. The concentrations of melittin used were 0, 0.5, 1 and 2
µg/ml, in combination with 20 ng/ml EGF. The lower chamber was
filled with DMEM. Cells in the chamber were incubated for 24 h at
37°C and cells that invaded the lower membrane surface were removed
using a cotton swab, fixed with methanol and stained with crystal
violet for 30 min at room temperature. The cells that passed
through the Matrigel and were located on the underside of the
filter were counted. A total of 6 random fields were counted by
light microscopy (13).
Wound-healing assays
The wound-healing assay is an effective tool for the
investigation of the migration characteristics of cultured cells.
Non-small cell lung cancer cells were seeded onto 6-well plates at
a density of 5×105 cells/well. Following reaching 80%
confluence, monolayers were scratched to create a wound, and then
were washed twice with PBS to remove non-adherent cells. Media were
then replaced with fresh serum-free medium containing different
concentrations of melittin (0, 0.5, 1 or 2 µg/ml), and 20 ng/ml of
EGF for 24 h, prior to being photographed (12).
Animal experiments
A total of 18 BALB/C nude mice (six-weeks-old; male;
weight, 20±1 g) were purchased from the Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). Mice were kept under standard
animal housing conditions (12-h light/dark cycle) with food and
water ad libitum at a temperature of 20±2°C and a relative
humidity of 40–50%. BALB/C nude mice were subcutaneously injected
with 0.1 ml Matrigel-containing non-small cell lung cancer cells
(1.5×106 cells/ml) into the left and right flank. A
total of 2 weeks following inoculation, tumors grew to 150–200
mm3 and the mice were randomly divided into the
following three groups (n=6 in each): Group A, administration with
a subcutaneous injection of melittin (1 mg/kg) every day for 24
days; group B, administration with a subcutaneous injection of
melittin (10 mg/kg) every day for 24 days; control, control group
treated with vehicle control of PBS. Tumor growth was recorded
daily until day 24. Following subcutaneous injections of melittin
for 24 days, the mice were sacrificed, and the tumors were
harvested and weighed using an electronic scale (CR5501; Care
Electronic Scale Co., Ltd., Zhongshan, China). The tumor volume was
calculated using the following formula: Tumor volume (V) = [length
(L) × width (W)]2/2, in which the length is greater than the width.
The average tumor volume in mm3 was plotted for each
third day, from day 9 until day 24. Data are presented as the mean
± standard deviation (15).
Measurement of VEGF levels using an
enzyme-linked immunosorbent assay (ELISA)
Non-small cell lung cancer cells (1,000 cells/well)
were seeded onto 96-well plates 6-well dishes. Following a 24 h
attachment period, the cells were treated using different
concentrations of melittin (0, 0.5, 1 or 2 µg/ml) for 24 h. The
culture supernatant of cells was collected individually, and the
concentration of VEGF was measured using an ELISA kit (cat no.
DVE00; R&D Systems, Inc., Minneapolis, MN, USA) according to
the manufacturer's protocol.
Western blot analysis
Cultured cells were used in western blotting, as
previously described (16),
followed by homogenization in lysis buffer (8 M urea, 0.2% SDS,
0.8% Triton X-100, 3% 2-mercaptoethanol), and the protein
concentration was measured using a protein assay kit (cat no.
5000002) with bovine serum albumin as the standard (both from
Bio-Rad Laboratories, Inc., Hercules, CA, USA). A total of 50 µg
protein/lane was separated by SDS-PAGE on a 10% gel and transferred
onto nitrocellulose membranes. Membrane were blocked in 5% milk in
TBS-Tween-20 for 1 h at room temperature. Western blot analysis was
performed using the following diluted primary antibodies against:
(HIF)-1α (cat no. sc-10790; 1:200), VEGF (cat no. A-20; 1:250),
β-actin (cat no. C-4; 1:500) (all from Santa Cruz Biotechnology,
Dallas, TX, USA) and biotinylated anti-mouse IgG and biotinylated
goat anti-rabbit IgG secondary antibodies (cat nos. 115–065-003 and
111-067-003; 1:100; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA). The β-actin served as the internal control.
Membranes were incubated with the primary antibodies for 1 h at
room temperature, and subsequently with the secondary antibodies
for 1 h at room temperature. The immunoreactivity was visualized
using the Immobilone western blotting detection system (cat no.
WBKLS0050; EMD Millipore, Billerica, MA, USA). Films were developed
and scanned, and bands were quantified using Scion Image Analysis
software, version 4.02 (Scion Corporation, Frederick, MD, USA).
Statistical analysis
One-way analysis of variance followed by the Tukey
post-hoc test was performed to analyze the differences between
different groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell viability
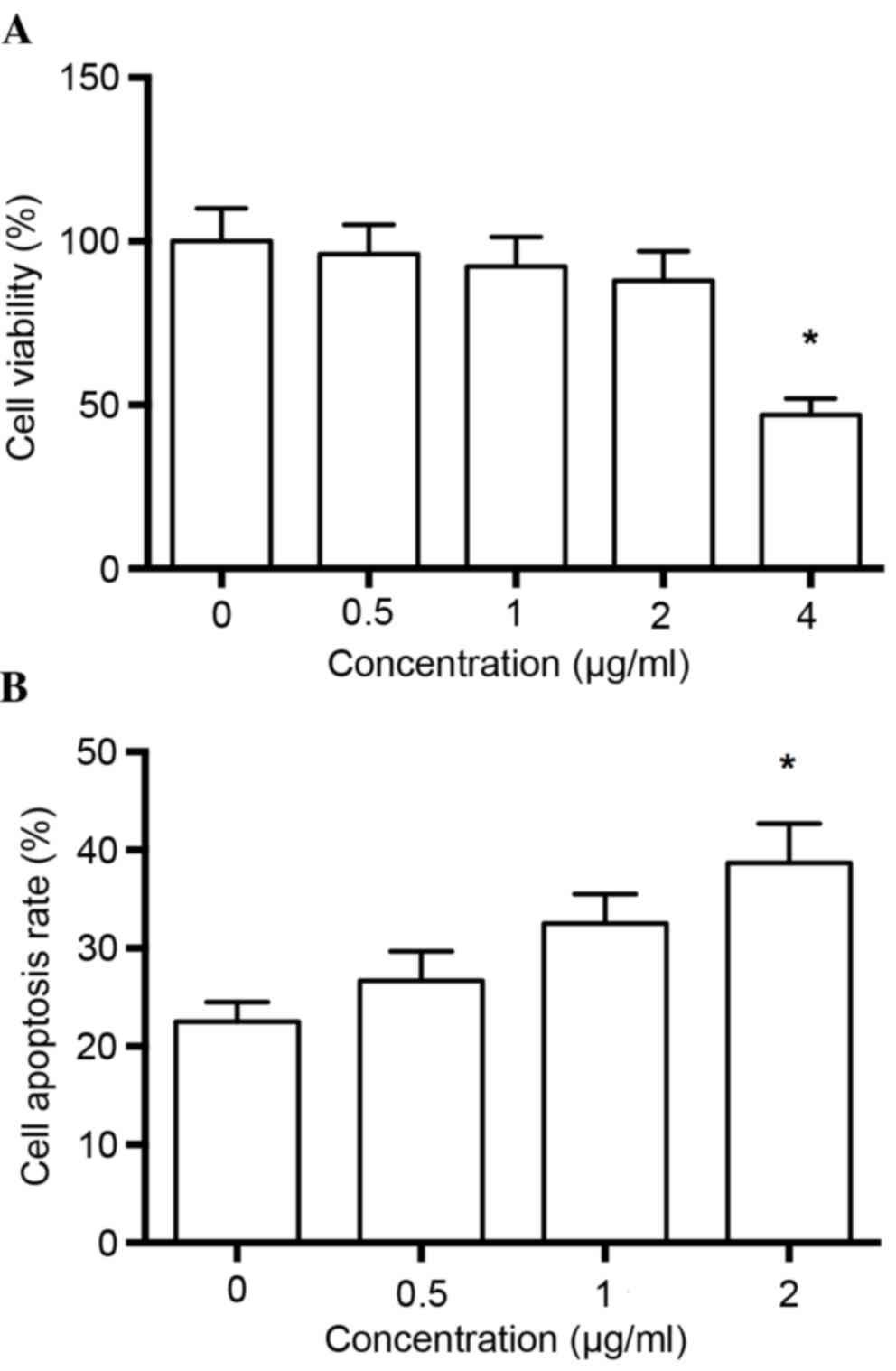
Prior to evaluating the pharmacological potential of
melittin on human non-small cell lung cancer cells, the cytotoxic
effects of the drug were examined via a CCK cell viability assay.
Each concentration of melittin was compared with the control group,
and it was observed that melittin did not significantly inhibit
cell viability at concentrations of up to 2 µg/ml (P>0.05;
Fig. 1A), therefore drugs at 2
µg/ml were used in the following experiments.
Flow cytometric analysis of apoptosis
rate
The authors detected the effects of melittin on
apoptosis of non-small cell lung cancer cells. The results
demonstrated that the apoptosis rate was significantly greater at 2
µg/ml melittin, when compared with the control group (0 µg/ml;
P<0.01; Fig. 1B).
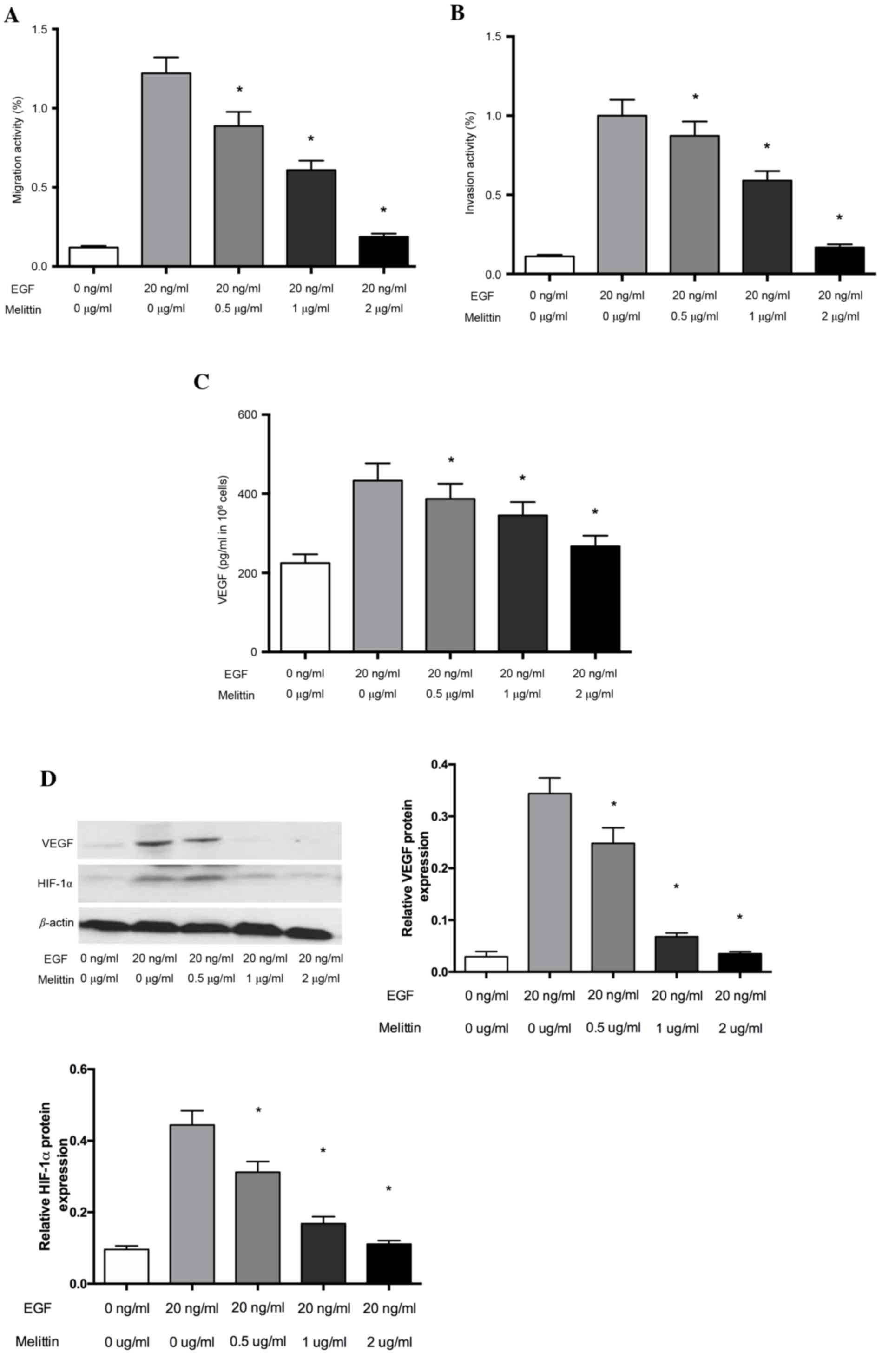
Melittin inhibits the migration and
invasion of non-small cell lung cancer cells
The cytotoxic effects of melittin were examined
using the CCK-8 kit prior to evaluating the pharmacological
potential of melittin on non-small cell lung cancer cells.
Cell Transwell invasion assays were used to evaluate
the inhibitory effects of melittin on the EGF-induced migration and
invasion of non-small cell lung cancer cells. As demonstrated in
Fig. 2, melittin inhibited
EGF-induced (20 ng/ml) cell migration of non-small cell lung cancer
cells. This inhibition was significant at melittin concentrations
of 0.5, 1 and 2 µg/ml, when compared with the control group
(P<0.05; Fig. 2A). Furthermore,
the inhibitory effect of melittin on cell invasion was similar to
that on migration, with melittin significantly inhibiting the
EGF-induced invasion in the Transwell invasion assays at melittin
concentrations of 0.5, 1 and 2 µg/ml (P<0.05; Fig. 2B).
Melittin decreases levels of VEGF in
non-small cell lung cancer cells
Tumor growth and metastasis depend on angiogenesis
triggered by chemical signals from tumor cells in the rapid growth
phase. VEGF performs a critical role in the development of
angiogenesis. To determine whether the inhibitory effect of
melittin affects VEGF expression, the secretion of VEGF protein was
examined via ELISA analysis under EGF-conditions in non-small cell
lung cancer cells. As presented in Fig. 2C, the effects of melittin on the
secretion of the VEGF protein increased significantly under the
EGF-induced conditions, whereas melittin at concentrations of 0.5,
1 and 2 µg/ml significantly decreased the secreted VEGF levels, as
induced by EGF (P<0.05). VEGF expression is primarily regulated
by HIF-1α, so the authors further evaluated HIF-1α and VEGF protein
expression using western blot analysis. The results suggested that
melittin decreased HIF-1α and VEGF protein expression, which
indicated that melittin may regulate VEGF levels by inhibiting
HIF-1α (Fig. 2D).
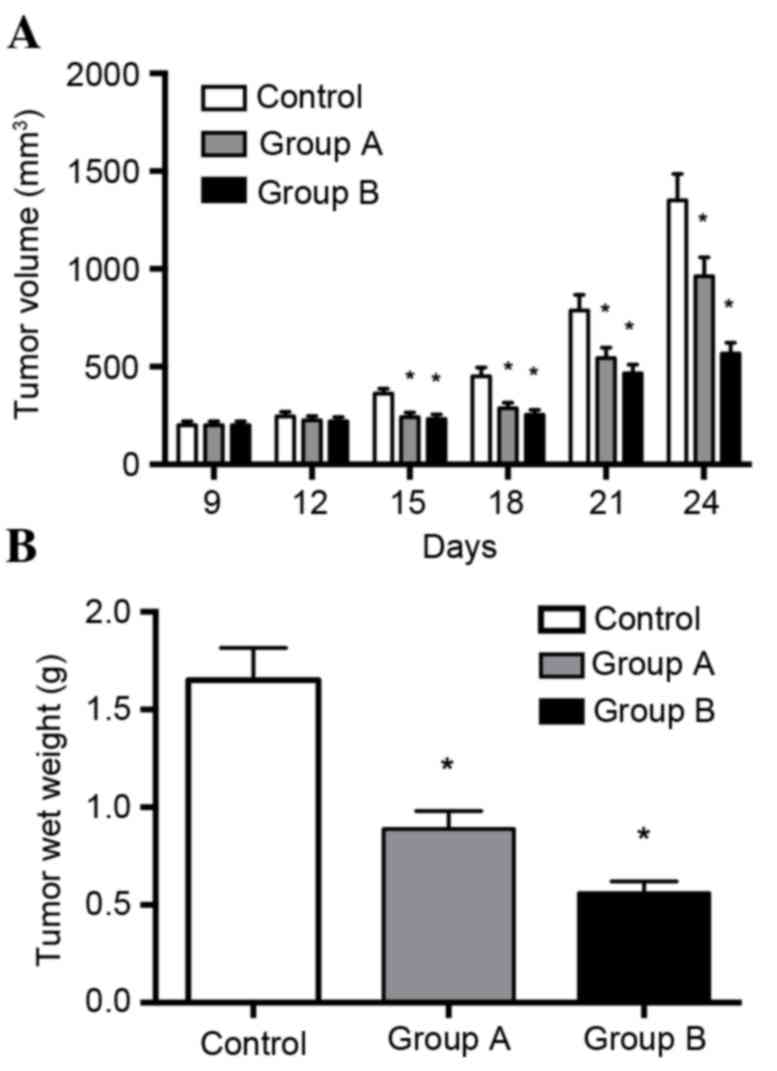
Melittin inhibits tumor growth in
xenograft models of lung cancer
The in vitro data demonstrated that melittin
exerts a tumor suppressor role on non-small cell lung cancer cells.
To explore this role in vivo, tumor growth was assessed
using tumor xenograft mouse models. As presented in Fig. 3A, melittin (at concentrations of 1
or 10 mg/kg) inhibited tumor growth of non-small cell lung cancer
cells. Tumor growth was significantly decreased by the two
concentrations of melittin when recordings were taken on days 15,
18, 21 and 24, when compared with the control group (P<0.05).
Tumors in experimental groups were smaller compared with those in
the control group, and tumors in the 10 mg/kg melittin group were
smaller than those in the 1 mg/kg melittin group. Melittin
significantly inhibited tumor growth by 27% (1 mg/kg/day) and 61%
(10 mg/kg/day).
Discussion
Lung cancer is a type of cancer that is initiated in
lung tissue. There are three primary types of lung cancer,
including non-small cell lung cancer, small cell lung cancer and
lung carcinoid tumor (17). Of
these, non-small cell lung cancer is the most frequently occurring
form, responsible for ~85% of all lung cancer diagnoses (18). Squamous cell carcinoma,
adenocarcinoma and large cell carcinoma are all subtypes of
non-small cell lung cancer. Lung cancer claims more lives each year
than colon, prostate, ovarian and breast cancers combined (19). As a result, it is crucial to
develop a novel drug for the treatment of lung cancer (20).
Melittin is a major peptide constituent of bee venom
that has been proposed as one of the potential drugs for anticancer
therapy (21). Certain studies
have suggested that melittin inhibits the melanotropin receptor in
M2R melanoma cell membranes (22),
whereas other studies have demonstrated that melittin kills
malignant cells by acting as a pore-forming agent (23).
Angiogenesis is known to serve a crucial role in
tumor growth, tumor propagation and metastasis formation (24). Therefore, as anti-angiogenesis is
an efficient method in antitumor treatment, the present study
tested the anti-angiogenesis potential of melittin. The results
indicated that it is effective, as the results of western blot
analysis presented a decrease in VEGF when cells were treated with
doses of melittin. It is reported in previous studies that melittin
exerts an effect on proliferation and induces growth inhibition and
apoptosis via several cancer cell death mechanisms. These
mechanisms include the activation of caspases and matrix
metalloproteinases in different types of cancer cells (25,26).
Melittin has been suggested to suppress tumor growth by inhibiting
VEGFR-2, thereby indicating that the antitumor activity of melittin
may be associated with anti-angiogenic actions via inhibition of
VEGFR-2 and inflammatory mediators involved in mitogen-activated
protein kinase signaling (12).
The results of the in vivo tumorigenicity
tests demonstrated that the melittin injection suppressed tumor wet
weight and tumor size. Melittin significantly inhibited tumor
growth by 27% (1 mg/kg/day) and 61% (10 mg/kg/day) over the study
period.
In conclusion, the findings suggested that melittin
exerts an antitumor effect on lung cancer cells in vitro and
in vivo, indicating the potential of melittin for the
treatment of lung cancer.
Acknowledgements
The present study was supported by the Shanghai
Xinglin Talent Doctor Training Program (grant no. ZY3-RCPY-2-2039),
the Shanghai TCM 3-year Action Plan (grant no. ZY3-JSFC-1-1001),
and the Program of Science and Technology Commission of Shanghai
Municipality (grant nos. 15401934500 and 16401932900).
References
|
1
|
Atlanta: Cancer Facts & Figures 2015.
American Cancer Society; 2015:
|
|
2
|
Henley SJ, Richards TB, Underwood JM,
Eheman CR, Plescia M, McAfee TA, et al: Centers for Disease Control
and Prevention (CDC): Lung cancer incidence trends among men and
women-United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 63:1–5.
2014.PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liam CK, Andarini S, Lee P, Ho JC, Chau NQ
and Tscheikuna J: Lung cancer staging now and in the future.
Respirology. 20:526–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsao AS and Papadimitrakopoulou V: The
importance of molecular profiling in predicting response to
epidermal growth factor receptor family inhibitors in
non-small-cell lung cancer: Focus on clinical trial results. Clin
Lung Cancer. 14:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heinen TE and da Veiga AB: Arthropod
venoms and cancer. Toxicon. 57:497–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song HS, Ko MS, Jo YS, Whang WK and Sim
SS: Inhibitory effect of acteoside on melittin-induced
catecholamine exocytosis through inhibition of Ca(2+)-dependent
phospholipase A2 and extracellular Ca(2+) influx in PC12 cells.
Arch Pharm Res. 38:1913–1920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mulder KC, Lima LA, Miranda VJ, Dias SC
and Franco OL: Current scenario of peptide-based drugs: The key
roles of cationic antitumor and antiviral peptides. Front
Microbiol. 4:3212013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Yu M, He Y, Xiao L, Wang F, Song C,
Sun S, Ling C and Xu Z: Melittin prevents liver cancer cell
metastasis through inhibition of the Rac1-dependent pathway.
Hepatology. 47:1964–1973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raghuraman H and Chattopadhyay A:
Melittin: A membrane-active peptide with diverse functions. Biosci
Rep. 27:189–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orsolic N: Bee venom in cancer therapy.
Cancer Metastasis Rev. 31:173–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang
JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, et al: Melittin
suppresses EGF-induced cell motility and invasion by inhibiting
PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem
Toxicol. 68:218–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin JM, Jeong YJ, Cho HJ, Park KK, Chung
IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, et al: Melittin
suppresses HIF-1α/VEGF expression through inhibition of ERK and
mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS One.
8:e693802013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JY, Hong WG, Cho JH, Kim EM, Kim J,
Jung CH, Hwang SG, Um HD and Park JK: Podophyllotoxin acetate
triggers anticancer effects against non-small cell lung cancer
cells by promoting cell death via cell cycle arrest, ER stress and
autophagy. Int J Oncol. 47:1257–1265. 2015.PubMed/NCBI
|
|
15
|
Yang X, Zhu H, Ge Y, Liu J, Cai J, Qin Q,
Zhan L, Zhang C, Xu L, Liu Z, et al: Melittin enhances
radiosensitivity of hypoxic head and neck squamous cell carcinoma
by suppressing HIF-1α. Tumour Biol. 35:10443–10448. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samarajeewa NU, Yang F, Docanto MM,
Sakurai M, McNamara KM, Sasano H, Fox SB, Simpson ER and Brown KA:
HIF-1α stimulates aromatase expression driven by prostaglandin E2
in breast adipose stroma. Breast Cancer Res. 15:R302013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schnabel PA and Junker K: Neuroendocrine
tumors of the lungs. From small cell lung carcinoma to diffuse
idiopathic pulmonary neuroendocrine cell hyperplasia. Pathologe.
35:557–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mahmood S, Bilal H, Faivre-Finn C and Shah
R: Is stereotactic ablative radiotherapy equivalent to sublobar
resection in high-risk surgical patients with stage I
non-small-cell lung cancer? Interact Cardiovasc Thorac Surg.
17:845–853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mokdad AH, Dwyer-Lindgren L, Fitzmaurice
C, Stubbs RW, Bertozzi-Villa A, Morozoff C, Charara R, Allen C,
Naghavi M and Murray CJ: Trends and patterns of disparities in
cancer mortality among US counties, 1980–2014. JAMA. 317:388–406.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spira A, Halmos B and Powell CA: Update in
lung cancer 2014. Am J Respir Crit Care Med. 192:283–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gajski G and Garaj-Vrhovac V: Melittin: A
lytic peptide with anticancer properties. Environ Toxicol
Pharmacol. 36:697–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerst JE and Salomon Y: Inhibition by
melittin and fluphenazine of melanotropin receptor function and
adenylate cyclase in M2R melanoma cell membranes. Endocrinology.
121:1766–1772. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duke RC, Witter RZ, Nash PB, Young JD and
Ojcius DM: Cytolysis mediated by ionophores and pore-forming
agents: Role of intracellular calcium in apoptosis. FASEB J.
8:237–246. 1994.PubMed/NCBI
|
|
24
|
Prager GW, Poettler M, Unseld M and
Zielinski CC: Angiogenesis in cancer: Anti-VEGF escape mechanisms.
Transl Lung Cancer Res. 1:14–25. 2012.PubMed/NCBI
|
|
25
|
Holle L, Song W, Holle E, Wei Y, Wagner T
and Yu X: A matrix metalloproteinase 2 cleavable melittin/avidin
conjugate specifically targets tumor cells in vitro and
in vivo. Int J Oncol. 22:93–98. 2003.PubMed/NCBI
|
|
26
|
Moon DO, Park SY, Heo MS, Kim KC, Park C,
Ko WS, Choi YH and Kim GY: Key regulators in bee venom-induced
apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells
through downregulation of ERK and Akt. Int Immunopharmacol.
6:1796–1807. 2006. View Article : Google Scholar : PubMed/NCBI
|