Introduction
Osteosarcoma (OS), an aggressive primary sarcoma of
bone, is the most common malignant bone tumor in children and
adolescents, comprising ~60% of malignant bone tumors in the first
2 decades of life (1–3). It predominantly arises from the
metaphysis of the long bones with active bone growth and
reparation, including the knee joint, lower femur and upper tibia
(4). A previous study demonstrated
that OS is induced by genetic and epigenetic alterations that cause
mesenchymal stem cells to differentiate into osteoblasts (5). Currently, the standard therapeutic
strategy for OS is surgery followed by radiotherapy and/or
chemotherapy (6). Although
advances have been made in OS treatment, the prognosis for OS
remains poor and the 5-year survival rate for OS patients with
aggressive metastases is only 10–30% (7). The majority of patients eventually
develop local relapse or metastatic disease, which is the major
cause of mortality (8). Therefore,
identification of the effective therapeutic targets that contribute
to growth and metastasis of OS is essential for improving the
prognosis of OS.
microRNAs (miRNAs) are a type of non-coding, highly
conserved and small (21–23 nucleotides) RNA molecules which
primarily suppress gene expression through binding to the 3′
untranslated region (3′UTR) of their target genes, eventually
leading to translational repression or degradation (9,10).
Increasing evidence indicates that these small molecules have
important functions in a wide range of physiological and
pathological processes, including growth, differentiation,
apoptosis, metastasis, migration and invasion (11,12).
Abnormal expression of miRNAs is significantly associated with
multiple human diseases, including obesity, cardiovascular diseases
and cancer (9,13). Previous studies have also
demonstrated that downregulation or upregulation of miRNAs is
associated with multiple types of human cancer, where they function
as tumor suppressors or oncogenes depending on the function of
their target genes (14–16). Notably, miRNAs have been reported
to be involved in the carcinogenesis and development of OS, and may
be prognostic markers or therapeutic targets for patients with OS
(17–19). Therefore, miRNAs may be promising
targets for OS treatment.
In the present study, the data indicated that
miR-130a expression was lower in OS tissues and cell lines compared
with normal bone tissues. Low miR-130a expression levels were
significantly associated with clinical stage and metastasis.
Furthermore, upregulation of miR-130a inhibited growth, migration
and invasion of OS cells by directly targeting zinc finger E-box
binding homeobox 1 (ZEB1). Overall, miR-130a may be involved
in the suppression of OS growth and metastasis through ZEB1
downregulation, suggesting that it may be a promising novel
therapeutic target for OS.
Materials and methods
Tissue samples, cell lines, cell
culture and cell transfection
Primary OS tissues (n=62) and their matched
non-cancerous bone tissues (n=62) were obtained from patients with
OS who underwent surgery at the Weifang People's Hospital (Weifang,
China). The matched non-cancerous bone tissues were obtained 5 cm
from the tumor margin. None of these patients had received any
therapeutic treatments prior to surgery. OS tissues and
non-cancerous bone tissues were immediately snap frozen in liquid
nitrogen and stored at −80°C. Use of these sample tissues was
approved by the Ethics Committee of Weifang People's Hospital
(Weifang, China), and written informed consent was collected from
the patients with OS.
Human OS cell lines (HOS, 143B, MG63, Saos-2, U2OS)
and a normal osteoblast cell line (NHOst) were obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) or RPMI 1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in a humidified atmosphere with 5% CO2.
The miR-130a mimics, the negative control miRNA
mimics (NC), ZEB1 small interfering RNA (si-ZEB1) and
control siRNA (si-control) were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). The sequence of the miR-130a mimic
was 5′-CAGUGCAAUGUUAAAAGGGCAU-3′. The sequence of the NC mimic was
5′-UUCUCCGAACGUGUCACGUTT-3′. The sequence of the si-ZEB1 was
5′-AACUGAACCUGUGGAUUAU-3′. The si-control sequence was
5′-AACAGGCACACGTCCCAGCGT-3′. Transfection was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
when cells were grown to 80% confluence, according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared from frozen tissues and
culture cells using the mirVana miRNA Isolation kit (Ambion; Thermo
Fisher Scientific, Inc.), following the manufacturer's protocol.
For miRNA expression, TaqMan miRNA reverse transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
synthesize cDNA from total RNA. The TaqMan miRNA assay kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to detect
miR-130a expression, with U6 small nuclear RNA used as an internal
control. This stage was performed using 40 cycles of denaturation
at 95°C for 15 sec and annealing/extension at 60°C for 60 sec. For
mRNA expression, cDNA was synthesized using the PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd., Dalian, China),
followed by RT-qPCR with Real-time PCR Mixture Reagent (Takara
Biotechnology Co., Ltd.), according to the manufacturer's protocol.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was measured as an
internal control for mRNA expression. This stage was performed as
follows: 42°C for 5 min; 95°C for 10 sec; and 40 cycles of 95°C for
5 sec, 55°C for 30 sec and 70°C for 30 sec. The primer sequences
used were as follows: miR-130a, forward 5′-AGGATGAGAGGAAGGCTGTG-3′
and reverse 5′-AGAAAACAGTGACGCTGAGG-3′; U6, forward
5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse 5′-CCAGTGCAGGGTCCGAGGT-3′;
ZEB1, forward 5′-CTCGAGCATTTAGACACAAGCG-3′ and reverse
5′-TTGCCCTTCCTTTCCTGTGT-3′; and GAPDH, forward
5′-CCCCCAATGTATCCGTTGTG-3′ and reverse 5′-TAGCCCAGGATGCCCTTTAGT-3′.
Relative expression fold changes were calculated using the
2−ΔΔCq method (20).
Cell proliferation analysis
The effect of miR-130a on OS cell proliferation was
assessed by Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Cells were seeded into
96-well plates at a density of 3,000 cells per well. Following
incubation overnight, cells were transfected with the miR-130a
mimic or NC miRNA. CCK-8 solution (10 µl) was added into each well
of the 96-well plates at different time points post-transfection
(24–96 h) and incubated for another 2 h. The optical density at 450
nm was detected with a microplate reader. The experiment was
performed in triplicate.
Transwell cell migration and Matrigel
cell invasion assays
The effect of miR-130a on OS cell metastasis was
evaluated using a hanging cell culture insert (Merck Millipore,
Darmstadt, Germany). For the cell migration assay, 5×104
transfected cells in 400 µl serum-free DMEM were transferred to the
upper insert. For the cell invasion assay, 5×104 transfected cells
in 400 µl serum-free medium were seeded into the upper chamber
coated with Matrigel (BD Biosciences, San Jose, CA, USA). For both
assays, the lower insert was filled with 500 µl complete medium
containing 20% FBS (Gibco; Thermo Fisher Scientific, Inc.). Cells
that had not migrated or invaded to the membrane of the culture
insert following 48 h incubation were removed from the upper
surface of the membrane with cotton swabs. Cells that had
penetrated the membrane were then fixed in 100% methanol, stained
with 0.5% crystal violet and the number of cells was counted in
five randomly selected fields (magnification, ×100) under an
inverted microscope (CKX41; Olympus Corporation, Tokyo, Japan).
miR-130a target prediction
TargetScan 6.0 (http://www.targetscan.org/vert_60/) was used to
predicate the putative targets of miR-130a (21).
Western blot analysis
Transfected cells were harvested and lysed with RIPA
lysis buffer. Equal amounts of protein lysate (20 µg) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (Merck Millipore). The membranes were blocked
with 5% non-fat milk in Tris-buffered saline, then incubated with
primary antibodies overnight at 4°C and corresponding horseradish
peroxidase (HRP)-conjugated secondary antibodies at room
temperature for 2 h. The protein bands were detected with the
SuperSignal West Pico Chemiluminescent Substrate kit (Pierce,
Rockford, IL). The primary antibodies used in the present study
were as follows: Mouse anti-human monoclonal ZEB1 antibody (1:1,000
dilution; cat. no. sc-81428; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and anti-human monoclonal GADPH antibody (1:1,000
dilution; cat. no. sc-59540; Santa Cruz Biotechnology, Inc.). The
secondary antibody used in the present study was goat anti-mouse
IgG+IgM-HRP (1:3,000 dilution; cat. no. ab-47827; Abcam, Cambridge,
MA, USA). Protein levels were normalized to total GAPDH.
Dual-luciferase report assay
pmiR-ZEB1-3′UTR Wt and pmiR-ZEB1-3′UTR Mut plasmids
were purchased from Guangzhou RiboBio Co., Ltd. OS cells were
seeded into 24-well culture dishes and co-transfected with
pmiR-ZEB1-3′UTR Wt or pmiR-ZEB1-3′UTR Mut and the miR-130a mimic or
NC at room temperature. Cells were harvested 48 h following
transfection and luciferase activities were measured using the
Dual-Luciferase Reporter Assay system (Promega Corporation,
Madison, WI, USA). Renilla luciferase activities were detected as
an internal control for firefly luciferase activities.
Statistical analysis
The data were presented as the mean ± standard
deviation. The data were evaluated using SPSS 19.0 statistical
software package (IBM SPSS, Armonk, NY, USA). Differences between
all variables were analyzed using Student's t-tests or Chi-square
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-130a expression levels are
decreased in OS
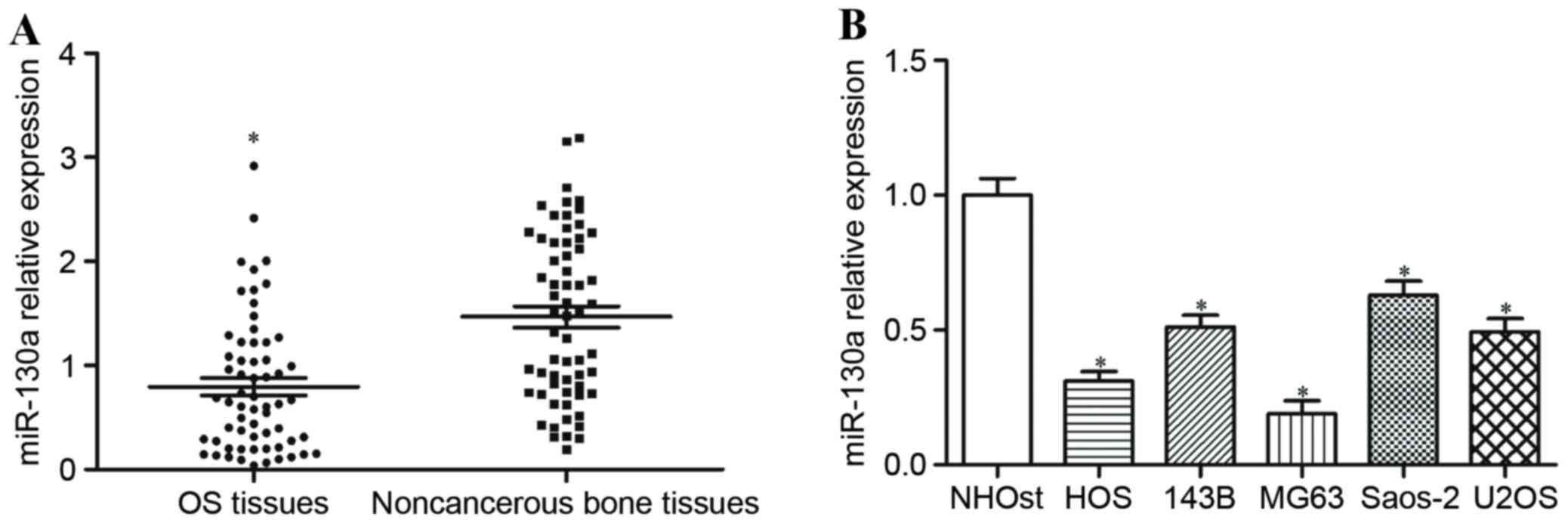
To explore whether miR-130a was deregulated in OS,
its expression level was measured in human OS tissues and matched
non-cancerous bone tissues using RT-qPCR. miR-130a expression
levels were significantly reduced in OS tissues compared with
non-cancerous bone tissues (P<0.05; Fig. 1A). miR-130a expression was also
determined in OS cell lines and a normal osteoblast cell line, and
miR-130a expression levels were significantly decreased in all five
OS cell lines in comparison with the normal osteoblast cell line
(P<0.05; Fig. 1B).
Correlations between miR-130a
expression and clinicopathological features in patients with
OS
Statistical analysis identified that miR-130a
expression in patients with OS was significantly negatively
correlated with clinical stage (P=0.040; Table I) and metastasis (P=0.002; Table I), but no significant associations
were observed between miR-130a expression and age, sex, tumor size
or location of the primary tumor (Table I).
 | Table I.Correlation of miR-130a expression
levels with clinicopathological features in osteosarcoma
patients. |
Table I.
Correlation of miR-130a expression
levels with clinicopathological features in osteosarcoma
patients.
|
|
| miR-130a
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Case no. (n=62) | Low (n=37) | High (n=25) | P-value |
|---|
| Age |
|
|
| 0.597 |
|
<13 | 38 | 24 | 14 |
|
| ≥13 | 24 | 13 | 11 |
|
| Sex |
|
|
| 1.000 |
| Male | 31 | 19 | 12 |
|
|
Female | 31 | 18 | 13 |
|
| Tumor size
(cm) |
|
|
| 0.799 |
| <8
cm | 36 | 22 | 14 |
|
| ≥8
cm | 26 | 15 | 11 |
|
| Location of the
primary tumor |
|
|
| 0.763 |
|
Tibia/femur | 47 | 29 | 18 |
|
|
Elsewhere | 15 | 8 | 7 |
|
| Clinical stage |
|
|
| 0.040 |
|
I-II | 28 | 12 | 16 |
|
|
III | 34 | 25 | 9 |
|
| Metastasis |
|
|
| 0.002 |
|
Present | 35 | 27 | 8 |
|
|
Absent | 27 | 10 | 17 |
|
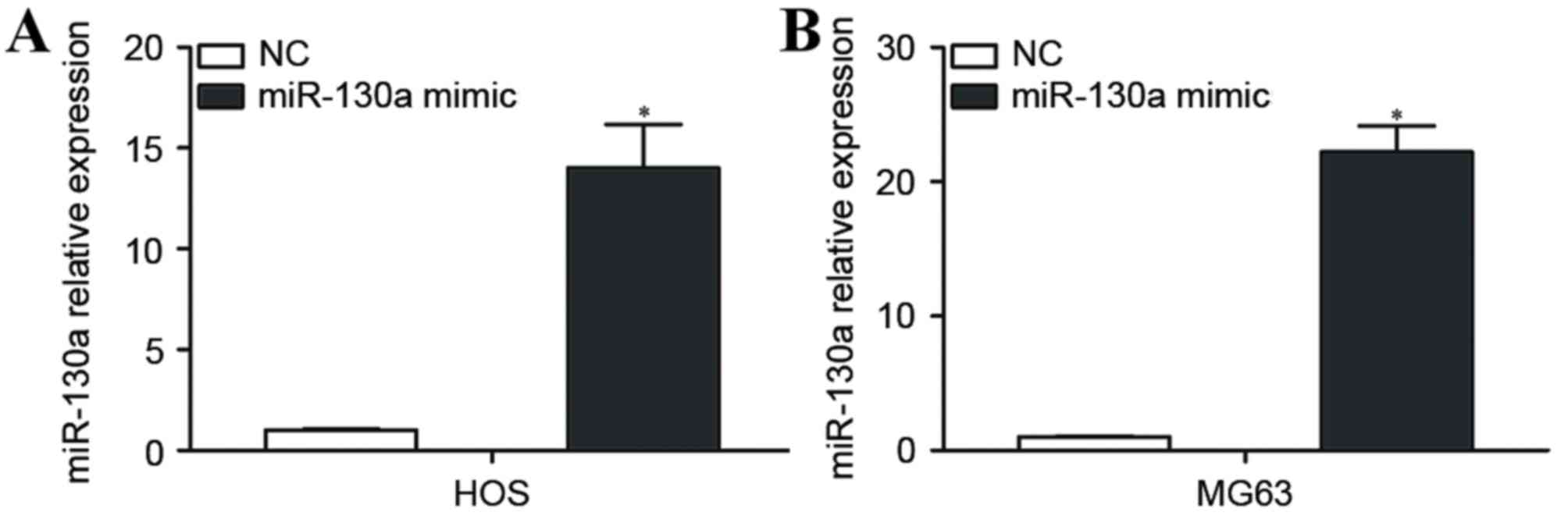
miR-130a is significantly upregulated
in OS cells transfected with an miR-130a mimic
HOS and MG63 cells, which expressed relatively low
levels of miR-130a, were selected for further studies, and were
transfected with the miR-130a mimic or NC. RT-qPCR was performed 48
h following transfection to measure miR-130a expression. miR-130a
was significantly upregulated in HOS and MG63 cells transfected
with the miR-130a-mimic compared with HOS and MG63 cells
transfected with NC (P<0.05; Fig.
2A).
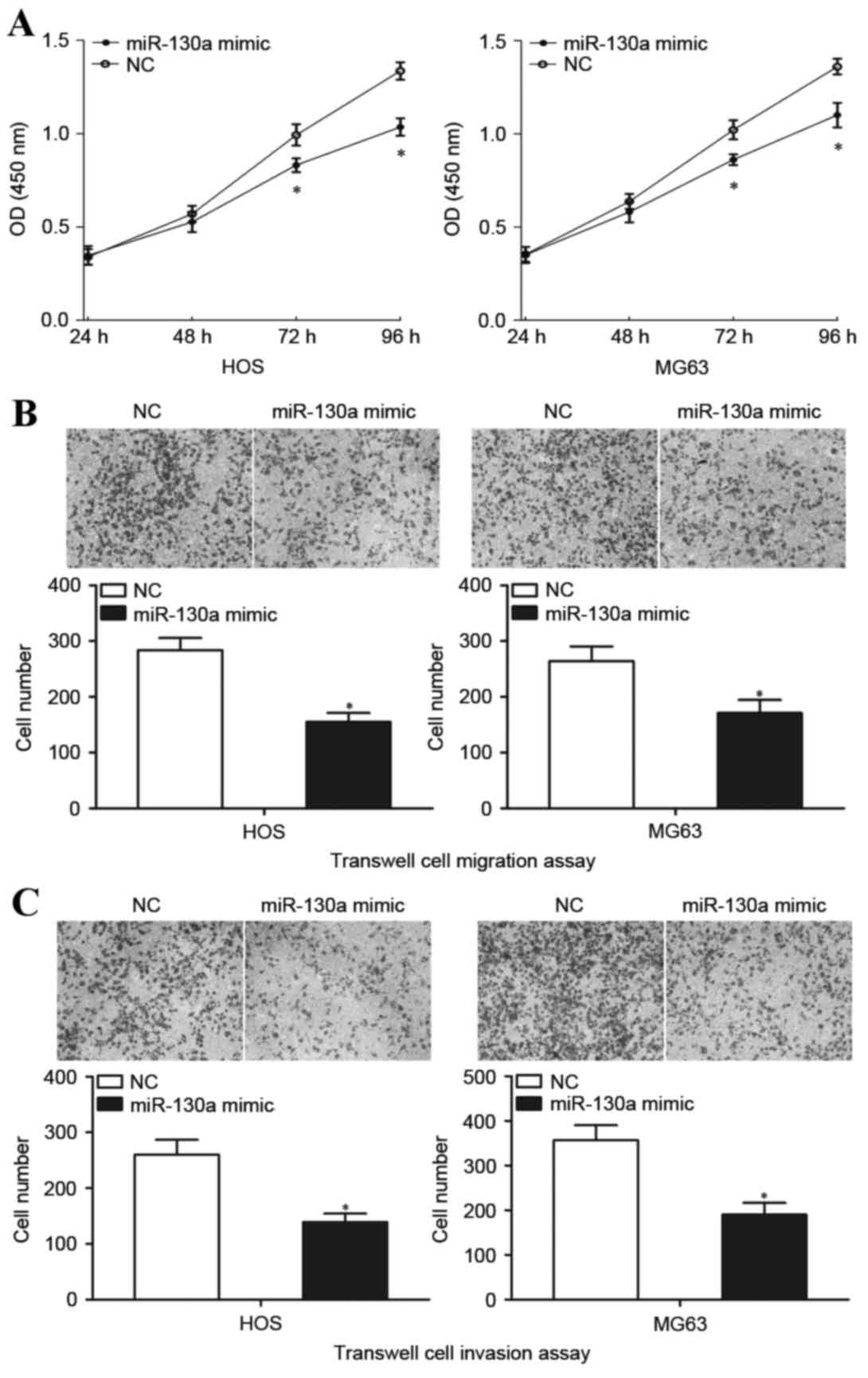
miR-130a inhibits OS cell growth,
migration and invasion
The cell proliferation assay was performed to
measure the involvement of miR-130a in OS cell growth
proliferation. Transfection with the miR-130a mimic significantly
inhibited the growth of HOS and MG63 cells at both 72 and 96 h
compared with transfection with NC (Fig. 3A). Transwell cell migration and
Matrigel cell invasion assays were performed to explore the effect
of miR-130a on the metastasis capacity of OS cells. HOS and MG63
cells transfected with the miR-130a mimic demonstrated
significantly decreased migratory capacity compared with the cells
transfected with NC (P<0.05; Fig.
3B). In addition, HOS and MG63 cells transfected with the
miR-130a mimic also demonstrated significantly decreased invasion
compared with the cells transfected with NC (P<0.05; Fig. 3C). These results indicated that
miR-130a functions as a tumor suppressor in OS, through inhibiting
growth and metastasis of OS cells.
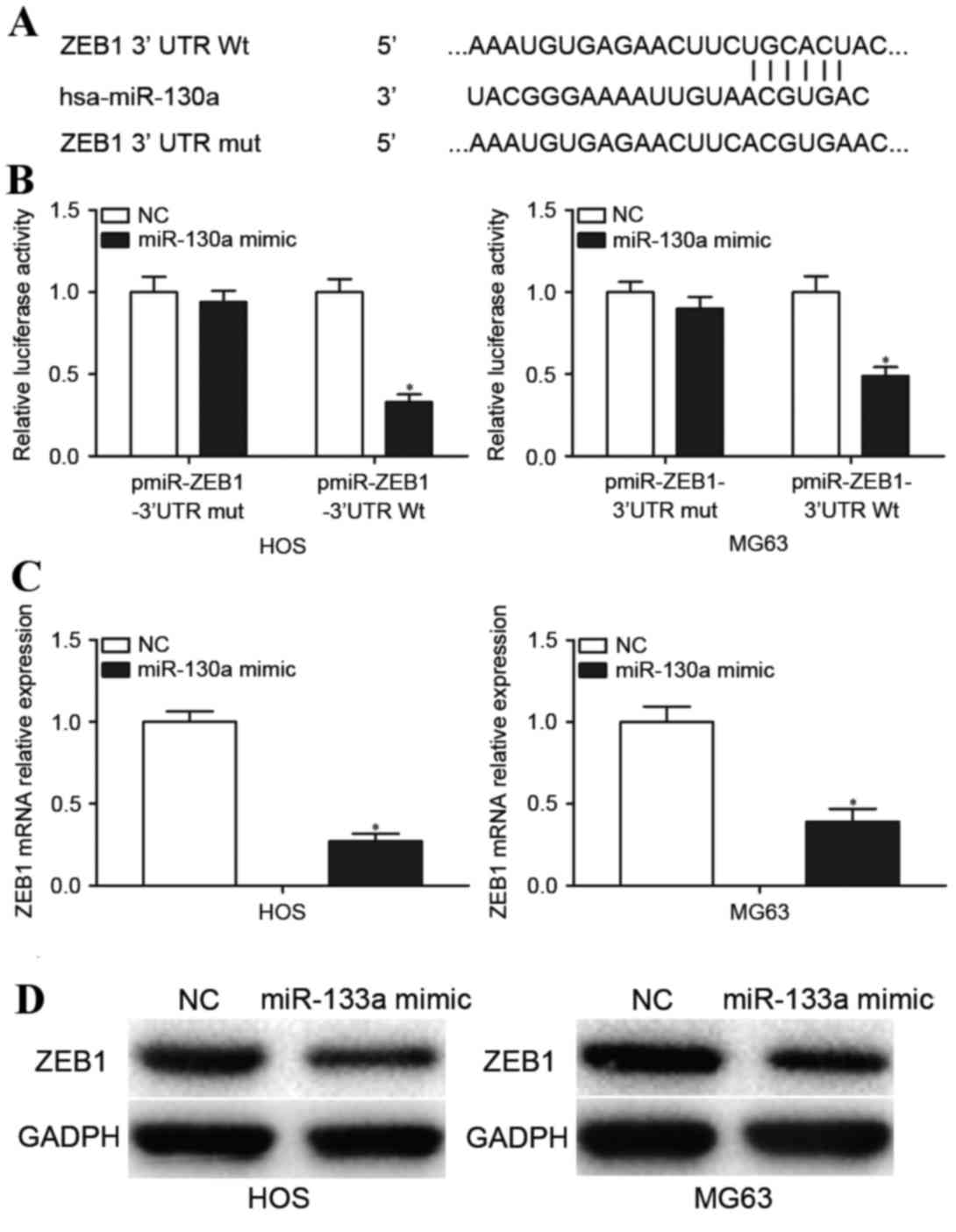
miR-130a downregulates ZEB1 expression
by directly targeting its 3′UTR
To explore the molecular mechanisms underlying the
involvement of miR-130a in OS, the miRNA target prediction tool
TargetScan was used to predict its target genes. Bioinformatics
analysis indicates a putative conserved binding site for miR-130a
in the 3′UTR of ZEB1 at 500–506 bp (Fig. 4A). Dual-Luciferase report assays
were performed to explore whether miR-130a directly targeted the
3′UTR of ZEB1. Transfection with the miR-130a mimic
significantly reduced the luciferase activity of pmiR-ZEB1-3′UTR Wt
compared with transfection with NC (P<0.05; Fig. 4B) but not the binding
pmiR-ZEB1-3′UTR Mut (P>0.05; Fig.
4B). To further validate that ZEB1 was a direct target
gene of miR-130a, RT-qPCR and western blotting analysis was
performed to determine ZEB1 mRNA and protein expression
levels in OS cells transfected with the miR-130a mimic and NC.
Transfection with the miR-130a mimic significantly decreased
ZEB1 mRNA expression levels (P<0.05; Fig. 4C) and visibly deceased ZEB1 protein
levels (P<0.05; Fig. 4D) in HOS
and MG63 cells compared with cells transfected with the NC. These
results indicated that miR-130a downregulates ZEB1
expression by directly targeting its 3′UTR.
Downregulation of ZEB1 mimicked the
effect of transfection with the miR-130a mimic in OS cells
To investigate the involvement of ZEB1 in OS,
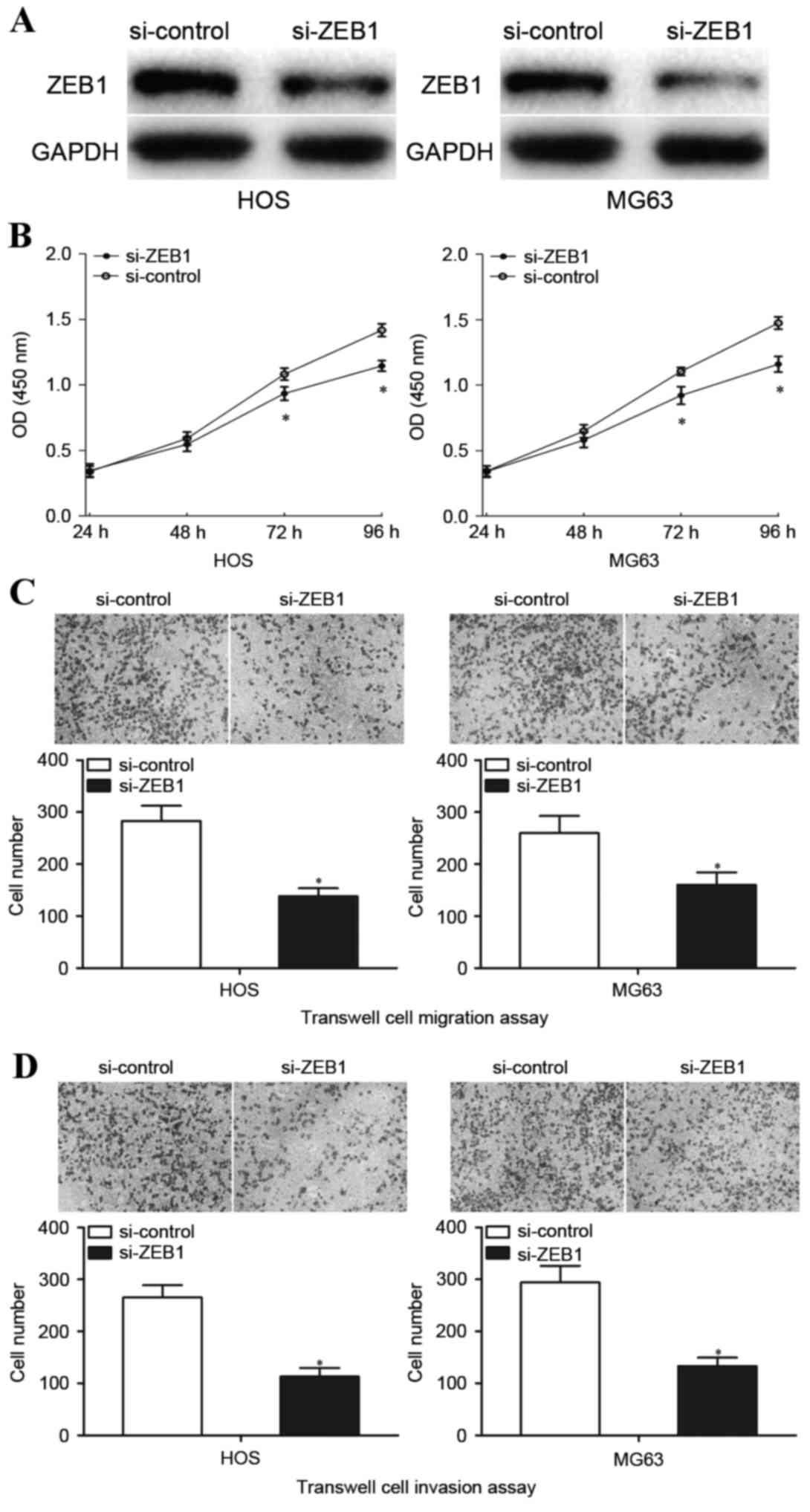
HOS and MG63 cells were transfected with si-ZEB1 or si-control, and
cell proliferation, Transwell cell migration and Matrigel cell
invasion assays were performed. Western blot analysis was performed
72 h following transfection to detect ZEB1 protein expression.
Transfection with si-ZEB1 visibly inhibited ZEB1 protein expression
in HOS and MG63 cells compared with cells transfected with
si-control (Fig. 5A). Transfection
with si-ZEB1 significantly inhibited HOS and MG63 cell
proliferation at both 72 and 96 h (Fig. 5B). Furthermore, downregulation of
ZEB1 by transfection with si-ZEB1 significantly decreased
HOS and MG63 cell migration (P<0.05; Fig. 5C) and invasion (P<0.05; Fig. 5D). These results indicated that
downregulation of ZEB1 mimicked the effects observed when
transfected with an miR-130a mimic in terms of inhibiting OS cell
proliferation, migration and invasion.
Discussion
OS, the most common primary bone tumor, rapidly
destroys the surrounding tissues (22). Current therapeutic strategies for
patients with OS are not sufficiently effective, therefore, novel
therapeutic targets are required. The abnormal expression of miRNAs
has been observed in multiple human cancers, and attention has been
focused on understanding the physiological and pathophysiological
mechanisms of miRNAs in cancer carcinogenesis and progression,
which may provide novel therapeutic targets for human cancers,
including OS (17). In the present
study, miR-130a expression was measured in OS tissue and cell
lines, and the correlations between the clinicopathological
features of OS and miR-130a expression levels were explored. The
results indicated that miR-130a was significantly downregulated in
OS tissues and cell lines compared with normal bone tissue and
normal osteoblasts, and low miR-130a expression levels were
significantly correlated with clinical stage and metastasis.
Further experiments demonstrated that increasing miR-130a
expression via transfection with a mimic significantly inhibited OS
cell proliferation, migration and invasion. ZEB1 was
identified as a direct target of miR-130a in OS, and knockdown of
ZEB1 using siRNA produced similar effects as observed
following transfection with an miR-130a mimic. Therefore, the
present study indicated that miR-130a may be a tumor suppressor in
OS via targeting ZEB1, and may be a potential novel target
for the treatment of patients with OS, to block rapid growth and
metastasis.
The abnormal expression of miR-130a has been
observed in multiple types of cancer. For example, in
hepatocellular carcinoma, miR-130a expression levels were decreased
in tumor tissues compared with adjacent non-tumor tissues.
Correlation analysis indicated that low miR-130a expression levels
were not associated with age, sex, tumor size or location of the
primary tumor, however miR-130a expression levels were
significantly correlated with clinical stage and metastasis. In
addition, Kaplan-Meier analysis identified that patients with low
miR-130a expression had a poorer overall survival than patients
with high miR-130a expression (23). Furthermore, multivariate Cox
regression analysis has previously indicated that miR-130a
expression is an independent prognostic factor for overall survival
(23). Low expression of miR-130a
has also been observed in prostate carcinoma (24) and breast cancer (25). However, in gastric cancer, miR-130a
was upregulated in tumor tissues. In addition, the low miR-130a
group had significantly improved overall survival in comparison
with the high miR-130a group. Furthermore, miR-130a was also
upregulated in the plasma of patients with gastric cancer, and the
diagnostic value of miR-130a for gastric cancer is more effective
than the tumor markers carcinoembryonic antigen and cancer antigen
19–9 (26). miR-130a was also
reported to be upregulated in cisplatin resistant ovarian cancer
(27), gefitinib-sensitive
non-small-cell lung cancer (28)
and cervical cancer (29). These
conflicting findings indicate that the effect of miR-130a
expression in human cancer has tissue specificity.
It has been previously reported that miR-130a has
multiple targets through which it regulates multiple human cancers.
For example, in breast cancer, miR-130a targeted RAB5A, member RAS
oncogene family to suppress cancer cell growth, migration and
invasion (25). Jiang et al
(26) and Lee et al
(30) reported that miR-130a
increased gastric cancer cell proliferation, migration, invasion
and angiogenesis via directly targeting runt-related transcription
factor 3. Zhou et al (28)
demonstrated that miR-130a suppressed cell proliferation and
increased apoptosis of non-small-cell lung cancer cells treated
with gefitinib through negative regulation of met. In the present
study, ZEB1 was identified as a direct target gene of
miR-130a in OS. Identification of miR-130a target genes is
essential for elucidating the functions of miR-130a in
carcinogenesis and progression of OS, and may provide effective
therapeutic targets for patients with OS.
ZEB1, an E-cadherin transcriptional
repressor, is a member of the zinc finger family and is located on
the short arm of human chromosome 10 (31). ZEB1 was upregulated in OS
tissues compared with normal bone tissues, in addition, ZEB1
expression in OS tissues with positive lung metastasis was
significantly increased compared with that from patients without
lung metastasis (32). Therefore,
ZEB1 may contribute to the progression of OS, and may be a
potential target for the treatment of OS. The present study
explored whether miR-130a inhibited the growth and metastasis of OS
by negative regulation ZEB1 in OS cell lines. miR-130a
significantly suppressed ZEB1 mRNA and protein expression
levels. In addition, downregulation of ZEB1 following transfection
with siRNA resulted in a similar effect as that observed following
transfection with an miR-130a mimic. It was confirmed that miR-130a
inhibited OS cell growth, migration and invasion by downregulating
ZEB1 expression.
In conclusion, the present study demonstrated that
miR-130a was downregulated in OS tissues and cell lines. In
addition, miR-130a expression levels were significantly negatively
correlated with clinical stage and metastasis. Overexpression of
miR-130a inhibited tumor aggressiveness by inhibiting OS cell
proliferation, migration and invasion, at least in part through the
targeting of ZEB1. These observations suggest that miR-130a
may be a potential novel candidate for the treatment of OS.
References
|
1
|
Salah Z, Arafeh R, Maximov V, Galasso M,
Khawaled S, Abou-Sharieha S, Volinia S, Jones KB, Croce CM and
Aqeilan RI: miR-27a and miR-27a* contribute to metastatic
properties of osteosarcoma cells. Oncotarget. 6:4920–4935. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han G, Wang Y and Bi W: C-Myc
overexpression promotes osteosarcoma cell invasion via activation
of MEK-ERK pathway. Oncol Res. 20:149–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang J, Shen L, Yang Q and Zhang C:
Overexpression of metadherin mediates metastasis of osteosarcoma by
regulating epithelial-mesenchymal transition. Cell Prolif.
47:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shang Y, Wang LQ, Guo QY and Shi TL:
MicroRNA-196a overexpression promotes cell proliferation and
inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int J Clin
Exp Pathol. 8:2461–2472. 2015.PubMed/NCBI
|
|
5
|
Liu Y, Li Y, Liu J, Wu Y and Zhu Q:
MicroRNA-132 inhibits cell growth and metastasis in osteosarcoma
cell lines possibly by targeting Sox4. Int J Oncol. 47:1672–1684.
2015.PubMed/NCBI
|
|
6
|
Gorlick R: Current concepts on the
molecular biology of osteosarcoma. Cancer Treat Res. 152:467–478.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyers PA: Muramyl tripeptide
(mifamurtide) for the treatment of osteosarcoma. Expert Rev
Anticancer Ther. 9:1035–1049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tahara H, Kay MA, Yasui W and Tahara E:
MicroRNAs in cancer: The 22nd Hiroshima cancer seminar/the 4th
Japanese Association for RNA Interference Joint International
Symposium, 30 August 2012, Grand Prince Hotel Hiroshima. Jpn J Clin
Oncol. 43:579–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caldas C and Brenton JD: Sizing up miRNAs
as cancer genes. Nat Med. 11:712–714. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Namlos HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XH, Cai P, Wang MH and Wang Z:
microRNA25 promotes osteosarcoma cell proliferation by targeting
the cell-cycle inhibitor p27. Mol Med Rep. 10:855–859.
2014.PubMed/NCBI
|
|
19
|
Zhuo W, Ge W, Meng G, Jia S, Zhou X and
Liu J: MicroRNA20a promotes the proliferation and cell cycle of
human osteosarcoma cells by suppressing early growth response 2
expression. Mol Med Rep. 12:4989–4994. 2015.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu R, Sun YF, Wu MF, Liu JB, Jiang JL,
Wang SH, Wang XL and Guo Q: Biological roles of microRNA-140 in
tumor growth, migration, and metastasis of osteosarcoma in vivo and
in vitro. Tumour Biol. 37:353–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Huang P, Qiu J, Liao Y, Hong J and
Yuan Y: MicroRNA-130a is down-regulated in hepatocellular carcinoma
and associates with poor prognosis. Med Oncol. 31:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boll K, Reiche K, Kasack K, Mörbt N,
Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn
F and Hackermüller J: MiR-130a, miR-203 and miR-205 jointly repress
key oncogenic pathways and are downregulated in prostate carcinoma.
Oncogene. 32:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long
G and Yang K: MicroRNA-130a inhibits cell proliferation, invasion
and migration in human breast cancer by targeting the RAB5A. Int J
Clin Exp Pathol. 8:384–393. 2015.PubMed/NCBI
|
|
26
|
Jiang H, Yu WW, Wang LL and Peng Y:
miR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncol Rep. 34:1153–1161. 2015.PubMed/NCBI
|
|
27
|
Yang LY, Wang HJ, Jia XB, Wang X, Luo J
and Zhang XY: Expression of miR-130a in cisplatin resistant cell
lines of ovarian cancer. Sichuan Da Xue Xue Bao Yi Xue Ban.
43:60–64. 2012.(In Chinese). PubMed/NCBI
|
|
28
|
Zhou YM, Liu J and Sun W: MiR-130a
overcomes gefitinib resistance by targeting met in non-small cell
lung cancer cell lines. Asian Pac J Cancer Prev. 15:1391–1396.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Wu H, Li P, Zhao Y, Liu M and
Tang H: NF-kB-modulated miR-130a targets TNF-α in cervical cancer
cells. J Transl Med. 12:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SH, Jung YD, Choi YS and Lee YM:
Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases
cell proliferation and tumor angiogenesis in gastric cancer cells.
Oncotarget. 6:33269–33278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Liu Y, Wu S, Shi X, Li L, Zhao J
and Xu H: Tumor-suppressing effects of miR-429 on human
osteosarcoma. Cell Biochem Biophys. 70:215–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen A, Zhang Y, Yang H, Xu R and Huang G:
Overexpression of ZEB1 relates to metastasis and invasion in
osteosarcoma. J Surg Oncol. 105:830–834. 2012. View Article : Google Scholar : PubMed/NCBI
|