Introduction
Systemic lupus erythematosus (SLE) is a chronic
autoimmune disease that can cause multiorgan and multisystemic
damage through the production of various autoantibodies, and the
development of lupus nephritis (LN) is one of the most important
factors influencing prognosis in SLE (1–3). A
number of factors, including genetic, hormonal, environmental and
presence of drugs, can influence the development of LN, which leads
to breakdown of immune tolerance and organ dysfunction (3). Although the precise pathogenesis of
LN remains to be elucidated, numerous clinical and animal
experiments have demonstrated that B cells serve an important
function in the pathogenesis of LN (4–6).
B-cell-activating factor (BAFF), member of the tumor
necrosis factor superfamily, is an important B cell-proliferation
and maturation factor, and is secreted by a variety of cell types
including monocytes, malignant B cells, macrophages, neutrophils
and activated T lymphocytes (7–9).
BAFF can act by binding a transmembrane activator coupled with a
calcium modulator, a cyclophilin ligand interactor transmembrane
activator and a calcium signal-modulating cyclophilin ligand
interactor, the BAFF receptor (BAFF-R) or the B cell maturation
protein, which are expressed on stimulated B cells (10). The binding of BAFF to a receptor
induces the proliferation and differentiation of B cells, which
serves a vital role in immunoglobulin class switching (11). Excessive amounts of BAFF result in
an abnormal autoimmune response mediated by activated B cells
(12). In addition, the
overexpression of BAFF not only causes B cell proliferation, but
also produces a lupus erythematosus-like syndrome in mice; however,
the development of lupus can be delayed in the SLE-spontaneous
mouse model if the mice are treated with a BAFF inhibitor (13–15).
The phosphatidylinositol 3-kinase (PI3K) P110δ
isoform enhances the BAFF-mediated cellular survival and maturation
of B cells (16), and this result
indicates an important role for the PI3K/protein kinase B (Akt)
signaling pathway in response to BAFF stimulation. Mammalian target
of rapamycin (mTOR) is a serine-threonine kinase that is the main
downstream target of PI3K/Akt. mTOR can regulate a variety of
cellular responses in mammalian cells, including cellular growth,
energy availability and protein synthesis (17). PI3K/Akt/mTOR activation serves a
vital role in the signaling pathways involved in the inhibition of
apoptosis, cell proliferation and expression of inflammatory
cytokines (18). Previous studies
have demonstrated that the PI3K/Akt/mTOR signaling pathway and
BAFF/BAFF-R mediated signaling is involved in the pathogenesis of
collagen-induced arthritis rats (19,20).
It is unknown whether BAFF is involved in the development of LN via
regulation of the PI3K/Akt/mTOR pathway. The present study
investigated the association between BAFF and PI3K/Akt/mTOR
signaling in order to investigate the pathogenesis in LN.
Materials and methods
Patient selection
A total of 18 patients (1 man and 17 women) who
fulfilled the criteria for LN at Binzhou Medical University
Hospital (Binzhou, China) and 20 controls were recruited into the
present study. Individuals (6 men and 14 women) aged between 25 and
65 years old who underwent side nephrectomy for renal benign tumors
were recruited as controls between December 2014 and December 2015.
Laboratory test data including the concentration of complement
proteins C3 and C4, proteinuria/24 h and presence of antinuclear,
anti-Smith, anti-ribonucleoprotein and anti-double-stranded DNA
antibodies were collected and are exhibited in Table I. The above indexes in the control
group were within the normal range. Patients and controls included
in the present study were excluded for primary and secondary
nephropathies, including Henoch-Schonlein purpura nephritis,
diabetic nephropathy and hypertensive nephropathy. The kidney
tissue of the patients with LN was obtained by renal biopsy. The
control tissue was normal renal tissue, taken from a site far
removed from that of the renal tumor. The present study was
approved by the Ethics Committee of Binzhou Medical University
Hospital (Binzhou, China). All the subjects were fully informed
about the characteristics and purpose of the present study, and
gave informed consent.
 | Table I.Clinical characteristics and
laboratory results of patients with LN. |
Table I.
Clinical characteristics and
laboratory results of patients with LN.
| Clinical
characteristic | Value |
|---|
| Sex, n
(male/female) | 1/17 |
| Age range, years | 15–47 |
| Range of LN duration,
months | 1–26 |
| SLEDAI | 15–32 |
| Proteinuria range,
g/24 h | 0.27–10.70 |
| Serum C3 range,
mg/dl | 16.0–56.7 |
| Serum C4 range,
mg/dl | 1.2–12.8 |
| ANA reactivity, n
(positive/negative) | 18/0 |
| Anti-dsDNA
reactivity, n (positive/negative) | 13/5 |
| Anti-Sm reactivity, n
(positive/negative) | 11/7 |
| Anti-RNP reactivity,
n (positive/negative) | 11/7 |
Determination of plasma BAFF
levels
Plasma BAFF levels were detected by ELISA using the
human BAFF kit (cat. no. BPE10132; Shanghai Langton Biotechnology,
Co., Ltd., Shanghai, China). According to the manufacturer's
protocol, the precoated microplate was covered with capture
antibody and incubated for 2 h following sample addition at room
temperature. Following four washes, BAFF conjugate (200 µl/well)
was added and agitated gently on a shaker for 30 min at room
temperature. The absorbance was measured on a microplate reader at
450 nm 10 min following the addition of the stop solution for
terminating the reaction.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Frozen kidney tissue stored at −80°C was sectioned
(100-µm thick) on a cryostat. RNA was extracted from frozen kidney
tissue samples using the RNAiso Plus (cat. no. 9108; Takara Bio,
Inc., Otsu, Japan), according to the manufacturer's protocol. RNA
(1 µg) was reverse transcribed into cDNA using the PrimeScript™ RT
reagent kit (cat. no. RR037A; Takara Bio Inc.,). qPCR was carried
out on a Real-Time PCR System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using the SYBR® Premix Ex Taq™ II
(cat. no. RR820A, Takara Bio Inc.). Thermocycling conditions were
as follows: Initial 1 cycle at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and at 60°C for 30 sec. All PCR reactions
were performed in triplicate. Primer sequences and the annealing
temperature of BAFF, phosphorylated (p)-PI3K, p-Akt, p-mTOR and
reference gene β-actin are presented in Table II. The 2−ΔΔCq (21) method was used to calculate the
expression levels of target genes.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
|
| Primer sequence
(5′-3′) |
|
|---|
| Gene | Forward | Reverse | Annealing
temperature, °C |
|---|
| β-actin |
CCTGTACGCCAACACAGTGC |
ATACTCCTGCTTGCTGATCC | 60 |
| BAFF |
TCTCCGGGAATCTCTGATGC |
TCTTGGTGGTCACCAGTTCAG | 60 |
| p-PI3K |
TCCCAGGTGGAATGAATGGC |
TTTAGCACCCTTTCGGCCTT | 60 |
| p-AKT |
GGACAAGGACGGGCACATTA |
CGACCGCACATCATCTCGTA | 60 |
| p-mTOR |
CTTATGGTGCGGTCCCTTGT |
GGTCACCTGAGGGTGAACTG | 60 |
Western blotting
Frozen kidney tissue samples were homogenized in a
cold radioimmunoprecipitation assay cell lysis buffer (Shanghai
Sunred Biological Technology Co., Ltd., Shanghai, China)
supplemented with a protease and phosphatase inhibitor cocktail,
and phenylmethane sulfonyl fluoride. The crude extract was
separated from the debris by centrifugation (800 × g; 10 min at
4°C) and the supernatant was separated by centrifugation (14,000 ×
g; 15 min at 4°C). The cryolysis product was collected and the
concentration of purified protein was measured by bicinchoninic
acid assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Equal amounts of the protein sample (50 µg) were denatured in 4X
Laemmli buffer [250 mM tris-hydrochloride (pH 6.8), 40% glycerol,
8% SDS, 0.01% bromphenol blue and 20% β-mercaptoethanol] and
separated via SDS-PAGE on 10% gels. Following that, the proteins
were transferred onto a nitrocellulose membrane (GE Healthcare,
Chicago, IL, USA). Subsequently, the membranes were blocked with 5%
milk at 4°C overnight in TBS with Tween-20 (TBS-T) and probed with
the primary antibody in TBS-T supplemented with 5% blocking milk at
a dilution of 1:1,000. The primary antibodies included: Rabbit
anti-BAFF (cat. no. 19944S; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-p-PI3K p85 (Tyr458; cat. no. 3821S; Cell
Signaling Technology, Inc.), anti-p-AKT (Thr308; cat. no. 4056S;
Cell Signaling Technology, Inc.), anti-p-mTOR (Ser2448; cat. no.
2976S; Cell Signaling Technology, Inc.), anti-GAPDH (cat. no.
2118L; Cell Signaling Technology, Inc.; 1:1,000), and incubated for
2 h at room temperature. An IRDye® 800CW goat anti-mouse
antibody (cat. no. 926-32210; LI-COR Biosciences, Lincoln, NE, USA;
1:10,000) was used as a secondary antibody for 2 h at room
temperature. Proteins were visualized at a wavelength of 800 nm on
an Odyssey Luminescent Image Analysis system (LI-COR Biosciences)
and analyzed with ImageJ software version 1.37v 51.52 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA) was used to perform all data analyses. The results were
expressed as the mean ± standard deviation. Differences between the
LN and control groups were analyzed using a Mann-Whitney U test.
The correlation between BAFF and PI3K/Akt/mTOR signaling was
determined using the Spearman's rank correlation coefficient.
P<0.05 was considered to indicate a statistically significance
difference.
Results
Clinical characteristics, laboratory
results and histological parameters of patients with LN
The clinical characteristics and laboratory test
data of recruited patients with LN are demonstrated in Table I, and histological parameters are
presented in Table III.
 | Table III.Histological parameters of patients
with LN. |
Table III.
Histological parameters of patients
with LN.
| ISN/RPS
classification | No. of
patients |
|---|
| I | 0 |
| II | 3 |
| III | 2 |
| IV | 10 |
| V | 0 |
| III+V | 2 |
| IV+V | 1 |
Plasma levels of BAFF
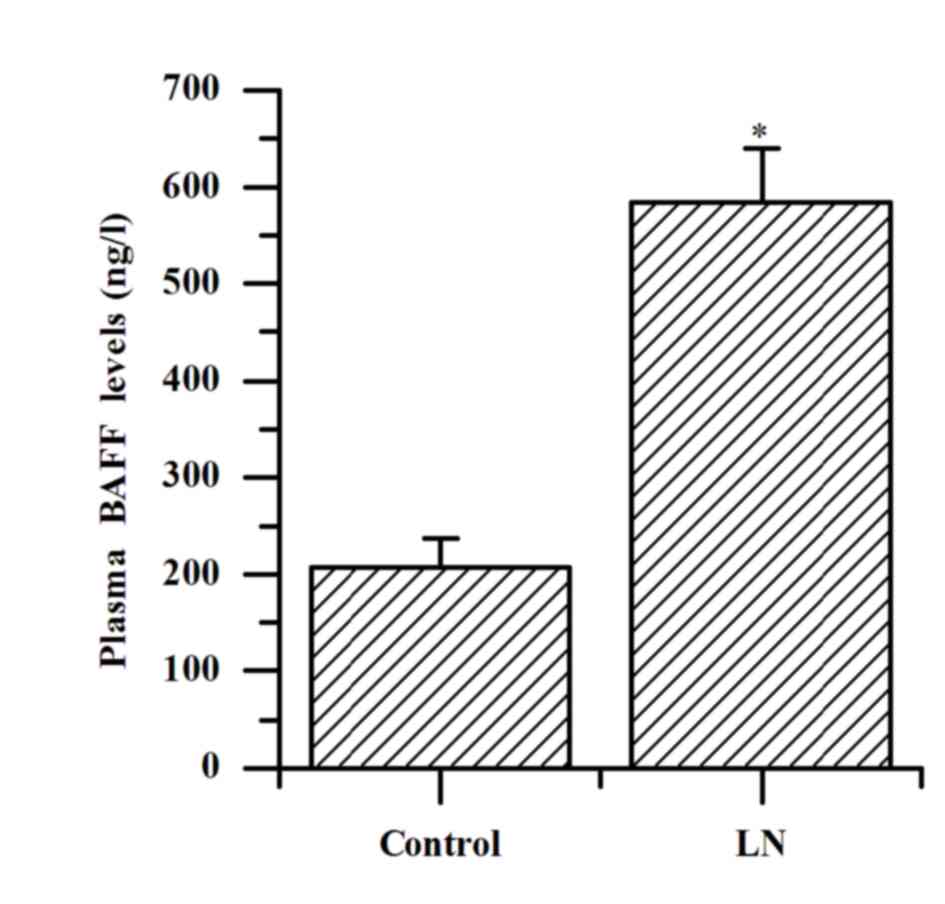
The plasma levels of BAFF in patients with LN were
increased significantly compared with the controls (584.07±55.57
vs. 207.73±29.77 ng/l; P<0.001; Fig. 1).
Analysis of mRNA expression of BAFF,
p-PI3K, p-Akt and p-mTOR in kidney tissues by RT-qPCR
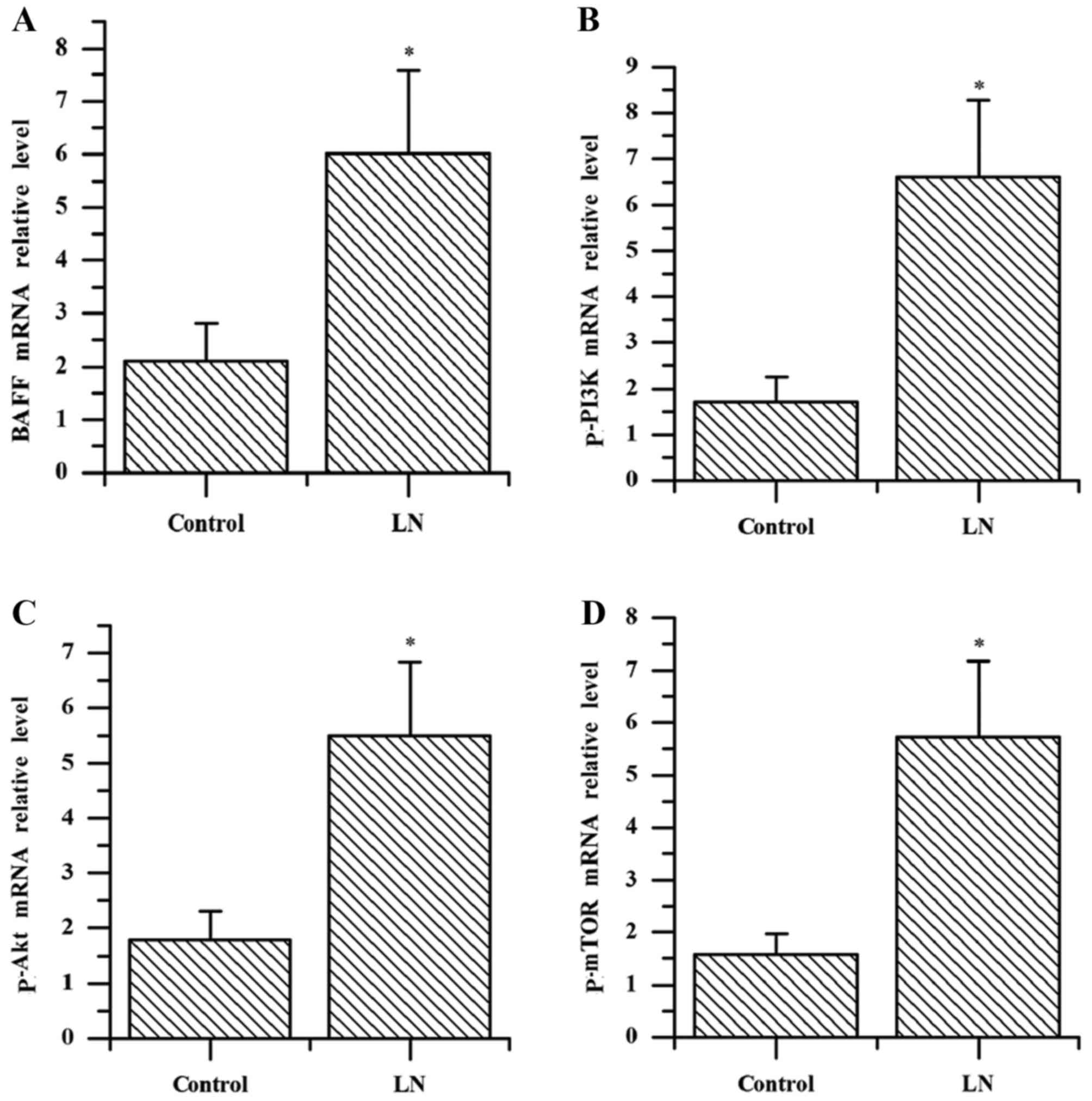
The results of the present study demonstrated that
the mRNA expression levels of BAFF, p-PI3K, p-Akt and p-mTOR in
kidney tissue of patients with LN were significantly increased
compared with the controls (all P<0.001; Fig. 2). The mRNA levels of BAFF in the
kidney tissue of patients with LN were positively correlated with
the expression levels of p-PI3K, p-Akt and p-mTOR (r=0.751, 0.810,
0.806; all P<0.001; data not shown).
Analysis of protein expression of
BAFF, p-PI3K, p-Akt and p-mTOR in kidney tissue by western
blotting
The protein expression levels of BAFF were
significantly increased compared with the controls, upregulation of
p-PI3K, p-AKT and p-mTOR was demonstrated in the patients with LN
compared with the control group (all P<0.001; Fig. 3A and B). The protein levels of BAFF
in the kidney tissue of patients with LN were positively correlated
with the expression levels of p-PI3K, p-Akt and p-mTOR (r=0.803,
0.738, 0.798; all P<0.001; data not shown). These results
indicate a correlation between BAFF and PI3K/Akt/mTOR signaling and
it is hypothesized that they are involved in the mechanism of LN
progression.
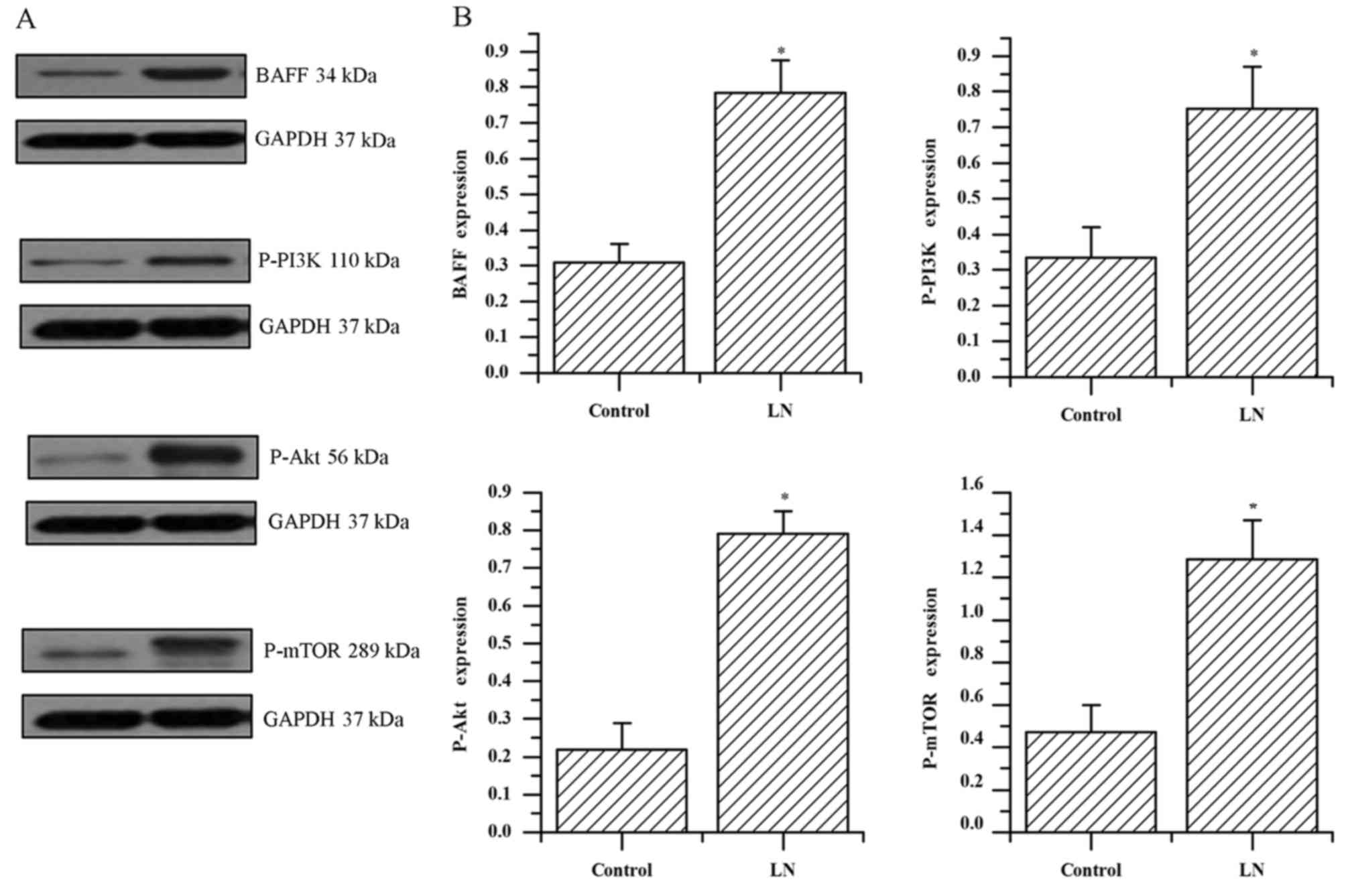 | Figure 3.Evaluation of protein expression in
the kidney of patients with LN. (A) Expression of BAFF, p-PI3K,
p-AKT, p-mTOR was detected by western blot analysis in patients
with LN and the control group. (B) Quantified protein levels of
BAFF, p-PI3K, p-AKT and p-mTOR. The data was expressed as the mean
± standard deviation from two independent experiments. *P<0.001
vs. the control group. LN, lupus nephritis; BAFF, B cell activating
factor; p-PI3K, phosphorylated-phosphoinositide 3-kinase; p-AKT,
phosphorylated-protein kinase B; p-mTOR, phosphorylated-mammalian
target of rapamycin. |
Discussion
LN is a common and serious complication of SLE,
which leads to increased mortality and decreased quality of life.
The mechanism of LN progression involves activation of polyclonal B
cells. BAFF is important to B lymphocyte survival and
differentiation. The overexpression of BAFF is correlated with the
pathogenesis and development of various autoimmune diseases,
including SLE (22). Furthermore,
a large number of studies have indicated that the level of BAFF is
increased significantly in SLE (23,24),
implying that it is a therapeutic target for SLE (25) and therefore BAFF has been indicated
as a prognostic marker in SLE. In the present study BAFF levels in
the plasma were also elevated in patients with LN. In addition, the
mRNA and protein levels of BAFF were elevated in the kidney tissue
of patients with LN. These results suggested that BAFF may serve a
role in kidney damage in patients with LN.
PI3K acts as a signaling molecule that can regulate
multiple cellular processes and Akt is a downstream effector of
PI3K. p-Akt is frequently formed as a result of PI3K activation. A
previous study revealed that B cells, under the influence of BAFF,
lead to prolonged phosphorylation of Akt, which serves an essential
role in regulating glucose metabolism and cell cycle progression
(26). It appears that Akt may be
a critical component of BAFF-mediated cellular survival and
metabolic fitness. In addition, further evidence demonstrates that
the activation of Akt by BAFF stimulation is inhibited by B cells
with the PI3K suppressant LY294002 (27,28).
Activation of the PI3K/Akt signaling pathway is important in the
biological function of normal and aberrant B lymphocytes in B cell
receptor signaling and the downstream pathways that regulate cell
proliferation, maturation and differentiation (29).
mTOR, a serine/threonine kinase, serves a pivotal
role in numerous cellular processes, including cellular
proliferation, apoptosis, migration, angiogenesis and protein
translation (30). A previous
study demonstrated that mTOR possesses extensive and diverse
physiological functions, particularly in cell metabolism (31) and could be more appropriately
described as an immune regulator (32). BAFF stimulation can result in the
activation of mTOR (27) and
PI3K/Akt signaling is the primary pathway that regulates mTOR
function in immune cells.
The PI3K/Akt/mTOR pathway contributes to a broad
range of biological processes including the cell cycle, glucose
metabolism and protein synthesis. An early study demonstrated that
B cells with protein kinase C-β deficiency exhibit altered
phosphorylation of Akt and restriction of cell growth when
stimulated by BAFF, and this demonstrated the significance of the
PI3K/Akt/mTOR signaling pathway in BAFF-mediated B cell growth
(28). PI3K/AKT/mTOR signaling can
provide B cells with resistance to apoptosis by increasing the
level of Bcl-2, leading to decreased cellular metabolism in B cells
(20).
The present study demonstrated that the expression
of BAFF was increased significantly in patients with LN. In
comparison with the controls, the results of RT-qPCR and western
blotting demonstrated that the expression levels of p-PI3K, p-Akt
and p-mTOR increased in patients with LN. Furthermore, mRNA and
protein levels of BAFF in kidney tissue of patients with LN were
positively correlated with the levels of p-PI3K, p-Akt and p-mTOR.
The results of the present study indicated that BAFF may be a
crucial upstream mediator of the PI3K/Akt/mTOR signaling pathway in
response to B cell activation. The present study demonstrated a
correlation between BAFF and the PI3K/Akt/mTOR pathway and
hypothesized that they are involved in the pathogenesis of LN in
patients.
In recent years, belimumab, a BAFF inhibitor, has
been developed which provides a promising, novel avenue for the
treatment of SLE and contributes to the progress of therapy
development. Meta-analysis indicates that combination therapy with
belimumab and the standard treatment is safer and more effective in
patients with SLE compared with using the standard therapy alone
(33). Furthermore, the
PI3K/Akt/mTOR pathway inhibitors were investigated to explore their
mode of action and therapeutic use against malignant tumors
(34–36) and have not been studied in patients
with SLE and LN. The present study revealed a correlation between
BAFF and the PI3K/Akt/mTOR pathway in patients with LN. As humans
were used in the present study, ethically they could not be treated
with BAFF and PI3K/Akt/mTOR pathway inhibitors. Therefore, animal
experiments would be required in the future to confirm whether BAFF
is involved in the mechanism of LN through regulation of the
PI3K/Akt/mTOR pathway, which may provide guidance for the
development of novel and effective therapies for patients with
LN.
Acknowledgements
The present study was funded by the Binzhou City
Science and Technology Development Program (grant no. 2014ZC0142),
and the Binzhou Medical University of Science and Technology Plan
Project (grant no. BY2013KJ29).
References
|
1
|
Crispín JC, Kyttaris VC, Terhorst C and
Tsokos GC: T cells as therapeutic targets in SLE. Nat Rev
Rheumatol. 6:317–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Zubiria Salgado A and Herrera-Diaz C:
Lupus nephritis: An overview of recent findings. Autoimmune Dis.
2012:8496842012.PubMed/NCBI
|
|
3
|
Tsokos GC: Systemic lupus erythematosus. N
Engl J Med. 365:2110–2121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen Y, Sun CY, Wu FX, Chen Y, Dai M, Yan
YC and Yang CD: Association of intrarenal B-cell infiltrates with
clinical outcome in lupus nephritis: A study of 192 cases. Clin Dev
Immunol. 2012:9675842012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun CY, Shen Y, Chen XW, Yan YC, Wu FX,
Dai M, Li T and Yang CD: The characteristics and significance of
locally infiltrating B cells in lupus nephritis and their
association with local BAFF expression. Int J Rheumatol.
2013:9542922013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heinemann K, Wilde B, Hoerning A, Tebbe B,
Kribben A, Witzke O and Dolff S: Decreased IL-10(+) regulatory B
cells (Bregs) in lupus nephritis patients. Scand J Rheumatol.
45:312–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schneider P and Tschopp J: BAFF and the
regulation of B cell survival. Immunol Lett. 88:57–62. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mackay F and Browning JL: BAFF: A
fundamental survival factor for B cell. Nat Rev Immunol. 2:465–475.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schneider P, MacKay F, Steiner V, Hofmann
K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S and
Acha-Orbea H: BAFF, a novel ligand of the tumor necrosis factor
family, stimulates B cell growth. J Exp Med. 189:1747–1756. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mayne CG, Amanna IJ, Nashold FE and Hayes
CE: Systemic autoimmunity in BAFF-R-mutant A/WySnJ strain mice. Eur
J Immunol. 38:587–598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackay F and Schneider P: Cracking the
BAFF code. Nat Rev Immunol. 9:491–502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thien M, Phan TG, Gardam S, Amesbury M,
Basten A, Mackay F and Brink R: Excess BAFF rescues self-reactive B
cells from peripheral deletion and allows them to enter forbidden
follicular and marginal zone niches. Immunity. 20:785–798. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross JA, Johnston J, Mudri S, Enselman R,
Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D and Lofton-Day
C: TACI and BCMA are receptors for a TNF homologue implicated in
B-cell autoimmune disease. Nature. 404:995–999. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khare SD, Sarosi I, Xia XZ, McCabe S,
Miner K, Solovyev I, Hawkins N, Kelley M, Chang D and Van G: Severe
B cell hyperplasia and autoimmune disease in TALL-1 transgenic
mice. Proc Natl Acad Sci USA. 97:3370–3375. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore PA, Belvedere O, Orr A, Pieri K,
LaFleur DW, Feng P, Soppet D, Charters M, Gentz R and Parmelee D:
BLyS: Member of the tumor necrosis factor family and B lymphocyte
stimulator. Science. 285:260–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henley T, Kovesdi D and Turner M: B-cell
responses to B-cell activation factor of the TNF family (BAFF) are
impaired in the absence of PI3K delta. Eur J Immunol. 38:3543–3548.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shor B, Cavender D and Harris C: A
kinase-dead knock-in mutation in mTOR leads to early embryonic
lethality and is dispensable for the immune system in heterozygous
mice. BMC Immunol. 10:282009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fruman DA: Phosphoinositide 3-kinase and
its targets in B-cell and T-cell signaling. Curr Opin Immunol.
16:314–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li PP, Liu DD, Liu YJ, Song SS, Wang QT,
Chang Y, Wu YJ, Chen JY, Zhao WD, Zhang LL and Wei W: BAFF/BAFF-R
involved in antibodies production of rats with collagen-induced
arthritis via PI3K-Akt-mTOR signaling and the regulation of
paeoniflorin. J Ethnopharmacol. 141:290–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Li P, Song S, Liu Y, Wang Q, Chang
Y, Wu Y, Chen J, Zhao W, Zhang L and Wei W: Pro-apoptotic effect of
epigallo-catechin-3-gallate on B lymphocytes through regulating
BAFF/PI3K/Akt/mTOR signaling in rats with collagen-induced
arthritis. Eur J Pharmacol. 690:214–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vincent FB, Morand EF, Schneider P and
Mackay F: The BAFF/APRIL system in SLE pathogenesis. Nat Rev
Rheumatol. 10:365–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salazar-Camarena DC, Ortiz-Lazareno PC,
Cruz A, Oregon-Romero E, Machado-Contreras JR, Muñoz-Valle JF,
Orozco-López M, Marín-Rosales M and Palafox-Sánchez CA: Association
of BAFF APRIL serum levels, BAFF-R, TACI and BCMA expression on
peripheral B-cell subsets with clinical manifestations in systemic
lupus erythematosus. Lupus. 25:582–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Figgett WA, Deliyanti D, Fairfax KA, Quah
PS, Wilkinson-Berka JL and Mackay F: Deleting the BAFF receptor
TACI protects against systemic lupus erythematosus without
extensive reduction of B cell numbers. J Autoimmun. 61:9–16. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jordan N and D'Cruz DP: Belimumab for the
treatment of systemic lupus erythematosus. Expert Rev Clin Immunol.
11:195–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Debnath I, Roundy KM, Weis JJ and Weis JH:
Analysis of the regulatory role of BAFF in controlling the
expression of CD21 and CD23. Mol Immunol. 44:2388–2399. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woodland RT, Fox CJ, Schmidt MR, Hammerman
PS, Opferman JT, Korsmeyer SJ, Hilbert DM and Thompson CB: Multiple
signaling pathways promote B lymphocyte stimulator dependent B-cell
growth and survival. Blood. 111:750–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patke A, Mecklenbräuker I,
Erdjument-Bromage H, Tempst P and Tarakhovsky A: BAFF controls B
cell metabolic fitness through a PKC beta- and Akt-dependent
mechanism. J Exp Med. 203:2551–2562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blachly JS and Baiocchi RA: Targeting
PI3-kinase (PI3K), AKT and mTOR axis in lymphoma. Br J Haematol.
167:19–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimobayashi M and Hall MN: Making new
contacts: The mTOR network in metabolism and signalling crosstalk.
Nat Rev Mol Cell Biol. 15:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomson AW, Turnquist HR and Raimondi G:
Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol.
9:324–337. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei LQ, Liang YG, Zhao Y, Liang HT, Qin DC
and She MC: Efficacy and safety of belimumab plus standard therapy
in patients with systemic lupus erythematosus: A meta-analysis.
Clin Ther. 38:1134–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JJ, Loh K and Yap YS: PI3K/Akt/mTOR
inhibitors in breast cancer. Cancer Biol Med. 12:342–354.
2015.PubMed/NCBI
|
|
36
|
Soares HP, Ming M, Mellon M, Young SH, Han
L, Sinnet-Smith J and Rozengurt E: Dual PI3K/mTOR inhibitors induce
rapid overactivation of the MEK/ERK pathway in human pancreatic
cancer cells through suppression of mTORC2. Mol Cancer Ther.
14:1014–1023. 2015. View Article : Google Scholar : PubMed/NCBI
|