Introduction
The major pathological basis of calcific
tendinopathy, the later stage of tendinopathy, is heterotopic
ossification (HO) in the tendon, which severely affects health and
quality of life, and can be career-ending for professional athletes
(1). Treatment options for
calcific tendinopathy are limited and tendon healing usually
requires long-term rehabilitation (2). Physical therapies using mechanical
loading as their functional element, such eccentric exercise, have
shown promising results in the treatment of calcific tendinopathy
(3,4). However, excessive mechanical loading
is also a major factor in the pathogenesis of calcific tendinopathy
and is associated with pain onset (5). The dual effects that mechanical
loading may have in the pathogenesis and rehabilitation of
calcified tendinopathy have not yet been fully clarified.
Mammalian target of rapamycin complex-1 (mTORC1), an
evolutionarily-conserved serine-threonine kinase, controls protein
synthesis, ribosome biogenesis and nutrient transport (6,7).
Several studies have demonstrated that mechanical loading is
sufficient for the activation of the mTORC1 signaling pathway
because it can induce phosphorylation of p70 S6 kinase (P70S6K), a
downstream protein in the mTORC1 signaling pathway (8–10).
Previous studies using the mTORC1 inhibitor rapamycin or
mTORC1 gene ablation demonstrated that mTORC1 signaling
exerts either stimulatory or inhibitory effects on osteogenic
differentiation, which leads to HO (11–14).
Thus, mechanical loading may regulate osteogenic differentiation of
tendon cells through the mTORC1 signaling pathway, however the
mechanism remains unclear.
In the present study, an Achilles tenotomy rat model
and a tendon cells stretch model were used to investigate the role
mechanical loading may have in regulating HO of the tendon through
the mTORC1 signaling pathway in calcific tendinopathy.
Materials and methods
Animals and treatment
A total of 50 male Sprague-Dawley rats [10 rats aged
2–3 weeks (50±5 g) and 40 rats aged 8 weeks (250±10 g)] were
purchased from the Experimental Animal Research Center of Southern
Medical University (Guangzhou, China) and housed in a specific
pathogen-free animal facility with standard food and water under
constant temperature (20–22°C), controlled humidity (45–55%) and a
standard 12-h light/dark cycle. All animal experimental protocols
were approved by the Animal Care and Use Committee of Southern
Medical University and performed according to the Southern Medical
University Guide for the Care and Use of Laboratory Animals.
Cell culture and mechanical loading
stimulation
The middle portions of tendons used for cell culture
were obtained from 10 Sprague-Dawley rats (aged 2–3 weeks, 50±5 g).
The rats were sacrificed and the middle portion of tendons was
obtained by cutting the tendon sample 5 mm from the tendon-muscle
junction to tendon-bone insertion. The tendons were minced into
small pieces (1 mm3) and digested with 0.1% collagenase
type I (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in 1 ml
phosphate-buffered saline at 37°C for 1 h. After centrifugation at
1,000 × g at 37°C for 5 min and removal of the supernatant, a
single-cell suspension was obtained, which was cultured in complete
medium [CM; Dulbecco's modified Eagle's medium (DMEM) plus 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA] at 37°C with 5% CO2. Passages 3 and 4
were used for experiments in this study. For osteogenic
differentiation, 1×105 cells were seeded into BioFlex
six-well plates (Flexcell International Corporation, Burlington,
NC, USA) in duplicate and incubated at 37°C in an atmosphere of 5%
CO2. After 24 h incubation, medium containing
non-adherent cells was removed and replaced with osteogenic
induction medium (OIM). OIM consisted of α-MEM (Gibco; Thermo
Fisher Scientific, Inc.), 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), 50 µM ascorbic acid (Sigma-Aldrich; Merck KGaA), 0.1 µM
dexamethasone (Sigma-Aldrich; Merck KGaA) and 10 mM
β-glycero-phosphate (Sigma-Aldrich; Merck KGaA).
Mechanical loading was applied using the Flexcell
FX-5000 Tension System (FX5K; Flexcell International Corporation).
When cultures reached 60–70% confluence, cells were serum-starved
for 24 h to arrest cells at G0. The cells were then
subjected to mechanical stimulation, which was applied for 8 h
daily for a week with the OIM replaced every 3rd day, using a
sinusoidal waveform at a frequency of 0.5 Hz and a strain of 4 or
12%, with or without 10 nM rapamycin (Sigma-Aldrich; Merck KGaA).
Control cultures were cultured under the same conditions without
mechanical stimulation (NS).
Total RNA isolation and RT-qPCR
analyses
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) followed by
cDNA synthesis performed with a StarScript II First-strand cDNA
Synthesis kit (GenStar BioSolutions Co., Ltd., Beijing, China). The
primers are listed in Table I. The
RT-qPCR reactions were performed using RealStar Green Power Mixture
with ROX (GenStar BioSolutions Co., Ltd) and an ABI Step One Plus
PCR Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling parameters were as follows:
Pre-denaturation at 95°C for 2 min, followed by 40 cycles of
denaturation at 94°C for 20 sec, annealing at 65°C for 20 sec and
extension at 72°C for 30 sec. All assays were performed in
triplicate and samples were normalized to GAPDH using the delta
delta Cq method (15).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence |
|---|
| RUNX2 | F
5′-CACAAACAACCACAGAACCAC-3′ |
|
| R
5′-TTGCTGTCCTCCTGGAGAAA-3′ |
| OSX | F
5′-CAAGAGTCGGATTCTAGGATTG-3′ |
|
| R
5′-GATCAAACTTGCTGCAGGCTGCT-3′ |
| GAPDH | F
5′-GAGAGGCTCTCTGTCGACTAC-3′ |
|
| R
5′-TAGTGTAGGTTGGGCGCTCAA-3′ |
Western blot analyses
Following the stretching procedure, cells were
rinsed with cold PBS and lysed in cold RIPA buffer [1×PBS, 1%
NP-40, 0.5% sodium dexoycholate, 0.1% SDS, protease inhibitor
cocktail tablet (Roche Diagnostics, Basel, Switzerland) and 1 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology)]. Lysates were incubated on ice for 20 min with
frequent vortexing and cleared twice by centrifugation (11,000 × g,
10 min, 4°C). Protein was subjected to SDS-PAGE and transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). Membranes were blocked for 60 min at room temperature in
5% non-fat milk/Tris-buffered saline/0.1% Tween-20 (TBST).
Following washing with TBST, the membranes were incubated overnight
at 4°C in primary antibodies, which were phospho-P70S6K (Thr389;
diluted 1:1,000 in 5% BSA; #9234; Cell Signaling Technology Inc.,
Danvers, MA, USA), runt-related transcription factor 2 (RUNX2;
1:1,000; #8486; Cell Signaling Technology, Inc.), osterix (OSX;
1:1,000; ab22552; Abcam, Cambridge, UK) or GAPDH (1:1,000; #2118;
Cell Signaling Technology, Inc.). Membranes were incubated with the
horseradish peroxidase-conjugated secondary antibody (1:2,000;
#7047; Cell Signaling Technology, Inc.) at room temperature for 1
h, and subsequently washed. Image detection was performed according
to the manufacturer's instructions using SignalFire enhanced
chemiluminescence reagent (#6883; Cell Signaling Technology, Inc.).
Data are representative of at least three experiments with similar
results performed in triplicate. Images were analyzed using Image
Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Establishment of animal model and drug
treatment
A total of 40 male Sprague-Dawley rats aged 8 weeks
and weighing 250±10 g were randomly distributed into 8 groups of 5
animals per group: Sham; Sham + rapamycin (Rapa); Control (Ctrl);
Ctrl + Rapa; low elongation (LE); LE + Rapa; high elongation (HE);
HE + Rapa. Rats were anesthetized with 10% chloral hydrate [300
mg/kg, intraperitoneally (i.p.)]. The rats in groups Ctrl, Ctrl +
Rapa, LE, LE + Rapa, HE and HE + Rapa underwent midpoint Achilles
tenotomy on the right leg through a posterior approach under
aseptic conditions; rats in the Sham and Sham + Rapa groups
received an incision only as a sham operation. Incisions were
routinely closed with an interrupted 4-0 silk suture. Subsequently,
the operated hind limbs of rats in Ctrl and Ctrl + Rapa groups and
the non-operated hind limbs of the rats in the HE and HE + Rapa
groups were fixed in plaster casts from the toes up to ~2.5 cm
above the knee. The operated hind limbs of the rats in the LE and
LE + Rapa groups were fixed in a plaster cast from ~1 cm below the
ankle up to ~2.5 cm above the knee, allowing the toes to touch the
ground directly. The ankle and knee were fixed at functional
positions. The outer surface of the cast was coated with black
pepper powder for protection (16). Thus, the animal models were
established with three tendon stretch levels: Totally fixed (groups
Ctrl and Ctrl + Rapa), low elongation stretch (groups LE and LE +
Rapa) and high elongation stretch (groups HE and HE + Rapa). The
rats in groups Sham + Rapa, Ctrl + Rapa, LE + Rapa and HE + Rapa
received rapamycin (20 mg/kg/day, i.p.) every day for 6 weeks after
operation (Table II).
 | Table II.Establishment of animal model and drug
treatments. |
Table II.
Establishment of animal model and drug
treatments.
| Group | Achilles
tenotomy | Right leg total
fixation | Right leg partial
fixation | Left leg total
fixation | Rapa injection |
|---|
| Sham | − | − | − | − | − |
| Sham + Rapa | − | − | − | − | + |
| Ctrl | + | + | − | − | − |
| Ctrl + Rapa | + | + | − | − | + |
| LE | + | − | + | − | − |
| LE + Rapa | + | − | + | − | + |
| HE | + | − | − | + | − |
| HE + Rapa | + | − | − | + | + |
Histological analyses
Following the operation, the tendon samples were
immediately fixed in 4% buffered paraformaldehyde at 4°C for 24 h.
The samples were then decalcified, embedded in paraffin, cut into 5
µm sections and placed on poly-L-lysine-coated slides for further
procedures. For hematoxylin and eosin staining, the sections were
stained with hematoxylin and eosin carried out according to the
protocol in the product manual. For immunohistochemical staining,
antigen retrieval of the sections was performed using the microwave
method (17). Sections were
incubated in 3% H2O2 at room temperature for
30 min to quench endogenous peroxidase activity, the sections were
subsequently incubated with 10% normal goat serum (Gibco; Thermo
Fisher Scientific, Inc.) at room temperature for 30 min to block
nonspecific binding. The sections were then incubated with antibody
against RUNX2 (diluted 1:100 in 5% BSA; ab23981; Abcam) and OSX
(diluted 1:100; ab22552; Abcam), followed by incubation with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(diluted 1:100; #7074; Cell Signaling Technology, Inc.). The
protein of interest was visualized using 3,3′-diaminobenzidine
using a light microscope and the images were analyzed by applying
Image Pro-Plus 6.0 software (Media Cybernetics, Inc.).
Micro-computed tomography (µCT)
analyses
µCT analysis of the right Achilles tendons was
performed using a high-resolution µCT (Skyscan 1172; Bruker
Corporation, Ettlingen, Germany). Hind limb samples were collected
and fixed overnight at 4°C in 4% paraformaldehyde, then scanned
using the settings 60 kV, 150 µA with a mean 20 µm slice thickness.
The reconstructed tendon images were selected for quantification of
bone mass. Bone volume density [bone volume/total volume (BV/TV)%],
trabecular number (Tb.N; l/mm) and trabecular thickness (Tb.Th; mm)
were calculated.
Statistical analyses
Data were analyzed using one-way analysis of
variance while homogeneity of variance tests were used to evaluate
data homogeneity (SPSS 21.0 software; IBM SPSS, Armonk, NY, USA).
If the variances were equal, least-significant difference tests
were employed; otherwise, Dunnett's tests were used. Results are
presented as the mean ± standard deviation unless otherwise
indicated. P<0.05 was considered to indicate a statistically
significant difference.
Results
Low elongation mechanical loading
attenuates HO while high elongation mechanical loading accelerates
HO in vivo
In order to evaluate the effects of mechanical
loading on HO in the Achilles tenotomy rat model, µCT analysis was
employed (Fig. 1A and B). Compared
with the ctrl group, lower BT/VT, Tb.N and Tb.Th were observed in
the LE group; whereas the HE group exhibited higher BT/VT, Tb.N and
Tb.Th compared with the control group. Hematoxylin and eosin
staining revealed the same phenomenon (Fig. 1C). Therefore, these data suggested
that the HO in rats was attenuated by low elongation mechanical
loading, but enhanced by high elongation mechanical loading.
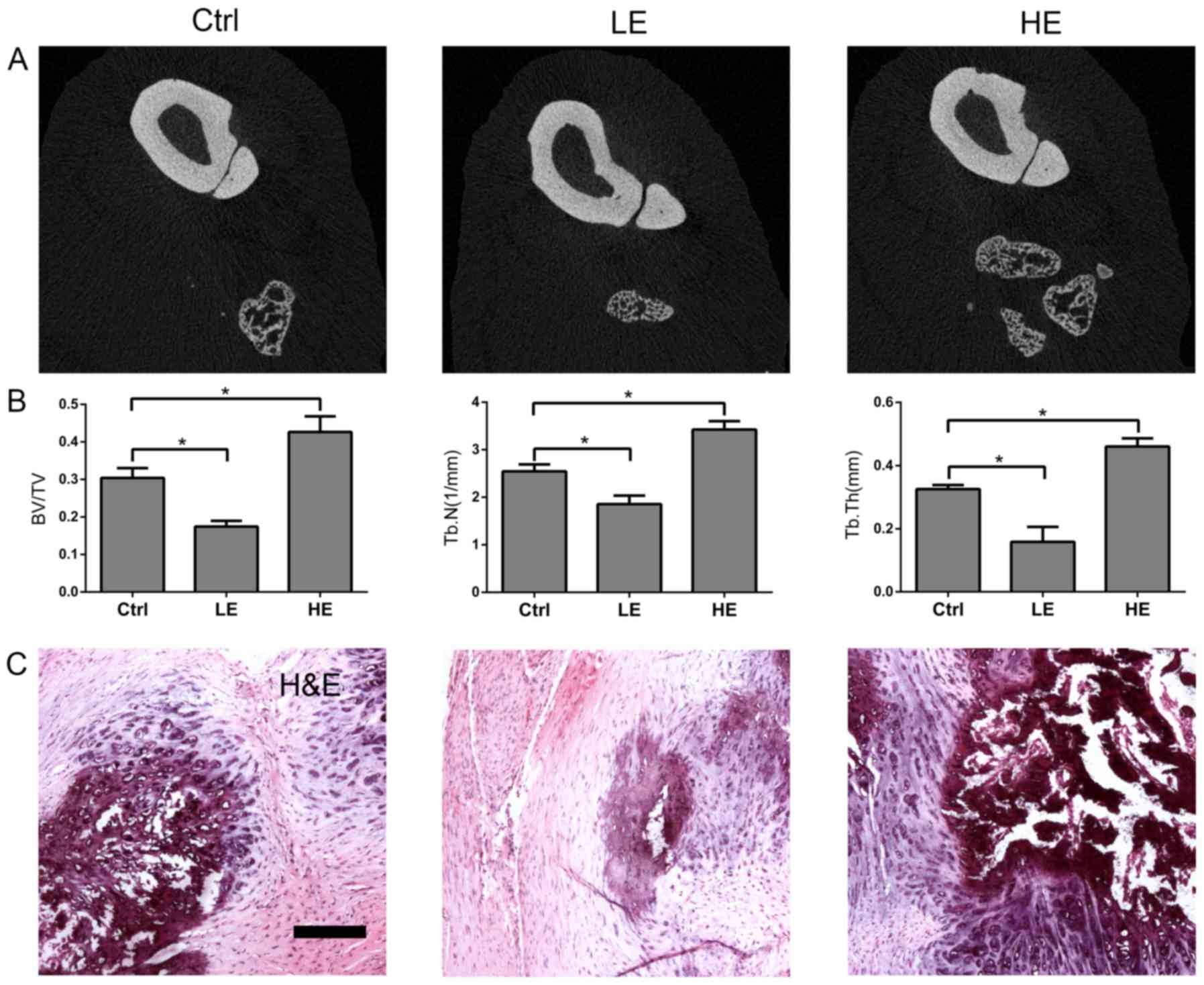 | Figure 1.Effects of mechanical loading on HO of
Achilles tendon in rats. The rats in Ctrl, LE and HE groups
underwent midpoint Achilles tenotomy on the right hind limbs;
operated hind limbs of rats in the Ctrl group and the non-operated
hind limbs of the rats in the HE group were fixed in plaster casts
from the toes up to ~2.5 cm above the knee. The operated hind limbs
of the rats in the LE group were fixed in a plaster cast from ~1 cm
below the ankle up to ~2.5 cm above the knee, allowing the toes to
touch the ground directly. The ankle and knee were fixed at
functional positions. At 6 weeks after operation, the heterotopic
ossification of the operated tendons were detected by using (A) µCT
analysis. Scale bar, 100 µm. (B) BV/TV (bone volume density), Tb.N
and Tb.Th were measured for each group. (C) Hematoxylin and eosin
staining was performed on tendon sections from each group. Data
represent the mean ± standard error, n=5. *P<0.05 vs. ctrl
group. Ctrl, control; LE, low elongation; HE, high elongation; µCT,
micro-computed tomography; BV/TV, bone volume/total volume; Tb.N,
trabecular number; Tb.Th, trabecular thickness. |
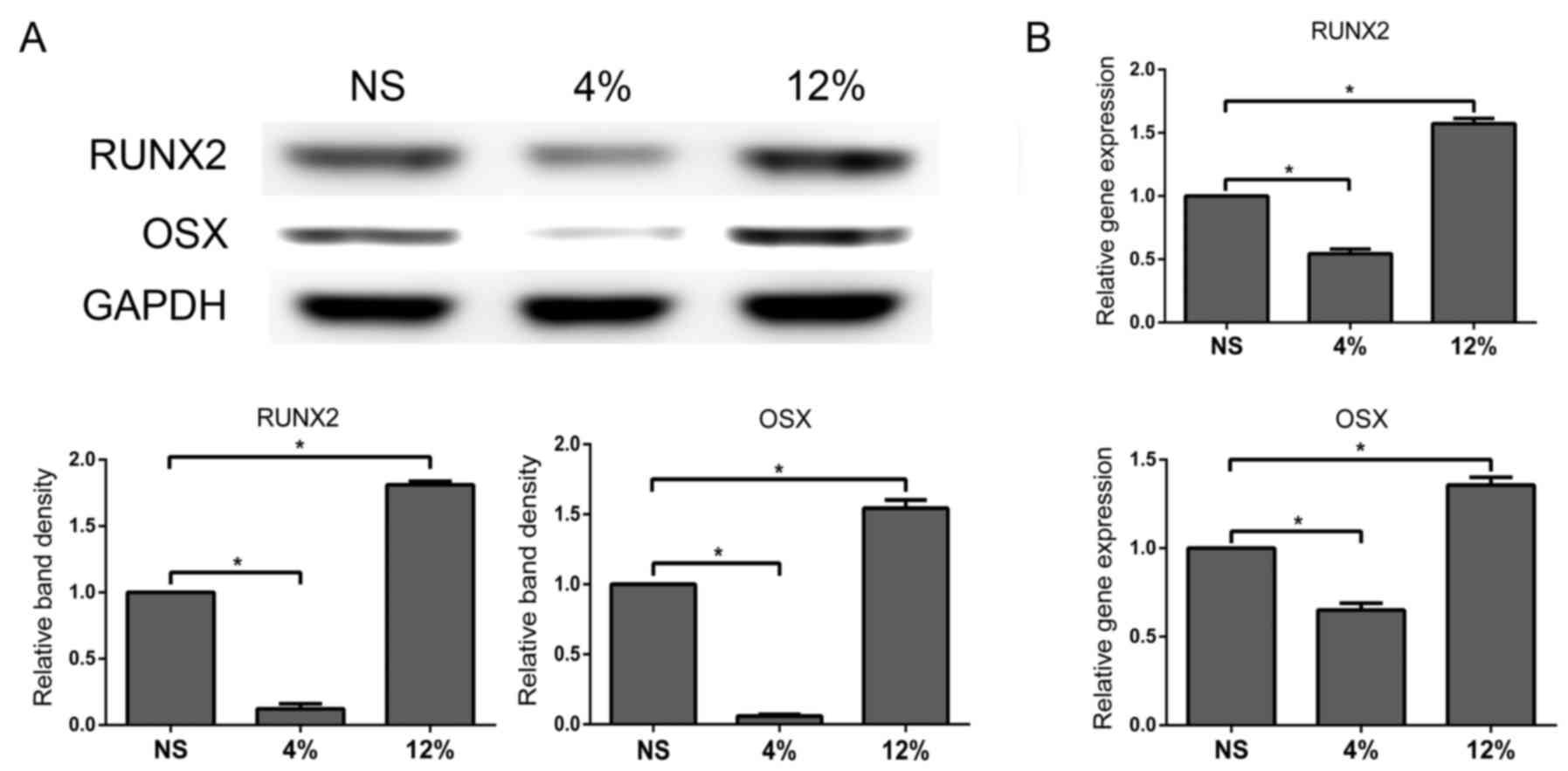
Low elongation mechanical loading
reduces osteogenic differentiation and high elongation mechanical
loading enhances osteogenic differentiation in vitro
Tendon cells in OIM were subjected to 4% elongation
mechanical loading and protein and mRNA expression of RUNX2 and OSX
were measured using western blot and RT-qPCR analyses,
respectively. Protein and mRNA expression of RUNX2 and OSX were
significantly decreased in the 4% stretch group compared with the
NS group (P<0.05; Fig. 2A and
B) while the 12% stretch group exhibited the opposite result
(P<0.05; Fig. 2A and B). These
data suggested that osteogenic differentiation in tendon cells was
reduced by low elongation mechanical loading and enhanced by high
elongation mechanical loading.
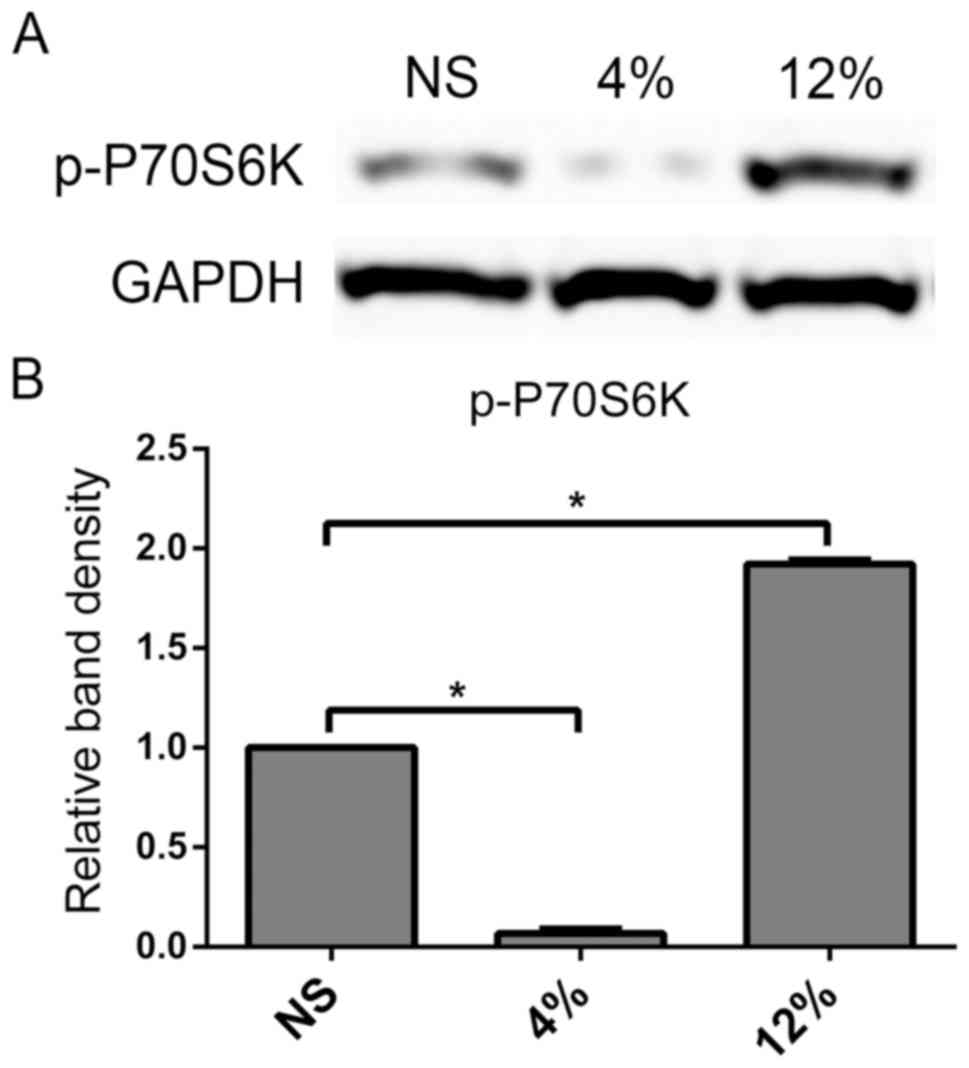
mTORC1 signaling pathway was modulated
by different levels of elongation mechanical loading in tendon
cells
To examine the effects of mechanical loading on the
mTORC1 signaling pathway in rat tendon cells, a downstream protein
in the mTORC1 signaling pathway, p-P70S6K, was investigated using
western blot analyses. Mechanical loading at 4% elongation
decreased the protein expression of p-P70S6K, while mechanical
loading at 12% elongation significantly increased p-P70S6K levels
in rat tendon cells compared with the NS group (P<0.05; Fig. 3). These data suggested that low
elongation mechanical loading suppressed the mTORC1 signaling
pathway, while high elongation had the opposite effect on tendon
cells.
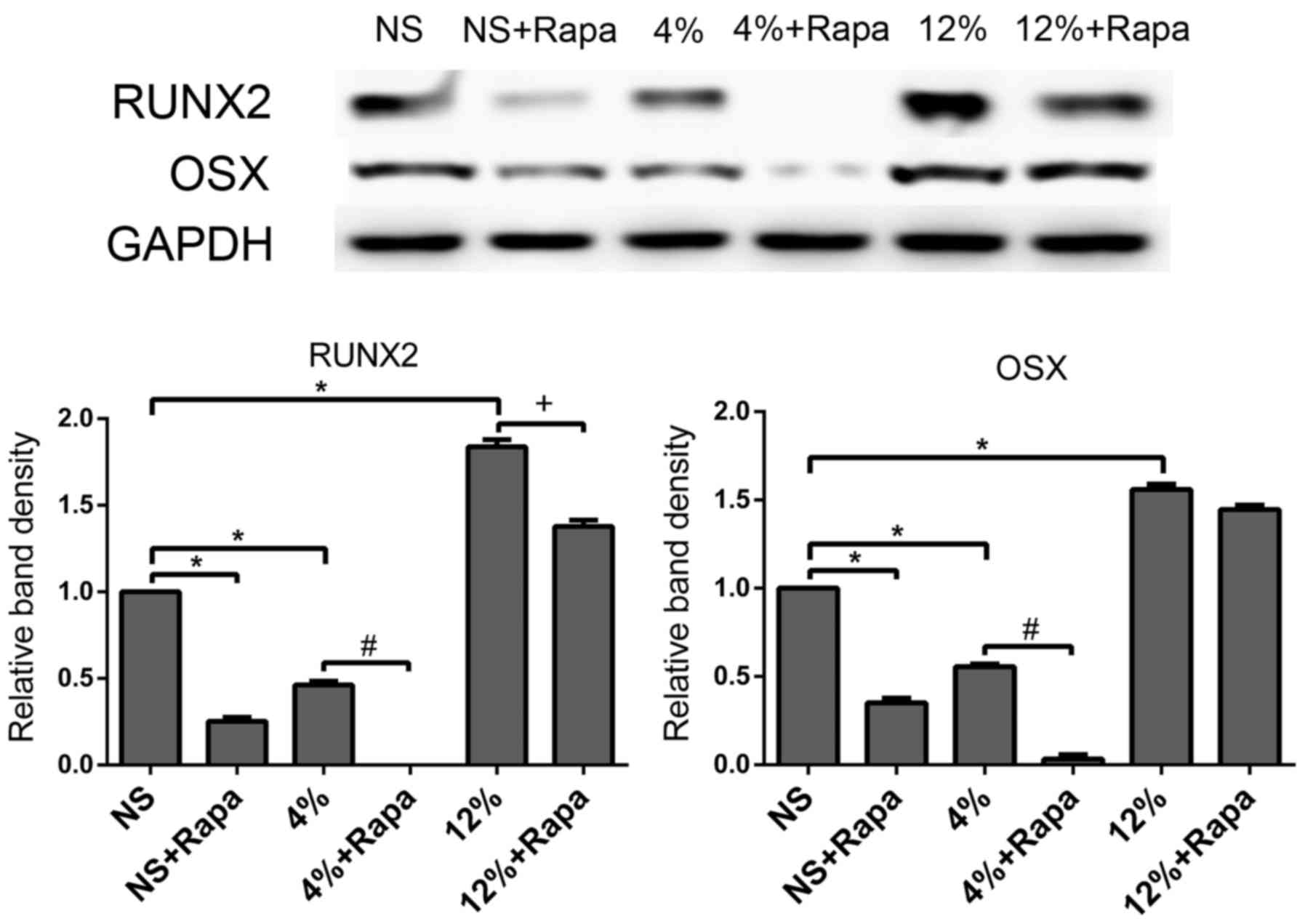
mTORC1 inhibition blocks the effect of
mechanical loading on osteogenic differentiation of tendon
cells
In the in vitro tendon cells stretch model,
the presence of rapamycin decreased protein expression of RUNX2 and
OSX in the NS + Rapa group compared with the NS group (P<0.05;
Fig. 4). Rapamycin-treated cells
at 4% elongation mechanical loading decreased protein expression of
RUNX2 and OSX compared with the untreated 4% group (P<0.05;
Fig. 4). Additionally, the
presence of rapamycin decreased 12% elongation mechanical
loading-induced protein expression of RUNX2 compared with the
untreated 12% group (P<0.05; Fig.
4). These data suggested that the osteogenic differentiation of
tendon cells was mTORC1-dependent and that mechanical loading
regulated the osteogenic differentiation of rat tendon cells
through the mTORC1 signaling pathway.
mTORC1 inhibition attenuates HO in
rats
In order to investigate the roles the mTORC1
signaling pathway plays in mechanical loading-induced HO of the
tendon, the mTORC1 selective inhibitor rapamycin was employed. The
presence of rapamycin decreased expression of RUNX2 and OSX in rats
in the Ctrl + Rapa group compared with the Ctrl group (Fig. 5). Rapamycin treated rats in the LE
+ Rapa group had decreased expression of RUNX2 and OSX compared
with the untreated LE group (Fig.
5). Additionally, rapamycin decreased the HE mechanical
loading-induced expression of RUNX2 and OSX in the HE + Rapa group
compared with the untreated HE group (Fig. 5). These data suggested that HO in
tendon was mTORC1-dependent and that mechanical loading regulated
HO in rat tendon through the mTORC1 signaling pathway.
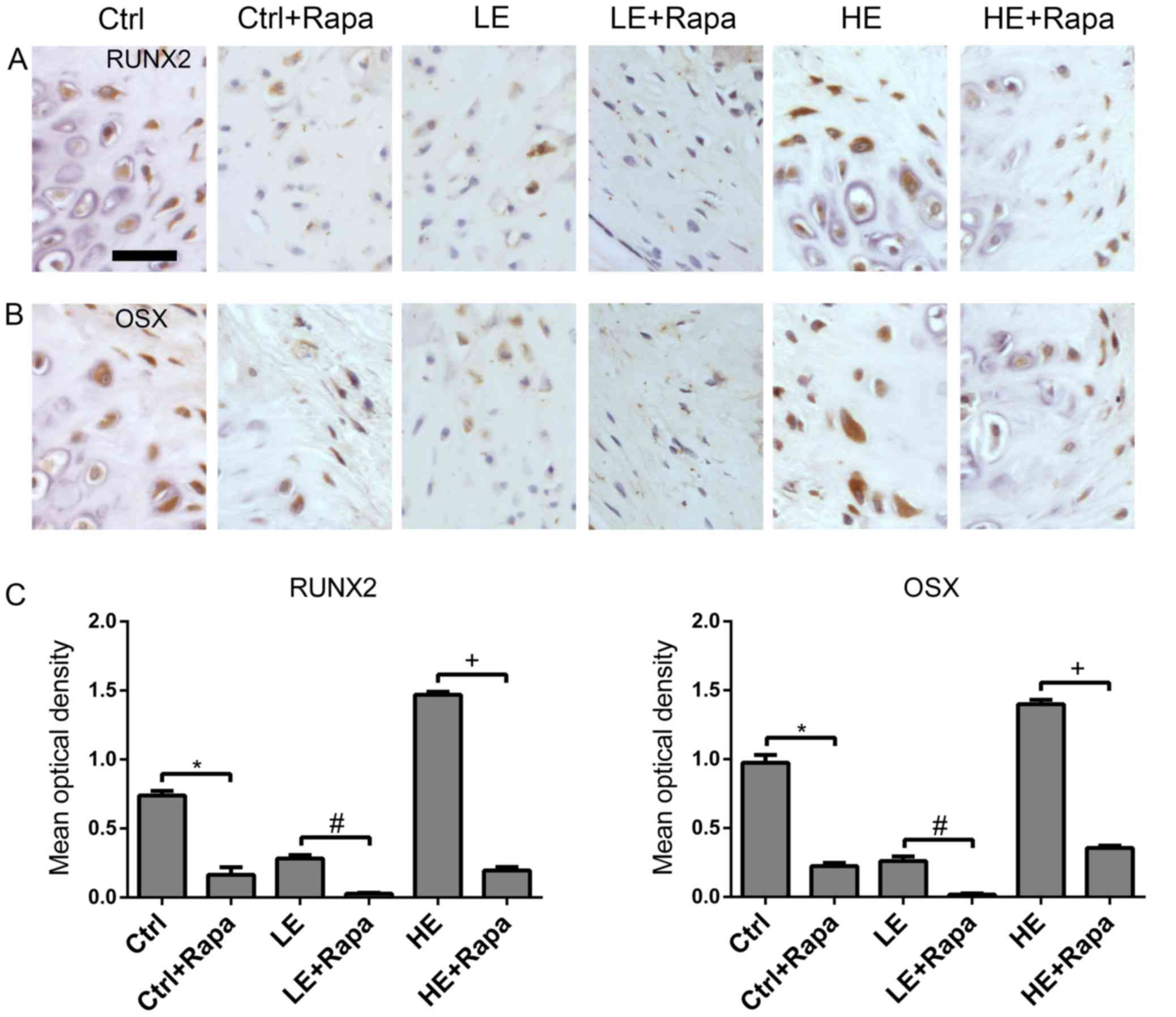 | Figure 5.Rapa attenuates HO of Achilles tendon
in rats. The rats in groups Ctrl, Ctrl + Rapa, LE, LE + Rapa, HE
and HE + Rapa underwent midpoint Achilles tenotomy on the right
leg; operated hind limbs of rats in groups Ctrl and Ctrl + Rapa and
the non-operated hind limbs of the rats in groups HE and HE + Rapa
were fixed in plaster casts from the toes up to ~2.5 cm above the
knee. The operated hind limbs of the rats in groups LE and LE +
Rapa were fixed in a plaster cast from ~1 cm below the ankle up to
~2.5 cm above the knee, allowing the toes to touch the ground
directly. The ankle and knee were fixed at functional positions.
The rats in groups Ctrl + Rapa, LE + Rapa and HE + Rapa received 20
mg/kg/day Rapa intraperitoneally every day for 6 weeks after
operation. Images presenting the immunohistochemical staining of
(A) RUNX2 and (B) OSX at 6 weeks after operation. Scale bar, 50 µm.
(C) Optical density data from RUNX2 and OSX stained sections. Data
represent the mean ± standard error, n=5. *P<0.05 vs. Ctrl
group; #P<0.05 vs. LE group; +P<0.05
vs. HE group. Ctrl, control; Rapa, rapamycin; LE, low elongation;
HE, high elongation; RUNX, runt-related transcription factor 2;
OSX, osterix. |
Discussion
It was recently demonstrated that excessive
mechanical loading may be a major factor involved in HO (5), which is the main pathological
alteration of calcific tendinopathy; however, few studies have
investigated the underlying mechanism. Therefore, the present study
was established to investigate the underlying molecular mechanism
of mechanical loading on heterotopic ossification in tendons.
Tendon stem cells or mesenchymal stem cells (MSCs)
can be induced to differentiate into the osteogenic lineage
following mechanical loading treatment, as evidenced by increased
protein and mRNA expression levels of RUNX2 and OSX (18–20).
In contrast, a previous study demonstrated that MSCs subjected to
mechanical loading applied using an FX-4000T system had
significantly decreased RUNX2 protein and mRNA expression (21). These contradictory results may have
resulted from the different cell types and different parameters of
the stretch equipment used in each experiment. In order to
investigate the effects of relatively low and high elongation
mechanical loading on osteogenic differentiation in tendon cells, 4
and 12% elongation were chosen to represent low and high elongation
of mechanical loading, respectively. Runx2 and OSX, the primary
regulators of osteogenic differentiation (22), were measured. The results indicated
that low elongation mechanical loading promoted osteogenic
differentiation of rat tendon cells. By contrast, high elongation
mechanical loading suppressed osteogenic differentiation of rat
tendon cells.
The Achilles tenotomy rat model has been used to
investigate HO prophylaxis and treatment based on its relative
simplicity and predictability (23–25).
In the present study, the Achilles tenotomy rat model was employed
to investigate how mechanical loading modulates HO in the tendon.
The model was established with certain modifications by using
plaster casts to simulate different levels of mechanical loading.
Micro CT analysis of the BT/VT, Tb.N and Tb.Th parameters of tendon
tissues suggested that HO in rats was attenuated by low elongation
mechanical loading, but enhanced by high elongation mechanical
loading. Through immunohistochemical and histological staining, the
present study also revealed that low elongation mechanical loading
downregulated RUNX2 and OSX by weakening mineralization, whereas
high elongation mechanical loading exhibited the opposite effect.
These results may illustrate that low elongation mechanical loading
prohibited HO, while high elongation mechanical loading accelerated
HO in the animal model.
Eccentric exercise, with mechanical loading as its
functional element, has exhibited promising results in the
treatment of calcific tendinopathy (3,4);
however in other studies, eccentric exercise demonstrated
relatively poor therapeutic effects (26,27).
The present findings may explain how eccentric exercise has a
therapeutic role through mechanical loading. The healing of tendons
is a physiological process involving the dynamic equilibrium of
micro-damage and micro-repair. This physiological process of
healing may be changed into a pathologic process when the balance
is broken. Thus, low elongation mechanical loading is preferred and
excessive training should be avoided when receiving physical
therapy.
In the present study, rapamycin, a selective mTORC1
signaling pathway inhibitor, exhibited capacity to inhibit
osteogenic differentiation in vitro and attenuate HO in
vivo. These results suggested that the mTORC1 signaling pathway
may have a crucial role in the occurrence and development of HO.
Previous studies have shown that mTORC1 signaling has an important
role in modulating protein metabolism and energy metabolism
(5,7,28).
Therefore, the mTORC1 signaling pathway may modulate HO through the
regulation of protein metabolism and energy metabolism. Thus,
rapamycin may be a potential drug treatment for calcific
tendinopathy.
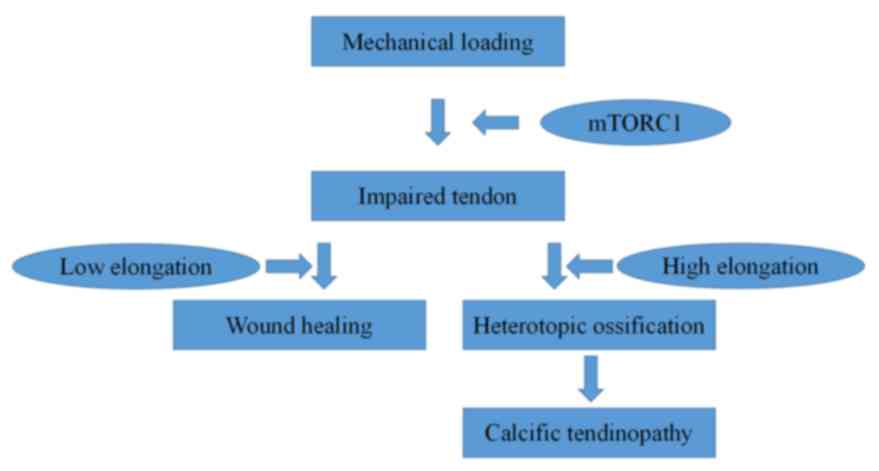
In conclusion, the present study provided novel
insights into the effects of mechanical loading on heterotopic
ossification. The results demonstrated that low elongation
mechanical loading attenuated HO while high elongation mechanical
loading accelerated HO. Furthermore, mTORC1 signaling may be
involved (Fig. 6) as an underlying
mechanism in this process.
Acknowledgements
This work was supported by National Natural Science
Foundation of China (NSFC; grant no. 31370985) and Natural Science
Foundation of Guangdong Province of China (grant no.
2015A030310416).
References
|
1
|
Kannus P and Józsa L: Histopathological
changes preceding spontaneous rupture of a tendon. A controlled
study of 891 patients. J Bone Joint Surg Am. 73:1507–1525. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greis AC, Derrington SM and McAuliffe M:
Evaluation and nonsurgical management of rotator cuff calcific
tendinopathy. Orthop Clin North Am. 46:293–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saithna A, Gogna R, Baraza N, Modi C and
Spencer S: Eccentric exercise protocols for patella tendinopathy:
Should we really be withdrawing athletes from sport? A systematic
review. Open Orthop J. 6:553–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larsson ME, Käll I and Nilsson-Helander K:
Treatment of patellar tendinopathy-a systematic review of
randomized controlled trials. Knee Surg Sports Traumatol Arthrosc.
20:1632–1646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaida JE, Cook JL, Bass SL, Austen S and
Kiss ZS: Are unilateral and bilateral patellar tendinopathy
distinguished by differences in anthropometry, body composition, or
muscle strength in elite female basketball players? Br J Sports
Med. 38:581–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DH, Sarbassov DD, Ali SM, King JE,
Latek RR, Erdjument-Bromage H, Tempst P and Sabatini DM: mTOR
interacts with raptor to form a nutrient-sensitive complex that
signals to the cell growth machinery. Cell. 110:163–175. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burry M, Hawkins D and Spangenburg EE:
Lengthening contractions differentially affect p70s6k
phosphorylation compared to isometric contractions in rat skeletal
muscle. Eur J Appl Physiol. 100:409–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haddad F and Adams GR: Aging-sensitive
cellular and molecular mechanisms associated with skeletal muscle
hypertrophy. J Appl Physiol (1985). 100:1188–1203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hornberger TA, Stuppard R, Conley KE,
Fedele MJ, Fiorotto ML, Chin ER and Esser KA: Mechanical stimuli
regulate rapamycin-sensitive signalling by a phosphoinositide
3-kinase-, protein kinase B- and growth factor-independent
mechanism. Biochem J. 380:795–804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong Y, Feng W, Wu Y, Lv H, Jia Y and
Jiang D: Mechano-growth factor accelerates the proliferation and
osteogenic differentiation of rabbit mesenchymal stem cells through
the PI3K/AKT pathway. BMC Biochem. 16:12015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Akiyama K, Wang D, Xu X, Li B,
Moshaverinia A, Brombacher F, Sun L and Shi S: mTOR inhibition
rescues osteopenia in mice with systemic sclerosis. J Exp Med.
212:73–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rolfing JH, Baatrup A, Stiehler M, Jensen
J, Lysdahl H and Bunger C: The osteogenic effect of erythropoietin
on human mesenchymal stromal cells is dose-dependent and involves
non-hematopoietic receptors and multiple intracellular signaling
pathways. Stem Cell Rev. 10:69–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gharibi B, Farzadi S, Ghuman M and Hughes
FJ: Inhibition of Akt/mTOR attenuates age-related changes in
mesenchymal stem cells. Stem Cells. 32:2256–2266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bring DK, Kreicbergs A, Renstrom PA and
Ackermann PW: Physical activity modulates nerve plasticity and
stimulates repair after Achilles tendon rupture. J Orthop Res.
25:164–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu L, Cong J, Zhang J, Tian YY and Zhai
XY: A microwave antigen retrieval method using two heating steps
for enhanced immunostaining on aldehyde-fixed paraffin-embedded
tissue sections. Histochem Cell Biol. 145:675–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Chen W, Zhou Y, Tang K and Zhang J:
Mechanical tension promotes the osteogenic differentiation of rat
tendon-derived stem cells through the Wnt5a/Wnt5b/JNK signaling
pathway. Cell Physiol Biochem. 36:517–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li R, Liang L, Dou Y, Huan Z, Mo H, Wang Y
and Yu B: Mechanical strain regulates osteogenic and adipogenic
differentiation of bone marrow mesenchymal stem cells. Biomed Res
Int. 2015:8732512015.PubMed/NCBI
|
|
20
|
Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang
T, Zhang X and Dai K: Uniaxial mechanical tension promoted
osteogenic differentiation of rat tendon-derived stem cells
(rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem. 113:3133–3142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Y, Li H, Zhang X, Fu Y, Huang Y, Lui
PP, Tang T and Dai K: Continuous cyclic mechanical tension
inhibited Runx2 expression in mesenchymal stem cells through
RhoA-ERK1/2 pathway. J Cell Physiol. 226:2159–2169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HW, Suh JH, Kim HN, Kim AY, Park SY,
Shin CS, Choi JY and Kim JB: Berberine promotes osteoblast
differentiation by Runx2 activation with p38 MAPK. J Bone Miner
Res. 32:1227–1237. 2008. View Article : Google Scholar
|
|
23
|
Zhang K, Wang L, Zhang S, Yu B, Liu F, Cui
Z, Jin D and Bai X: Celecoxib inhibits the heterotopic ossification
in the rat model with Achilles tenotomy. Eur J Orthop Surg
Traumatol. 23:145–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin L, Shen Q, Xue T and Yu C: Heterotopic
ossification induced by Achilles tenotomy via endochondral bone
formation: Expression of bone and cartilage related genes. Bone.
46:425–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McClure J: The effect of diphosphonates on
heterotopic ossification in regenerating Achilles tendon of the
mouse. J Pathol. 139:419–430. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rompe JD, Furia J and Maffulli N:
Eccentric loading compared with shock wave treatment for chronic
insertional achilles tendinopathy. A randomized, controlled trial.
J Bone Joint Surg Am. 90:52–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fahlström M, Jonsson P, Lorentzon R and
Alfredson H: Chronic Achilles tendon pain treated with eccentric
calf-muscle training. Knee Surg Sports Traumatol Arthrosc.
11:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng Z, Jing D, Zhang X, Duan Y and Xue F:
Cyclic mechanical stretch promotes energy metabolism in
osteoblast-like cells through an mTOR signaling-associated
mechanism. Int J Mol Med. 36:947–956. 2015. View Article : Google Scholar : PubMed/NCBI
|