Introduction
Glomerular nephritis is a type of diffuse or focal
autoimmune disease characterized by glomerular damage (1). Clinical manifestations include edema,
abnormal urine (proteinuria, hematuria), anemia, renal dysfunction
and difficult treatment; with disease migration, most patients may
develop chronic renal failure, and the prognosis is poor (2). Current research has indicated that
the prevalence of chronic kidney disease will increase rapidly in
the next 20 years; the proportion of adults with chronic kidney
disease will increase from current 13.2 to 14.1% in 2020, and to
16.7% in 2030 (3). The development
of effective therapies and treatment methods for the prevention and
treatment of kidney disease is particularly important, and the
studies have suggested that kidney-associated diseases will be an
important direction for future research of novel drugs (4).
Present research into nephritis has suggested that
inflammatory responses are associated with genetic, immune and
cytokine immune complex deposition factors (2). Both natural and acquired immune
responses against pathogens are mediated by Toll-like receptors
(TLRs) (5). After TLRs recognize
pathogen-associated molecular patterns, signals are transmitted
into the cells via the Toll-interleukin (IL)-1 receptor homology
region (TIR), to activate nuclear factor (NF)-κB transcription
factors, causing the release of various immune-associated
cytokines, including IL-1, IL-6, IL-8 and TNF-α, thus causing an
inflammatory response (5). Apart
from exogenous factors, the products decomposed by the
extracellular matrix (ECM), including hyaluronic acid, acetyl
heparin, fibrinogen or fibronectin EDA domain, and other endogenous
ligands, may act via TLR4 to trigger immune and inflammatory
responses (6).
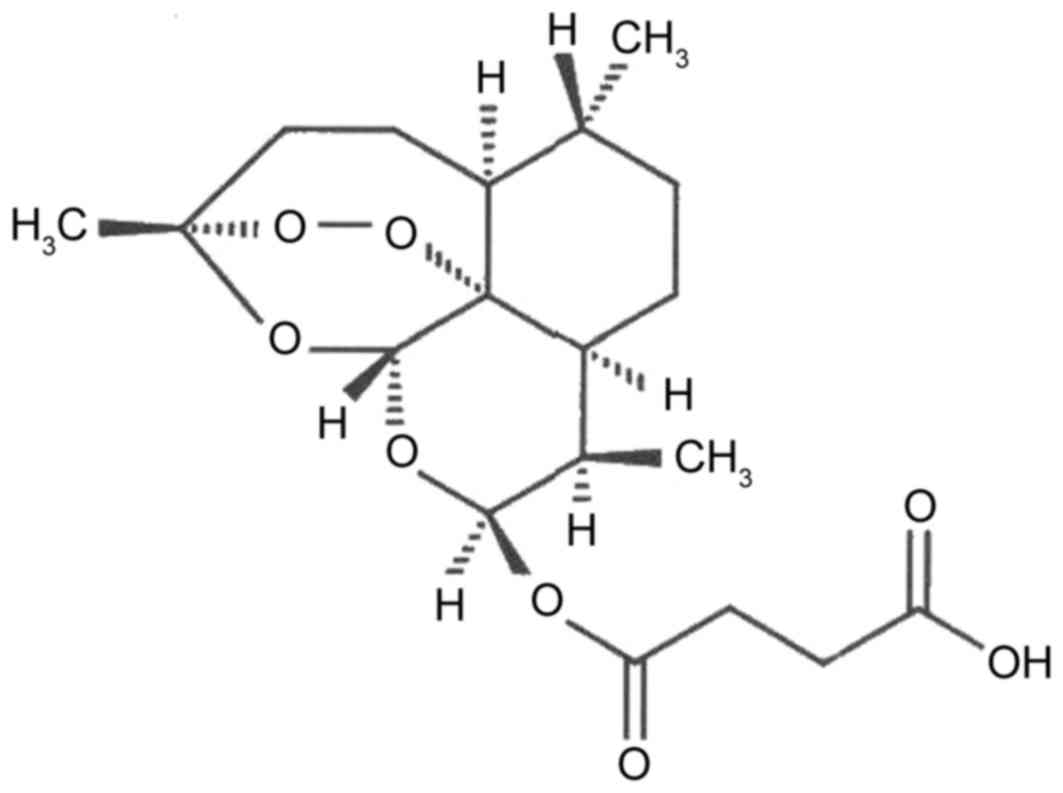
In previous years, research on Artesunate (Fig. 1) has made great progress in
anti-inflammatory immune activity (7). Artesunate exhibits significant
anti-inflammatory activity and immunosuppressive effects in
vivo and in vitro (7).
Artesunate reduces the generation of various inflammatory cytokines
including tumor necrosis factors (TNFs), namely α-induced IL-1β,
IL-6 and IL-8 generated in synovial cells; IL-4, IL-5, IL-13 and
eosinophil activating chemokines secreted in BALB/c mice
broncho-alveolar caused by ovalbumin; interferon-γ, IL-17 and TNF-α
induced by trinitrobenzene sulfonic acid in mice colitis; the
expression levels of IL-1β, IL-17 and TNF-α, and the activity of
metalloproteinase-9 induced by collagen protein in a mouse
arthritis model (8). Artesunate
may inhibit the immune response mediated by T auxiliary cells 1/T
and 17, to reduce the expression levels of TLR 4 and TLR 9. The
anti-inflammatory activity and immune inhibition function of
Artesunate suggest therapeutic prospects for chronic inflammation
and autoimmune diseases (9).
Clinical and animal experiments have demonstrated Artesunate has
good therapeutic effects on rheumatoid arthritis, asthma and
systemic lupus erythematosus (10). Artesunate is a compound with the
best solubility among all the artemisinin derivatives, which is
simple to use clinically, so research on its pharmacological
activity is extensive, and has been in relation to anti-tumor,
anti-fibrosis and anti-parasitic disease effects (10). These results suggest that
Artesunate may be a promising therapeutic agent for the prevention
of nephritis by inactivation of the TLR4/NF-κB signaling pathway in
mice.
Materials and methods
Animal model of nephritis
Female BALB/c mice (weight, 11–14 g; age, 5 weeks;
n=66) were purchased from the Experimental Animal Centre of
Laboratory Animal Sciences, Tianjin Medical University (Tianjin,
China) and housed in a specific pathogen-free facility at 23±2°C
and 50–60% relative humidity, with a 12-h day/night cycle and free
access to food and water. The experimental protocol was proved by
the Ethics Committee of Tianjin Nankai Hospital. After being
acclimated for 1 week, mice were injected intraperitoneally (i.p.)
with 500 µl pristine (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) as the nephritis model (n=60), or were injected i.p. with
500 µl PBS as sham (n=6). After three months, nephritis model mice
were randomized into two groups: Nephritis model (n=30) and 28.8
mg/kg artesunate (n=30). The nephritis model and artesunate groups
received either, 100 µl of PBS or 28.8 mg/kg artesunate,
respectively, orally by gavage each day for 6 weeks.
Proteinuria and renal function
Blood samples (50 µl) were obtained from the tail
tip under ether anesthesia (35 mg/kg pentobarbital; Sigma-Aldrich;
Merck KGaA) to examine the levels of blood urea nitrogen (BUN) and
serum creatinine with a commercial autoanalyzer (Beckman Coulter,
Inc., Brea, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Following animal sacrifice using decollation under
35 mg/kg pentobarbital anesthesia, kidney tissues from the three
groups were harvested, washed with PBS, then homogenized using
radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) for 30 min at 4°C. Homogenates
were centrifuged at 1,000 × g for 10 min at 4°C and collected to
measure TNF-α (cat. no. H052) and IL-6 (cat. no. H007) levels using
commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China). Optical densities were measured at a wavelength of
450 nm with an ELISA plate scanner (CA94089; Molecular Devices,
LLC, Sunnyvale, CA, Canada).
Western blot analysis
Kidney tissues from the three groups were harvested,
washed with PBS, homogenized using RIPA buffer (Cell Signaling
Technology, Inc.) for 30 min at 4°C and homogenates were
centrifuged at 1,000 × g for 10 min at 4°C. Protein was quantified
with a bicinchoninic acid assay following centrifugation at 1,000 ×
g for 10 min at 4°C. Proteins (50–80 µg) were separated by 10%
SDS-PAGE and transferred onto a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 5%
bovine serum albumin (Beyotime Institute of Biotechnology, Haimen,
China) or 5% skimmed milk powder in TBS with Tween-20 and incubated
with the following rabbit primary antibodies at a 1:500 dilution:
Anti-mouse α-smooth muscle actin (SMA; sc-53142), TLR4 (sc-10741),
myeloid differentiation primary response gene 88 (MyD88; sc-11356),
NF-κB p65, transforming growth factor (TGF)-β1 (sc-9043) and GAPDH
(sc-367714), all purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) at 4°C overnight. Following this, membranes were
washed with TBST (TBS with 0.1% Tween-20) and incubated with
anti-rabbit or anti-mouse IgG, horseradish peroxidase-conjugated
antibodies (cat. nos. 7074 and 7076, respectively; 1:5,000; Cell
Signaling Technology, Inc.) for 1 h at 37°C. Proteins were
visualized using an Enhanced Chemiluminescence detection system
(EMD Millipore). Protein expression was quantified using ImageJ
analysis software version 1.37 (National Institutes of Health,
Bethesda, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way analysis of variance and a post hoc Tukey test were used
for multiple comparisons using SPSS software version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
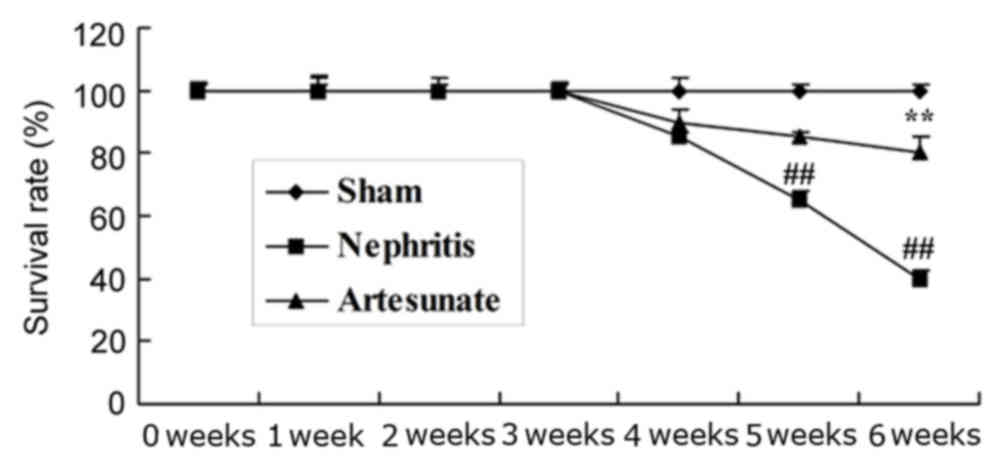
Artesunate enhances survival rate
The survival rate of mice in the sham, nephritis and
Artesunate groups was assessed over a 6-week period. As presented
in Fig. 2, the survival rate of
nephritis model group was reduced compared with the sham group.
However, treatment with 28.8 mg/kg Artesunate significantly
increased the survival rate of the nephritis mice, compared with
the nephritis model group (Fig.
2).
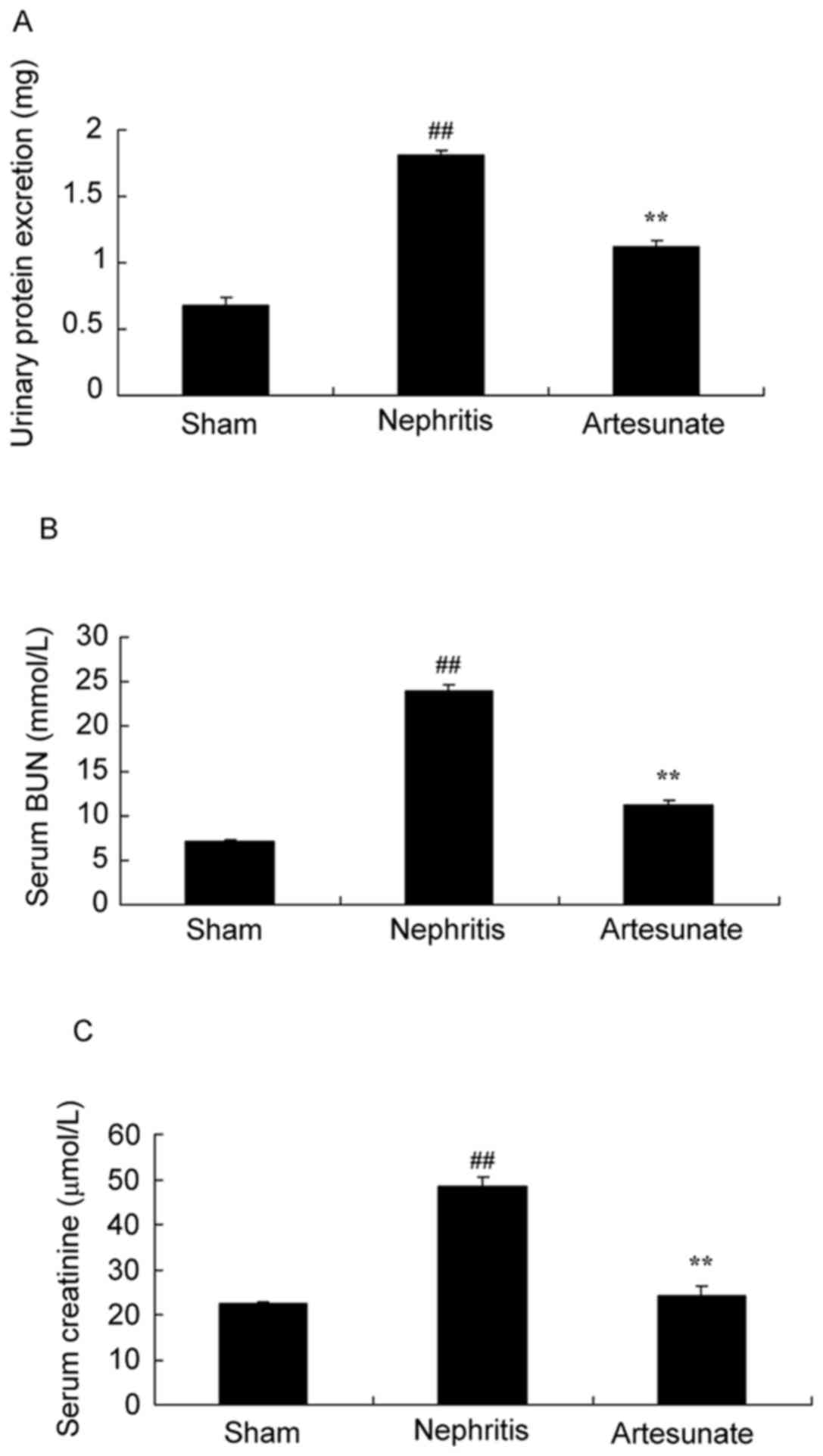
Artesunate prevents nephritis and
improves renal function
The effect of Artesunate on nephritis was
investigated by blood content analysis. Urinary protein (Fig. 3A), serum BUN (Fig. 3B) and creatinine (Fig. 3C) levels in the nephritis model
group were significantly increased compared with the sham group.
However, 28.8 mg/kg Artesunate treatment significantly inhibited
urinary protein, serum BUN and creatinine levels in nephritis mice
(Fig. 3).
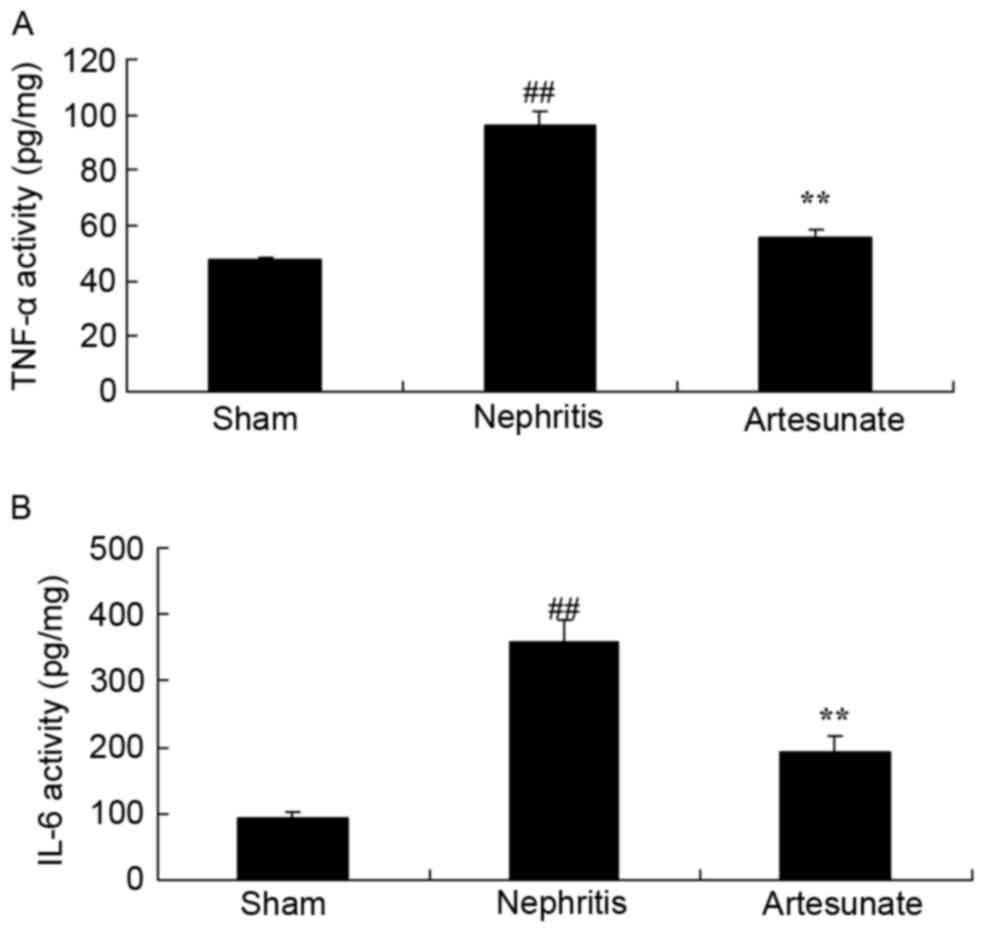
Artesunate inhibits TNF-α and IL-6
levels
The effect of Artesunate on inflammation in
nephritis mice was investigated by ELISA. As expected, a
significant increase in TNF-α (Fig.
4A) and IL-6 (Fig. 4B) levels
were observed in the nephritis model group compared with the sham
group. Treatment with Artesunate significantly decreased TNF-α and
IL-6 levels in nephritis mice, compared with non-treated animals
(Fig. 4).
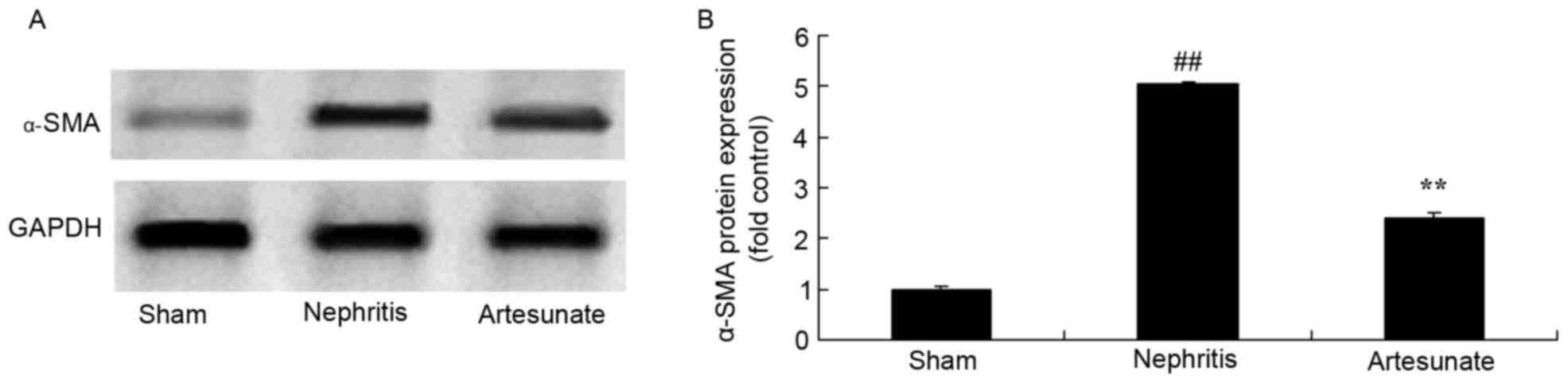
Artesunate inhibits α-SMA protein
expression
The effect of Artesunate on α-SMA protein expression
in nephritis mice was investigated by western blotting. The protein
expression of α-SMA in the nephritis model group was significantly
increased compared with the sham group (Fig. 5). However, a notable reduction of
α-SMA protein expression following Artesunate treatment was
observed in nephritis mice, compared with the nephritis model group
(Fig. 5).
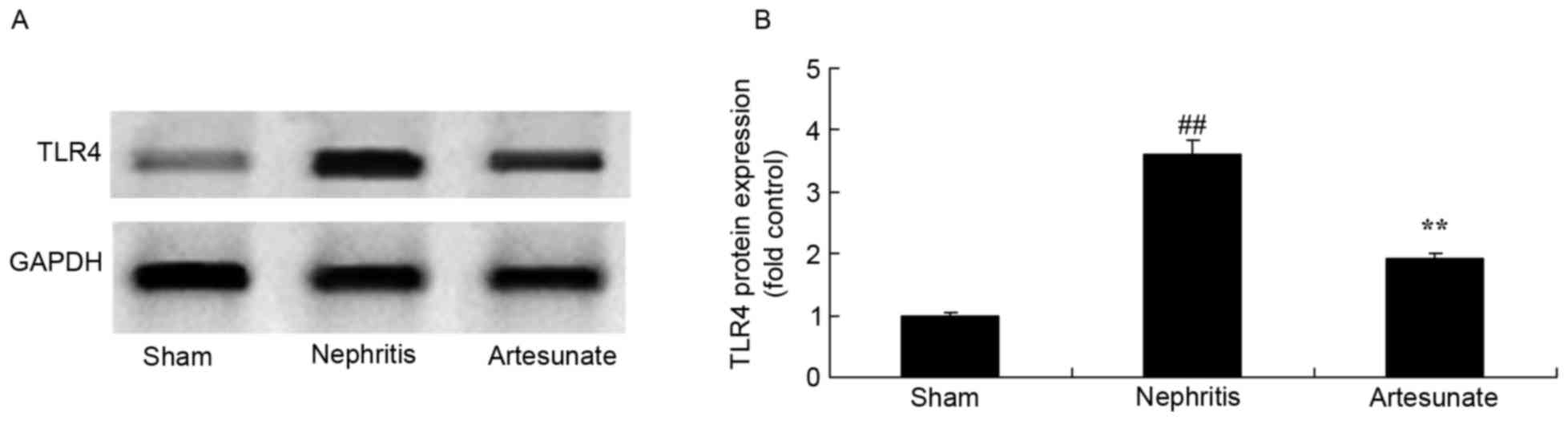
Artesunate inhibits TLR4 protein
expression
A similar effect on TLR4 protein expression by
Artesunate treatment was observed. The results demonstrated a
significant increase in TLR4 protein expression in the nephritis
model group compared with the sham group (Fig. 6). Treatment with Artesunate
significantly suppressed TLR4 protein expression in nephritis mice
compared with the nephritis model group (Fig. 6).
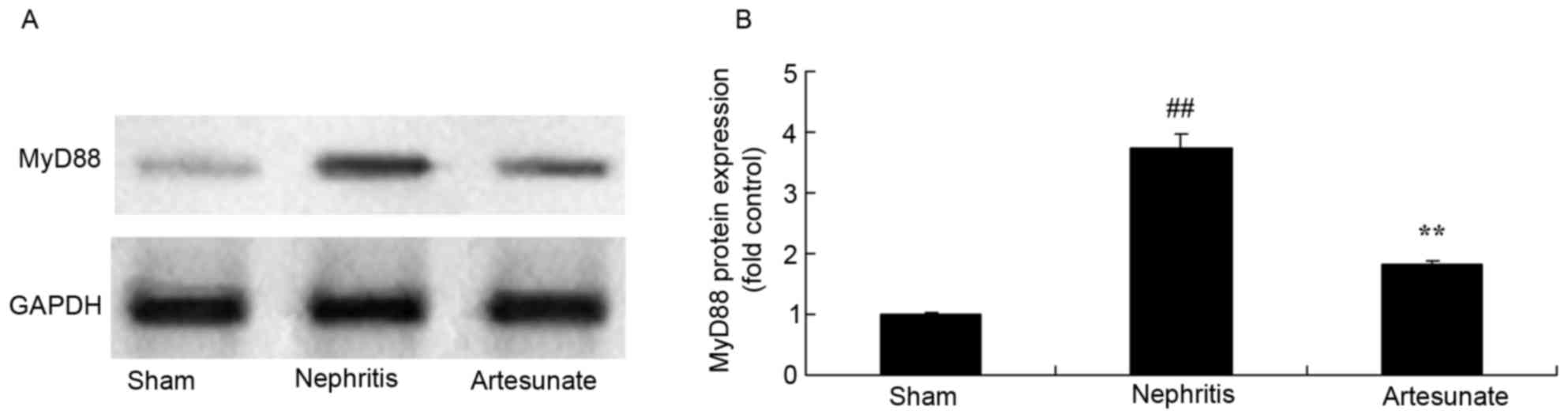
Artesunate inhibits MyD88 protein
expression
Artesunate was administrated in order to observe its
influence on MyD88 protein expression in nephritis mice. The
results demonstrated that MyD88 protein expression in the nephritis
model group markedly increased compared with the sham group
(Fig. 7). Artesunate (28.8 mg/kg)
treatment obviously decreased MyD88 protein expression in nephritis
mice compared with the nephritis model group (Fig. 7).
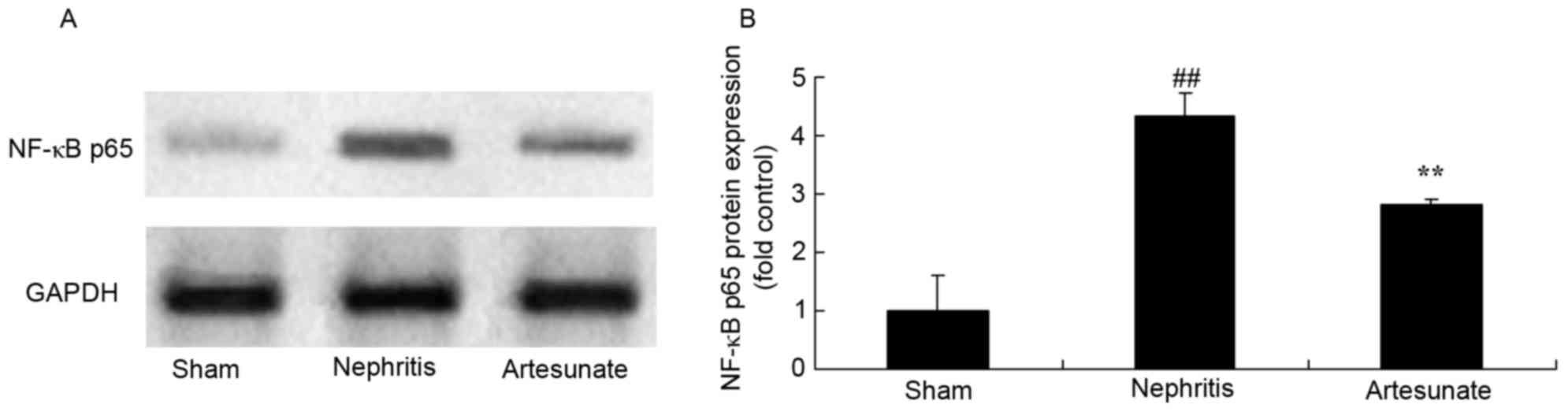
Artesunate inhibits NF-κB p65 protein
expression
NF-κB p65 protein expression levels are closely
associated with pro-inflammatory cytokine levels in nephritis
models. The results demonstrated that NF-κB p65 protein expression
markedly increased in nephritis the model group compared with the
sham group (Fig. 8). Artesunate
(28.8 mg/kg) treatment significantly decreased NF-κB p65 protein
expression in nephritis mice compared with the nephritis model
group (Fig. 8).
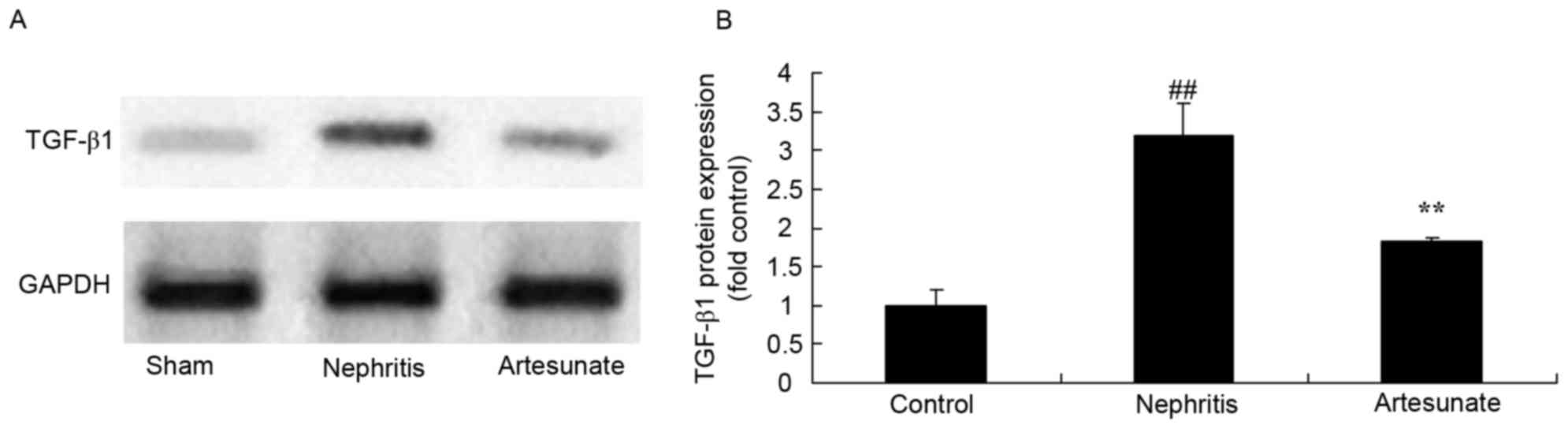
Artesunate inhibits TGF-β1 protein
expression
The effects of Artesunate on TGF-β1 protein
expression in a nephritis model were examined by western blot
analysis. The results demonstrated that TGF-β1 protein expression
markedly increased in the nephritis model group compared with the
sham group (Fig. 9). Artesunate
treatment significantly inhibited TGF-β1 protein expression in
nephritis mice compared with the nephritis model group (Fig. 9).
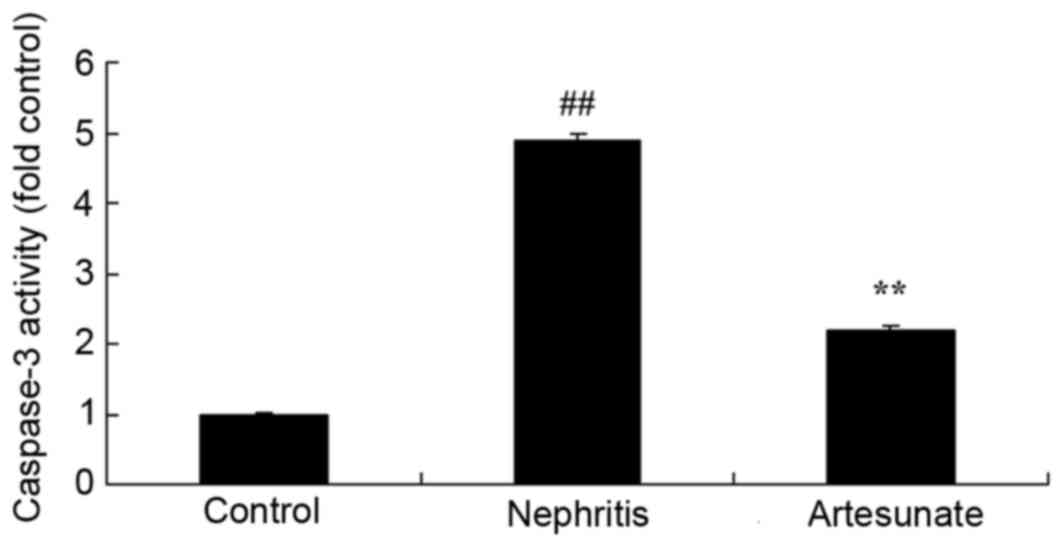
Artesunate inhibits caspase-3
activity
To further evaluate the effects of Artesunate on
apoptosis in a nephritis model, caspase-3 activity was measured by
ELISA. Caspase-3 activity in the nephritis model group as
significantly increased compared with the sham group. Artesunate
treatment significantly decreased caspase-3 activity in nephritis
mice compared with the nephritis model group (Fig. 10).
Discussion
Glomerular nephritis, also known as chronic
nephritis, occurs in bilateral kidneys, and has complex etiology
and multiple pathology types. It is a diffuse or focal autoimmune
disease primarily manifested by glomerular damage (11). Clinical manifestations include
edema, abnormal urine (proteinuria, hematuria), high blood
pressure, anemia, renal dysfunction and difficult treatment. As the
disease migrates, most patients develop chronic renal failure, and
the prognosis is poor (12). For
early chronic nephritis, appropriate treatment should be given
according to the pathological type, to suppress immune-mediated
inflammation, inhibit mesangial cell proliferation and alleviate
the kidney hardening, in order to prevent or slow the progressive
deterioration of renal function, and improve or relieve symptoms
and complications. The following measures may be adopted for
comprehensive treatment (13). The
results of the present study indicated that Artesunate
significantly increased the survival rate, inhibited urinary
protein, serum BUN and creatinine levels, and decreased TNF-α and
IL-6 levels in nephritis mice. Luo et al (8) indicated that Artesunate inhibits the
effects of cigarette smoke and inflammation.
The distribution of α-SMA is closely associated with
the development, occurrence and prognosis of kidney disease
(14). Mesangial cells (MCs)
expressing α-SMA are able to contract to reduce the capillary area
to influence glomerular hemodynamics, resulting in the occurrence
of glomerular sclerosis; in addition, α-SMA induces ECM synthesis
and secretion, leading to glomerulosclerosis (15). A previous study demonstrated that
MC proliferation, and mRNA and protein expression levels of
intracellular α-SMA, are increased significantly in an anti-Thy-1
nephritis model. However, removal of platelets may successfully
inhibit these effects (16). A
previous glomerulonephritis study has indicated that the expression
level of α-SMA is proportional to the degree of MC proliferation
(16). Therefore, α-SMA may be an
indicator of transformation of MCs from the stationary phenotype to
the proliferative/secretory phenotype (14). The results of the present study
indicated that Artesunate significantly suppressed α-SMA protein
expression levels in nephritis mice. Xu et al (17) suggested Artesunate ameliorates
hepatic fibrosis via regulating α-SMA expression in mice.
In pathological process of mesangial proliferative
glomerulonephritis, there are many cytokines involved. MCs are
stimulated through autocrine and paracrine pathways, and tissue
hypertrophy, cell proliferation, and synthesis and accumulation of
ECM components, eventually leads to glomerulosclerosis (18). The TGF-β1 signaling pathway is the
most active. TGF-β is a multifunctional polypeptide growth factor
that regulates cell proliferation, differentiation and apoptosis by
complex receptor signaling pathways in the cell surface (19). TGF-β inhibits proliferation of
glomerular epithelial cells and endothelial cells; however, it has
a dual role on MCs: It stimulates proliferation at a low
concentration, and inhibits proliferation at a high concentration
(20). MCs have been revealed to
secrete TGF-β, and TGF-β regulates the proliferation rate and
synthesis of ECM components by binding to the corresponding
receptors on MCs (21). The most
marked effect of TGF-β on MCs is to stimulate secretion of the ECM,
so that ECM components accumulate (21). A previous study in animals and
human kidney disease confirmed TGF-β1 may increase ECM synthesis,
reduce ECM decomposition, increase and coordinate the expression of
integrin, and promote the excessive accumulation of the ECM in the
kidneys, eventually leading to glomerulosclerosis (22). Jiang et al (23) reported that Artesunate attenuates
lung injury through TGF-βl and anti-inflammatory activities.
Consistent with this, the results of the present study demonstrated
that Artesunate significantly suppresses TGF-β protein expression
in nephritis mice.
Previous studies have indicated TLRs are involved in
immune and inflammatory response (6,24).
In TLR family, TLR4 mediates inflammation, and serves an important
role in non-infectious inflammations in liver, lung and kidney
ischemic reperfusion injury. Organs may be protected by inhibiting
the expression of TLR4 (25). One
of the main signal transduction pathways involves binding with the
TIR region within TLR cells through an adapter protein (MyD88),
which leads to activation of transcription factors (such as NF-κB),
thus contributing to synthesis and transcriptional expression of a
large number of cellular activity factors, including IL-1, IL-6 and
IL-8, and adhesion molecules and inflammatory cytokines, which
ultimately affects the immune and inflammatory response (25). There has been increased focus on
the role of TLR4 in kidney disease; glomerular disease is a type of
immune-mediated inflammation response, and TLR4 is closely
associated with inflammation regulation (24). TLR4 expression is increased in
glomeruli, which activates renal parenchymal cells and immune cells
to promote secretion of large amounts of cytokines and inflammatory
mediators, causing persistent inflammation, leading to
significantly faster glomerulosclerosis. NF-κB activity is
significantly increased, which is induced by MyD88 signal
transduction pathways, and activated NF-κB regulates gene
transcription of inflammatory cytokines and a variety of
fiber-associated factors, including TGF2β1. Fibroblast
proliferation and differentiation may also be directly mediated by
NF-κB, thereby affecting the development of glomerulosclerosis
(26). A previous study confirmed
that TLR4 is an important regulatory factor in the inflammatory
response activation pathway, and diabetic kidney injury may be
associated with increased expression of TLR4 (24). The results of the present study
demonstrated that Artesunate significantly decreased TLR4, MyD88
and NF-κB p65 protein expression in nephritis mice. Wang et
al (27) indicated that
Artesunate attenuates lipopolysaccharide-induced inflammation via
the TLR4/MyD88/NF-κB signaling pathways in microglial cells
(27). The present study indicated
that the protective effects of Artesunate on nephritis are
partially attributed to inhibition of the TLR4/MyD88/NF-κB p65
signaling pathway.
In conclusion, Artesunate treatment attenuated
nephritis by its anti-inflammatory effect, and by inhibiting the
TLR4/MyD88/NF-κB p65 signaling pathway in mice. Therefore,
Artesunate may be a potential therapeutic agent to prevent
nephritis.
References
|
1
|
Rabrenović V, Poskurica M, Kovačević Z,
Nešić V, Savin M, Mitić B, Dimković N, Čučković Č, Vujić D, Plješa
S, et al: Treatment of lupus nephritis by mycophenolate mofetil.
Kidney Blood Press Res. 33:297–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Hu W, Xie H, Zhang H, Chen H, Zeng
C, Liu Z and Li L: Induction therapies for class IV lupus nephritis
with non-inflammatory necrotizing vasculopathy: Mycophenolate
mofetil or intravenous cyclophosphamide. Lupus. 16:707–712. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok CC, Ying KY, Yim CW, Siu YP, Tong KH,
To CH and Ng WL: Tacrolimus versus mycophenolate mofetil for
induction therapy of lupus nephritis: A randomised controlled trial
and long-term follow-up. Ann Rheum Dis. 75:30–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petersson L, Dexlin-Mellby L, Bengtsson
AA, Sturfelt G, Borrebaeck CA and Wingren C: Multiplexing of
miniaturized planar antibody arrays for serum protein profiling-a
biomarker discovery in SLE nephritis. Lab Chip. 14:1931–1942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anders HJ, Banas B and Schlöndorff D:
Signaling danger: Toll-like receptors and their potential roles in
kidney disease. J Am Soc Nephrol. 15:854–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding Y, Liao W, He X, Xiang W and Lu Q:
CSTMP exerts anti-inflammatory effects on LPS-induced human renal
proximal tubular epithelial cells by inhibiting TLR4-mediated NF-κB
pathways. Inflammation. 39:849–859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Z, Ding J, Yang C, Gao Y, Li X, Chen
X, Peng Y, Fang J and Xiao S: Immunomodulatory and
anti-inflammatory properties of artesunate in experimental colitis.
Curr Med Chem. 19:4541–4551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo Q, Lin J, Zhang L, Li H and Pan L: The
anti-malaria drug artesunate inhibits cigarette smoke and ovalbumin
concurrent exposure-induced airway inflammation and might reverse
glucocorticoid insensitivity. Int Immunopharmacol. 29:235–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee IS, Ryu DK, Lim J, Cho S, Kang BY and
Choi HJ: Artesunate activates Nrf2 pathway-driven anti-inflammatory
potential through ERK signaling in microglial BV2 cells. Neurosci
Lett. 509:17–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bethell D, Se Y, Lon C, Tyner S, Saunders
D, Sriwichai S, Darapiseth S, Teja-Isavadharm P, Khemawoot P,
Schaecher K, et al: Artesunate dose escalation for the treatment of
uncomplicated malaria in a region of reported artemisinin
resistance: A randomized clinical trial. PLoS One. 6:e192832011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito T, Iwano M, Matsumoto K, Mitarai T,
Yokoyama H, Yorioka N, Nishi S, Yoshimura A, Sato H, Ogahara S, et
al: Significance of combined cyclosporine-prednisolone therapy and
cyclosporine blood concentration monitoring for idiopathic
membranous nephropathy with steroid-resistant nephrotic syndrome: A
randomized controlled multicenter trial. Clin Exp Nephrol.
18:784–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kopp JB, Winkler CA, Zhao X, Radeva MK,
Gassman JJ, D'Agati VD, Nast CC, Wei C, Reiser J, Guay-Woodford LM,
et al: Clinical features and histology of apolipoprotein
L1-associated nephropathy in the FSGS clinical trial. J Am Soc
Nephrol. 26:1443–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z,
Chen J, Lin H, Liu F, He Y, et al: Multitarget therapy for
induction treatment of lupus nephritis: A randomized trial. Ann
Intern Med. 162:18–26. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Han C, Sun C, Meng H, Ye F, Na S,
Chen F, Zhang D and Jin X: Serum levels of alpha-smooth muscle
actin and c-Met as biomarkers of the degree of severity of
Henoch-Schonlein purpura nephritis. Transl Res. 161:26–36. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawasaki Y, Suzuki J, Sakai N, Tanji M and
Suzuki H: Predicting the prognosis of renal dysfunction by renal
expression of alpha-smooth muscle actin in children with MPGN type
1. Am J Kidney Dis. 42:1131–1138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu CY: Wakayama Symposium:
Notch-FoxL2-α-SMA axis in eyelid levator muscle development and
congenital blepharophimosis. Ocul Surf. 10:221–223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Liu W, Fang B, Gao S and Yan J:
Artesunate ameliorates hepatic fibrosis induced by bovine serum
albumin in rats through regulating matrix metalloproteinases. Eur J
Pharmacol. 744:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu S, Sun G, Zhang Y, Li X and Wang R:
Involvement of the NF-κB signaling pathway in the renoprotective
effects of isorhamnetin in a type 2 diabetic rat model. Biomed Rep.
4:628–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong F, Wu N, Ge Y, Zhou Y, Shen T, Qiang
Q, Zhang Q, Chen M, Wang Y, Wang L and Hong J: Nanosized titanium
dioxide resulted in the activation of TGF-β/Smads/p38MAPK pathway
in renal inflammation and fibration of mice. J Biomed Mater Res A.
104:1452–1461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Susianti H, Iriane VM, Dharmanata S,
Handono K, Widijanti A, Gunawan A and Kalim H: Analysis of urinary
TGF-β1, MCP-1, NGAL, and IL-17 as biomarkers for lupus nephritis.
Pathophysiology. 22:65–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang W, Wang J, Shi L, Yu L, Qian Y, Liu
Y, Wang W and Cheng S: Podocyte injury and overexpression of
vascular endothelial growth factor and transforming growth
factor-beta 1 in adriamycin-induced nephropathy in rats. Cytokine.
59:370–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capuano A, Costanzi S, Peluso G, Zannoni
G, Vellone VG, Gremese E, Zoli A, Scott C, Beltrami CA, Romano G
and Ferraccioli G: Hepatocyte growth factor and transforming growth
factor beta1 ratio at baseline can predict early response to
cyclophosphamide in systemic lupus erythematosus nephritis.
Arthritis Rheum. 54:3633–3639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang L, Zhang Y, Sun Y, Hu L and Gao D:
Artesunate attenuates lung injury in paraquat-intoxicated rats via
downregulation of inflammatory cytokines. Clin Lab. 61:1601–1607.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chassin C, Goujon JM, Darche S, du Merle
L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguénec C,
Buzoni-Gatel D and Vandewalle A: Renal collecting duct epithelial
cells react to pyelonephritis-associated Escherichia coli by
activating distinct TLR4-dependent and -independent inflammatory
pathways. J Immunol. 177:4773–4784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding Y, Yang H, Xiang W, He X, Liao W and
Yi Z: CD200R1 agonist attenuates LPS-induced inflammatory response
in human renal proximal tubular epithelial cells by regulating
TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway. Biochem Biophys
Res Commun. 460:287–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Correa-Costa M, Braga TT, Semedo P,
Hayashida CY, Bechara LR, Elias RM, Barreto CR, Silva-Cunha C,
Hyane MI, Gonçalves GM, et al: Pivotal role of Toll-like receptors
2 and 4, its adaptor molecule MyD88, and inflammasome complex in
experimental tubule-interstitial nephritis. PLoS One. 6:e290042011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang D, Shi J, Lv S, Xu W, Li J, Ge W,
Xiao C, Geng D and Liu Y: Artesunate attenuates
lipopolysaccharide-stimulated proinflammatory responses by
suppressing TLR4, MyD88 expression, and NF-κB activation in
microglial cells. Inflammation. 38:1925–1932. 2015. View Article : Google Scholar : PubMed/NCBI
|