Introduction
Macrophages are important phagocytic and
antigen-presenting cells in the body and they serve an important
role in handling external pathogenic microorganisms and endogenous
danger signals. Macrophages are widely distributed in various
tissues and organs and are important in maintaining homeostasis,
body defense, regulating inflammation and promoting wound healing
(1).
As important immune cells in human body, macrophages
serve an important role in antigen presentation and inflammatory
response. A recent study demonstrated that macrophages are a group
of heterogeneous and flexible cells with diverse and varied immune
functions, and they can polarize into different phenotypes in
different microenvironments or under different stimuli, playing
different roles in inflammatory response of tissues (2). Depending on the activation and immune
functions, they can be divided into the classically activated type
(M1 type) and the alternatively activated type (M2 type). M1 type
macrophages are activated by T helper (h)1 cytokines of helper T
lymphocytes, such as interferon (IFN), tumor necrosis factor (TNF)
and bacterial lipopolysaccharides (LPS), and the activated cells
can induce the expression of pro-inflammatory cytokines, such as
interleukin (IL)-6, TNF-α and IL-1β, resist pathogen invasion, and
participate in the inflammatory response, but they can also cause
body injury, which is manifested as high antigen-presenting ability
(3). M2 type macrophages are
activated by Th2 cytokines and, following activation, they can
induce high expression of anti-inflammatory cytokines including
IL-10, transforming growth factor-β and arginase, and low
expression of pro-inflammatory cytokines, thereby inhibiting the
inflammatory response, protecting surrounding tissues from the harm
caused by immune response and promoting repair of tissue injuries
(4). Therefore, a deep study into
the internal mechanism of macrophages polarity differentiation will
be of great importance for a better understanding occurrence,
development and treatment of various immune-related diseases.
The suppressor of cytokine signaling (SOCS) family
is a collection of negative regulatory factors that are generated
by cells and can block cytokine signaling in feedback (5). As one important member of the family,
SOCS1 is involved in various cytokine signal transductions and the
differentiation of immune cells, and serves an important role in
innate and adaptive immune response. As an important determinant
for the activity and function of differentiated macrophages, SOCS1
is not only a feedback inhibitor of inflammation, but is also an
important molecular switch that can effectively regulate different
aspects of macrophage balance by regulating crucial signaling
pathways (6). In the case of
tissue inflammation, macrophages will have high expression of SOCS1
or SOCS3, but the probability of simultaneous expression of both is
small. By using IFN-γ or LPS to culture mouse bone marrow-derived
macrophages, they could express SOCS1 and SOCS3, yet following
stimulation with IL-4, macrophages only expressed SOCS1 (6,7). In
contrast, when both IFN-γ and LPS were used for culturing,
expression of SOCS1 in macrophages was inhibited, and these cells
gradually polarized into M1 macrophages that only express SOCS3.
Following the knock out of SOCS3 in macrophages, culturing with
IFN-γ and LPS led to an upregulation of SOCS1 expression. The cells
restored the reactivity for IL-4, thereby polarizing into M2
macrophages and inhibiting the generation of M1 macrophages and
inflammatory mediators. The results demonstrated that SOCS3 is of
great importance for the activation of M1 macrophages, while SOCS1
can control macrophages' reactivity for IFN-γ, as well as signaling
pathways of TLR4 and TLR9 activation; it is also an endogenous
inhibitor of the STAT1 pathway. Upregulated SOCS1 expression can
promote the polarization of macrophages into M2 type, indicating
that SOCS1 may be involved in the polarization process into M2 type
macrophages (8–10).
Although there currently is a certain understanding
of SOCS1, its specific mechanism remains to be further explored so
as to better guide our clinical practice. In addition, Xuebijing
(XBJ) injection is an intravenous preparation made from traditional
Chinese medicines. Previous studies and clinical trials have
indicated that it is a good treatment of SIRS/MODS (systemic
inflammatory response syndrome/multiple organ dysfunction syndrome)
(11,12). In addition, current fundamental
research has suggested that it could restrain the release of
inflammatory mediators, eliminate endotoxin and reduce the
mortality of septic animals and patients (11,12).
Nevertheless, its effect on STATs and SOCSs has not been explored
until now. Thus, the present study will make a discussion to
provide a clinical basis for determining the mechanism of
Xuebijing.
Materials and methods
Isolation and culture of mouse
macrophages
Animal care and use followed the ethical guidelines
of the Chinese Council on Animal Care and were reviewed and
approved by the Institutional Animal Care and Use Committee. The
mice (purchased from the Institute of Laboratory Animal Sciences,
Chinese Academy of Medical Sciences and Peking Union Medical
College, Beijing, China) were sacrificed by cervical dislocation. A
total of 5 ml chilled RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was intraperitoneally injected.
At 5 min, a pipette was used to draw peritoneal lavage fluid, and
the lavage was repeated once. The lavage fluid recycled twice was
centrifuged at 4°C, 8,000 × g, and the obtained cell pellet was
washed twice with pre-cooled RPMI1640 medium, and pre-cooled
RPMI1640 medium (penicillin 100 U/ml, streptomycin 100 µg/ml and
10% FBS) was added for resuspension. The cells were inoculated in
25 mm2 culture flasks (BD Biosciences, Franklin Lakes,
NJ, USA) and incubated at 37°C with 5% CO2. Following 4
h incubation, the medium was changed and was rinsed with RPMI1640
medium twice, non-adherent cells were discarded and the adherent
cells obtained were monolayer macrophages.
Construction and transfection of short
hairpin (sh)RNA vector
shRNA targeting SOCS1 was synthesized, and was
cloned into pSilencer 2.1-U6 neo vector (Qiagen GmbH, Hilden,
Germany) following double enzyme digestion by BamHI and
HindIII (Takara Biotechnology Co., Ltd., Dalian, China). The
cells were seed into a six-well plate at 1×105 cells/ml
and incubated for 24 h. The plasmid transfection was carried out
following the instruction of Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) with a concentration of 4 µg/well.
Following transfection, fludarabine (2 µM; Selleck Chemicals,
Shanghai, China) or Xuebijing injection (50 mg/ml; Tianjin Chase
Sun Pharmaceutical Co., Ltd., Tianjin, China) was added, and cells
were cultured for 48 h prior to being analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting.
RT-qPCR
Total RNA was extracted with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instruction, and then reverse transcription was performed with One
Step SYBR PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.).
The reaction was performed with ABI PRISM 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) at 42°C for 5
min, 95°C for 10 sec; then at 40 cycles of 95°C for 5 sec, 55°C for
30 sec and 72°C for 30 sec. Primers used for RT-qPCR are presented
in Table I. Three independent
experiments were conducted for each sample. Data were analyzed by
comparing the 2−ΔΔCq value (13).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′-3′) |
|---|
| JAK1-m-F |
TCAAACCTGTGTCTCGCTCT |
| JAK1-m-R |
ACGCTGTTAGTTTTCTGTGTCAG |
| SOCS1-m-F |
CTCCTTGGGGTCTGTTGGC |
| SOCS1-m-R |
GCGTGCTACCATCCTACTCG |
| STAT1-m-F |
GACCTGTCATCCCGCAGAGA |
| STAT1-m-R |
GGAGCAGAGCTGAAACGACC |
Western blotting
Total cellular proteins were extracted by incubating
cells in radioimmunoprecipitation assay buffer with protease
inhibitors (Pierce; Thermo Fisher Scientific, Inc.). The protein
concentrations in the lysates were determined by Quick Start
Bradford Protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). SDS-PAGE was conducted using 12% gels (Bio-Rad Laboratories,
Inc.) loading equal amount of proteins (20 µg) per lane. Following
electrophoresis, separated proteins were transferred to a
polyvinylidene difluoride membrane (Pierce; Thermo Fisher
Scientific, Inc.) and blocked with 5% non-fat milk. Following this,
the membranes were incubated with anti-SOCS1 antibody (dilution,
1:500; cat. no. ab62584), anti-JAK1 antibody (dilution, 1:400; cat.
no. ab133666), anti-STAT1 antibody (dilution, 1:500; cat. no.
ab99415), anti-p-STAT1 antibody (dilution, 1:500; cat. no.
ab109461) and anti-GAPDH antibody (dilution, 1:1,000; cat. no.
ab181603; all from Abcam, Cambridge, MA, USA) in 5% non-fat milk
overnight at 4°C, and then goat anti-rabbit IgG monoclonal antibody
(dilution, 1:5,000; cat. no. sc-2004; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) conjugated with horseradish peroxidase was
incubated with the membranes for 1 h at room temperature. Protein
bands were detected using the West Femto system (Pierce; Thermo
Fisher Scientific, Inc.), and gray values of the bands were
measured by Gel-Pro Analyzer software version 6.3 (Media
Cybernetics, Rockville, MD, USA).
Flow cytometry
Following digesting macrophages with trypsin,
RPMI1640 medium was added and cells were collected by pipetting,
then the cells were centrifuged at 6,000 × g for 5 min at room
temperature and washed once with PBS. PBS was used for
resuspension, and fluorescein isothiocyanate-labeled CD206 antibody
(dilution, 1:10; cat. no. EL921283; EterLife, London, UK) was
added, and the cells were incubated in the dark for 30 min.
Following this, the phycoerythrin-labeled anti-mouse CD197 (CCR7)
(1 µg; cat. no. 12-1971-63; eBioscience, Inc., San Diego, CA, USA)
was added, and cells were incubated for 30 min. Following washing
twice with PBS, and resuspended by PBS, the cells were tested on BD
Accuri™ C6 flow cytometer (BD Biosciences). The negative control
(NC) group was transfected with an empty pSilencer 2.1-U6 neo
vector. The control group was not transfected.
ELISA
IL-4 (cat. no. M4000B), IL-10 (cat. no. M1000B),
TNF-α (cat. no. MTA00B) and IFN-γ (cat. no. MIF00) were detected
according to the manufacturer's protocol of the ELISA kits (R&D
Systems China Co., Ltd. (Shanghai, China).
Statistical analysis
SPSS statistical software (version, 17.0; SPSS,
Inc., Chicago, IL, USA) was used for data processing, and
measurement data were presented as mean ± standard deviation and
processed with t-test. Differences were analyzed by non-parametric
statistical analysis (Mann-Whitney U tests) between control and
treated groups, P<0.05 was considered to indicate a
statistically significant difference.
Results
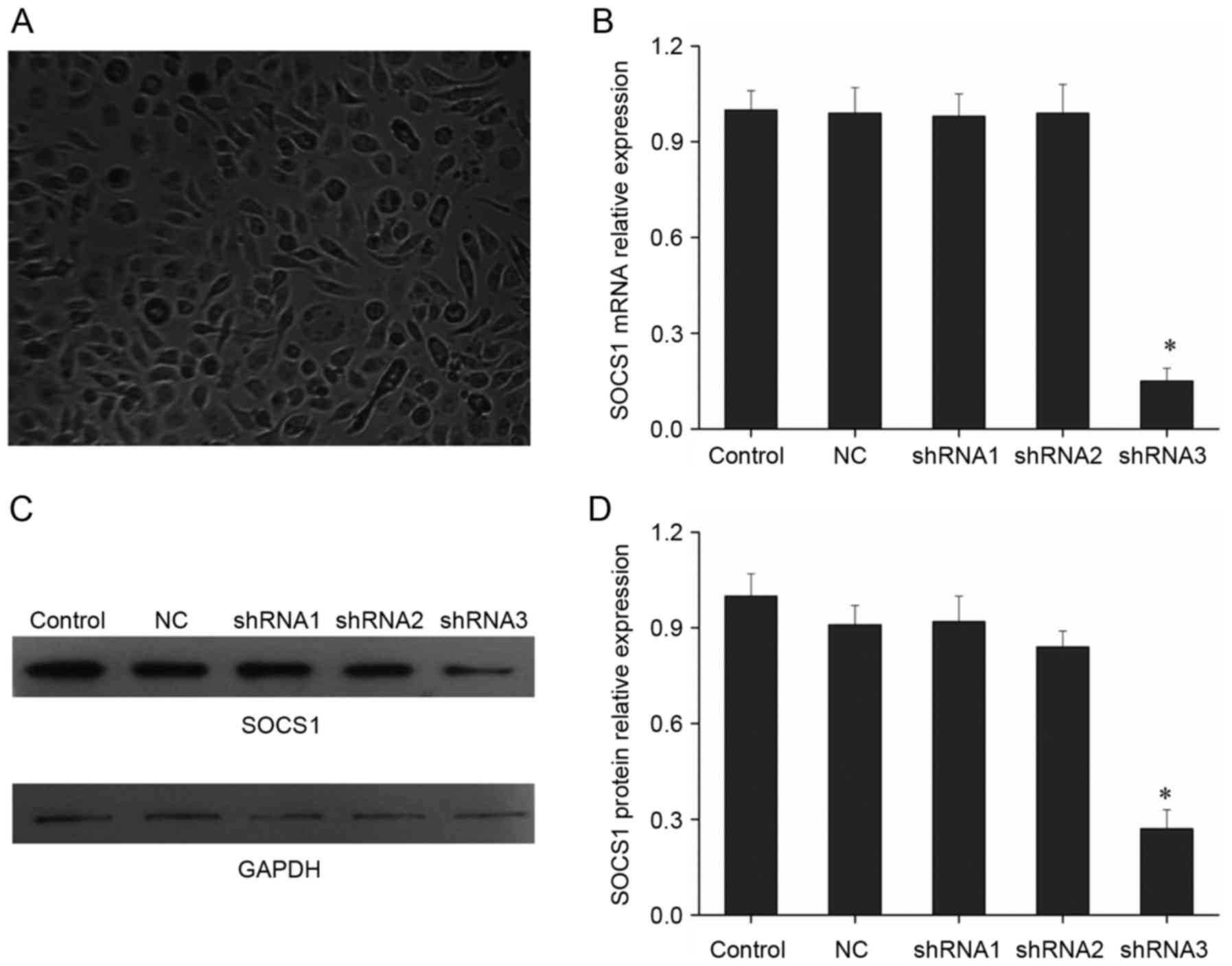
SOCS1 shRNA screening
Studies have suggested that SOCS1 upregulation would
promote macrophages to polarize into M2 type (6). In order to investigate how SOCS1
downregulation would affect the polarization of macrophages, the
present study was conducted. First, shRNA targeting SOCS1 was
screened. In the study, three SOCS1 shRNAs were designed,
synthesized and cloned into pSilencer 2.1-U6 neo vector. Following
transfecting shRNAs into mouse macrophage cells, SOCS1 expression
was detected. Results indicated that only shRNA3 had a significant
inhibitory effect, with the inhibition efficiency up to 85%, while
the inhibitory effect of the other two shRNAs was not obvious
(Fig. 1B). SOCS1 expression was
further detected by Western blot, and results showed that only
shRNA3 could significantly inhibit SOCS1 expression, while the
other two shRNAs did not substantially affect the protein levels of
SOCS1 (Fig. 1C and D). Thus,
shRNA3 has obvious effect of suppressing SOCS1 expression and it
can be used for subsequent research.
Influence of SOCS1 downregulation and
Xuebijing on the JAK/STAT pathway
The JAK/STAT signaling pathway is a stress response
pathway that was only recently discovered (14,15).
It is widely involved in processes such as cell proliferation,
differentiation, maturation, apoptosis and immune regulation, and
is one of the many important ways for cytokine signal transduction
(14,15). As a main feedback regulatory
protein of the JAK/STAT signaling pathway, SOCS1 conducts negative
feedback inhibition (5,16). After downregulating SOCS1
expression using shRNA, the mRNA levels of JAK1 and STAT1 both
increased significantly. When STAT1 activation inhibitor,
fludarabine, was added, the mRNA levels of SOCS1, JAK1 and STAT1
presented no significant changes (Fig.
2A). Meanwhile, western blotting indicated that, following the
downregulation of SOCS1 expression, expression levels of JAK1,
STAT1 and p-STAT1 increased significantly. Fludarabine can inhibit
STAT1 phosphorylation and SOCS1 expression, but it cannot affect
the expression of JAK1 and STAT1. In addition when SOCS1 shRNA
transfection and fludarabine was added, the inhibition effect of
fludarabine was weakened, but p-STAT1 expression remained
upregulated (Fig. 2B and C).
Furthermore, studies have reported that the compound traditional
Chinese medicine, Xuebijing, can promote LPS to stimulate
macrophages differentiation into M2 type (17). Therefore, the current study also
explored the underlying mechanism of Xuebijing. Given that that
mRNA and protein levels of SOCS1, JAK1 and STAT1 presented no
significant changes when Xuebijing was administered (Fig. 2), Xuebijing may not serve its role
through SOCS1, JAK1 and STAT1.
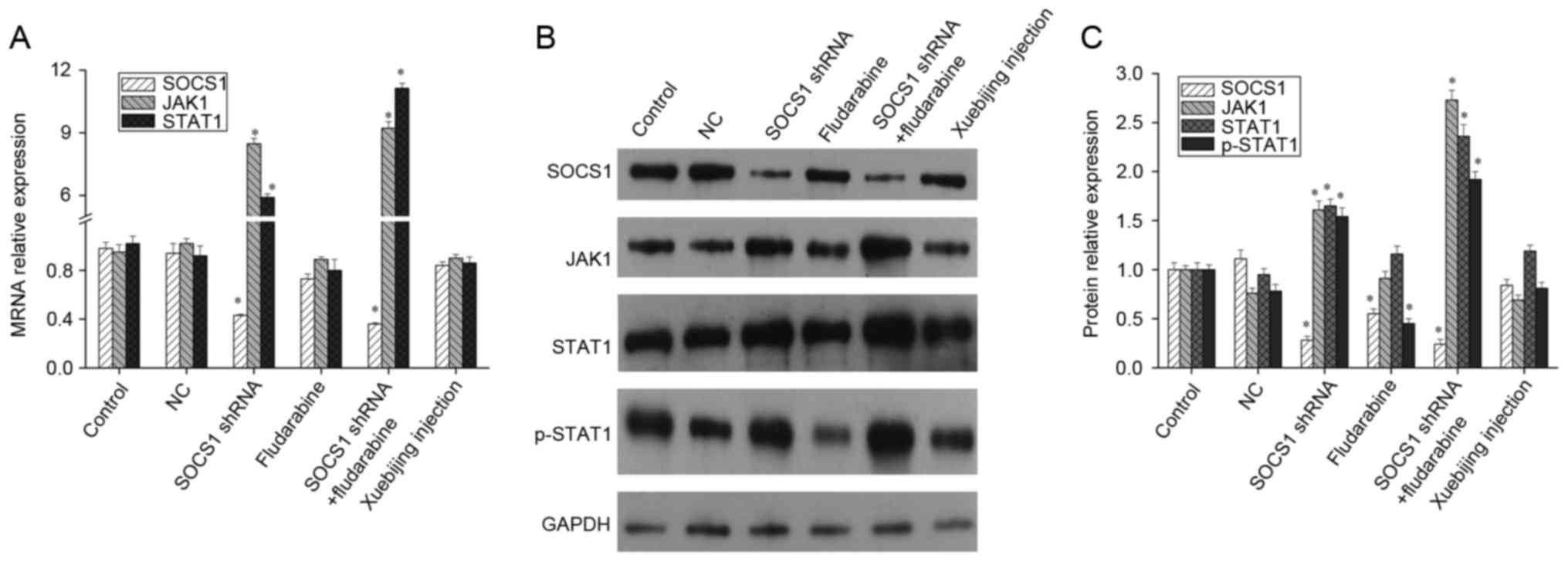 | Figure 2.Expression of SOCS1, JAK1 and STAT1 in
macrophages. Mouse macrophages were transfected with SOCS1 shRNA,
added with fludarabine or Xuebijing, respectively, and then the
expression of SOCS1, JAK1 and STAT1 was measured by (A) reverse
transcription-quantitative polymerase chain reaction or (B and C)
western blot analysis. Experiments were carried out at least in
triplicate and the results were expressed as the mean ± standard
deviation. *P<0.01 vs. control. SOCS1, suppressor of cytokine
signaling-1; JAK1, Janus kinase 1; STAT1, signal transducer and
activator of transcription; shRNA, short hairpin RNA; NC, negative
control. |
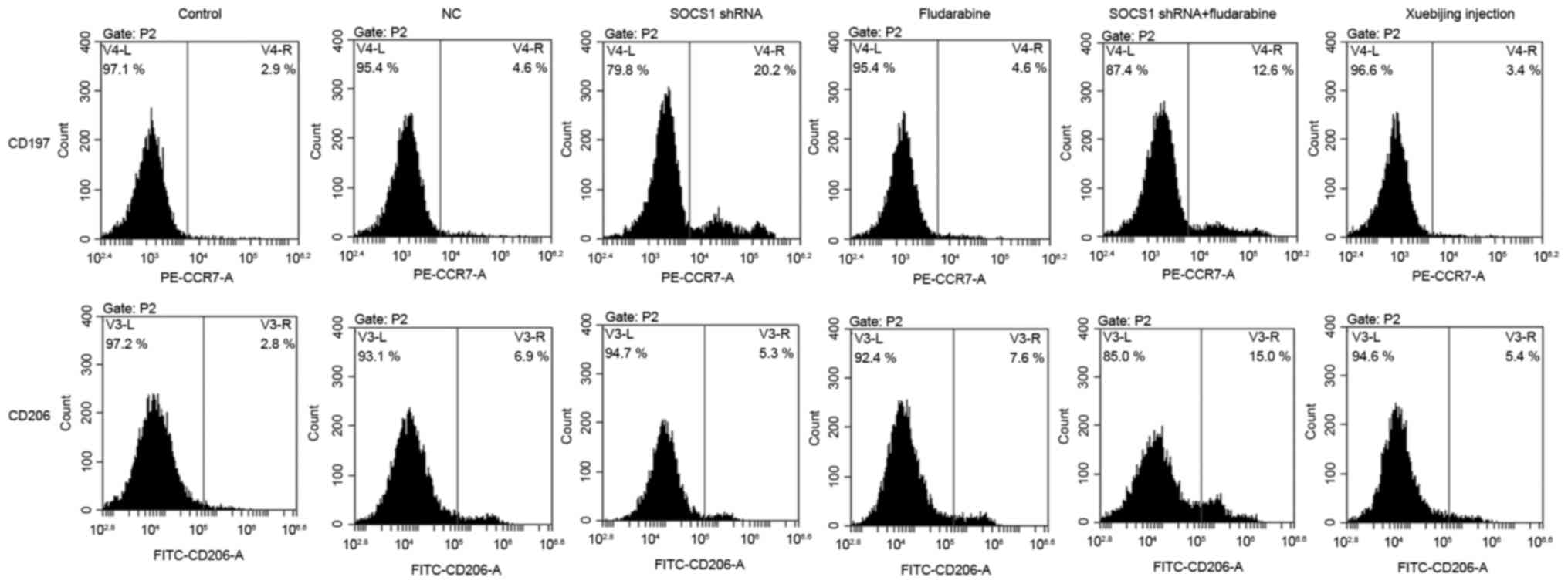
Influence of SOCS1 downregulation and
Xuebijing on polarization of macrophages
Macrophages will polarize into M1 or M2 phenotypes
under different conditions, and CCR7 (CD197) is the surface marker
for M1 type cells, and CD206 is the surface marker for M2 type
cells (18). Following the
downregulation of SOCS1 expression, flow cytometry was conducted
and the results are presented in Fig.
3. Results suggested that, following downregulation of SOCS1,
the proportion of M1 type cells was significantly increased, while
that of M2 type cells did not change significantly, indicating that
downregulated SOCS1 expression is conducive to the polarization of
macrophages into M1 phenotype. Adding fludarabine or Xuebijing
alone did not affect macrophage polarization. However, when SOCS1
shRNA transfection and fludarabine was administered, macrophages
could polarize into M1 and M2 phenotypes, and the ratio of two
types was significantly increased; but the proportion of M1 type
cells slightly decreased while compared to the shRNA transfection
group.
Influence of SOCS1 downregulation and
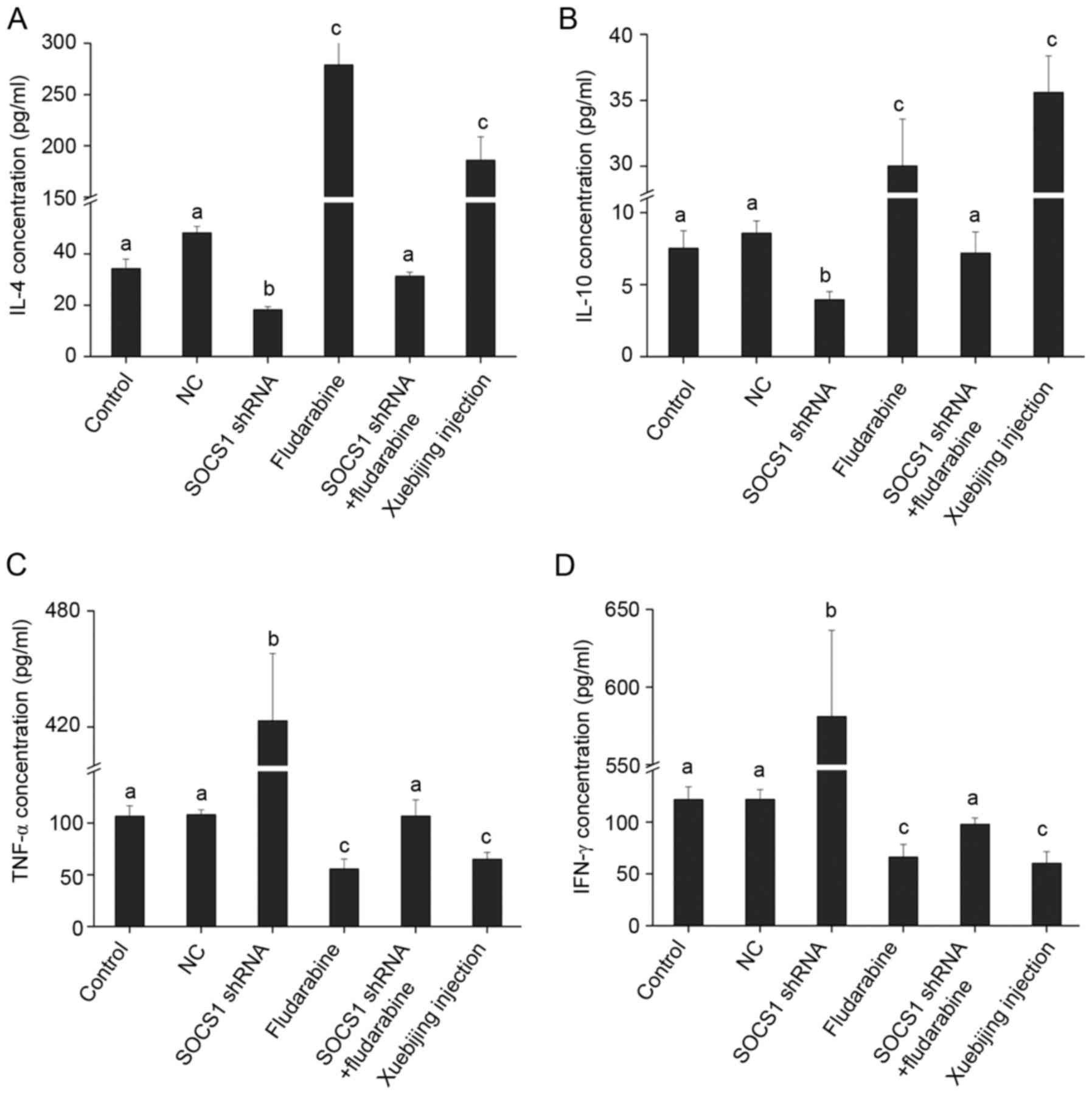
Xuebijing on the expression of inflammatory cytokines
Changes in SOCS1 expression affect macrophage
polarization, and different phenotypes of macrophages secrete and
express different inflammatory cytokines. Activated M1 macrophages
can induce expression of pro-inflammatory cytokines, such as TNF-α
and IFN-γ, while activated M2 macrophages can induce high
expression of anti-inflammatory cytokines, such as IL-4 and IL-10
(19). The present study attempted
to detect the influence of SOCS1 on inflammatory cytokine
expression. ELISA results indicated that, when downregulating SOCS1
expression, IL-4 and IL-10 expression was downregulated and TNF-α
and IFN-γ expression was significantly upregulated (Fig. 4), indicating that inhibited SOCS1
expression is not conducive to the secretion of anti-inflammatory
cytokines, but is conducive to the secretion of pro-inflammatory
cytokines. These findings are consistent with the result that, when
SOCS1 expression is inhibited, macrophages polarize into M1 type
(Fig. 3). Furthermore, when adding
fludarabine or Xuebijing, IL-4 and IL-10 expression significantly
increased, and TNF-α and IFN-γ expression significantly decreased.
When SOCS1 shRNA transfection and fludarabine was added, expression
of IL-4, IL-10, TNF-α and IFN-γ reported no significant changes
(Fig. 4), indicating that the two
have a mutually antagonistic effect.
Discussion
Macrophage polarization is involved in various
signaling pathways and in the action of transcription factors, and
polarized macrophages are associated with the occurrence and
development of many diseases (1,2).
Therefore, research on the plasticity mechanism of macrophages
polarization is of great significance. In addition, research on
macrophage polarization can give us a better understanding of the
mechanisms of cells, especially immune cells adapting to different
micro-environments, and serve as important theoretical basis for
treatment various diseases.
SOCS is generated under inducement of cytokines. As
a crucial negative regulatory protein of the JAK/STAT signaling
pathway, it regulates the body's over-stimulation for cytokines by
inhibiting signal transduction of cytokines (5). Previous studies have indicated that
SOCS is an important physiological regulator of the innate and
adaptive immune response; it can promote or inhibit activation of
macrophages and dendritic cells, participate in T cells
differentiation and immune regulation (5,16).
SOCS1 is an important member, and there have been some progress in
the study of its function at present, but there were few studies
about the effect of its downregulation on macrophages. In the
present study, shRNA targeting SOCS1 was screened. Results
demonstrated that, among the three synthesized shRNAs, one had a
significant inhibitory effect, with the inhibition efficiency up to
85%, and western blot detection also demonstrated that it could
significantly inhibit the expression of SOCS1. Therefore, this
shRNA can be used for subsequent research.
SOCS regulates the intensity and duration of
cytokines, hormones and growth factors primarily through inhibition
of JAK/STAT signal transduction (5). Through the JAK/STAT pathway,
macrophages will make appropriate response to >20 types of
cytokine (20). Therefore, the
influence of SOCS1 on the JAK/STAT pathway was first detected.
Following downregulating SOCS1 expression in macrophages using
shRNA, expression levels of JAK1 and STAT1 both increased
significantly, and phosphorylation levels of STAT1 also increased
significantly. Fludarabine did not affect the mRNA levels of SOCS1,
JAK1 and STAT1, but it could downregulate protein levels of SOCS1
and inhibit phosphorylation of STAT1. Furthermore, when Xuebijing
was administered, the mRNA and protein levels of SOCS1, JAK1 and
STAT1 had no significant changes, indicating that it may not play
its role through SOCS1, JAK1 and STAT1. SOCS1 was previously
indicated to act on the JH1 domain of JAKs, inhibiting the kinase
activity, and thereby inhibited the conduction of JAK/STAT
signaling pathway (21). The
current study demonstrated that SOCS1 downregulation is conducive
to the activation of the JAK/STAT pathway. But SOCS is not only a
negative regulator for JAK/STAT signaling pathway but also a target
gene of the pathway. Therefore, the result that fludarabine could
downregulate SOCS1 expression is consistent with current
findings.
Further flow cytometry detection demonstrated that,
after the downregulation of SOCS1 expression, the proportion of
M1-type cells significantly increased, while that of M2-type cells
did not change significantly. Adding fludarabine or Xuebijing alone
did not affect macrophage polarization. However, when SOCS1 shRNA
transfection and fludarabine was added, macrophages could polarize
into M1 and M2 phenotypes, but the proportion of M1 type cells
slightly decreased while compared to the shRNA transfection group.
It indicated that downregulated SOCS1 expression is conducive to
the polarization of macrophages into the M1 phenotype, while
fludarabine had an inhibitory effect. Studies on mice knocked out
of SOCS1 also suggested that SOCS1 has an inhibitory effect on
STAT1 (22). Activated M2
macrophages in vitro demonstrated a selective and
IL-4-dependent upregulation of SOCS1 (6). The test using small interfering RNA
to knockout SOCS1 in bone marrow-derived macrophage cells indicated
that SOCS1 expression is of great importance for IL-4-induced
M2-type characteristics (6).
Spence et al (23)
stimulated the mice LPS to knockdown SOCS2 and SOCS3, and reported
that SOCS2 and SOCS3 are important regulatory molecules for
polarization into M1 and M2 type macrophages and inflammatory
responses. The SOCS2 knockout macrophages highly expressed M1-type
markers, while SOCS3 knockout macrophages highly expressed M2-type
markers, indicating that SOCS2 plays an important role in
polarization into M2 type macrophages and inhibition on
inflammatory responses. However, while SOCS3 is involved in
polarization into M1 type macrophages and promotes the occurrence
of inflammatory responses (8). The
present study and above results all demonstrate that SOCS1 serve a
key role in the regulation of macrophage polarization.
Different phenotypes of macrophages secrete and
express various inflammatory cytokines, so ELISA was used to
further detect the expression of iconic factors such as IL-4,
IL-10, TNF-α and IFN-γ. Results demonstrated that, after SOCS1
expression was downregulated, expression of IL-4 and IL-10 was
downregulated, while expression of TNF-α and IFN-γ was
significantly upregulated. When adding fludarabine or Xuebijing,
expression of IL-4 and IL-10 significantly increased, while that of
TNF-α and IFN-γ significantly decreased. When SOCS1 shRNA was
transfected and fludarabine was added, expression of above factors
reported no significant changes. The above results indicated that
inhibited SOCS1 expression is not conducive to the secretion of
anti-inflammatory cytokines, but is conducive to the secretion of
pro-inflammatory cytokines, and fludarabine had the role of
blocking the function of SOCS1 shRNA. Studies have suggested that
SOCS1 serves an important role in the negative feedback regulation
of the JAK2/STAT3 signaling pathway (5,24).
It was further indicated that SOCS1 inhibited JAK2 phosphorylation
through the central SH-2 region and inhibited activation of the
STAT3 signaling pathway, thus regulating the secretion of
inflammatory cytokines by macrophages (25,26).
Function loss of SOCS1 may lead to JAK2/STAT3 activation and
cytokine accumulation. In contrast, an increase in SOCS1 expression
would inhibit JAK2/STAT3 activation and reduce secretion of
cytokines (27,28). Therefore, downregulation of SOCS1
could activate the JAK/STAT pathway and thereby promote
polarization of macrophages into M1 type.
In summary, changes in SOCS1 expression serve an
important regulatory role in macrophage polarization. With a clear
understanding of its polarization mechanism, we will be able to
intervene reasonably certain key steps of macrophage polarization
to reverse the imbalance of macrophage polarization, and thereby
treat various immune-related diseases from new angles.
Acknowledgements
The present study was supported by grants from
Science and Technology Planning Project of Guangdong Province
(grant no. 2012B031800291), the Science and Technology Planning
Project of Guangzhou City (grant no. 201300000160). This study was
supported by funding from Tianjin Chase Sun Pharmaceutical Co.,
Ltd.
References
|
1
|
Zhou D, Huang C, Lin Z, Zhan S, Kong L,
Fang C and Li J: Macrophage polarization and function with emphasis
on the evolving roles of coordinated regulation of cellular
signaling pathways. Cell Signal. 26:192–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verreck FA, de Boer T, Langenberg DM,
Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, Waal-Malefyt R
and Ottenhoff TH: Human IL-23-producing type 1 macrophages promote
but IL-10-producing type 2 macrophages subvert immunity to
(myco)bacteria. Proc Natl Acad Sci USA. 101:4560–4565. 2004;
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bouhlel MA, Derudas B, Rigamonti E,
Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx
N, et al: PPARgamma activation primes human monocytes into
alternative M2 macrophages with anti-inflammatory properties. Cell
Metab. 6:137–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamiya T, Kashiwagi I, Takahashi R,
Yasukawa H and Yoshimura A: Suppressors of cytokine signaling
(SOCS) proteins and JAK/STAT pathways: Regulation of T-cell
inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol.
31:980–985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whyte CS, Bishop ET, Rückerl D,
Gaspar-Pereira S, Barker RN, Allen JE, Rees AJ and Wilson HM:
Suppressor of cytokine signaling (SOCS)1 is a key determinant of
differential macrophage activation and function. J Leukoc Biol.
90:845–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stoiber D, Kovarik P, Cohney S, Johnston
JA, Steinlein P and Decker T: Lipopolysaccharide induces in
macrophages the synthesis of the suppressor of cytokine signaling 3
and suppresses signal transduction in response to the activating
factor IFN-gamma. J Immunol. 163:2640–2647. 1999.PubMed/NCBI
|
|
8
|
Liu Y, Stewart KN, Bishop E, Marek CJ,
Kluth DC, Rees AJ and Wilson HM: Unique expression of suppressor of
cytokine signaling 3 is essential for classical macrophage
activation in rodents in vitro and in vivo. J Immunol.
180:6270–6278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold CE, Whyte CS, Gordon P, Barker RN,
Rees AJ and Wilson HM: A critical role for suppressor of cytokine
signalling 3 in promoting M1 macrophage activation and function in
vitro and in vivo. Immunology. 141:96–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prêle CM, Woodward EA, Bisley J,
Keith-Magee A, Nicholson SE and Hart PH: SOCS1 regulates the IFN
but not NFkappaB pathway in TLR-stimulated human monocytes and
macrophages. J Immunol. 181:8018–8026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He XD, Wang Y, Wu Q, Wang HX, Chen ZD,
Zheng RS, Wang ZS, Wang JB and Yang Y: Xuebijing protects rats from
sepsis challenged with Acinetobacter baumannii by promoting Annexin
A1 expression and inhibiting proinflammatory cytokines secretion.
Evid Based Complement Alternat Med. 2013:8049402013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Hu Z, Zhou B, Li X and Tao R:
Chinese herbal preparation Xuebijing potently inhibits inflammasome
activation in hepatocytes and ameliorates mouse liver
ischemia-reperfusion injury. PLoS One. 10:e01314362015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 8:539–552. 2009. View Article : Google Scholar
|
|
15
|
Murray PJ: The JAK-STAT signaling pathway:
Input and output integration. J Immunol. 178:2623–2629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimura A: Regulation of cytokine
signaling by the SOCS and Spred family proteins. Keio J Med.
58:73–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YC, Yao FH, Chai YF, Dong N, Sheng ZY
and Yao YM: Xuebijing injection promotes M2 polarization of
macrophages and improves survival rate in septic mice. Evid Based
Complement Alternat Med. 2015:3526422015.PubMed/NCBI
|
|
18
|
Chen Z, Li F, Yang W, Liang Y, Tang H, Li
Z, Wu J, Liang H and Ma Z: Effect of rTsP53 on the M1/M2 activation
of bone-marrow derived macrophage in vitro. Int J Clin Exp Pathol.
8:13661–13676. 2015.PubMed/NCBI
|
|
19
|
Ouedraogo R, Daumas A, Ghigo E, Capo C,
Mege JL and Textoris J: Whole-cell MALDI-TOF MS: A new tool to
assess the multifaceted activation of macrophages. J Proteomics.
75:5523–5532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Chen J, Wang L and Ivashkiv LB:
Crosstalk among Jak-STAT, toll-like receptor and ITAM-dependent
pathways in macrophage activation. J Leukoc Biol. 82:237–243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krebs DL and Hilton DJ: SOCS proteins:
Negative regulators of cytokine signaling. Stem Cells. 19:378–387.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wormald S and Hilton DJ: Inhibitors of
cytokine signal transduction. J Biol Chem. 279:821–824. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spence S, Fitzsimons A, Boyd CR, Kessler
J, Fitzgerald D, Elliott J, Gabhann JN, Smith S, Sica A, Hams E, et
al: Suppressors of cytokine signaling 2 and 3 diametrically control
macrophage polarization. Immunity. 38:66–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davey GM, Heath WR and Starr R: SOCS1: A
potent and multifaceted regulator of cytokines and cell-mediated
inflammation. Tissue Antigens. 67:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tajiri K, Imanaka-Yoshida K, Matsubara A,
Tsujimura Y, Hiroe M, Naka T, Shimojo N, Sakai S, Aonuma K and
Yasutomi Y: Suppressor of cytokine signaling 1 DNA administration
inhibits inflammatory and pathogenic responses in autoimmune
myocarditis. J Immunol. 189:2043–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashimoto M, Ayada T, Kinjyo I, Hiwatashi
K, Yoshida H, Okada Y, Kobayashi T and Yoshimura A: Silencing of
SOCS1 in macrophages suppresses tumor development by enhancing
antitumor inflammation. Cancer Sci. 100:730–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Capello D, Gloghini A, Baldanzi G, Martini
M, Deambrogi C, Lucioni M, Piranda D, Famà R, Graziani A, Spina M,
et al: Alterations of negative regulators of cytokine signalling in
immunodeficiency-related non-Hodgkin lymphoma. Hematol Oncol.
31:22–28. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Souma Y, Nishida T, Serada S, Iwahori K,
Takahashi T, Fujimoto M, Ripley B, Nakajima K, Miyazaki Y, Mori M,
et al: Antiproliferative effect of SOCS-1 through the suppression
of STAT3 and p38 MAPK activation in gastric cancer cells. Int J
Cancer. 131:1287–1296. 2012. View Article : Google Scholar : PubMed/NCBI
|