Introduction
Hepatic disorder has become a major disease
endangering human health and exhibits a high morbidity and fatality
rate (1). As the mechanisms of
severe hepatic failure are poorly understood, there remains a lack
of effective treatment. An increasing number of studies have
suggested that the production of inflammatory cytokines serves
important roles in various types of liver damage (2,3).
Lipopolysaccharide (LPS), a component of Gram-negative bacteria,
can activate liver macrophages, which produce inflammatory
cytokines including tumor necrosis factor-α (TNF-α), interleukin
(IL)-1β and IL-6 (4–6). In addition, LPS possesses
pro-oxidative action via the induction of excessive production of
reactive oxygen species (ROS). ROS are the major cause of damage to
macromolecules, including protein and DNA, and to the cell
membrane, which leads to mitochondrial dysfunction (7,8). A
further study demonstrated that ROS are involved in modulation of
the inflammatory response (9).
Various types of liver damage, including ischemia-reperfusion and
liver cancer, are associated with LPS (10,11).
Therefore, LPS-induced liver injury is used as an animal model of
liver disorder.
Taurine, a sulfur-containing β-amino acid, is a
metabolic product of L-cysteine and is abundant in a number of
mammalian tissues. Taurine is not involved in the synthesis of
protein; however, considerable evidence has demonstrated that
taurine serves a number of vital roles in physiological processes,
including regulation of calcium concentration (12), stabilization of the cell membrane
(13), regulation of blood
pressure and protection of endothelial cells (14). The antioxidant properties of
taurine have been confirmed by a number of results, although
taurine itself is not able to scavenge ROS. Taurine exerts its
antioxidant action by inhibiting the production of ROS, which
result from the increasing activities of antioxidases (15). Certain studies have indicated that
taurine protects cells against oxidative stress (16,17).
Taurine is changed into taurine chloramine (TauCl) in vivo,
which inhibits secretion of pro-inflammatory cytokines including
IL-6, IL-1β, TNF-α and IL-8 (18,19).
It is suggested that taurine is a potent anti-inflammatory
factor.
The present study examined the beneficial effects of
taurine on LPS-induced liver injury in rats. The results suggested
that administration of taurine may be beneficial for patients with
hepatopathy.
Materials and methods
Animals and experimental design
A total of 30 healthy male Sprague-Dawley rats
(weighing 280±20 g) were obtained from the Animal Center at West
Anhui Health Vocational College and housed in a standard facility
at 22°C and 50–70% humidity with a 12-h light/dark cycle.
Experimental rats received a standard pellet diet and water ad
libitum. The study was approved by the Ethics Committee of West
Anhui Health Vocational College (Lu'an, China). After a week, the
animals were randomly divided into three groups (n=10 per group):
i) Normal saline group (NS), ii) LPS control group (LPS) and iii)
taurine + LPS group (TL). Rats from NS and LPS were treated with
sterile saline by intravenous injection and animals from TL were
intravenously injected with taurine (100 mg/kg body weight,
dissolved in sterile saline; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). After 4 h, rats from LPS and TL groups were
intraperitoneally injected with LPS (10 mg/kg body weight,
dissolved in sterile saline; Sigma-Aldrich; Merck KGaA) and NS rats
received sterile saline. At 6 h following administration of LPS,
the animals were anesthetized with sodium pentobarbital (50 mg/kg,
i.p.; Sigma-Aldrich; Merck KGaA) to collect blood samples prior to
animal sacrifice. Fasting blood samples were collected by artery
catheterization for biochemical analyses and liver tissues were
obtained for histological analyses.
Determination of liver function
Fasting blood samples were centrifuged at 3,000 × g
to separate the serum. Markers of liver function, serum aspartate
transaminase (AST) and alanine transaminase (ALT) were determined
using an enzymatic colorimetric method (Diagnostica Stago S.A.S.,
Paris, France) according to the manufacturer's protocols and
analyzed by a semi-automatic analyzer, with the results being
expressed as U/l.
Measurement of inflammatory
cytokines
Serum levels of TNF-α, procalcitonin (PCT) and IL-6
were detected with rat TNF-α (cat. no. ELS-2855-1), PCT (cat. no.
ELS-3485-1) and IL-6 (cat. no. ELS-2866-1) specific ELISA kits
(Hefei Bomei Biotechnology Co., Ltd., Hefei, China) according to
manufacturer's protocol. The levels of TNF-α and IL-6 were
expressed as ng/l.
Estimation of antioxidant effects
To estimate changes in antioxidant effects, the
activity of the antioxidase, superoxide dismutase (SOD) was
determined using xanthine oxidase methods, and the content of lipid
peroxidation production, malonaldehyde (MDA), was measured using
thiobarbituric acid methods, according to the manufacturer's
protocol (both from Nanjing Jiancheng Bioengineering Institute,
Nanjing, China).
Histological analysis
Liver tissues were harvested and fixed in 4%
phosphate-buffered formalin for pathological analysis. Fixed
tissues were dehydrated in ethanol, embedded in paraffin and 5 µm
sections were cut. After drying overnight, sections were dewaxed,
rehydrated and stained with hematoxylin and eosin (H&E) for
histomorphological observation under a light microscope.
Western blot analysis
Liver tissues (0.2 g) were harvested, lysed and
homogenized in 2 ml lysis buffer with 10 mM Tris-buffered saline, 1
mM EDTA, 1 mM EGTA, 2 mM PMSF and 1% Triton X-100 (v/v) for 20 min.
Lysates were centrifuged at 13,000 × g for 15 min at 4°C. Protein
concentration was measured using a Quick Start™ Bradford protein
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Denatured
proteins in supernatants were separated by 10% SDS-PAGE and
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% non-fat milk in TBS with Tween-20 (10 mM Tris-HCl,
150 mM NaCl and 1% Tween-20) for 2 h. The membranes were
subsequently incubated with primary polyclonal antibodies against
β-actin (1:1,000), heme oxygenase-1 (HO-1; 1:1,000),
cyclooxygenase-2 (COX-2), nuclear factor (NF)-κB, phosphorylated
(p)-NF-κB, extracellular signal-regulated kinase (ERK) and p-ERK1/2
(Bio Basic Inc., Markham, ON, Canada) overnight at 4°C. Following
an extensive wash with TBST, the membranes were incubated with a
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:10,000; cat. no. A9169; Sigma-Aldrich; Merck KGaA) for
2 h at room temperature. The membranes were washed three times and
visualized with 3,3′-diaminobenzidine (Bio Basic Inc.).
Statistical analysis
All values are expressed as mean ± standard
deviation. The data were analyzed using SPSS version 16.0 (SPSS,
Inc., Chicago, IL, USA). Statistical difference was determined by
Tukey's test for unpaired data or one-way analysis of variance with
least significant difference-t and/or Tamhane's T2 post
hoc tests for multiple comparisons. P<0.05 was considered
statistically to indicate a statistically significant
difference.
Results
Ameliorative effects of taurine on
liver damage
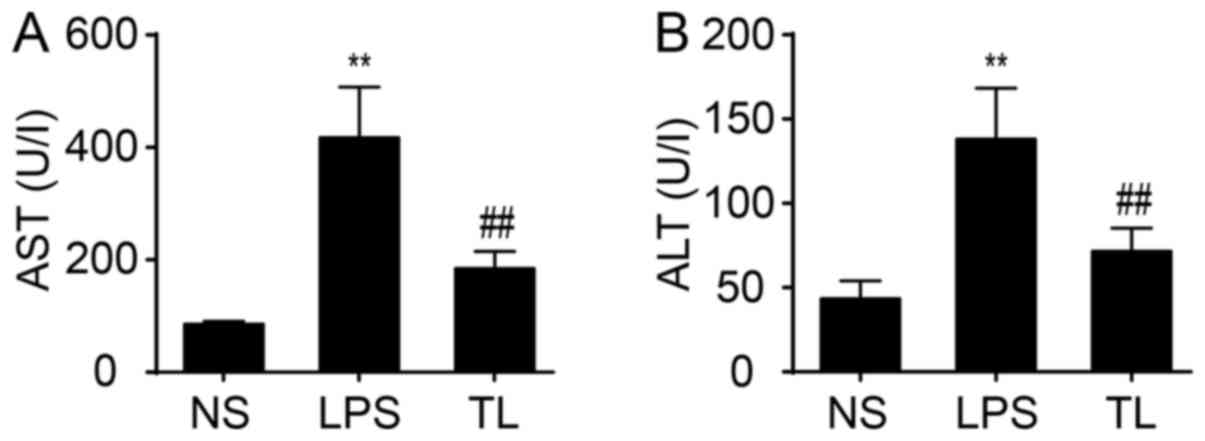
To examine liver function, serum AST and ALT
transaminases were determined. The result demonstrated a
significant increase in activity of AST and ALT in LPS rats
compared with NS rats (P<0.01; Fig.
1). Administration of taurine reduced the increase in activity
of AST and ALT (P<0.01; Fig.
1).
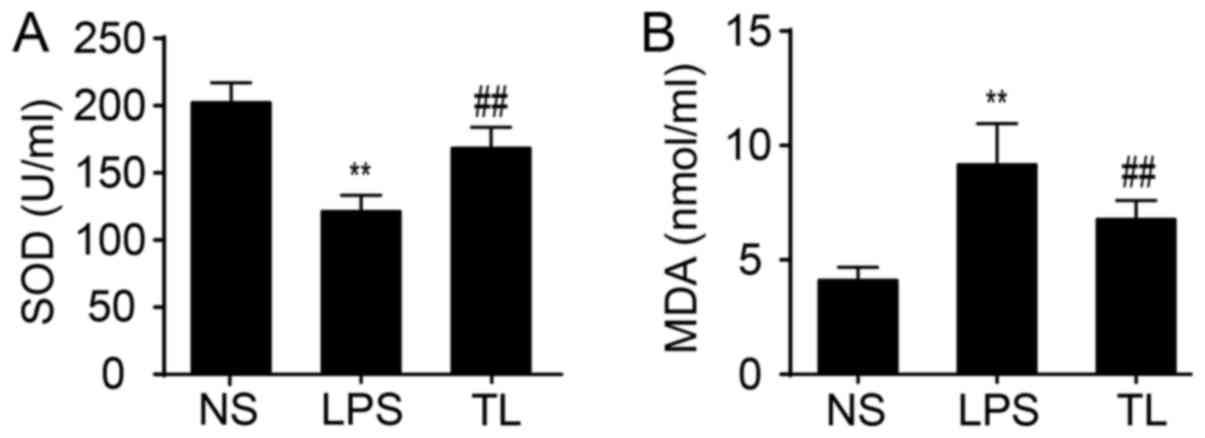
Change of antioxidant effects
The activity of serum SOD, an antioxidase, was
reduced and the content of serum MDA, a product of lipid
peroxidation, was increased in LPS-treated rats compared with NS
rats (P<0.01; Fig. 2). Taurine
significantly enhanced SOD activity and decreased the concentration
of MDA (P<0.01; Fig. 2).
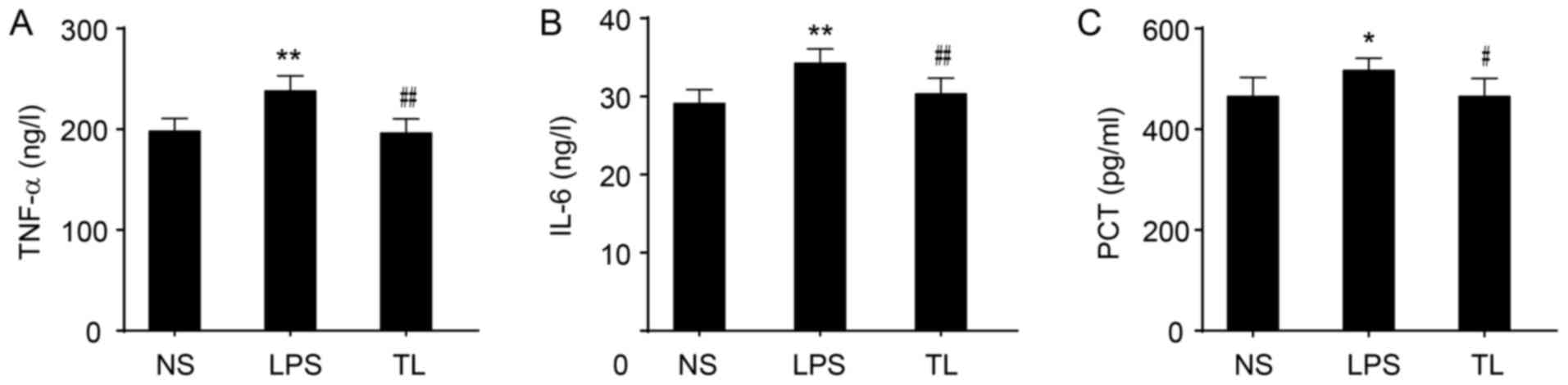
Effects of taurine on pro-inflammatory
cytokines
The levels of TNF-α and IL-6 in serum were
significantly increased in LPS rats (P<0.01; Fig. 3A and B) and an increased level of
PCT was determined (P<0.05; Fig.
3C). Taurine treatment prior to LPS significantly reduced the
levels of TNF-α and IL-6 (P<0.01; Fig. 3A and B) and decreased the
concentration of PCT (P<0.05; Fig.
3C).
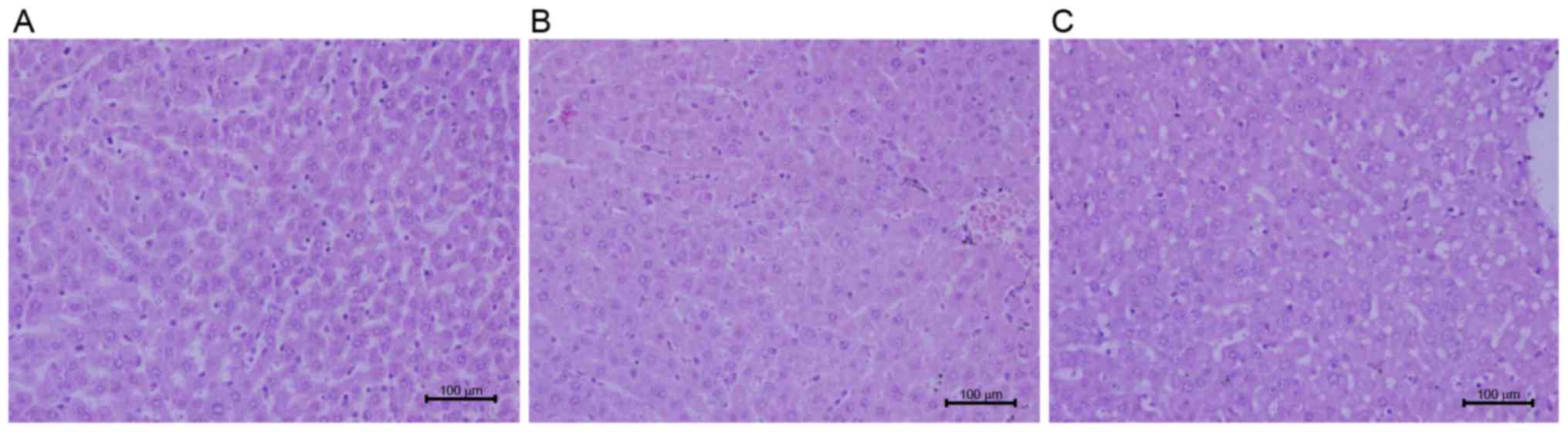
Effects of taurine on hepatic
histopathology
Liver sections stained with H&E were observed
under a light microscope for hepatic morphology. Recruitment of
inflammatory cells and release of inflammatory factors in liver are
involved in liver injury (20).
Exposure to LPS resulted in an increase in the infiltration of
inflammatory cells and hepatocyte edema (Fig. 4). Administration of taurine prior
to LPS attenuated congestion in liver tissues, abated the number of
the infiltration of inflammatory cells and intact lobular structure
was observed (Fig. 4).
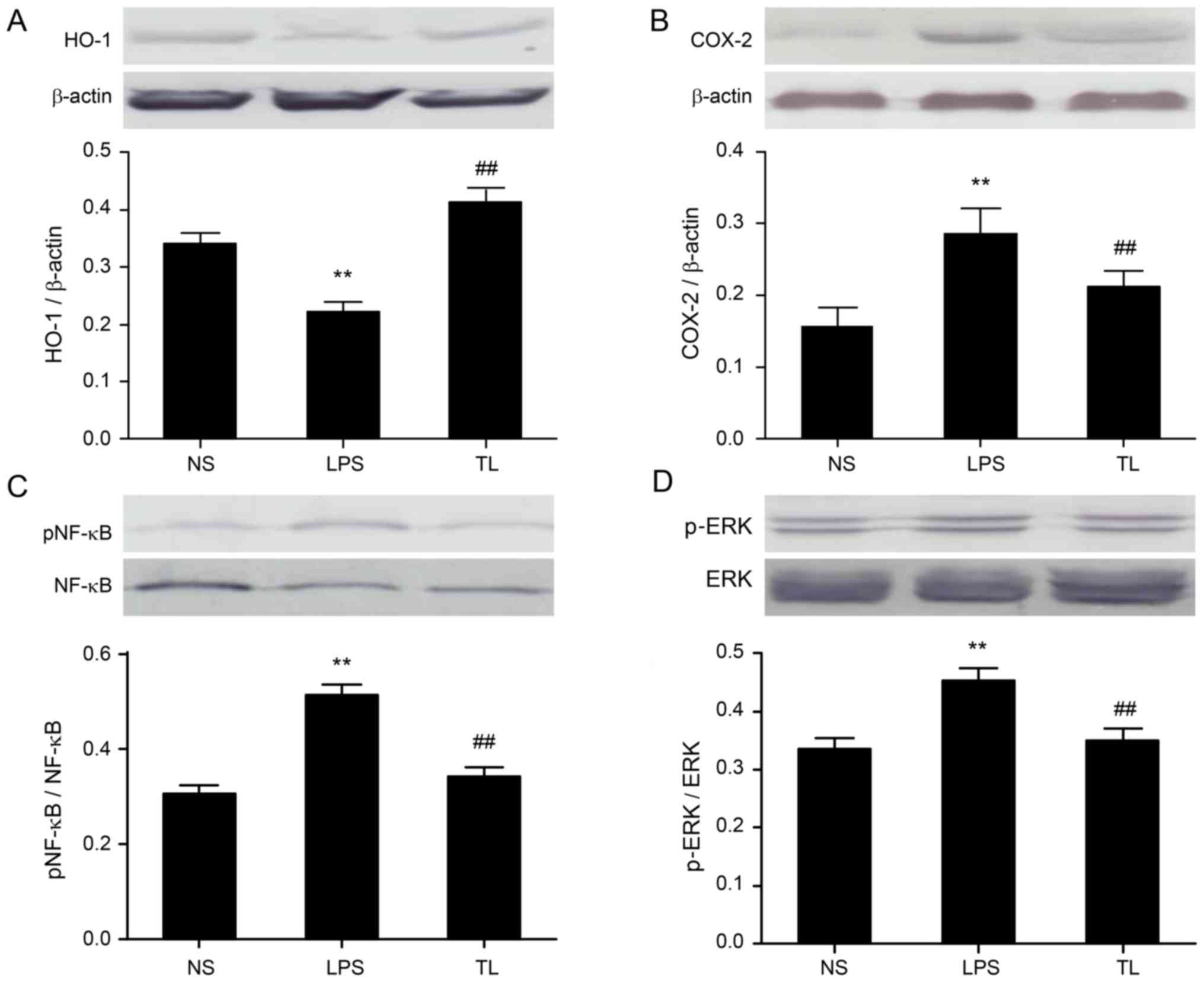
Effects of taurine on liver ERK1/2,
COX-2, HO-1 and NF-κB protein expression
Antioxidant alterations were examined; taurine
pretreatment elevated HO-1 protein expression (Fig. 5A). Furthermore, the protein
expression levels of COX-2 (Fig.
5B), NF-κB (Fig. 5C) and p-ERK
(Fig. 5D) were investigated to
evaluate inflammation signaling. Taurine pretreatment reduced the
protein expression of NF-κB, COX-2 and p-ERK.
Discussion
It is well-known that activation of macrophages and
release of inflammatory cytokines serve important roles in organ
damage, including acute and chronic liver injury (21,22).
The present study aimed to investigate effects of taurine
pretreatment on LPS-induced liver injury. The results demonstrated
that taurine pretreatment by intravenous injection reduced the
activity of plasma AST and ALT, and decreased the level of serum
inflammatory cytokines including TNF-α, IL-6 and MDA. Serum SOD
activity and HO-1 protein expression in liver was significantly
increased in taurine-pretreated rats, while COX-2, pNF-κB (E) and
p-ERK protein expression levels in liver were reduced. In addition,
taurine pretreatment alleviated the infiltration of inflammatory
cells in liver tissues and hepatic congestion. The present study
suggested that taurine pretreatment protected the liver against
LPS-induced injury.
LPS-induced tissue injury results from an increase
in the release of cytokines, oxidative stress and impairment of
mitochondrial function (23). LPS
induces excessive release of pro-inflammatory cytokines including
TNF-α and IL-6 and the production of ROS by binding with Toll-like
receptor 4 on the surface of Küpffer cells (24,25),
stimulating the apoptosis of hepatic cells and necrosis (26).
Oxidative stress results from excessive generation
of ROS and/or deletion of antioxidants including reduced activities
of the antioxidant enzymes, imbalance of glutathione redox status
(27) and increased products of
lipid peroxidation (28), which
damages cells via macromolecules and mitochondrial dysfunction,
further harming various tissues including the liver (7,29).
Release of ROS is a mechanism of LPS-induced hepatic injury,
therefore reduction of ROS signaling relieves such damage (30). Studies have indicated that
treatment with antioxidant and anti-inflammatory agents is
beneficial in LPS-induced hepatic injury (31,32),
and that taurine can reduce oxidative stress (33,34)
and relieve tissue injuries by its antioxidative properties
(35,36). The results of the present study
indicated that administration of LPS resulted increased ALT and AST
activities in serum, considered as markers of liver injury. Taurine
pretreatment reduced the increases of ALT and AST, and decreased
the concentration of MDA, a marker of lipid peroxidation; it also
elevated the activity of SOD and the protein expression of
antioxidant enzyme HO-1.
Inflammatory response serves an important role in
various liver disorders. LPS initiates inflammation by recruiting
neutrophils to the liver and subsequently stimulating the
expression of inflammatory factors, including TNF-α, which provoke
the release of ROS (37). The
results of the present study demonstrated that LPS elevated the
levels of TNF-α and IL-6 in serum and expression of COX-2 and NF-κB
protein. Taurine pretreatment reduced the expression of
pro-inflammatory proteins including COX-2 and NF-κB.
It is reported that taurine is converted into
taurine chloramine in vivo and that this reduces the
inflammatory response (38).
Taurine increases its antioxidative effects by increasing the
expression of HO-1 protein (39),
which is reported to inhibit expression of COX-2 (40). COX-2 induces the production of
prostaglandin, which is involved in inflammation and pain and
results in cellular injury (41,42).
HO-1, an inducible rate-limiting enzyme, catalyzes heme into
equimolar amounts of carbon monoxide (CO), biliverdin and free
iron. Induction of HO-1 may protect against oxidative
stress-related cell and tissue injury (40,43).
Biliverdin has been confirmed to be a potent antioxidant (44). CO, a catalytic product of HO-1,
exerts antioxidative, anti-inflammatory and anti-apoptotic effects
(45). Increasing evidence has
confirmed that the HO/CO signaling exerts a vital role in
regulation of anti-inflammation and cytoprotection (46–48).
NF-κB is involved in LPS signaling. Activation of
NF-κB by LPS upregulates the expression of COX-2 (49). It is reported that the
anti-inflammatory effect of CO is associated with its regulation on
transcription factors including NF-κB (50,51).
A previous study demonstrated that CO alleviates LPS-induced
inflammation by suppression of NF-κB (48). The results suggested that taurine
inhibited NF-κB/COX-2 signaling via induction of HO-1. In addition,
the mitogen-activated protein kinase (MAPK)/ERK pathway is
implicated in LPS-induced inflammation (52) and is an upstream mediator of NF-κB
nuclear translocation (53,54).
Another study demonstrated that reduced MAPK/ERK1/2 signaling
downregulates NF-κB in LPS-activated hepatocytes (54). Recent studies have indicated that
increased levels of phosphorylated ERK and c-Jun N-terminal kinases
promote COX-2 protein expression (55,56).
In conclusion, the results of the present study
indicated that taurine pretreatment protected the liver against
LPS-induced injury by increasing its antioxidation and
anti-inflammation ability, which were associated with the increased
expression of HO-1 protein and reduced expression levels of NF-κB,
COX-2 and p-ERK proteins. The findings suggested that taurine
reduced NF-κB/COX-2 signaling by activation of HO-1/CO.
Acknowledgements
The present study was supported by the Outstanding
Young Talent Fund Key Project of Anhui Province (grant no.
2013SQRL146ZD).
References
|
1
|
Mizuhara H, O'Neill E, Seki N, Ogawa T,
Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H and Fujiwara H: T
cell activation-associated hepatic injury: Mediation by tumor
necrosis factors and protection by interleukin 6. J Exp Med.
179:1529–1537. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gasparini C and Feldmann M: NF-κB as a
target for modulating inflammatory responses. Curr Pharm Des.
18:5735–5745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Josephs MD, Bahjat FR, Fukuzuka K,
Ksontini R, Solorzano CC, Edwards CK III, Tannahill CL, MacKay SL,
Copeland EM III and Moldawer LL: Lipopolysaccharide and
D-galactosamine-induced hepatic injury is mediated by TNF-alpha and
not by Fas ligand. Am J Physiol Regul Integr Comp Physiol.
278:R1196–R1201. 2000.PubMed/NCBI
|
|
4
|
Enomoto N, Ikejima K, Bradford BU, Rivera
CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, et
al: Role of Kupffer cells and gut-derived endotoxins in alcoholic
liver injury. J Gastroenterol Hepatol. 15 Suppl:D20–D25. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uesugi T, Froh M, Arteel GE, Bradford BU,
Wheeler MD, Gäbele E, Isayama F and Thurman RG: Role of
lipopolysaccharide-binding protein in early alcohol-induced liver
injury in mice. J Immunol. 168:2963–2969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enomoto N, Schemmer P, Ikejima K, Takei Y,
Sato N, Brenner DA and Thurman RG: Long-term alcohol exposure
changes sensitivity of rat Kupffer cells to lipopolysaccharide.
Alcohol Clin Exp Res. 25:1360–1367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cadenas S and Cadenas AM: Fighting the
stranger-antioxidant protection against endotoxin toxicity.
Toxicology. 180:45–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mallis RJ, Buss JE and Thomas JA:
Oxidative modification of H-ras: S-thiolation and S-nitrosylation
of reactive cysteines. Biochem J. 355:145–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuo Y, Xiang B, Yang J, Sun X, Wang Y,
Cang H and Yi J: Oxidative modification of caspase-9 facilitates
its activation via disulfide-mediated interaction with Apaf-1. Cell
Res. 19:449–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colletti LM and Green M: Lung and liver
injury following hepatic ischemia/reperfusion in the rat is
increased by exogenous lipopolysaccharide which also increases
hepatic TNF production in vivo and in vitro. Shock. 16:312–319.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong
W, Tang L, Lin Y, He YQ, Zou SS, et al: Endotoxin accumulation
prevents carcinogen-induced apoptosis and promotes liver
tumorigenesis in rodents. Hepatology. 52:1322–1333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992.PubMed/NCBI
|
|
13
|
Pasantes-Morales H, Wright CE and Gaull
GE: Taurine protection of lymphoblastoid cells from iron-ascorbate
induced damage. Biochem Pharmacol. 34:2205–2207. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maia AR, Batista TM, Victorio JA, Clerici
SP, Delbin MA, Carneiro EM and Davel AP: Taurine supplementation
reduces blood pressure and prevents endothelial dysfunction and
oxidative stress in post-weaning protein-restricted rats. PLoS One.
9:e1058512014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Jang J, Piao S, Cha YN and Kim C:
Taurine chloramine activates Nrf2, increases HO-1 expression and
protects cells from death caused by hydrogen peroxide. J Clin
Biochem Nutr. 45:37–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaffer SW, Azuma J and Mozaffari M: Role
of antioxidant activity of taurine in diabetes. Can J Physiol
Pharmacol. 87:91–99. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erdamar H, Turközkan N, Ekremoğlu M, Kurt
Y and Yaman H: The effect of taurine on polymorphonuclear leukocyte
functions in endotoxemia. Amino Acids. 33:581–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kontny E, Plebanczyk M, Lisowska B,
Olszewska M, Maldyk P and Maslinski W: Comparison of rheumatoid
articular adipose and synovial tissue reactivity to proinflammatory
stimuli: Contribution to adipocytokine network. Ann Rheum Dis.
71:262–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marcinkiewicz J and Kontny E: Taurine and
inflammatory diseases. Amino Acids. 46:7–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ambade A, Catalano D, Lim A and Mandrekar
P: Inhibition of heat shock protein (molecular weight 90 kDa)
attenuates proinflammatory cytokines and prevents
lipopolysaccharide-induced liver injury in mice. Hepatology.
55:1585–1595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rossignol DP and Lynn M: TLR4 antagonists
for endotoxemia and beyond. Curr Opin Investig Drugs. 6:496–502.
2005.PubMed/NCBI
|
|
22
|
Nolan JP: The role of intestinal endotoxin
in liver injury: A long and evolving history. Hepatology.
52:1829–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lowes DA, Webster NR, Murphy MP and Galley
HF: Antioxidants that protect mitochondria reduce interleukin-6 and
oxidative stress, improve mitochondrial function, and reduce
biochemical markers of organ dysfunction in a rat model of acute
sepsis. Br J Anaesth. 110:472–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun S, Zhang H, Xue B, Wu Y, Wang J, Yin Z
and Luo L: Protective effect of glutathione against
lipopolysaccharide-induced inflammation and mortality in rats.
Inflamm Res. 55:504–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohsaki Y, Shirakawa H, Hiwatashi K,
Furukawa Y, Mizutani T and Komai M: Vitamin K suppresses
lipopolysaccharide-induced inflammation in the rat. Biosci
Biotechnol Biochem. 70:926–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Gao LN, Cui YL and Jiang HL:
Protective effect of danhong injection on acute hepatic failure
induced by lipopolysaccharide and d-galactosamine in mice. Evid
Based Complement Alternat Med. 2014:1539022014.PubMed/NCBI
|
|
27
|
Davies KJ: Protein damage and degradation
by oxygen radicals. I. General aspects. J Biol Chem. 262:9895–9901.
1987.PubMed/NCBI
|
|
28
|
Klein T, Neuhaus K, Reutter F and Nüsing
RM: Generation of 8-epi-prostaglandin F(2alpha) in isolated rat
kidney glomeruli by a radical-independent mechanism. Br J
Pharmacol. 133:643–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sewerynek E, Melchiorri D, Chen L and
Reiter RJ: Melatonin reduces both basal and bacterial
lipopolysaccharide-induced lipid peroxidation in vitro. Free Radic
Biol Med. 19:903–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsing CH, Lin MC, Choi PC, Huang WC, Kai
JI, Tsai CC, Cheng YL, Hsieh CY, Wang CY, Chang YP, et al:
Anesthetic propofol reduces endotoxic inflammation by inhibiting
reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS
One. 6:e175982011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ajuwon OR, Oguntibeju OO and Marnewick JL:
Amelioration of lipopolysaccharide-induced liver injury by aqueous
rooibos (Aspalathus linearis) extract via inhibition of
pro-inflammatory cytokines and oxidative stress. BMC Complement
Altern Med. 14:3922014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takata J, Ito S, Karube Y, Nagata Y and
Matsushima Y: Water-soluble prodrug of vitamin E for parenteral use
and its effect on endotoxin-induced liver toxicity. Biol Pharm
Bull. 20:204–209. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliveira MW, Minotto JB, de Oliveira MR,
Zanotto-Filho A, Behr GA, Rocha RF, Moreira JC and Klamt F:
Scavenging and antioxidant potential of physiological taurine
concentrations against different reactive oxygen/nitrogen species.
Pharmacol Rep. 62:185–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeon SH, Lee MY, Rahman MM, Kim SJ, Kim
GB, Park SY, Hong CU, Kim SZ, Kim JS and Kang HS: The antioxidant,
taurine reduced lipopolysaccharide (LPS)-induced generation of ROS,
and activation of MAPKs and Bax in cultured pneumocytes. Pulm
Pharmacol Ther. 22:562–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Motawi TK, Abd Elgawad HM and Shahin NN:
Modulation of indomethacin-induced gastric injury by spermine and
taurine in rats. J Biochem Mol Toxicol. 21:280–288. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimizu M, Zhao Z, Ishimoto Y and Satsu H:
Dietary taurine attenuates dextran sulfate sodium (DSS)-induced
experimental colitis in mice. Adv Exp Med Biol. 643:265–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McDonald B, Jenne CN, Zhuo L, Kimata K and
Kubes P: Kupffer cells and activation of endothelial TLR4
coordinate neutrophil adhesion within liver sinusoids during
endotoxemia. Am J Physiol Gastrointest Liver Physiol.
305:G797–G806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weiss SJ, Klein R, Slivka A and Wei M:
Chlorination of taurine by human neutrophils. Evidence for
hypochlorous acid generation. J Clin Invest. 70:598–607. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang GG, Li W, Lu XH, Zhao X and Xu L:
Taurine attenuates oxidative stress and alleviates cardiac failure
in type I diabetic rats. Croat Med J. 54:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shih RH and Yang CM: Induction of heme
oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2
expression in mouse brain endothelial cells. J Neuroinflammation.
7:862010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fletcher JR: Eicosanoids. Critical agents
in the physiological process and cellular injury. Arch Surg.
128:1192–1196. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Williams JA and Shacter E: Regulation of
macrophage cytokine production by prostaglandin E2. Distinct roles
of cyclooxygenase-1 and −2. J Biol Chem. 272:25693–25699. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen QY, Wang GG, Li W, Jiang YX, Lu XH
and Zhou PP: Heme oxygenase-1 promotes delayed wound healing in
diabetic rats. J Diabetes Res. 2016:97265032016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stocker R, Yamamoto Y, McDonagh AF, Glazer
AN and Ames BN: Bilirubin is an antioxidant of possible
physiological importance. Science. 235:1043–1046. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ryter SW, Otterbein LE, Morse D and Choi
AM: Heme oxygenase/carbon monoxide signaling pathways: Regulation
and functional significance. Mol Cell Biochem 234–235. 1–263.
2002.
|
|
47
|
Wang XM, Kim HP, Nakahira K, Ryter SW and
Choi AM: The heme oxygenase-1/carbon monoxide pathway suppresses
TLR4 signaling by regulating the interaction of TLR4 with
caveolin-1. J Immunol. 182:3809–3818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chhikara M, Wang S, Kern SJ, Ferreyra GA,
Barb JJ, Munson PJ and Danner RL: Carbon monoxide blocks
lipopolysaccharide-induced gene expression by interfering with
proximal TLR4 to NF-kappaB signal transduction in human monocytes.
PLoS One. 4:e81392009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakao S, Ogata Y, Shimizu-Sasaki E,
Yamazaki M, Furuyama S and Sugiya H: Activation of NFkappaB is
necessary for IL-1beta-induced cyclooxygenase-2 (COX-2) expression
in human gingival fibroblasts. Mol Cell Biochem. 209:113–118. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morse D, Pischke SE, Zhou Z, Davis RJ,
Flavell RA, Loop T, Otterbein SL, Otterbein LE and Choi AM:
Suppression of inflammatory cytokine production by carbon monoxide
involves the JNK pathway and AP-1. J Biol Chem. 278:36993–36998.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sarady JK, Otterbein SL, Liu F, Otterbein
LE and Choi AM: Carbon monoxide modulates endotoxin-induced
production of granulocyte macrophage colony-stimulating factor in
macrophages. Am J Respir Cell Mol Biol. 27:739–745. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang SI, Kim HJ, Kim YJ, Jeong SI and You
YO: Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW
264.7 cells: Possible involvement of the NIK-IKK, ERK1/2, p38 and
JNK pathways. Eur J Pharmacol. 542:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chiu WT, Lin YL, Chou CW and Chen RM:
Propofol inhibits lipoteichoic acid-induced iNOS gene expression in
macrophages possibly through downregulation of toll-like receptor
2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB. Chem Biol
Interact. 181:430–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jawan B, Kao YH, Goto S, Pan MC, Lin YC,
Hsu LW, Nakano T, Lai CY, Sun CK, Cheng YF, et al: Propofol
pretreatment attenuates LPS-induced granulocyte-macrophage
colony-stimulating factor production in cultured hepatocytes by
suppressing MAPK/ERK activity and NF-kappaB translocation. Toxicol
Appl Pharmacol. 229:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen WC, Tseng CK, Chen YH, Lin CK, Hsu
SH, Wang SN and Lee JC: HCV NS5A Up-regulates COX-2 expression via
IL-8-mediated activation of the ERK/JNK MAPK pathway. PLoS One.
10:e01332642015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gu W, Song L, Li XM, Wang D, Guo XJ and Xu
WG: Mesenchymal stem cells alleviate airway inflammation and
emphysema in COPD through down-regulation of cyclooxygenase-2 via
p38 and ERK MAPK pathways. Sci Rep. 5:87332015. View Article : Google Scholar : PubMed/NCBI
|