Introduction
Osteoporosis, which usually leads to bone fractures
due to fragility, is a major public health problem, especially in
the elderly population. Osteoporosis is generally characterized by
low bone mass and microarchitectural deterioration of bone tissue
(1) as a result of metabolic bone
disorders. Studies focusing on the etiology, prevention, and
treatment of osteoporosis have been widely conducted, and
significant progress has been made during the last two decades
(2–8). A number of key effectors such as
NF-κB, RANKL, Wnt5a/Ror2, and PPARβ/δ have been identified to be
pivotal in the regulation of bone metabolism (9–12).
On the other hand, bone is a highly vascularized
tissue, and blood vessels participate in bone development,
remodeling, and homeostasis (13).
Bone vasculature not only supplies bone tissue with oxygen,
nutrients, and growth factors but also removes waste products and
delivers osteoprogenitors to fracture sites (14,15).
Activation of the hypoxia-inducible factor (HIF) pathway can
accelerate bone regeneration and improve bone healing in
vitro and in vivo (16). This suggests intercellular
signaling between osteoprogenitors and endothelial precursor cells,
which has been also termed as a coupling of osteogenesis and
angiogenesis (17,18). Recently, a distinct capillary
subtype termed the type H vessel was found in murine bone
metaphysis and periosteum, which couples osteogenesis and
angiogenesis. Type H vessels, strongly positive for endothelial
cell surface markers, CD31 and Endomucin
(CD31hiEmcnhi), can mediate local bone
vascular growth and also maintain perivascular osteoprogenitors
(19).
Desferrioxamine (DFO) can promote the
differentiation of mesenchymal stem cells (MSCs) by activating a
β-catenin signaling pathway (20).
DFO also enhances normal fracture healing when injected locally
into a fracture callus by increasing endothelial tubule formation
(21). Recent reports also
indicated that DFO administration can prevent age-dependent loss of
type H vessels thus to improve the bone quality in aged mice
(19). Given all these facts, we
propose that DFO treatment theoretically have potential to activate
angiogenesis thus to help maintain bone mass, which would be
especially beneficial to prevent or treat osteoporosis. To verify
the hypothesis, ovariectomized (OVX) mice were used as animal model
here in this study to investigate the effect of DFO on OVX-induced
osteoporosis.
Materials and methods
Mice
The C57BL/6 mice used in this study were provided by
National Resource Center of Model Mice of Nanjing University and
housed in the specific pathogen free barrier system in the animal
facility of Soochow University. All animal-related study was
performed in compliance with the relevant laws and internal
guidelines of the Ethics Committee of the Second Affiliated
Hospital of Soochow University (approval no. sdfey160215). All
surgery was performed under avertin anesthesia and all efforts were
made to minimize suffering.
Osteoporotic mouse model
Eight-week-old female mice were randomly divided
into 3 groups. All mice were anesthetized and subjected to
bilateral OVX or a sham operation. After a 1-week recovery period,
the sham group mice were administrated intraperitoneal saline (sham
group) while the OVX mice were administered saline (OVX group) or
DFO (OVX + DFO group) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). For DFO treatment, freshly prepared DFO in 0.9% saline
[15 mg/ml per mouse as previous reported (19)] was administrated every other day
for 4 weeks. Mouse tissues were collected at week 1, 2, 3 and 4 for
investigation of vascular and osseous changes by immunostaining,
hematoxylin and eosin staining, and micro-computed tomography
(micro-CT) scanning.
Micro-CT analysis
Mouse femora and lumbar vertebrae were dissected at
several points in time, and any attached soft tissue was completely
removed. Collected bone specimens were then fixed in 4%
paraformaldehyde (PFA) and analyzed by micro-CT (SkyScan 1176;
Kontich, Belgium). The scanners were set at a voltage of 50 kV, a
current of 500 µA, and a resolution of 9 µm. Image software (NRecon
v1.6) and data analysis software (CTAn v1.13.11.0) were used for
three-dimensional (3D) reconstruction of the trabecular bone.
After scanning, regions of interest (ROI) were
defined by analysis software and limited to the trabecular regions.
3D trabecular bone images were reconstructed based on the ROI.
Mouse femoral ROI were drawn starting from 540 µm proximally to the
distal growth plate over 1.35 mm toward the diaphysis. Lumbar
vertebra ROI was drawn in the middle trabecular area with 2 mm of
thickness. Trabecular bone parameters were calculated, including
BMD (g/cm3), BV/TV (%), Tb.Th (mm), Tb.N (per mm) and
Tb.Sp (mm).
Bone tissue processing and
immunostaining
For staining of bone sections, freshly collected
specimens were fixed in 4% PFA overnight at 4°C.
Ethylenediaminetetraacetic acid solution was then applied for
decalcification for at least 4 weeks. Tissues were then dehydrated
using 20% sucrose and 2% polyvinylpyrrolidone solution for 2 weeks
at 4°C and embedded in O.C.T. compound (Tissue-Tek). Cryosections
(10 µm) were prepared using a freezing microtome (Leica, Wetzlar,
Germany) for immunostaining.
For immunostaining, bone sections were washed 3
times with 0.3% phosphate-buffered saline with Triton X-100 (PBST)
and blocked with 5% bovine serum albumin in PBST. Sections were
incubated with primary antibodies at 4°C overnight. After washing 3
times with 0.3% PBST, sections were then incubated with fluorescein
conjugated secondary antibodies together with nuclear
counterstaining dye (DAPI) at room temperature. After another 3
thorough washes, slides were sealed with 50% glycerol and nail
polish.
Antibodies
Primary antibodies used include rabbit-anti-mouse
osterix (sc-22536-R) and rat-anti-mouse endomucin (sc-65495) (both
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and Alexa
Fluor 488-conjugated anti-mouse CD31 (FAB3628 G; R&D Systems,
Inc., Minneapolis, MN, USA). Cy3- or Alexa 647-conjugated secondary
antibodies (Molecular Probes, Eugene, OR, USA) were used for
visualization.
Statistical analysis
All data are expressed as mean ± SD. Statistical
analyses were carried out using one-way analysis of variance with
Student-Newman-Keuls post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference (P<0.01; P<0.0001). All statistical analyses were
performed using SAS 8.2 software (SAS Institute Inc., Cary, NC,
USA).
Results
Partial recovery of
ovariectomy-induced osteoporosis in mouse femora by DFO
administration
OVX female mice are frequently used as animal models
for osteoporosis studies. In this study, we also used OVX female
mice to investigate the detailed etiology and the therapeutic
effect of DFO on osteoporosis. All mice were anesthetized and
subjected to bilateral OVX or a sham operation (sham). After
recovery, the Sham group mice were administrated with saline
intraperitoneally (sham group), while the OVX mice were
administered with saline (OVX group) or deferoxamine mesylate (OVX
+ DFO group; 15 mg/ml per mouse) every other day for 4 weeks. Mice
were sacrificed at week 1, 2, 3 and 4 for µCT and histological
evaluation. Based on µCT and quantification results, OVX induced
remarkable reduction of bone mineral density (BMD) in mice femora,
beginning at the first week. As shown in the reconstructed µCT
images, the microarchitecture of the femora in OVX mice at week 1
was already significantly impaired (Fig. 1A and B). On the other hand, mice
treated with deferoxamine (OVX + DFO group) showed slower reduction
of BMD. The OVX + DFO group mice maintained comparable femoral BMD
with the sham group at week 1, which began to decrease at week 2
and reached a similar BMD to the OVX group at week 3 (Fig. 1C). Quantification of BMD and other
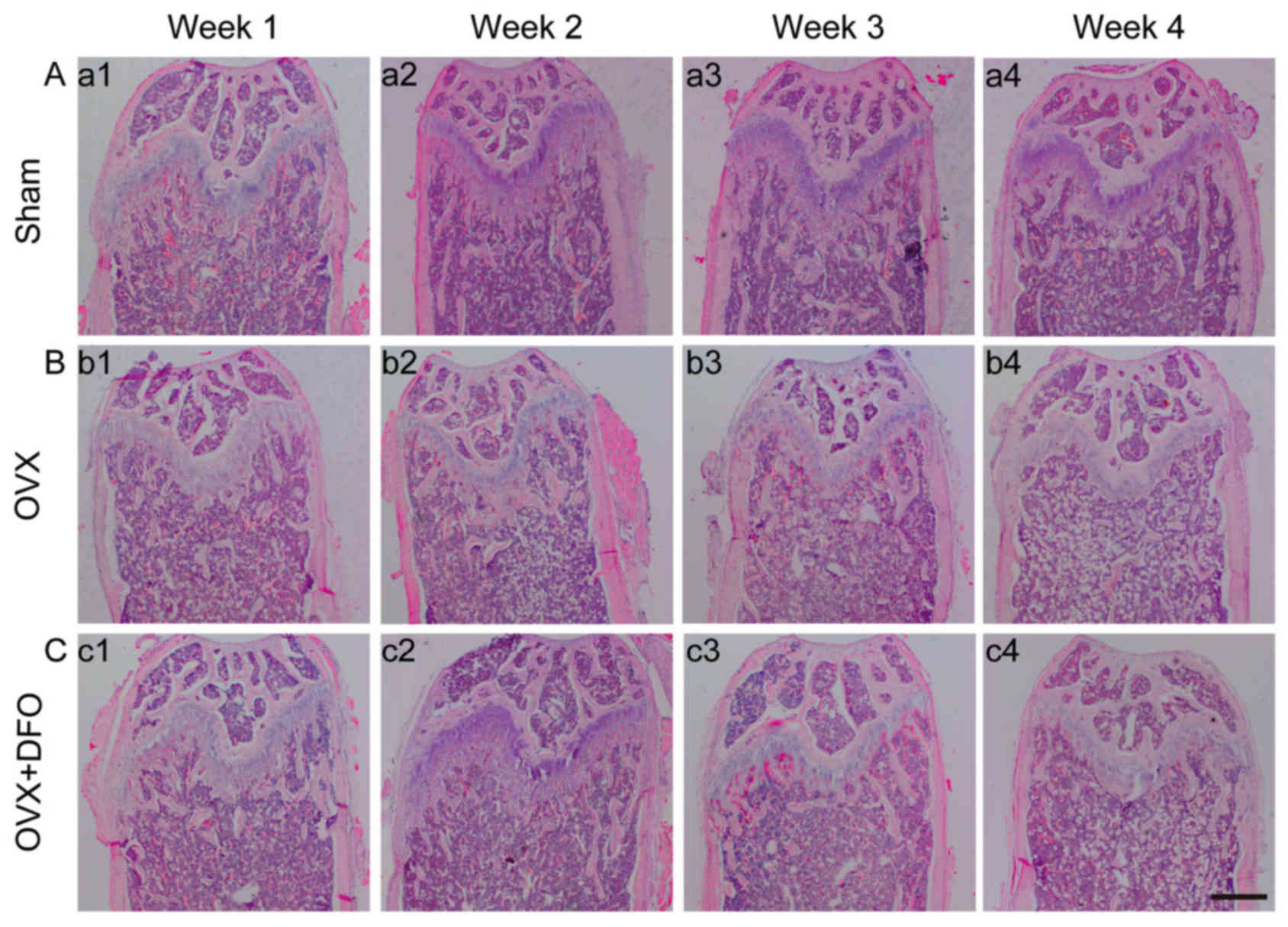
parameters also showed consistent results (Fig. 1D-H; Table I). In addition, the
histomorphological changes of the distal femora were evaluated by
hematoxylin and eosin staining. OVX mice showed less trabecular
density at the distal ends of the femora compared to the sham
group, and DFO treatment attenuated this effect at week 1, which
showed similar results comparable with µCT scanning (Fig. 2). The change of bone density in the
OVX + DFO group indicated a temporary protective effect of DFO
against OVX-induced osteoporosis.
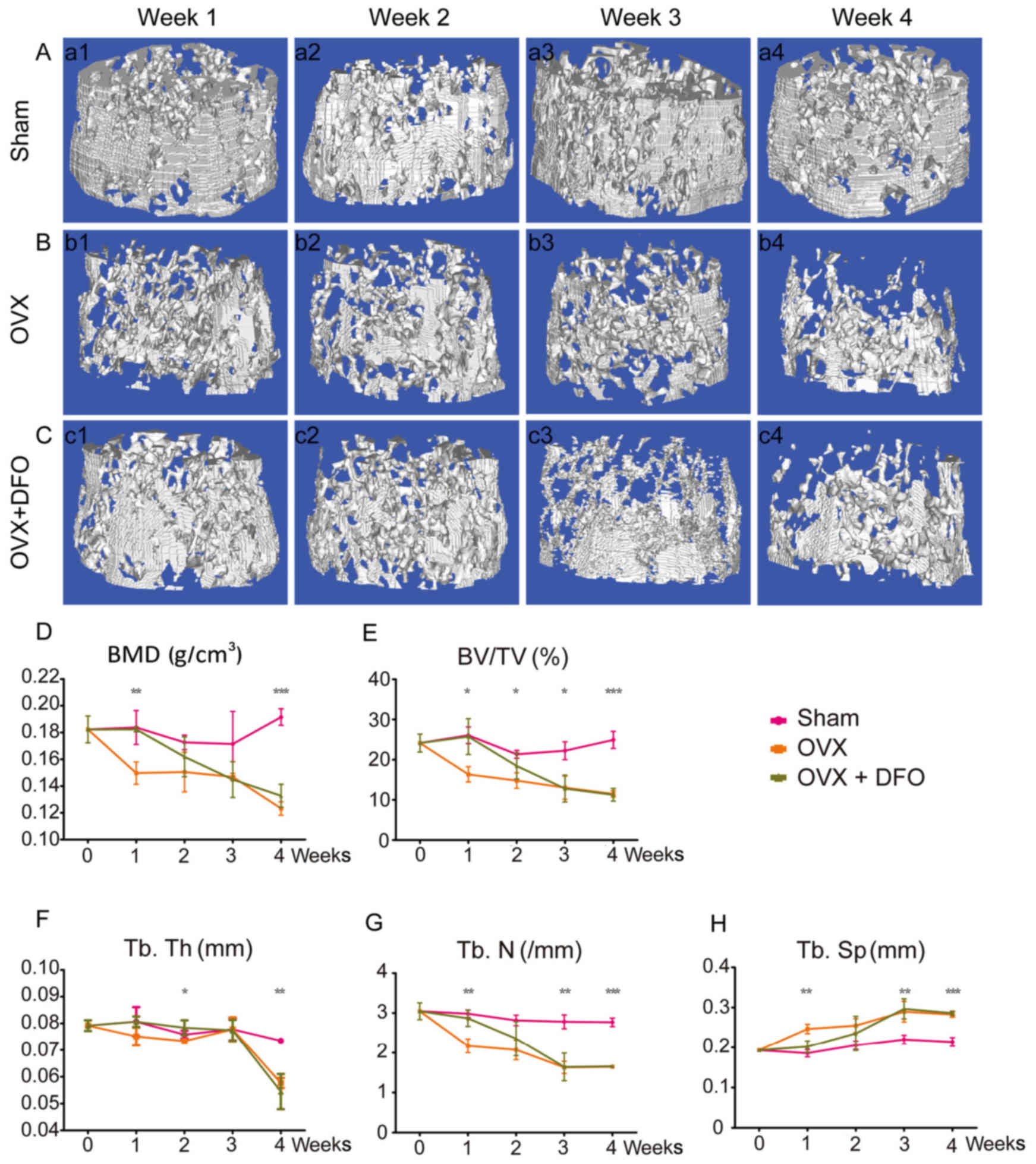 | Figure 1.Micro-CT and quantification of bone
microarchitecture in distal femur. (A-C) 3D micro-CT reconstructed
images of the distal femora from sham group (A-a1-a4), OVX group
(B-b1-b4) and OVX + DFO group (C-c1-c4) at different time-points
(week 1, 2, 3 and 4). (D-H) Quantification of BMD (D), BV/TV (E),
Tb.Th (F), Tb.N (G) and Tb.Sp (H) from the sham, OVX and OVX + DFO
mice. *P<0.05; **P<0.01; ***P<0.0001. Micro-CT,
micro-computed tomography; OVX, ovariectomy; DFO, desferrioxamine;
BMD, bone mineral density; BV/TV, bone volume/tissue volume; Tb.Th,
trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular
separation. |
 | Table I.BMD and trabecular microarchitecture
of femur at different time-points. |
Table I.
BMD and trabecular microarchitecture
of femur at different time-points.
| Parameters | Group | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 |
|---|
| BMD
(g/cm3) | Sham |
0.182±0.010 |
0.184±0.013b |
0.173±0.005 |
0.171±0.024 |
0.192±0.006b |
|
| OVX |
0.182±0.010 |
0.149±0.008a |
0.150±0.014 |
0.147±0.002 |
0.123±0.005a |
|
| OVX + DFO |
0.182±0.010 |
0.182±0.002b |
0.162±0.015 |
0.145±0.013 |
0.133±0.008a |
| BV/TV (%) | Sham |
24.135±2.221 |
26.054±2.055b |
21.332±0.967b |
22.213±2.209b |
24.919±2.114b |
|
| OVX |
24.135±2.221 |
16.345±1.889a |
14.798±1.929a |
13.025±2.879a |
11.467±0.793a |
|
| OVX + DFO |
24.135±2.221 |
25.746±4.458b |
18.438±3.256 |
12.805±3.333a |
11.247±1.564a |
| Tb.Th (mm) | Sham |
0.079±0.002 |
0.081±0.005 |
0.076±0.001 |
0.078±0.004 |
0.073±0.000b |
|
| OVX |
0.079±0.002 |
0.075±0.003 |
0.073±0.000 |
0.078±0.004 |
0.058±0.002a |
|
| OVX + DFO |
0.079±0.002 |
0.081±0.002 |
0.078±0.003b |
0.077±0.004 |
0.054±0.007a |
| Tb.N (/mm) | Sham |
3.045±0.210 |
2.985±0.096b |
2.815±0.131 |
2.781±0.172b |
2.768±0.104b |
|
| OVX |
3.045±0.210 |
2.172±0.173a |
2.071±0.248 |
1.631±0.154a |
1.646±0.037a |
|
| OVX + DFO |
3.045±0.211 |
2.868±0.203b |
2.355±0.428 |
1.643±0.347a |
1.659±0.020a |
| Tb.Sp (mm) | Sham |
0.193±0.004 |
0.186±0.009b |
0.206±0.010 |
0.219±0.011b |
0.214±0.011b |
|
| OVX |
0.193±0.005 |
0.247±0.012a |
0.255±0.017 |
0.289±0.026a |
0.283±0.006a |
|
| OVX + DFO |
0.193±0.005 |
0.202±0.014b |
0.235±0.042 |
0.297±0.025a |
0.286±0.005a |
Differential effect of DFO in the
vertebrae of OVX mice
As indicated by a slight decline in BMD, OVX also
induced osteoporosis in mouse lumbar vertebrae similar to that in
the femur (Fig. 3A and B). The BMD
and trabecular microarchitecture of lumbar vertebrae remarkably
deteriorated in OVX mice compared to the sham group, which also
began from week 1 but significantly changed at week 3. However, DFO
failed to protect the bone mass loss induced by OVX (Fig. 3C). The lumbar vertebrae BMD and
microarchitecture of the OVX + DFO group mice also began to
decrease at week 1, with a similar trend to the OVX group mice
(Fig. 3D-H; Table II). The BMD and microarchitecture
results of the OVX + DFO group are similar to that of the OVX
group, suggesting that DFO at this dose had no effect at weeks
3–4.
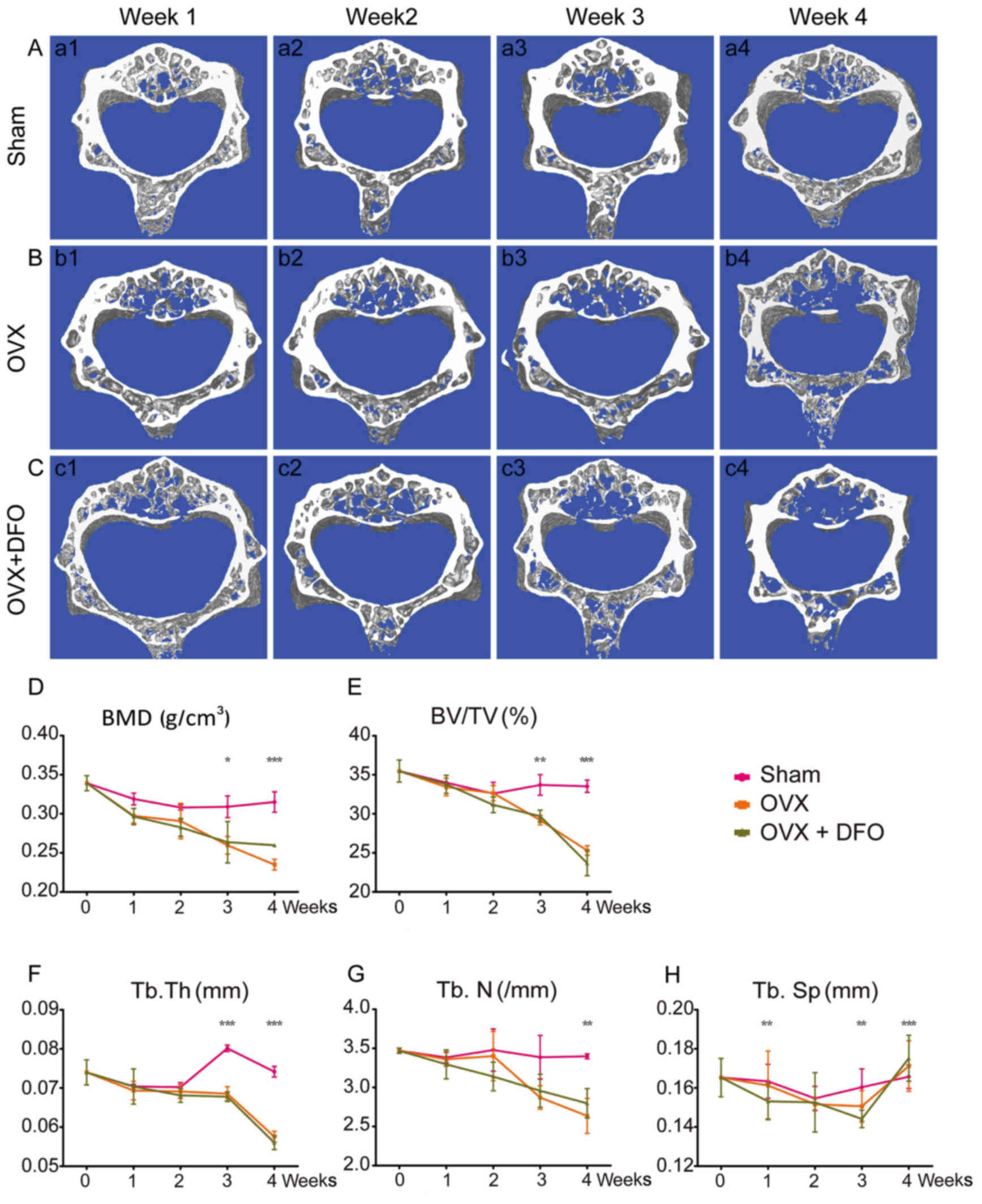 | Figure 3.Micro-CT and quantification of bone
microarchitecture in the lumbar vertebra. (A-C) 3D micro-CT
reconstructed images of the lumbar vertebrae from sham group
(A-a1-a4), OVX group (B-b1-b4) and OVX + DFO group (C-c1-c4) at 4
time-points. (D-H) Quantification of BMD (D), BV/TV (E), Tb.Th (F),
Tb.N (G) and Tb.Sp (H) from the sham, OVX and OVX + DFO mice.
*P<0.05; **P<0.01; ***P<0.0001. Micro-CT, micro-computed
tomography; OVX, ovariectomy; DFO, desferrioxamine; BMD, bone
mineral density; BV/TV, bone volume/tissue volume; Tb.Th,
trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular
separation. |
 | Table II.BMD and trabecular microarchitecture
of lumbar at different time-points. |
Table II.
BMD and trabecular microarchitecture
of lumbar at different time-points.
| Parameters | Group | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 |
|---|
| BMD
(g/cm3) | Sham |
0.339±0.010 |
0.319±0.007 |
0.308±0.003 |
0.309±0.014b |
0.315±0.013b |
|
| OVX |
0.339±0.010 |
0.297±0.009 |
0.291±0.023 |
0.260±0.011a |
0.235±0.007a |
|
| OVX + DFO |
0.339±0.010 |
0.297±0.010 |
0.283±0.011 |
0.264±0.027a |
0.260±0.002a,b |
| BV/TV (%) | Sham |
35.472±1.409 |
33.994±0.217 |
32.583±1.452 |
33.693±1.314b |
33.526±0.788b |
|
| OVX |
35.472±1.409 |
33.431±1.126 |
32.642±0.972 |
29.180±0.603a |
25.301±0.610a |
|
| OVX + DFO |
35.472±1.409 |
33.775±1.163 |
31.146±1.002 |
29.696±0.772a |
23.684±1.609a |
| Tb.Th (mm) | Sham |
0.074±0.003 |
0.071±0.000 |
0.070±0.001 |
0.080±0.000b |
0.074±0.001b |
|
| OVX |
0.074±0.003 |
0.069±0.002 |
0.069±0.002 |
0.069±0.002a |
0.058±0.001a |
|
| OVX + DFO |
0.074±0.003 |
0.070±0.004 |
0.068±0.002 |
0.068±0.001* |
0.056±0.002* |
| Tb.N (/mm) | Sham |
3.469±0.033 |
3.384±0.069 |
3.479±0.270 |
3.387±0.278 |
3.398±0.032b |
|
| OVX |
3.469±0.033 |
3.360±0.087 |
3.401±0.314 |
2.871±0.150 |
2.636±0.224a |
|
| OVX + DFO |
3.469±0.033 |
3.295±0.184 |
3.140±0.183 |
2.957±0.211 |
2.797±0.189a |
| Tb.Sp (mm) | Sham |
0.165±0.009 |
0.163±0.008b |
0.155±0.006 |
0.160±0.009b |
0.166±0.006b |
|
| OVX |
0.165±0.010 |
0.161±0.018a |
0.152±0.003 |
0.151±0.008a |
0.171±0.013a |
|
| OVX + DFO |
0.165±0.010 |
0.153±0.009b |
0.153±0.015 |
0.144±0.004a |
0.175±0.012a |
Vascular change correlated with
osseous changes in the osteoporotic model
As reported, blood vasculature is essential during
bone mass maintenance and fracture repair (13–15).
A recent study characterized a distinct subtype of blood vessel,
the type H vessel, which plays a pivotal role in combining
angiogenesis and osteogenesis (19). Herein, we tried to investigate the
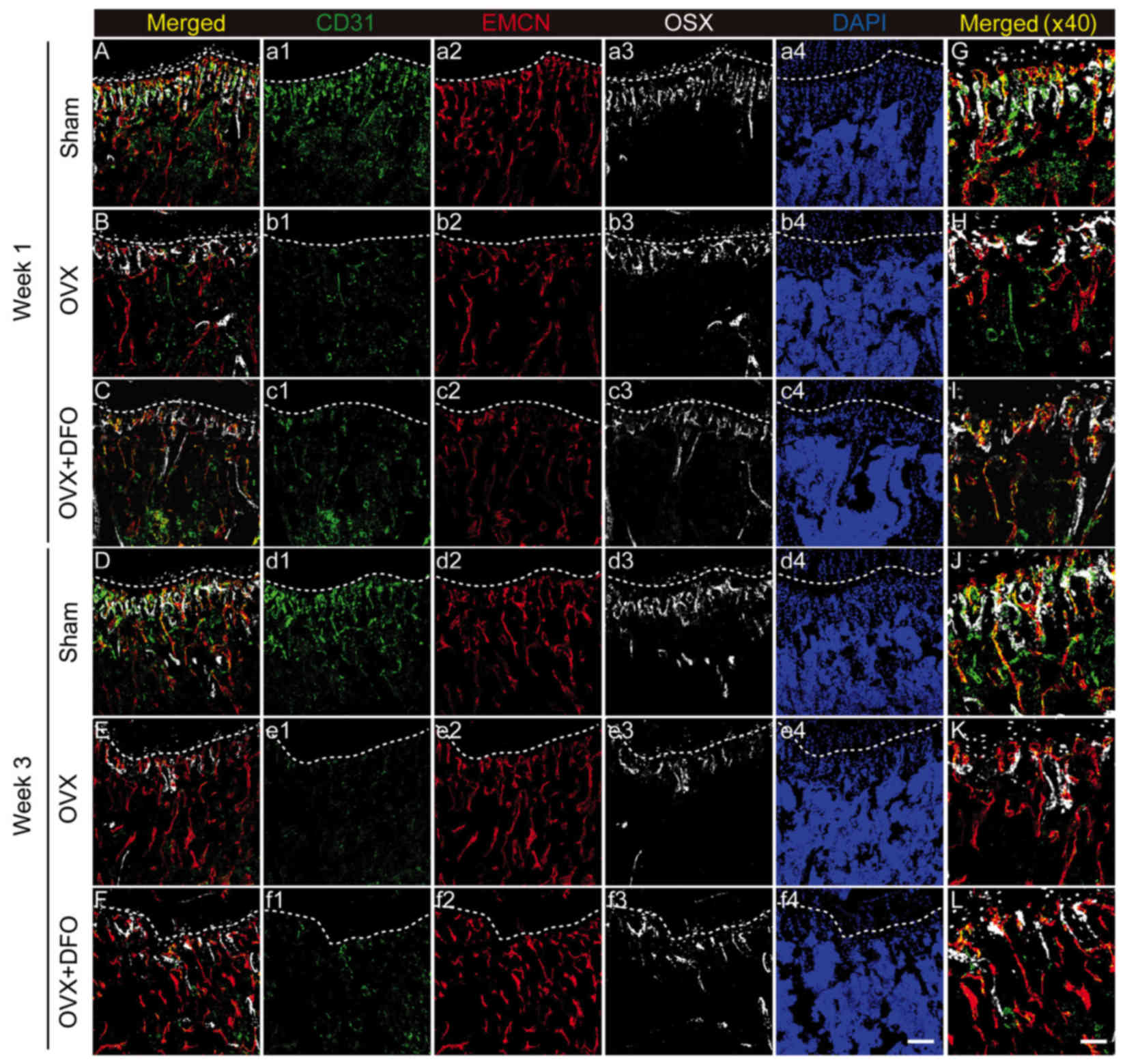
vascular changes during our experiment at week 1 and 3. By
immunostaining, we observed that the vascular changes in the tibia
also showed a similar trend to the bone mass changes (Fig. 4A-L).
CD31+Emcn+ type H blood vessels in OVX mice
decreased dramatically at week 1 (Fig.
4B and H), while the OVX + DFO group still maintained a small
portion of type H vessels (Fig. 4C and
I). However, type H vessels were rarely observed in either the
OVX or OVX + DFO groups at week 3 after OVX (Fig. 4E, F, K and L), suggesting that DFO
failed to increase the number of type H vessels at the dose
administered. Correlation between vascular and osseous changes
implies that blood vessels may be a potential target for
osteoporosis therapy.
Discussion
In this present study, DFO showed a temporary
protective effect on OVX-induced osteoporosis, especially in femur.
We noticed that the femur showed a greater and sooner response
compared to the spine, which is consistent with previous study
(22). By immunostaining, we also
discovered that type H vessels and bone mass reduced post-OVX in a
time-dependent manner. DFO treatment helped maintain trabecular
bone density, restore microarchitecture, and increase the number of
type H vessels in OVX mice at only week 1 and these effects were
reversed at weeks 2–4. In addition, our results also revealed that
DFO administration had no significant effect on the improvement of
spine BMD in OVX mice. One possible cause for this effect is that
the concentration of DFO administrated is not able to completely
withstand the bone loss caused by continual estrogen loss. It will
be our future direction to make in-depth investigation in order to
study the dynamics of osteogenesis and angiogenesis using DFO
gradients and to determine the underlying mechanism.
DFO has been shown to increase angiogenesis via the
HIF pathway in bone (23). DFO
therapy can optimize bone regeneration in mandibular distraction
osteogenesis by increasing the number of osteocytes and vessels
(24,25). DFO released from poly
lactic-co-glycolic acid promotes healing of osteoporotic bone
defects by enhancing the differentiation of MSCs and endothelial
tubule formation (26). These
results show that DFO can accelerate fracture healing and bone
regeneration by inducing new blood vessel formation. In our study,
DFO increased bone mass and enhanced type H vessels at week 1 in
the femur, which may be attributed to HIF pathways. However, at
week 3, DFO failed to increase the number of type H vessels and
restore BMD. One cause may be that OVX-induced estrogen loss
overwhelms the osteogenic and angiogenic effect of DFO.
Accumulating evidence suggests that local blood
supply or decreased angiogenesis play pivotal roles in osteoporosis
(27–29). Osteoporotic fracture is one of the
most frequent complications of bone loss, easily leading to
disability and death in the elderly. Healing of fragility fractures
would be prolonged with the decreased levels of angiogenesis and
MSCs caused by OVX (30).
Low-magnitude high-frequency vibration treatment could enhance
fracture healing by increasing blood flow and angiogenesis in the
OVX mouse model (31). This has
great clinical significance in the improvement of osteoporosis and
prevention of fragility fractures by promoting blood vessel
formation in bone. Type H vessels combine angiogenesis and
osteogenesis in murine bone, which decreases along with ageing
(19,32,33).
DFO could increase type H vessel formation and improve BMD in aged
mice (19). When looked
separately, CD31+ ECs showed more significant change
than EMCN+ ECs, resulting in dramatic change of type H
vessels. The vessel changes we observed in OVX mice models are
quite similar. EMCN+ ECs appeared to be comparable at
week-3 after OVX while CD31+ ECs decreased dramatically.
As a result, type H vessel was significantly reduced in OVX mice
and DFO treatment attenuated the effect temporarily. This would be
due to type H vessels (CD31 expression) are more sensitive to
physiological and/or pathological stress and would be considered as
an early marker for bone status. Studies need to be pursued to
investigate the relationship between type H vessels and bone
formation/maintenance, especially in pathological situations.
As an angiogenic agent, DFO has potential to active
certain vascular pathways. How these two molecular effects
coordinate to achieve a protective effect against osteoporotic
process is still unknown. Our study offers preliminary information
for the clinical therapeutic treatment of osteoporosis. Coupling
different pathways, e.g., vascular and osseous, may achieve a
better therapeutic effect. Scientists and clinicians need to
further test specific therapeutic options, e.g., DFO, to fully
understand their functions.
Acknowledgements
We thank all the staffs of the animal facility for
maintaining our experimental animals. This study was supported by
the National Natural Science Foundation of China (grant nos.
81273090 and 81572179), the Clinical Special Program of Jiangsu
Province (grant no. BL2014044), Clinical Key Diseases' Diagnosis
and Treatment of Suzhou (grant no. LCZX201305), Science and
Technology Project of Suzhou (grant no. SYS201637).
References
|
1
|
Kanis JA: Assessment of fracture risk and
its application to screening for postmenopausal osteoporosis:
Synopsis of a WHO report. WHO Study Group. Osteoporos Int.
4:368–381. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanis JA, McCloskey EV, Johansson H and
Oden A: Approaches to the targeting of treatment for osteoporosis.
Nat Rev Rheumatol. 5:425–431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harvey N, Dennison E and Cooper C:
Osteoporosis: Impact on health and economics. Nat Rev Rheumatol.
6:99–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garnero P: New developments in biological
markers of bone metabolism in osteoporosis. Bone. 66:46–55. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harvey N, Dennison E and Cooper C:
Osteoporosis: A lifecourse approach. J Bone Miner Res.
29:1917–1925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendrickx G, Boudin E and Van Hul W: A
look behind the scenes: The risk and pathogenesis of primary
osteoporosis. Nat Rev Rheumatol. 11:462–474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reid IR: Short-term and long-term effects
of osteoporosis therapies. Nat Rev Endocrinol. 11:418–428. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang J, Wang Z, Tang E, Fan Z, McCauley
L, Franceschi R, Guan K, Krebsbach PH and Wang CY: Inhibition of
osteoblastic bone formation by nuclear factor-kappaB. Nat Med.
15:682–689. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewiecki EM: New targets for intervention
in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol.
7:631–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maeda K, Kobayashi Y, Udagawa N, Uehara S,
Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et
al: Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scholtysek C, Katzenbeisser J, Fu H,
Uderhardt S, Ipseiz N, Stoll C, Zaiss MM, Stock M, Donhauser L,
Böhm C, et al: PPARβ/δ governs Wnt signaling and bone turnover. Nat
Med. 19:608–613. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomlinson RE and Silva MJ: Skeletal blood
flow in bone repair and maintenance. Bone Res. 1:311–322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colnot C: Cellular and molecular
interactions regulating skeletogenesis. J Cell Biochem. 95:688–697.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brandi ML and Collin-Osdoby P: Vascular
biology and the skeleton. J Bone Miner Res. 21:183–192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan C, Gilbert SR, Wang Y, Cao X, Shen X,
Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC,
et al: Activation of the hypoxia-inducible factor-1alpha pathway
accelerates bone regeneration. Proc Natl Acad Sci USA. 105:686–691.
2008; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schipani E, Maes C, Carmeliet G and
Semenza GL: Regulation of osteogenesis-angiogenesis coupling by
HIFs and VEGF. J Bone Miner Res. 24:1347–1353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dirckx N, Van Hul M and Maes C: Osteoblast
recruitment to sites of bone formation in skeletal development,
homeostasis and regeneration. Birth Defects Res C Embryo Today.
99:170–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu ZH, Zhang XL, Tang TT and Dai KR:
Promotion of osteogenesis through beta-catenin signaling by
desferrioxamine. Biochem Biophys Res Commun. 370:332–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donneys A, Weiss DM, Deshpande SS, Ahsan
S, Tchanque-Fossuo CN, Sarhaddi D, Levi B, Goldstein SA and Buchman
SR: Localized deferoxamine injection augments vascularity and
improves bony union in pathologic fracture healing after
radiotherapy. Bone. 52:318–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu XL, Li CL, Lu WW, Cai WX and Zheng LW:
Skeletal site-specific response to ovariectomy in a rat model:
Change in bone density and microarchitecture. Clin Oral Implants
Res. 26:392–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drager J, Harvey EJ and Barralet J:
Hypoxia signalling manipulation for bone regeneration. Expert Rev
Mol Med. 17:e62015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farberg AS, Sarhaddi D, Donneys A,
Deshpande SS and Buchman SR: Deferoxamine enhances bone
regeneration in mandibular distraction osteogenesis. Plast Reconstr
Surg. 133:666–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donneys A, Farberg AS, Tchanque-Fossuo CN,
Deshpande SS and Buchman SR: Deferoxamine enhances the vascular
response of bone regeneration in mandibular distraction
osteogenesis. Plast Reconstr Surg. 129:850–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia P, Chen H, Kang H, Qi J, Zhao P, Jiang
M, Guo L, Zhou Q, Qian ND, Zhou HB, et al: Deferoxamine released
from poly(lactic-co-glycolic acid) promotes healing of osteoporotic
bone defect via enhanced angiogenesis and osteogenesis. J Biomed
Mater Res A. 104:2515–2527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Senel K, Baykal T, Seferoglu B, Altas EU,
Baygutalp F, Ugur M and Kiziltunc A: Circulating vascular
endothelial growth factor concentrations in patients with
postmenopausal osteoporosis. Arch Med Sci. 9:709–712. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanczler JM and Oreffo RO: Osteogenesis
and angiogenesis: The potential for engineering bone. Eur Cell
Mater. 15:100–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Griffith JF, Wang YX, Zhou H, Kwong WH,
Wong WT, Sun YL, Huang Y, Yeung DK, Qin L and Ahuja AT: Reduced
bone perfusion in osteoporosis: Likely causes in an ovariectomy rat
model. Radiology. 254:739–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheung WH, Miclau T, Chow SK, Yang FF and
Alt V: Fracture healing in osteoporotic bone. Injury. 47 Suppl
2:S21–S26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheung WH, Sun MH, Zheng YP, Chu WC, Leung
AH, Qin L, Wei FY and Leung KS: Stimulated angiogenesis for
fracture healing augmented by low-magnitude, high-frequency
vibration in a rat model-evaluation of pulsed-wave doppler, 3-D
power Doppler ultrasonography and micro-CT microangiography.
Ultrasound Med Biol. 38:2120–2129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
le Noble F and le Noble J: Bone biology:
Vessels of rejuvenation. Nature. 507:313–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kusumbe AP and Adams RH: Osteoclast
progenitors promote bone vascularization and osteogenesis. Nat Med.
20:1238–1240. 2014. View
Article : Google Scholar : PubMed/NCBI
|