Introduction
In the last two decades, the demand for functional
foods and nutraceuticals has increased significantly in China. In
China, individuals are beginning to pay more attention to their own
health, and self-health care awareness is increasing. Therefore, in
order to satisfy consumer needs, bioactive peptides from various
foods are isolated by enzymatic hydrolysis and their functional
activities are being investigated (1,2).
Bioactive peptide hydrolysates have gained increasing attention due
to their physiologic effects on cardiovascular, gastrointestinal,
nervous and immune systems (3).
The hydrolysates of numerous marine organisms, such as
Pardachirus marmoratus (P. marmoratus; also known as the Red
Sea Moses sole), have been demonstrated to possess significant
antitumor activities (4). Cancer
is a disease of worldwide importance. Its incidence in developed
countries is increasing, and cancer is the second most common cause
of mortality (5). In 2017, 1.69
million new cancer cases and 59,000 mortalities from cancer are
projected to occur in the United States (6). Therefore, there is an urgent
requirement for the development of novel tumor-targeted therapies
that specifically and effectively target tumor cells with low
toxicity to normal tissues (7–9).
Prostate cancer is one of the world's most commonly
diagnosed cancer, and is second to lung cancer as the leading cause
of cancer-associated mortality in men (6). In China, prostate cancer is currently
the most common malignancy diagnosed in men, and is one of the ten
most common cancers in urban areas (10). Prostate cancer has the seventh
highest incidence rate, and it is the tenth leading cause of
cancer-associated mortality in males residing in urban areas, with
a mortality rate of 3.67/100,000 males in 2010 (11). During the early development of
prostate carcinomas, the growth of prostate epithelial cells is
androgen-dependent (5). Therefore,
hormone therapy is a primary method used to treat patients with
prostate cancer. However, a proportion of tumor cells become
androgen-independent (12).
Despite the improved efficacy of chemotherapy for the majority of
cancer types over the last 30 years, the highly toxic effects of
chemotherapeutic drugs, such as doxorubicin, have lead to a
significant reduction in the quality of life of patients, which
remains a formidable problem in clinical medicine (13). Therefore, the discovery and
development of novel potent anticancer agents with minimal toxicity
are in high demand.
In the present study, hydrolysates derived from the
enzymatic hydrolysis of the common marine organism, Sinonovacula
constricta (S. constricta), were evaluated as a source of
antitumor peptides. S. constricta is a commercially
important bivalve that is used as food in China. In order to
optimize its use for human consumption, and potentially gain higher
value-added advantages, the authors of the present study focused on
the utilization of S. constricta to obtain bioactive
peptides and investigate their antitumor effects, with the aim of
increasing the survival rates from cancer and enhancing the quality
of life of patients. A number of bioactive peptides derived from
marine organisms, such as the marine sponge, Jaspis jonhstoni,
P. marmoratus, and somocystinamide A isolated from a Lyngbya
majuscula/Schizothrix spp. marine cyanobacteria, have been
demonstrated to possess significant antitumor activities (4,14–16).
For instance, jasplakinolide (also known as jaspamide), a cyclic
depsipeptide, inhibited human Jurkat T cell growth by inducing
apoptosis (17). In addition, a
short peptide isolated from the heated products of half-fin anchovy
(Setipinna taty) peptide hydrolysates demonstrates
antiproliferative activities against human PC-3 prostate cancer
cells (18). However, the
bioactivities of hydrolysates derived from S. constricta,
particularly with regards to its nutraceutical and pharmaceutical
applications, have not yet been reported. Therefore, the aim of the
present study was to determine whether S. constricta might
be a useful source of antitumor peptides obtained by enzymatic
hydrolysis. Enzymatic hydrolysis was used to generate complex
mixtures of similar molecular weight (MW) fractions, which were
subsequently subject to ultrafiltration, and high-performance
liquid chromatography (HPLC) was employed to obtain purified
peptide fractions. The acquired peptides were evaluated for their
antiproliferative properties using the colorimetric MTT assay.
Peptides confirmed to possess potent antiproliferative activities
were selected for identification of their amino acid composition
and sequence. In addition, the mechanisms underlying the
antiproliferative effects of identified peptides were investigated
further.
Materials and methods
Materials
A total of 10 kg S. constricta were obtained
from Beimen market in Zhoushan City, (Zhejiang, China). The muscles
were rapidly separated, and stored at −80°C. Trypsin, pepsin,
papain, alcalase, acetonitrile and trifluoroacetic acid (TFA) were
purchased from YTHX Biotechnology Co., Ltd. (Beijing, China;
www.ythxbio.com). The MTT assay reagent was
purchased from Beyotime Institute of Biotechnology (Nanjing,
China). All additional reagents used were of analytical grade.
Hormone-dependent DU-145 and PC-3 cell lines were obtained from the
China Cell Bank of the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). The annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis Detection and cell cycle
staining kits were obtained from YTHX Biotechnology Co., Ltd.
Cell culture conditions
DU-145 and PC-3 cells were cultured in Ham's F-12
medium supplemented with 10% fetal calf serum (FBS) (both Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin (10,000 U/ml penicillin G sodium and 10,000
mg/ml streptomycin sulfate) (Thermo Fisher Scientific, Inc.). Cells
were cultured in an incubator at 37°C and 5% CO2 in a
humidified atmosphere.
Preparation of S. constricta
hydrolysates (SCH)
Hydrolysis of S. constricta muscles was
performed using trypsin, pepsin, papain and alcalase enzymes. S.
constricta meal (500 g) was homogenized using a tissue
homogenizer and suspended in distilled water at a ratio of 1:2
(m/m). The homogenate was adjusted to the optimum conditions for
digestion with trypsin, pepsin, papain or alcalase using 1.0 N NaOH
or 1.0 N HCI. The S. constricta homogenate was hydrolyzed
using the four proteases at a concentration of 3% (m/m,
enzyme/substrate), and at the pH and temperatures listed in
Table I. The enzymatic hydrolysate
mixtures were incubated for 5 h at 37°C in a thermostatic water
bath. The enzymatic reactions were inactivated by heating the
mixtures for 15 min at 100°C. The mixture was centrifuged at 4,472
× g for 15 min at 4°C, and the soluble fraction was collected. The
resulting SCH were lyophilized and stored at −80°C for downstream
analysis.
 | Table I.Optimum conditions for the enzymatic
digestion of Sinonovacula constricta using four
proteinases. |
Table I.
Optimum conditions for the enzymatic
digestion of Sinonovacula constricta using four
proteinases.
|
| Optimum
conditions |
|---|
|
|
|
|---|
| Proteinase | pH | Temperature
(°C) |
|---|
| Trypsin | 7.5 | 37 |
| Pepsin | 2.0 | 37 |
| Papain | 6.0 | 37 |
| Alcalase | 8.0 | 37 |
Membrane fractionation
The SCH was isolated using the Amicon 8400 Stirred
Ultrafiltration Unit (EMD Millipore, Billerica, MA, USA) with MW
thresholds of 3, 5 and 8 KDa. Briefly, the freeze-dried SCH samples
were diluted in distilled water (1:3) and the following fractions
were collected: SCH-I, MW ≥8 kDa; SCH-II, 5≤ MW <8 kDa; SCH-III,
3≤ MW <5 kDa; and SCH-IV, MW <3 kDa. The SCH group was the
unfractionated sample. All SCH fractions were lyophilized and their
antitumor activities were determined using DU-145 and PC-3
cells.
Gel filtration chromatography
The SCH fraction (500 mg/ml) exhibiting the highest
antitumor activity following ultrafiltration, was further separated
using Sephadex G-25 gel filtration media (YTHX Biotechnology Co.,
Ltd) and a gel filtration column (2.5×80 cm; 75–180 µm), eluted
with distilled water at a flow rate of 1 ml/min at room
temperature. Each fraction was detected at 280 nm. The SCH-IV
fraction was further sub-fractionated into 5 groups, using G-25, by
molecular weight. G-25 may be used to fractionate the molecules
between 1 and 5 kDa. Bioactive fractions were collected by a
computerized automatic collector (Shanghai Qingpu Huxi Instrument
Factory, Shanghai, China) and lyophilized for an antitumor activity
assay.
HPLC
The sample obtained from the Sephadex G-25 gel which
demonstrated the highest antitumor activity was further purified by
reverse-phase (RP)-HPLC using an Agilent 1260 Infinity system
(Agilent Technologies, Inc., Santa Clara, USA) and a Zorbax SB-C18
column (4.6×250 mm; 5 µm; Agilent Technologies, Inc.) and the
column temperature was 30°C. The mixture of acetonitrile (A) and
0.06% TFA (B) was used as mobile phase in linear gradient mode
(5–30% of A for 0–50 min; 30–75% of A for 50–80 min), with a
flowrate of 0.8 ml/min. Elution peaks were detected at 280 nm, and
were collected for determination of antitumor activity. The amino
acid sequences of purified peptides were then determined.
Amino acid sequence analysis and
determination of molecular mass
A total of 200 µg protein was mixed with 250 µl
rehydration solution and rehydrated for 14 h using an IPGphor (GE
Healthcare, Beijing, China). The isoelectric focusing was achieved
with four steps, then carried out by two-dimensional gel
electrophoresis (19). Following
pretreatment, the samples (50 pmol/20 µl) were directly sequenced
using a Shimadzu PPSQ-31A automated gas phase protein sequencer
(Shimadzu Corporation, Kyoto, Japan). Briefly, samples were
dissolved in 20 µl CH3CN (37%; v/v) solution and applied
to TFA-treated glass fiber membranes pre-treated with polybrene
(Shimadzu Corporation). Data were recorded using Shimadzu PPSQ
version 31A software (Shimadzu Corporation). An accurate molecular
mass of SCH-P9 and SCH-P10 peptides was determined using an LTQ FT
Ultra mass spectrometer (Thermo Fisher Scientific, Inc.). The
1H nuclear magnetic resonance (NMR) and 13C NMR spectra
were recorded in d-dimethyl sulfoxide (DMSO) on a Bruker AV-400
spectrometer (Bruker Corporation, Billerica, MA, USA) at working
frequencies 400 and 100 MHz. Chemical shifts are expressed in parts
per million (δ) values and coupling constants (J) in Hz. High
resolution electrospray ionization mass spectrometry (HRESI-MS)
were measured on the LTQ FT Ultra instrument. The above procedure
was performed by Wuhan Moon Biosciences Co., Ltd. (Wuhan,
China).
MTT assay
The effects of SCH on cell viability were determined
using a colorimetric MTT assay. Briefly, DU-145 and PC-3 cells were
seeded at a density of 1×104 cells/well in 96-well
plates, and incubated for 24 h to allow cells to adhere to the
surface of the plates in a humidified atmosphere, in a 5%
CO2 incubator at 37°C (Forma; Thermo Fisher Scientific,
Inc.). The DU-145 and PC-3 cells were then treated with SCH at 1,
5, 10 mg/ml for 24 h. DU-145 and PC-3 control cells were treated
with F-12 culture medium. Cell viability was detected by adding MTT
reagent. A total of 20 µl MTT solution was added to each well for
an additional 4–5 h at 37°C. Following the addition of the DMSO
solution (150 µl) to each well to dissolve the formazan crystals,
the optical density (OD) was read at a wavelength of 450 nm using a
Model 680 Microplate Reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Three independent experiments were performed, and the
cell inhibitory rate (A value) was determined using the following
equation: Inhibition (%) =
[(ODcontrol-ODtreated)/(ODcontrol-ODblank)
× 100].
Cell cycle analysis
Cell cycle analysis and measurement of cellular DNA
content were performed following synchronization. Synchronization
was achieved by incubating DU-145 and PC-3 cells in 0.5% FBS F-12
medium for 48 h in a humidified atmosphere with 5% CO2
at 37°C. DU-145 and PC-3 cells were seeded in six-well plates
(1×105 cells/well) and allowed to adhere overnight.
DU-145 cells were treated with SCH-P9 (1.21 mg/ml), SCH-P10 (1.41
mg/ml) and fluorouracil (5-FU; 2.02 µg/ml) for 24 h. PC-3 cells
were treated with SCH-P9 (1.09 mg/ml), SCH-P10 (0.91 mg/ml) and
5-FU (2.14 µg/ml) for 24 h. For cell cycle analysis, cells were
first collected by trypsinization, fixed in 70% ethanol, washed in
cold phosphate-buffered saline (PBS; pH 7.4) and then stained with
PI solution (50 µg/ml PI, 100 µg/ml RNase and 0.1% Triton X-100 in
PBS) for 30 min in the dark at room temperature. The DNA content of
DU-145 and PC-3 cells was analyzed using a Guava easyCyte Flow
Cytometer (EMD Millipore). Histograms showing the population of
cells in each cell cycle phase based on DNA content were
constructed using GuavaSoft software (version, 4.20; EMD
Millipore). A total of three independent experiments were
performed, and the results are presented as the mean ± standard
deviation.
Cell apoptosis analysis
Cell apoptosis detection was performed using
fluorescence-activated cell sorting (FACS) analysis. The exposure
of phosphatidylserine on the extracellular side of the cell
membrane was quantified by annexin V-FITC/PI staining. DU-145 and
PC-3 cells were seeded in six-well plates (1×105
cells/well). DU-145 cells were treated with SCH-P9 (1.21 mg/ml),
SCH-P10 (1.41 mg/ml) or 5-FU (2.02 µg/ml) for 24 h. PC-3 cells were
treated with SCH-P9 (1.09 mg/ml), SCH-P10 (0.91 mg/ml) or 5-FU
(2.14 µg/ml) for 24 h. The cells were collected by trypsinization.
Following centrifugation at 213 × g at 4°C for 5 min, cell pellets
were washed twice with cold PBS. Cells were then incubated with 5
µl annexin V-FITC and 10 µl PI (Shanghai BestBio, Shanghai, China),
according to the manufacturer's protocol, at room temperature for
15 min in the dark. Binding buffer (400 µl; 1X) was subsequently
added to each tube and the cells were immediately analyzed using a
Guava easyCyte Flow Cytometer (EMD Millipore). The data were
analyzed using GuavaSoft software (version, 4.20; EMD
Millipore).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. Data were analyzed by
one-way repeated measures analysis of variance with Tukey test
using SPSS software (version 16; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Preparation of S. constricta enzymatic
hydrolysates
SCHs were produced by incubating the organisms with
trypsin, pepsin, papain or alcalase enzymes under optimal
conditions (Table I). The
antitumor activities of the hydrolysates were assessed using an MTT
assay and the results are shown in Table II. Out of all hydrolysates, the
pepsin hydrolysate exhibited the highest antiproliferative activity
against DU-145 and PC-3 human prostate cancer cell lines (Table II). Therefore, the pepsin
hydrolysate was selected for further study.
 | Table II.Effect of different enzymatic
Sinonovacula constricta hydrolysates on the percentage
growth inhibition of human prostate cancer cell lines. |
Table II.
Effect of different enzymatic
Sinonovacula constricta hydrolysates on the percentage
growth inhibition of human prostate cancer cell lines.
| Enzymatic
lysate | DU-145 (%) | PC-3 (%) |
|---|
| Trypsin | 33.67±2.3 | 48.23±6.3 |
| Pepsin | 86.24±3.5 | 88.21±7.3 |
| Papain | 45.26±5.4 | 44.75±1.4 |
| Alcalase | 69.32±5.6 | 67.28±7.1 |
Ultrafiltration of SCH
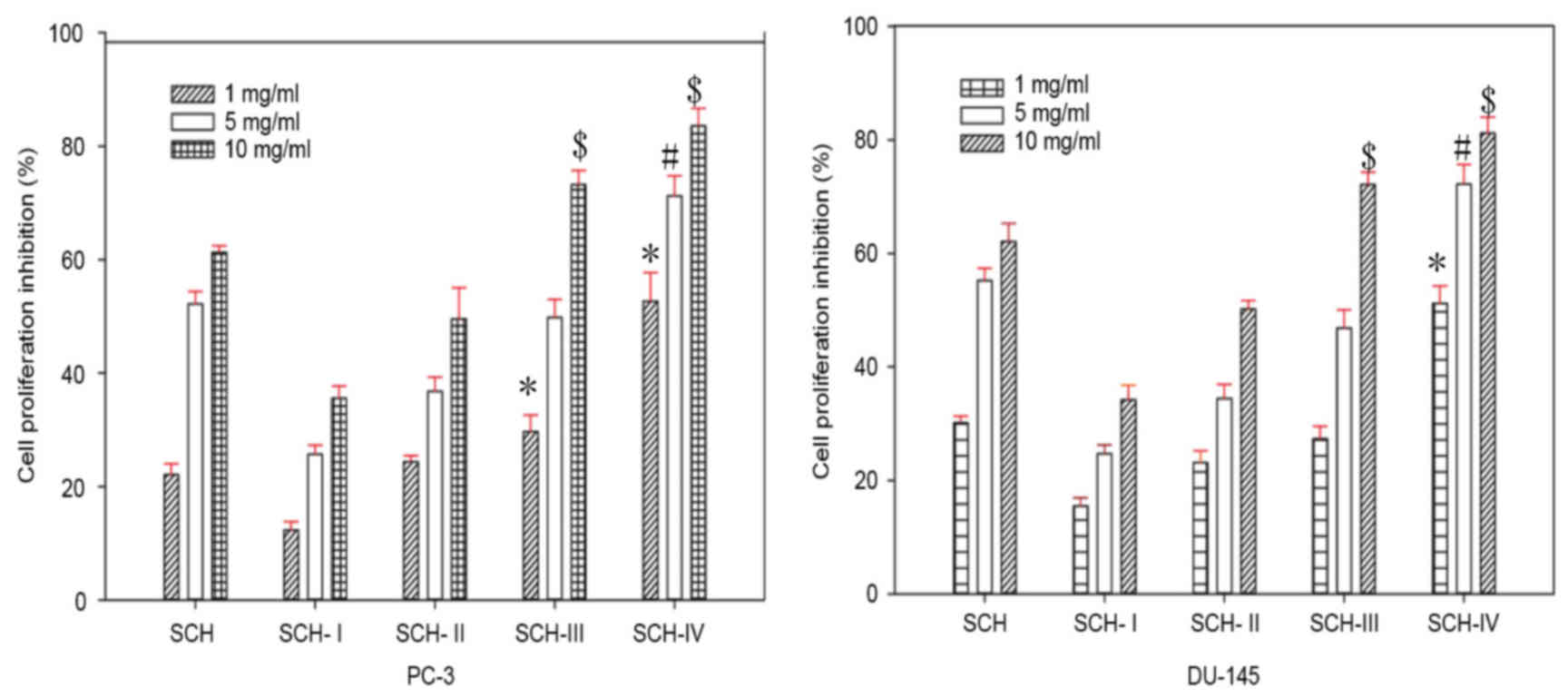
SCHs were fractionated by MW using an
ultrafiltration membrane bioreactor system, with MW thresholds of
8, 5 and 3 kDa. Therefore, four different fractions were obtained
and divided into SCH-I, (MW ≥8 kDa), SCH-II (5≤ MW <8 kDa),
SCH-III (3≤ MW <5 kDa) and SCH-IV, (MW <3 kDa) groups. The
SCH group was treated with the unfractionated sample. SCH, SCH-I,
SCH-II, SCH-III and SCH-IV inhibited the growth of DU-145 and PC-3
cells in a dose-dependent manner (Fig.
1). In addition, SCH-IV exhibited the highest antiproliferative
activity among all of the fractions (Fig. 1). Therefore, SCH-IV was selected
for further purification by gel filtration chromatography.
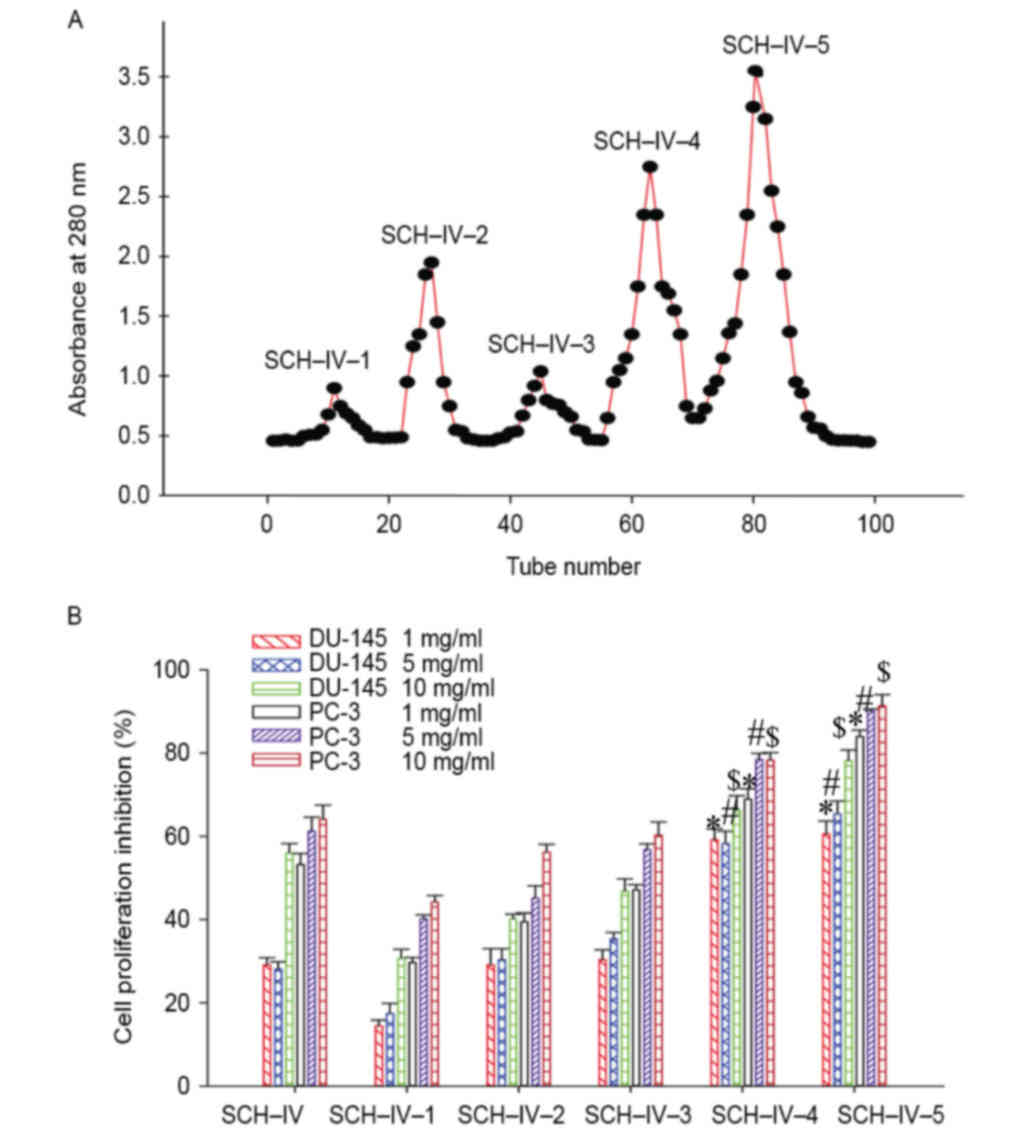
Gel filtration chromatography of the
SCH-IV fraction
As shown in Fig.
2A, SCH-IV was further separated into five fractions, termed
SCH-IV-1, SCH-IV-2, SCH-IV-3, SCH-IV-4 and SCH-IV-5. Out of all
fractions examined, SCH-IV-5 demonstrated the greatest inhibitory
effect on the growth of DU-145 and PC-3 cells (Fig. 2B). Therefore, SCH-IV-5 was selected
for further purification by HPLC to isolate and characterize the
peptides responsible for the observed antiproliferative
effects.
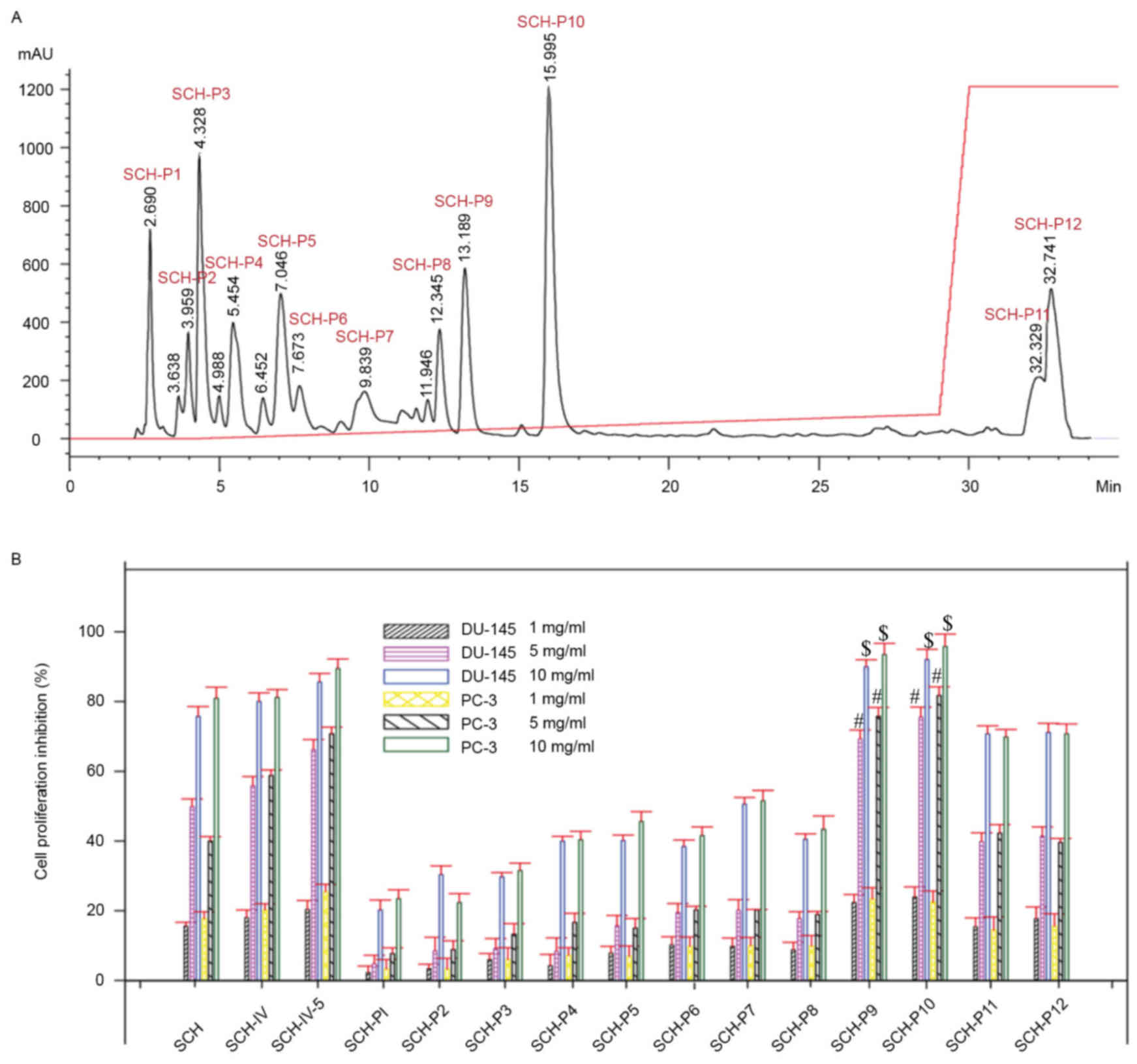
Isolation of peptides from SCH-IV-5 by
RP-HPLC
SCH-IV-5 was further purified by RP-HPLC. As shown
in Fig. 3, SCH-IV-5 was separated
into 12 fractions, termed SCH-P1, SCH-P2, SCH-P3, SCH-P4, SCH-P5,
SCH-P6, SCH-P7, SCH-P8, SCH-P9, SCH-P10, SCH-P11 and SCH-P12, and
their antiproliferative effects on DU-145 and PC-3 cells were
investigated. The antiproliferative activities of SCH-P9 and
SCH-P10 peptides were greater when compared with the remaining 10
peptides. Therefore, SCH-P9 and SCH-P10 were considered to be the
peptides exhibiting the greatest antitumor activity, and their
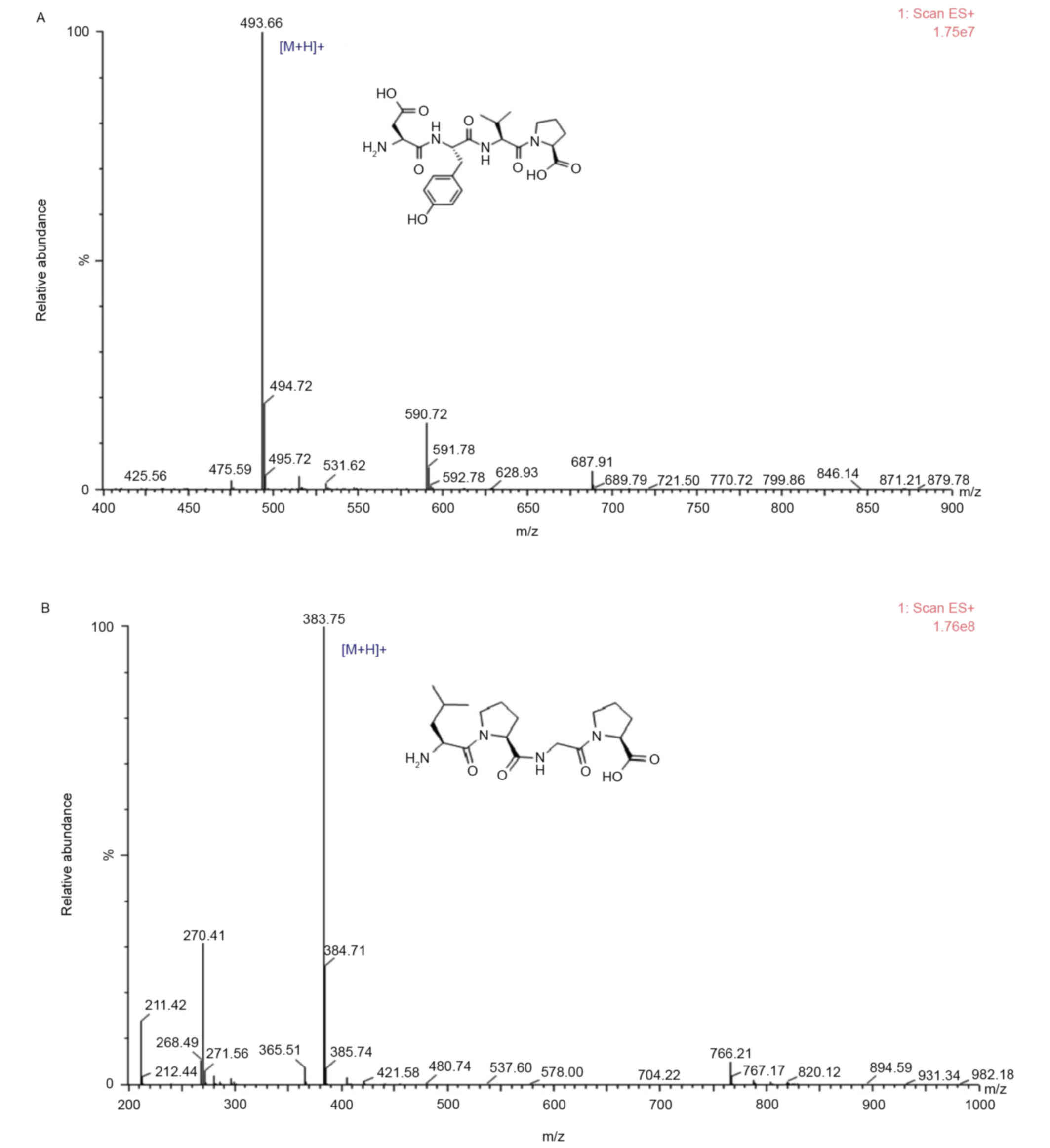
sequences were identified as Leu-Pro-Gly-Pro and Asp-Tyr-Val-Pro,
respectively (Fig. 4). The purity
of SCH-P9 and SCH-P10 were 95.99 and 96.99%, and the yields were
5.79 and 8.92%, respectively.
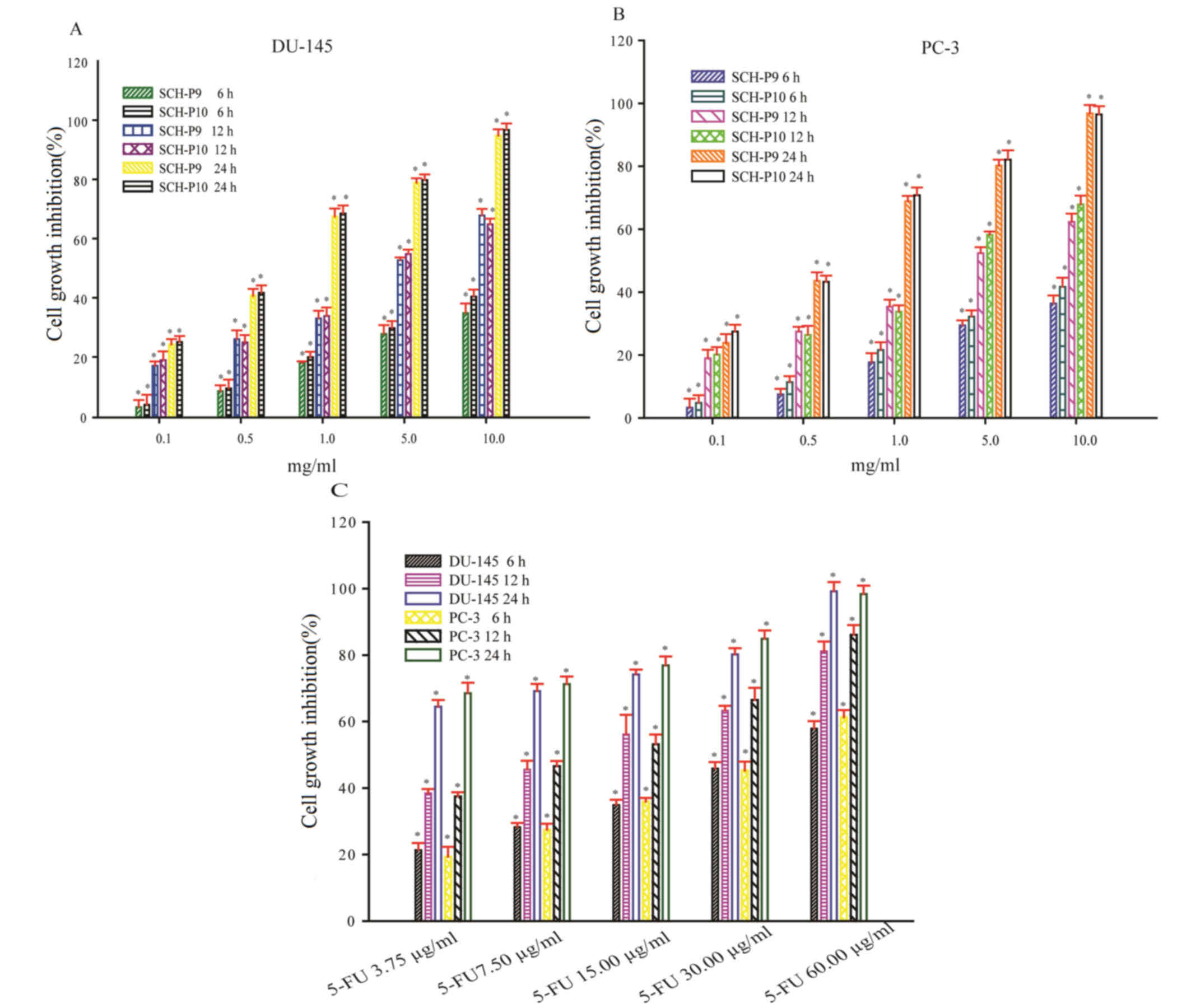
Growth inhibition rate of DU-145 and
PC-3 cells following treatment with SCH-P9 and SCH-P10
DU-145 and PC-3 cells were exposed to 0.1, 0.5, 1.0,
5.0, and 10 mg/ml SCH-P9 or SCH-P10 for 6, 12 or 24 h. At all doses
examined, SCH-P9 and SCH-P10 effectively inhibited the growth of
DU-145 and PC-3 cells at 6, 12, and 24 h (Fig. 5A and B). A time- and dose-dependent
increase in the growth inhibition rate of DU-145 and PC-3 cells
treated with SCH-P9 and SCH-P10 was observed (Fig. 5A and B). The maximal inhibitory
concentration (IC50) of SCH-P9 on DU-145 cells was
12.66, 5.45 and 1.21 mg/ml at 6, 12 and 24 h, respectively. The
IC50 of SCH-P10 on DU-145 cells was 11.28, 5.49 and 1.41
mg/ml at 6, 12 and 24 h, respectively. The IC50 of
SCH-P9 on PC-3 cells was 12.09, 5.96 and 1.09 mg/ml at 6, 12 and 24
h, respectively. The IC50 of SCH-P10 on PC-3 cells was
10.94, 5.12 and 0.91 mg/ml at 6, 12 and 24 h, respectively. Of
particular note, SCH-P9 and SCH-P10 did not inhibit the growth of
DU-145 and PC-3 cells to a greater extent when compared with 5-FU
under a similar growth inhibition rate (Fig. 5C).
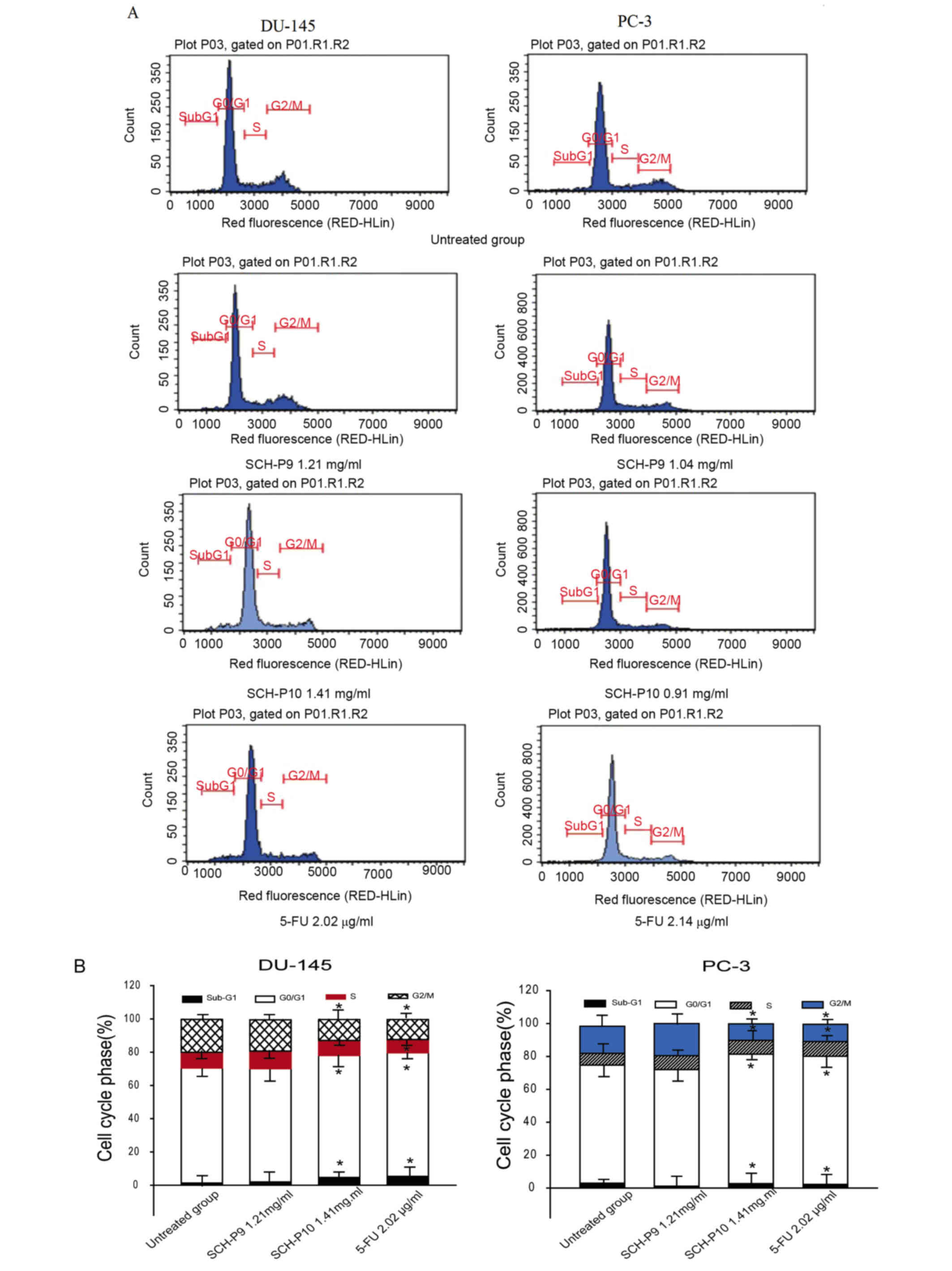
Effect of SCH-P9 and SCH-P10 on the
cell cycle distribution of DU-145 and PC-3 cells
In order to investigate the mechanisms responsible
for SCH-P9- and SCH-P10-mediated cell growth inhibition, the cell
cycle distribution of DU-145 and PC-3 cells were evaluated by flow
cytometry. A representative example indicating the effect of SCH-P9
and SCH-P10 treatment for 24 h on cell cycle phase distribution is
shown in Fig. 6. When compared
with the untreated cells, treatment with SCH-P10 was associated
with an increase in the number of DU-145 cells in the sub-G1 phase,
together with a concomitant increase in the proportion of cells in
the G0/G1 phase, and a decrease in the number
of cells in S and G2/M phases (Fig. 6). However, there were no
significant changes in the cell cycle distribution of DU-145 cells
following treatment with SCH-P9. When compared with untreated
controls, SCH-P9 reduced the number of PC-3 cells in
subG1 and G0/G1 phases, whereas an
increased number were observed in the G2/M phase;
however, these alterations did not reach statistical significance
(Fig. 6). SCH-P10 treatment
significantly reduced the number of PC-3 cells in G2/M
phase, and significantly increased the number in
G0/G1 phase when compared with untreated
controls (Fig. 6).
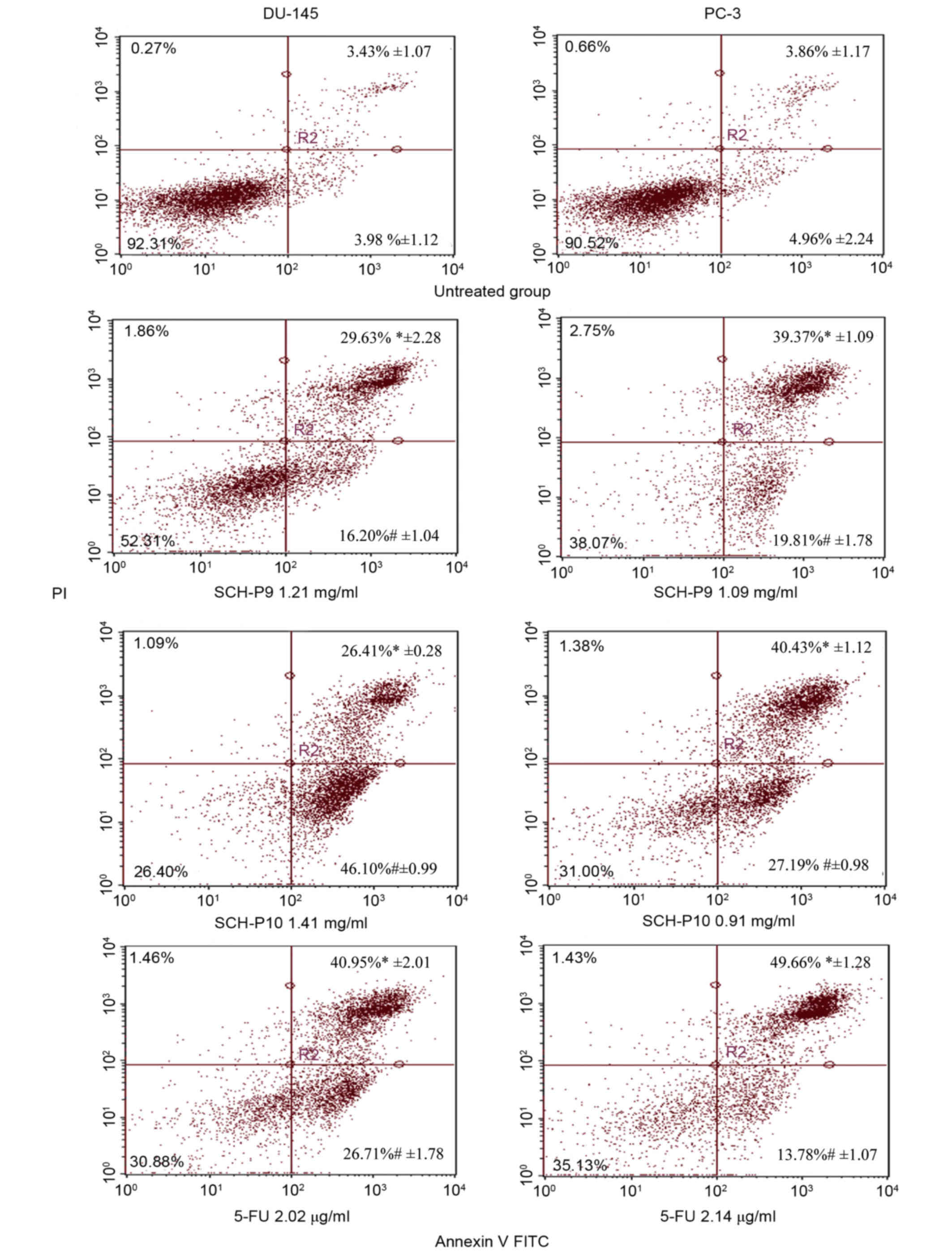
SCH-P9 and SCH-P10 induced apoptosis
of DU-145 and PC-3 cells
In order to determine whether the observed
alterations in prostate cancer cell cycle distributions following
treatment with SCH-P9 and SCH-P10 were due to increased levels of
apoptosis, treated DU-145, and PC-3 cells were stained with annexin
V-FITC/PI and analyzed by flow cytometry. As shown in Fig. 7, treatment of DU-145 and PC-3 cells
with SCH-P9 and SCH-P10 for 24 h was associated with a significant
increase in early apoptosis rates when compared with untreated
control cells. The percentage of DU-145 cells in early apoptosis
following treatment with 2.02 µg/ml 5-FU was 26.71%, which was
significantly lower than the percentage of DU-145 cells in early
apoptosis following treatment with 1.41 mg/ml SCH-P10 (Fig. 7). However, the percentage of DU-145
cells in late apoptosis following treatment with 2.02 µg/ml 5-FU
was 40.95%, which was higher than the number of cells in late
apoptosis following treatment with 1.41 mg/ml SCH-P10. Similar
alterations in the number of PC-3 cells in early and late apoptosis
following treatment with 5-FU and SCH-P10 were observed (Fig. 7).
Discussion
Bioactive peptides obtained by enzymatic hydrolysis
are gaining increased attention due to their unique amino acid
compositions, and they may hold promise for a wide range of
therapeutic applications (20).
Bioactive peptides may reduce disease risk and promote human health
(20). Peptides that induce
apoptosis of tumor cells are considered to be effective anticancer
agents.
Anticancer peptides derived from marine sources and
their potential use as alternative anticancer treatments has been
well established. For instance, a novel peptide derived from
Ruditapes philippinarum, with an N-terminal amino acid
sequence identified as Ala-Val-Leu-Val-Asp-Lys-Gln-Cys-Pro-Asp,
effectively induces apoptosis of prostate cancer cells (21). In addition,
Ala-Phe-Asn-Ile-His-Asn-Arg-Asn-Leu-Leu, a decapeptide derived from
the pepsin hydrolysates of the Mytilus coruscus shellfish,
has been demonstrated to exhibit anticancer properties (22).
In the present study, a low MW peptide derived from
the hydrolysate of S. constricta was observed to exhibit the
strongest inhibitory effects on the growth of DU-145 and PC-3
cells, and was therefore purified further. A number of previous
studies have demonstrated that bioactive peptides are primarily low
MW peptides (23–25). The sepia ink oligopeptide, a
tripeptide extracted from Sepia ink (Sepia esculenta),
induces apoptosis of prostate cancer cell lines via caspase-3
activation and elevation of the Bcl-2 like protein 4/B-cell
lymphoma-2 (Bcl-2) ratio (26).
In the current study, SCH-P9 and SCH-P10 fractions
inhibited the growth of PC-3 and DU-145 cells to a greater extent
when compared with the other SCH fractions. The peptide size and
composition of the amino acids in protein hydrolysates serve
crucial roles in their cell inhibitory activity (27). The N-terminal amino acid sequence
and molecular mass of SCH-P9 and SCH-P10 were determined using a
Shimadzu PPSQ-31A automated gas phase sequencer followed by a
Thermo Fisher Scientific LTQ FT Ultra mass spectrometer. The
sequences of SCH-P9 and SCH-P10 were identified as Leu-Pro-Gly-Pro
(MW, 382.46 Da) and Asp-Tyr-Val-Pro (MW, 492.53 Da), respectively.
The SCH-P9 and SCH-P10 peptides were effective against the growth
of PC-3 and DU-145 cells in a time and dose-dependent manner. Lower
MW peptides demonstrate greater molecular mobility and diffusivity
when compared with higher MW peptides, which contributes to
enhanced interactions with cancer cell components and therefore
increases anticancer activities (28).
A number of peptides obtained from marine animal
species have been demonstrated to exert strong antitumor
activities, including didemnin B, keenamide A, kahalalide F,
jasplakinolide, aurilide and theopedirn B (17). These antitumor compounds possess
tumor-targeting abilities, with lower toxicity in normal tissues.
The properties of these anticancer peptides make them promising
therapeutic agents, as functional food ingredients or antitumor
drugs that may be used for cancer prevention and/or treatment.
Therefore, investigating anticancer peptides, such as SCH-P9 and
SCH-P10, may be important for the development of novel cancer
prevention and treatment strategies. Future studies investigating
the molecular structure and antitumor mechanisms of SCH-P9 and
SCH-P10 may promote the emergence of tetrapeptides as novel
functional food ingredients or antitumor drugs.
In recent years, an increasing number of marine
anticancer peptides have been identified. For instance,
jasplakinolide, which is a cyclic depsipeptide with a 15-carbon
macrocyclic ring, was isolated from marine sponge Jaspis
splendens (29). This peptide
induced apoptosis HCT-116 and HeLa cells by activating caspase-3
and decreasing Bcl-2 protein expression (29). Analogs of jasplakinolide, such as
jasplakinolide B, C, and V, isolated from Jaspis. splendens
have demonstrated anticancer activities in NCI60 cells (29). It has been reported that dolastatin
10, isolated from the marine mollusk Dolabella auricularia,
is a linear pentapeptide that inhibits the proliferation of murine
L1210 leukemia cells (12,17,30).
In addition, the growth-inhibiting effects of this peptide were
associated with induction of apoptosis via increasing p53 and
decreasing Bcl-2 expression levels. Similarly, SCH-P9 and SCH-P10
were demonstrated to serve a role in increasing apoptosis of
prostate cancer cells. An increase in PI and annexin V staining in
DU-145 and PC-3 cells was observed following treatment with SCH-P9
and SCH-P10 for 24 h, which indicates that SCH-P9 and SCH-P10
induces apoptosis of prostate cancer cells.
The acquisition of novel anticancer peptides from
marine sources has gained significant attention due to potential
therapeutic applications. Foodstuffs that improve the state of
health and/or reduce the risk of disease are defined as functional
foods (31). Functional foods with
added health benefits, such as anticancer effects, are currently of
major interest to consumers. In the present study, SCH-P9 and
SCH-P10 demonstrated antitumor effects and possessed
apoptosis-inducing activities. S. constricta may therefore
present a source of functional food, and its derivatives, SCH-P9
and SCH-P10, may be useful as anticancer therapies.
In conclusion, the results of the current study
present the purification and characterization of two novel
anticancer peptides derived from the peptide hydrolysates of S.
constricta. The two peptides, SCH-P9 and SCH-P10, were
identified as Leu-Pro-Gly-Pro (MW, 382.46 Da) and Asp-Tyr-Val-Pro
(MW, 492.53 Da), respectively. SCH-P9 and SCH-P10 inhibited the
growth of DU-145 and PC-3 cells in a dose- and time-dependent
manner. These results indicate that SCH-P9 and SCH-P10 present a
potential source of functional food, which may promote the
emergence of tetrapeptides as novel functional food ingredients or
antitumor drugs.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant nos. LY13C200004,
LY15C200016, LY12C20005, LY12C20008, LQ16H300001, Y201534400 and
21136001315), the Science and Technology Department of Zhejiang
Province (grant no. 2013C03036), the Scientific and Technologic
Planning of Zhoushan (grant no. 2012C21013) and the Scientific
Research Foundation of Zhejiang Ocean University (grant no.
Q1408).
References
|
1
|
Chakrabarti S, Jahandideh F and Wu J:
Food-derived bioactive peptides on inflammation and oxidative
stress. Biomed Res Int. 2014:6089792014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mora L, Aristoy MC and Toldrá F: Bioactive
Peptides in Foods. Encyclopedia Food Health. 1–400. 2016.
|
|
3
|
Udenigwe CC and Aluko RE: Food
protein-derived bioactive peptides: Production, processing, and
potential health benefits. J Food Sci. 77:R11–R24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan WR, Chen PW, Chen YL, Hsu HC, Lin CC
and Chen WJ: Bovine lactoferricin B induces apoptosis of human
gastric cancer cell line AGS by inhibition of autophagy at a late
stage. J Dairy Sci. 96:7511–7520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong MC, Goggins WB, Wang HH, Fung FD,
Leung C, Wong SY, Ng CF and Sung JJ: Global Incidence and Mortality
for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36
Countries. Eur Urol. 70:862–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2016. Ca Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng L, Wang C, Liu H, Wang F, Zheng L,
Zhao J, Chu E and Lin X: A novel polypeptide extracted from Ciona
savignyi induces apoptosis through a mitochondrial-mediated pathway
in human colorectal carcinoma cells. Clin Colorectal Cancer.
11:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang TC, Lee JF and Chen JY: Pardaxin, an
antimicrobial peptide, triggers caspase-dependent and ROS-mediated
apoptosis in HT-1080 cells. Mar Drugs. 9:1995–2009. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Liu M, Cheng L, Wei J, Wu N, Zheng
L and Lin X: A novel polypeptide from Meretrix meretrix Linnaeus
inhibits the growth of human lung adenocarcinoma. Exp Biol Med
(Maywood). 237:442–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lyu P, Zhang SD, Yuen HF, McCrudden CM,
Wen Q, Chan KW and Kwok HF: Identification of TWIST-interacting
genes in prostate cancer. Sci China Life Sci. 60:386–396. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
12
|
Luesch H, Moore RE, Paul VJ, Mooberry SL
and Corbett TH: Isolation of dolastatin 10 from the marine
cyanobacterium Symploca species VP642 and total stereochemistry and
biological evaluation of its analogue symplostatin 1. J Nat Prod.
64:907–910. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Damiani RM, Moura DJ, Viau CM, Caceres RA,
Henriques JA and Saffi J: Pathways of cardiac toxicity: comparison
between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch
Toxicol. 90:2063–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mader JS, Richardson A, Salsman J, Top D,
de Antueno R, Duncan R and Hoskin DW: Bovine lactoferricin causes
apoptosis in Jurkat T-leukemia cells by sequential permeabilization
of the cell membrane and targeting of mitochondria. Exp Cell Res.
313:2634–2650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okumura K, Itoh A, Isogai E, Hirose K,
Hosokawa Y, Abiko Y, Shibata T, Hirata M and Isogai H: C-terminal
domain of human CAP18 antimicrobial peptide induces apoptosis in
oral squamous cell carcinoma SAS-H1 cells. Cancer Lett.
212:185–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saadi S, Saari N, Anwar F, Hamid AA and
Ghazali H Mohd: Recent advances in food biopeptides: Production,
biological functionalities and therapeutic applications. Biotechnol
Adv. 33:80–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng L, Lin X, Wu N, Liu M, Zheng Y,
Sheng J, Ji X and Sun M: Targeting cellular apoptotic pathway with
peptides from marine organisms. Biochim Biophys Acta. 1836:42–48.
2013.PubMed/NCBI
|
|
18
|
Song R, Wei RB, Luo HY and Yang ZS:
Isolation and identification of an antiproliferative peptide
derived from heated products of peptic hydrolysates of half-fin
anchovy (Setipinna taty). J Functional Foods. 10:104–111. 2014.
View Article : Google Scholar
|
|
19
|
Sato S, Ohta K, Kojima K, Kozeki Ohmachi
and Yoshida T: Isolation and characterization of two types of
xyloglucanases from a phytopathogenic fungus, Verticillium dahliae.
J App Glycoscience. 9:110–112. 2016.
|
|
20
|
Singh BP, Vij S and Hati S: Functional
significance of bioactive peptides derived from soybean. Peptides.
54:171–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim EK, Kim YS, Hwang JW, Lee JS, Moon SH,
Jeon BT and Park PJ: Purification and characterization of a novel
anticancer peptide derived from Ruditapes philippinarum. Pro Bioch.
48:1086–1090. 2013. View Article : Google Scholar
|
|
22
|
Kim EK, Joung HJ, Kim YS, Hwang JW, Ahn
CB, Jeon YJ, Moon SH and Park PJ: Purification of a novel
anticancer peptide from enzymatic hydrolysate of Mytilus coruscus.
J Microbiol Biotechnol. 22:1381–1387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke-Han XU, Shen XR and Chen GH:
Angiotensin I-converting enzyme (ACE) inhibitory activity of
enzymatic hydrolysate from three-spot hippocampus. Sci Technol Food
Industry. 36:96–99. 2015.(In Chinese).
|
|
24
|
Vermeirssen V and Jon JV: Verstraete:
Fractionation of angiotensin I converting enzyme inhibitory
activity from pea and whey protein in vitro gastrointestinal
digests. J Sci Food Agr. 85:399–405. 2005. View Article : Google Scholar
|
|
25
|
Zhu Z, Qiu N and Yi J: Production and
characterization of angiotensin converting enzyme (ACE) inhibitory
peptides from apricot (Prunus armeniaca L.) kernel protein
hydrolysate. Eur Food Res Technol. 231:13–19. 2010. View Article : Google Scholar
|
|
26
|
Huang F, Yang Z, Yu D, Wang J, Li R and
Ding G: Sepia ink oligopeptide induces apoptosis in prostate cancer
cell lines via caspase-3 activation and elevation of Bax/Bcl-2
ratio. Mar Drugs. 10:2153–2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song R, Wei RB, Luo HY and Yang ZS:
Isolation and identification of an antiproliferative peptide
derived from heated products of peptic hydrolysates of half-fin
anchovy (Setipinna taty). J Func Foods. 10:104–111. 2014.
View Article : Google Scholar
|
|
28
|
Jumeri and Kim SM: Antioxidant and
anticancer activities of enzymatic hydrolysates of solitary
tunicate (Styela clava). Food Sci Biotechnol. 20:10752011.
View Article : Google Scholar
|
|
29
|
Watts KR, Morinaka BI, Amagata T, Robinson
SJ, Tenney K, Bray WM, Gassner NC, Lokey RS, Media J, Valeriote FA
and Crews P: Biostructural features of additional jasplakinolide
(jaspamide) analogues. J Nat Prod. 74:341–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pettit GR, Kamano Y, Fujii Y, Herald CL,
Inoue M, Brown P, Gust D, Kitahara K, Schmidt JM, Doubek DL and
Michel C: Marine animal biosynthetic constituents for cancer
chemotherapy. J Nat Prod. 44:482–485. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiménez-Colmenero F: Potential
applications of multiple emulsions in the development of healthy
and functional foods. Food Res Int. 52:64–74. 2013. View Article : Google Scholar
|