Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by chronic, progressive and invasive
arthrosynovitis (1). RA affects
0.5–1% of the adult population (2). Its pathological features include
persistent synovitis, abnormal synovial hyperplasia, increased
angiogenesis and pannus formation. RA gradually expands into the
articular surface and articular cartilage to induce the progressive
destruction of cartilage and bones, eventually resulting in joint
deformity (3,4). There have been many recent and
relevant studies on RA; however, its pathogenic mechanism has not
yet been elucidated.
Lysyl oxidase (LOX), an extracellular
copper-dependent amine oxidase, participates in the catalysis of
cross-links between the lysine residues of collagen and elastin in
the extracellular matrix, and is important in the initial stage of
the conversion of soluble collagen and elastin monomers into
insoluble fibers. LOX is involved in numerous cellular
physiological and pathological processes including extracellular
matrix formation, cell proliferation, chemotaxis, inflammation,
angiogenesis and tumor formation (5). Abnormal LOX expression is associated
with the occurrence and development of various diseases. Decreased
expression, decreased activity and a lack of LOX are associated
with cutis laxa, emphysema and uterine prolapse (6,7),
whereas increased LOX expression is associated with scleroderma
(8), cirrhosis (9,10)
and tumor metastasis (11,12). A previous study of the authors
indicated that high concentrations of LOX are present in the
synovial membrane and synovial fluid of patients with RA (13); however, the role of LOX in joint
diseases associated with RA remains unclear.
The type II collagen-induced arthritis (CIA) model
is currently the most commonly used animal model for RA studies, as
it has immunological, pathological and arthritic presentations
similar to those observed in RA in humans (14,15).
In the present study, a rat CIA model was established and
β-aminopropionitrile (BAPN) was used to inhibit LOX activity
(16,17) in order to observe synovial
hyperplasia and angiogenesis, and to investigate the role and
mechanism of LOX in arthritic diseases of rats. The present study
aimed to provide theoretical bases for further studies
investigating pathogenic mechanism underlying RA and novel targets
for clinical treatment.
Materials and methods
Animals
A total of 30 6–8-week-old healthy male Sprague
Dawley rats (SPF grade) with body weights of 180–200 g were
purchased from Shanghai Sino-British SIPPR/BK Laboratory Animal
Co., Ltd. [Shanghai, China; permit no: SCXK (Hu) 2013–0016]. Rats
were housed in separate cages and were fed a standard diet every
day. Following adaptive feeding under the conditions of a 12:12 h
light:dark cycle, with a temperature of 22±2°C, and 55±5% humidity
for 1 week, experiments were performed in accordance with the
Guidelines for the Care and Use of Laboratory Animals. The present
study was approved by the Ethics Committee of the General Hospital
of Ningxia Medical University (Yinchuan, China; registration no.
2016-230).
Establishment of the CIA model and
drug administration
SD rats were randomly divided into a control group,
model group and intervention group (n=10/group). For rats in the
model group, 0.2 ml bovine collagen II (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) emulsified by complete Freund's adjuvant
(Sigma-Aldrich; Merck KGaA) was intradermally injected into the
back, tail and footpad; a booster was administered after 7 days
using the same method, in the same locations, and at the same dose.
The model establishment method for rats in the intervention group
was the same as that in the model group; in addition, BAPN
(Sigma-Aldrich; Merck KGaA) was intraperitoneally injected (100
mg/kg/days) for 40 days to inhibit LOX activity. Rats in the
control group were injected with an equal volume of normal saline
using the same method and at the same locations as in the model
group.
Assessment of the arthritis index
(AI)
From day 4 after the booster administration, the
conditions and extent of joint redness and swelling were evaluated
every 4 days using the AI scoring method of the left hind paw joint
of each rat. This was determined using a 5-grade scoring method: 0,
normal, without any macroscopic signs of arthritis; 1, mild redness
and swelling of the toe joint; 2, moderate redness and swelling of
the toe joint; 3, severe redness and swelling of the ankle; and 4,
extreme severe redness, swelling and deformation of the ankle
joint, including complete paw swelling and inability to bend the
joint and walk (18,19). Rats with an AI score of ≥2 were
selected for use in the subsequent experiments.
Evaluation of paw edema
The assessment of paw edema was performed one day
prior to the first injection and once every 4 days following the
booster injection. The left hind paw volume of each rat was
determined using the drainage volume method, as follows. A line was
drawn as a marker at the salient section of the left hind paw ankle
joint of each rat. A specific measuring cylinder was filled with
water and the paw was inserted to allow the water surface to reach
the marked level of the labeled ankle. A beaker was used to capture
the volume of the water displaced, which determined the paw volume
in ml. Each paw evaluation was repeated five times and the mean
value was recorded.
Sample collection
At the end of experiment, rats were anesthetized
with chloral hydrate (350 mg/kg; intraperitoneal) and sacrificed by
cervical dislocation. Blood, synovial fluid and synovial membrane
from the knee joint were collected. The collected blood samples
were incubated at room temperature for 2 h and then centrifuged at
2,000 × g for 20 min to obtain the serum. The synovial fluids were
centrifuged in order to obtain the supernatants. Serum and
supernatant samples were divided into aliquots and frozen at −80°C
for subsequent use. Synovial membranes were washed with sterile
normal saline, placed in a 0.5 ml tube, labeled and stored at
−80°C.
Hematoxylin and eosin (H&E)
staining
Rat synovial membranes were fixed using 4%
paraformaldehyde, dehydrated in graded alcohol, cleared with
xylene, embedded in paraffin and sectioned. H&E staining was
performed and hyperplasia of the synovial membrane was observed
under a light microscope. For each membrane section, five areas
were selected randomly and images were captured using a low-power
lens (magnification, ×100).
Inspection of microvascular density
(MVD)
Synovial membrane sections were blocked in 10% fetal
bovine serum at room temperature for 30 min and then incubated with
rabbit anti-rat CD34 monoclonal antibodies (1:200 dilution, cat.
no. ab81289; Abcam, Cambridge, MA, USA) at 4°C overnight. Following
washing, the membrane sections were processed with rabbit
horseradish peroxidase (HRP)-polymer kit (cat. no. PV-6001;
ZSGB-BIO, Beijing, China) at room temperature, developed using
3,3′-diaminobenzidine (DAB) for 4 min and mounted on slides. MVD
was determined by evaluating the number of CD34-stained vessels in
the membrane samples. In brief, the five hot spot areas with the
highest numbers of microvessels were selected from each membrane
section using a low-power lens (magnification, ×100), and numbers
of microvessels in each of these areas were determined using a
high-power lens (magnification, ×400). The mean microvessel number
represented the MVD in the synovial membrane.
Immunohistochemical (IHC)
analysis
Synovial membrane sections were blocked and then
incubated with rabbit anti-rat LOX polyclonal antibodies (1:100
dilution; cat. no. ab31238; Abcam), rabbit anti-rat MMP-2
polyclonal antibodies (1:400 dilution; cat. no. ab37150; Abcam) or
mouse anti-rat MMP-9 monoclonal antibodies (1:500 dilution; cat.
no. ab38898; Abcam) at 4°C overnight. Following washing, the
membrane sections were processed with rabbit HRP-polymer kit (cat.
no. PV-6001; ZSGB-BIO, Beijing, China), developed using DAB for 4
min, cleared and mounted on slides. Under a light microscope, five
or more hot spots in each section were observed. Images were
captured using DP controller software. Image-Pro Plus version 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA) was used for
image analysis to determine the positively stained surface areas
and the integrated optical densities (IODs) of captured images. The
relative expression level was calculated using the average optical
density (AOD): AOD = IOD/positive area.
ELISA
LOX, MMP-2 and MMP-9 concentrations in the synovial
fluid and serum were detected using rat ELISA kits (cat nos.
SEC580Ra, SEA100Ra and SEA553Ra), according to the manufacturer's
instructions (Cloud-Clone Corp., Houston, TX, USA). Synovial fluid
was diluted at 1:10, and serum was diluted at 1:5. The optical
density values were used to calculate the concentrations of LOX,
MMP-2 and MMP-9 in all of the samples. The concentration was
multiplied by the dilution fold in order to obtain the actual
concentration.
Amplex Red method
An Amplex Red reagent kit was purchased from Abcam
(cat. no. ab112139; Cambridge, MA, USA) and used to evaluate LOX
activities in the rat serum and synovial fluid. All experimental
procedures were performed according to the manufacturer's protocol.
Synovial fluid and serum were diluted at 1:5. The diluted serum and
synovial fluid samples were divided into the experimental group and
the parallel group. All wells in the parallel group were
supplemented with 0.3 mM BAPN to inhibit the activity of LOX.
Various concentrations of H2O2 were used as
the standards of oxidation in order to plot a standard curve and
obtain the corresponding calculation formula. Finally, the
oxidation activity value in the parallel group was subtracted from
the oxidation activity value in the experimental group to determine
the oxidation activity value of LOX in the samples.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS statistical software
(version, 20.0; IBM SPSS, Armonk, NY, USA). Normally distributed
continuous variables were compared using one-way analysis of
variance. Non-normally distributed data were compared using
nonparametric tests, including the Mann-Whitney U test or the
Kolmogorov-Smirnov test. Pearson correlation analysis was performed
to analyze the correlation between LOX expression level and MVD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
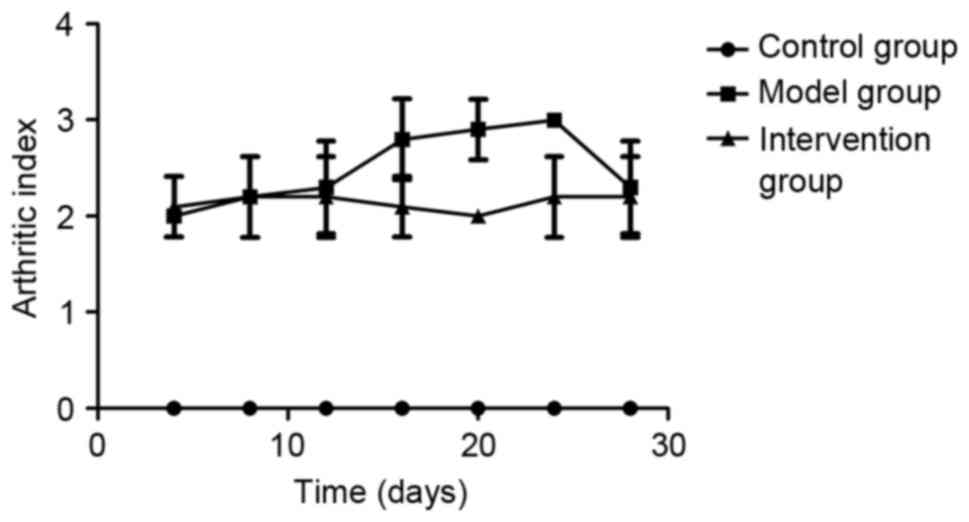
Inhibiting LOX with BAPN decreased the
AI scores in CIA rats
AIs were determined on days 4, 8, 12, 16, 20, 24 and
28 following the booster immunization with collagen. Rats in the
control group demonstrated no macroscopic signs of arthritis. AI
scores in the model group and the intervention group were higher
compared with those in the control group (P<0.05), and the
scores in the intervention group were lower compared with those in
the model group on days 16, 20 and 24 (P<0.05; Fig. 1). The redness and swelling of the
toe joint was markedly ameliorated following inhibition of LOX by
BAPN. An AI score of ≥2 for each rat in the model and intervention
group was considered to indicate successful model
establishment.
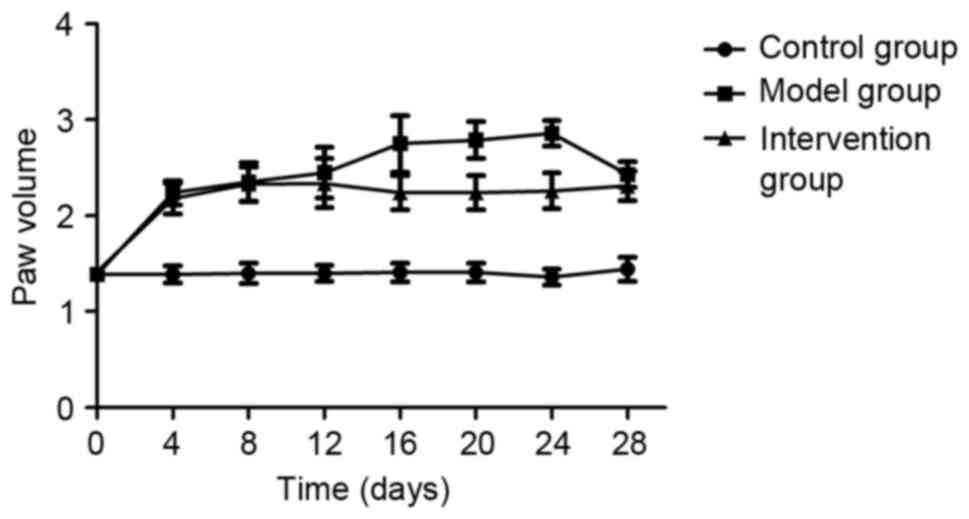
Inhibiting LOX with BAPN attenuated
the hind paw swelling in CIA rats
Prior to the first collagen injection (day 0), no
significant hind paw swelling in any of the rats of the three
experimental groups was observed (P>0.05). At day 4 following
the collagen booster, significantly increased levels of hind paw
swelling were observed in rats of the model and intervention groups
compared with those in the control group (P<0.05), however, the
paw swelling in rats of the intervention group was decreased on
days 16, 20 and 24 compared with in the model group (P<0.05;
Fig. 2).
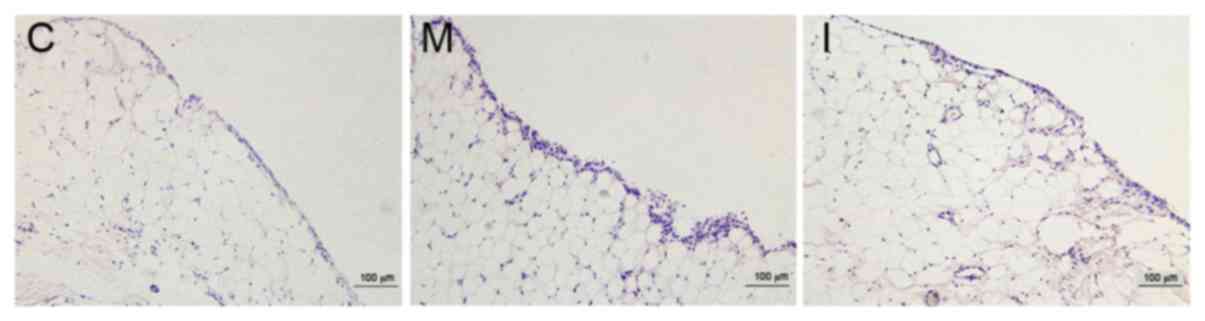
Inhibiting LOX with BAPN decreased
synovial hyperplasia in CIA rats
The synovial membrane surfaces of rats in the
control group were smooth and synovial cells were arranged in a
uniform manner as observed by light microscopy following H&E
staining. Following a longer inflammation induction in the model
and intervention groups, rough synovial tissue surfaces and
thickening of synovial tissues were observed; the number of
synovial cell layers increased and the layers exhibited a
disorderly arrangement, as presented in Fig. 3.
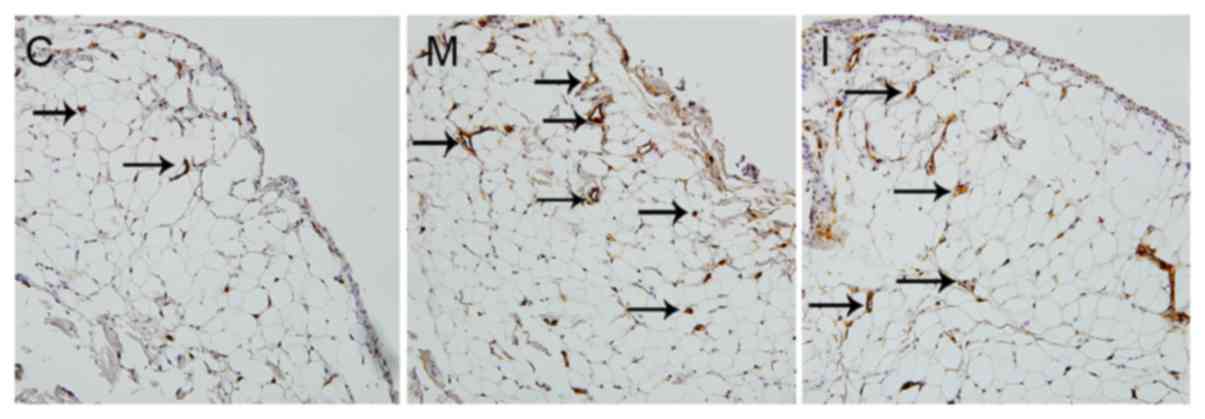
Inhibiting LOX with BAPN increases MVD
in CIA rats
CD34-positive staining, indicated by a yellow-brown
color, was localized in the cytoplasm of vascular endothelial
cells. CD34-positive cells surrounded a lumen-like structure,
forming microvessels (Fig. 4). The
MVDs were 13.44±2.94 in the model group and 11.22±1.67 in the
intervention group, and were higher compared with that in the
control group (9.10±1.94; P<0.05). The MVD in the intervention
group was lower compared with that in the model group
(P<0.05).
Expressions levels of LOX, MMP-2 and
MMP-9 in synovial membrane of rats
The IHC results indicated that LOX expression in the
rat synovial membrane was primarily distributed in the cytoplasm of
synovial cells and the extracellular matrix (Fig. 5A). MMP-2 and MMP-9 were also
primarily distributed in the cytoplasm of synovial cells (Fig. 5B and C). The AODs of LOX, MMP-2 and
MMP-9 in rat synovial membrane of the model group were higher
compared with those in the control group and the intervention group
(P<0.05), whereas the AODs of LOX, MMP-2 and MMP-9 in the
intervention group were lower compared with those in the control
group (P<0.05; Fig. 5D).
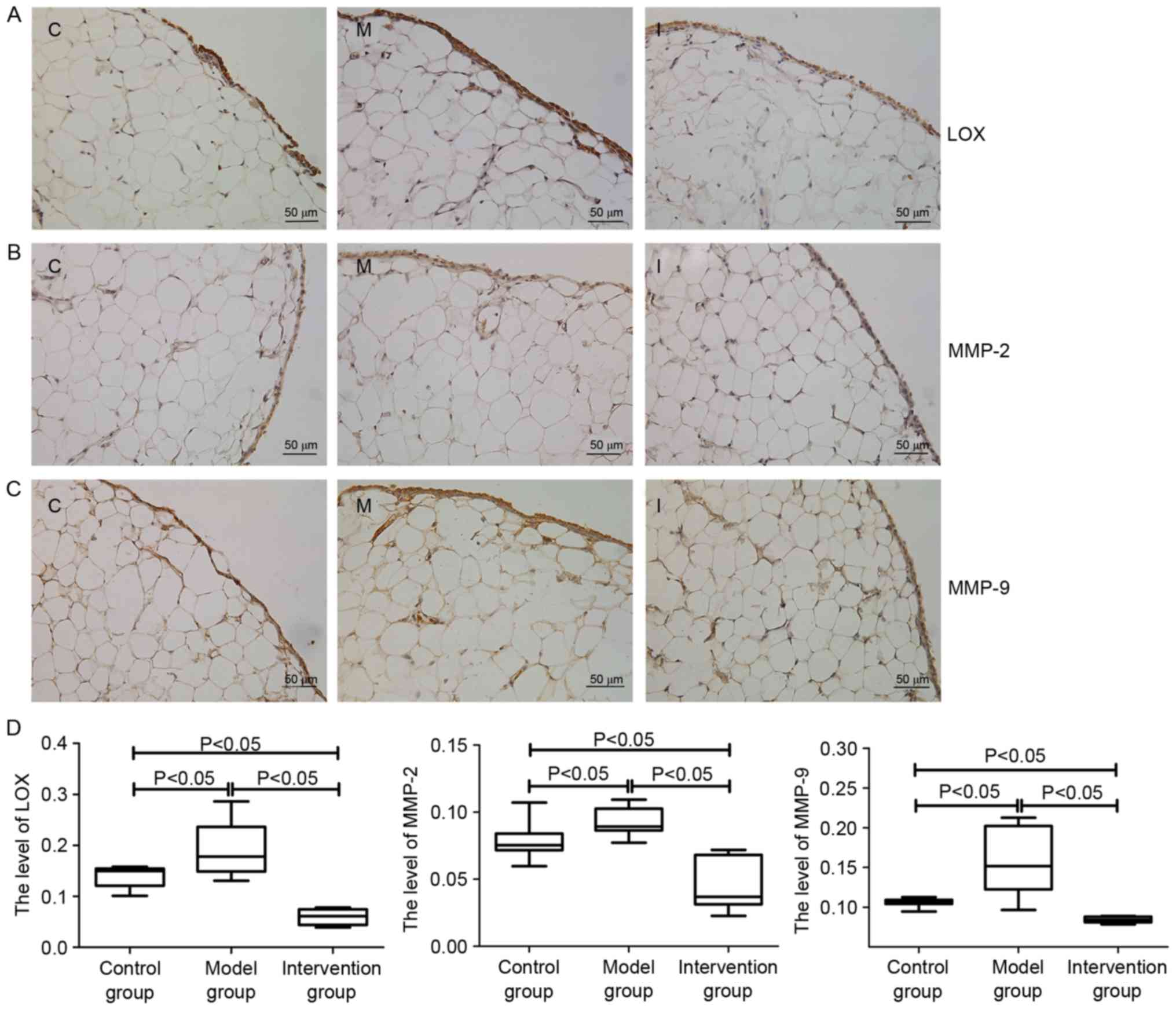 | Figure 5.Expressions levels of (A) LOX, (B)
MMP-2 and (C) MMP-9 in rats of the synovial membrane of the control
group (C), model group (M) and intervention group (I), as
determined by immunohistochemical staining. Following establishment
of the CIA model, the expressions levels of LOX, MMP-2 and MMP-9 in
the synovial membranes were increased compared with those in the
control group. However, following inhibition of LOX with BAPN, the
expression levels of MMP-2 and MMP-9 were decreased significantly
compared with those in (D) the model group. Data are presented as
the mean ± standard deviation. LOX, lysyl oxidase; MMP, matrix
metalloproteinase; CIA, collagen-induced arthritis; BAPN,
β-aminopropionitrile. |
Comparison of LOX, MMP-2 and MMP-9
expression levels in the synovial fluid and serum
ELISA results demonstrated that the concentrations
of LOX, MMP-2 and MMP-9 in the synovial fluid and serum of rats
from the model group were significantly higher compared with those
in the control and intervention groups (P<0.05). Concentrations
of LOX, MMP-2 and MMP-9 in the control group were significantly
higher compared with that in the intervention group (P<0.05;
Table I).
 | Table I.Comparison of the concentrations of
LOX, MMP-2, MMP-9 and LOX enzyme activity in the synovial fluid and
serum among three groups (n=10). |
Table I.
Comparison of the concentrations of
LOX, MMP-2, MMP-9 and LOX enzyme activity in the synovial fluid and
serum among three groups (n=10).
|
| Concentration of LOX
(ng/ml) | Concentration of
MMP-2 (ng/ml) | Concentration of
MMP-9 (ng/ml) | LOX enzyme
activity |
|---|
|
|
|
|
|
|
|---|
| Groups | Serum | Synovial fluid | Serum | Synovial fluid | Serum | Synovial fluid | Serum | Synovial fluid |
|---|
| Control | 8.92±0.78 | 8.35±2.39 | 38.66±2.41 | 20.07±1.56 | 110.05±34.02 | 130.71±51.08 | 0.20±0.04 | 0.17±0.05 |
| Model | 10.92±2.70 | 9.99±1.73 | 47.04±2.33 | 28.97±2.79 | 213.58±21.52 | 223.47±41.92 | 0.34±0.13 | 0.22±0.07 |
| Intervention | 7.16±0.59 | 6.76±0.26 | 29.84±2.27 | 13.91±2.20 | 23.92±12.69 | 31.83±19.11 | 0.10±0.07 | 0.11±0.04 |
| P-value | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 |
Comparison of LOX enzyme activity in
the serum and synovial fluid
The enzyme activity of LOX in the model group was
significantly higher compared with that in the control and
intervention groups (P<0.05), and the LOX activity in rats of
the control group was higher compared with that in the intervention
group (P<0.05; Table I).
Correlation of LOX expression levels
with MVD, MMP-2 and MMP-9 in the synovial membrane
Pearson correlation analysis revealed that the
expression level of LOX in the synovial membrane was positively
correlated with MVD (r=0.253; P<0.05), MMP-2 (r=0.741;
P<0.01) and MMP-9 (r=0.644; P<0.01).
Discussion
The pathogenic mechanism underlying RA is
complicated. Genetic, environmental, and immunological factors
affect its occurrence and development (20,21).
Increasing evidence indicates that various immune cells (including
T lymphocytes, B lymphocytes, and synovial fibroblasts), cytokines
(including tumor necrosis factor-α and interleukin-6), and enzymes
(MMPs) are involved in the mechanisms underlying RA (22,23).
However, whether LOX affects RA, and the potential underlying
mechanism of action of LOX remains unclear.
In the present study, CIA rats were selected as an
experimental animal model. During the process of model
establishment, rats in the model group and the intervention group
exhibited hind paw redness and swelling from day 2 following the
first injection. On day 24 following administration of the collagen
booster, the hind paw and ankle joint swelling was most severe. At
all evaluation time-points, the AI and joint swelling of rats in
the model and intervention groups were significantly higher
compared with those in rats of the control group; these findings
are in accordance with the disease pattern of the type II
collagen-induced CIA model. H&E staining of the synovial
membrane demonstrated that rats in the model and intervention
groups had rough and thickened synovial tissue surfaces and
increased numbers of disordered synovial cell layers. In addition,
the MVD in the synovial membrane was higher compared with that in
the control group. These results confirmed that the model was
successfully established.
LOX is an oxidase secreted by numerous types of
cells and is a key enzyme involved in the cross-linking of collagen
and elastin in extracellular matrix. LOX participates in the
stabilization and remodeling of the extracellular matrix (24). LOX has a 50 kDa zymogene form and a
32 kDa active form. The active form of LOX is secreted into the
serum and bodily fluids. The results of the present study
demonstrated that the concentrations of LOX in the synovial
membrane, synovial fluid and serum of rats in the model group were
significantly higher compared with those in the control group. The
enzymatic activity of LOX in the synovial fluid and serum of the
model group was higher compared with that in the control group; in
addition, the concentration of LOX was positively correlated with
the MVD in the synovial membranes. These results suggested that LOX
may be involved in diseases of the joints and angiogenesis in rats
of the model group.
BAPN is an irreversible inhibitor of LOX. Following
LOX inhibition by BAPN in rats of the intervention group, the
concentrations and activities of LOX in the serum, synovial fluid
and synovial membrane were significantly decreased compared with
those in the model and control groups. These results revealed that
BAPN inhibited but did not completely suppress LOX in rats. In the
present study, the AI, hind paw swelling, synovial hyperplasia and
MVD of rats in the intervention group were lower compared with
those in rats of the CIA model group. These results suggested that
the suppression of LOX may reduce arthritic inflammation and
angiogenesis in rats, and may attenuate and improve the
pathological process of arthritis. In addition, the results
revealed the involvement of LOX in diseases of the joint and
angiogenesis in rats of the model group. This suggested that LOX
inhibition may be a potential therapeutic target in such
diseases.
The MMP family is a group of zinc-dependent
endopeptidases that are present in the normal human body, degrade
almost all extracellular matrixes, and are major regulators of
tissue remodeling and extracellular matrix degradation (25,26).
High expression levels of MMP-2 (also known as gelatinase A) and
MMP-9 (also known as gelatinase B) promote the occurrence and
development of RA (27,28). The expression levels of MMP-2 and
MMP-9 are increased in the synovial membrane of patients with RA,
which is a major causative factor of the degradation of
extracellular matrix components, including collagen and
proteoglycan, in the bones and cartilage of diseased joints
(29–31). The results of the present study
also demonstrated that the expression levels of MMP-2 and MMP-9 in
the serum, synovial fluid and synovial membrane of rats in the
model group were significantly higher compared with those in the
control group, indicating that MMP-2 and MMP-9 are involved in the
development of arthritis in rats of the model group. However,
following the inhibition of LOX activity by BAPN, the expression
levels of MMP-2 and MMP-9 in the serum, synovial fluid and synovial
tissues in rats of the intervention group decreased to levels lower
than those in the model and control groups. Pearson's correlation
analysis revealed that LOX expression level in synovial tissues was
positively correlated with MMP-2 and MMP-9 expression levels,
suggesting that the inhibition of LOX may downregulate the
expression of MMP-2 and MMP-9. The functionality of LOX in the
attenuation or improvement of diseases in rats of the intervention
group may be mediate by this mechanism. However, the specific
pathway of MMP-2 and MMP-9 downregulation by LOX remains unclear.
The detailed mechanism underlying the action of LOX in CIA rats
requires further study.
Currently, there is no cure for RA. Hyperplasia of
the synovial membrane and angiogenesis serve important roles in the
occurrence and delay of RA; therefore, targeting this pathological
basis in further investigation of potential therapeutic targets is
expected to provide novel approaches for RA treatment (32). The results of the present study
suggested that the inhibition of LOX may attenuate synovial
hyperplasia and angiogenesis in type II collagen-induced CIA model
rats and ameliorate the damage induced by MMP-2 and MMP-9.
In summary, the present study demonstrated high
expressions levels of LOX in the synovial membranes, synovial fluid
and serum of CIA rats. Inhibition of LOX attenuated inflammation,
synovial hyperplasia, angiogenesis and expression of MMP-2 and
MMP-9, indicating that LOX promotes synovial hyperplasia and
angiogenesis in CIA rats. Therefore, LOX may be a potential target
for the treatment of RA.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant. no. 81260459) and the
Science and Technology Research Project of the Department of
Education in Ningxia (grant. no. NGY2012050).
References
|
1
|
Mclnnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bansback NJ, Regier DA, Ara R, Brennan A,
Shojania K, Esdaile JM, Anis AH and Marra CA: An overview of
economic evaluations for drugs used in rheumatoid arthritis: Focus
on tumour necrosis factor-alpha antagonists. Drugs. 65:473–496.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibofsky A: Overview of epidemiology,
pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag
Care. 18 13 Suppl:S295–S302. 2012.PubMed/NCBI
|
|
4
|
Scherer HU and Burmester GR: A clinical
perspective of rheumatoid arthritis. Eur J Immunol. 39:2044–2048.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kagan HM and Li W: Lysyl oxidase:
Properties, specificity, and biological roles inside and outside of
the cell. J Cell Biochem. 88:660–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khakoo A, Thomas R, Trompeter R, Duffy P,
Price R and Pope FM: Congenital cutis laxa and lysyl oxidase
deficiency. Clin Genet. 51:109–114. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Zhou J, Chen L, Luo Z and Zhao Y:
Lysyl oxidase, a critical intra- and extra-cellular target in the
lung for cigarette smoke pathogenesis. Int J Environ Res Public
Health. 8:161–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rimar D, Rosner I, Nov Y, Slobodin G,
Rozenbaum M, Halasz K, Haj T, Jiries N, Kaly L, Boulman N, et al:
Brief report: Lysyl oxidase is a potential biomarker of fibrosis in
systemic sclerosis. Arthritis Rheumatol. 66:726–730. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mesarwi OA, Shin MK, Drager LF,
Bevans-Fonti S, Jun JC, Putcha N, Torbenson MS, Pedrosa RP,
Lorenzi-Filho G, Steele KE, et al: Lysyl oxidase as a serum
biomarker of liver fibrosis in patients with severe obesity and
obstructive sleep apnea. Sleep. 38:1583–1591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baiocchini A, Montaldo C, Conigiaro A,
Grimaldi A, Correani V, Mura F, Ciccosanti F, Rotiroti N, Brenna A,
Montalbano M, et al: Extracellular matrix molecular remodeling in
human liver fibrosis evolution. PLoS One. 11:e01517362016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rankin EB and Giaccia AJ: Hypoxic control
of metastasis. Science. 352:175–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perryman L and Erler JT: Lysyl oxidase in
cancer research. Future Oncol. 10:1709–1717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu R, Sun B, Lin J, Song T, Li H, Wen P
and Han M: Comparison of lysyI oxidase expression between active
rheumatoid arthritis and active osteoarthritis. Chin J Rheumatol.
17:95–97. 2013.(In Chinese).
|
|
14
|
Rosloniec EF, Cremer M, Kang AH, Myers LK
and Brand DD: Collagen-induced arthritisCurrent Protocols in
Immunology. Coico R and Shevach E: John Wiley & Sons; New York:
pp. 15.5.1–15.5.25. 2010
|
|
15
|
Elhai M, Chiocchia G, Marchiol C, Lager F,
Renault G, Colonna M, Bernhardt G, Allanore Y and Avouac J:
Targeting CD226/DNAX accessory molecule-1 (DNAM-1) in
collagen-induced arthritis mouse models. J Inflamm. 12:92015.
View Article : Google Scholar
|
|
16
|
Miana M, Galán M, Martínez-Martínez E,
Varona S, Jurado-López R, Bausa-Miranda B, Antequera A, Luaces M,
Martínez-González J, Rodríguez C and Cachofeiro V: The lysyl
oxidase inhibitor β-aminopropionitrile reduces body weight gain and
improves the metabolic profile in diet-induced obesity in rats. Dis
Models Mech. 8:543–551. 2015. View Article : Google Scholar
|
|
17
|
Gao HY, Luo J, Li XF, Lv Q, Wen HY, Song
QZ, Zhao WP, Zhao XC, Zhang TT, Zhang SY and Zhi JM: Changes in
focal adhesion kinase expression in rats with collagen-induced
arthritis and efficacy of intervention with disease modifying
anti-rheumatic drugs alone or in combination. Int J Clin Exp
Pathol. 8:15573–15581. 2015.PubMed/NCBI
|
|
18
|
Amdekar S and Singh V: Lactobacillus
acidophilus maintained oxidative stress from reproductive organs in
collagen-induced arthritic rats. J Hum Reprod Sci. 9:41–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aciksari K, Yanar HT, Hepqul G, Ozucelik
DN, Yanar F, Agcaoglu O, Eser M, Tanriverdi G, Topacoglu H, Ayvaci
BM, et al: The effect of Beta-aminopropionitrile and prednisolone
on the prevention of fibrosis in alkali esophageal burns: An
experimental study. Gastroenterology Res Pract.
2013:5742602013.
|
|
20
|
Jutley G, Raza K and Buckley CD: New
pathogenic insights into rheumatoid arthritis. Curr Opin Rheumatol.
27:249–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haag S, Tuncel J, Thordardottir S, Mason
DE, Yau AC, Dobritzsch D, Bäcklund J, Peters EC and Holmdahl R:
Positional identification of RT1-B (HLA-DQ) as susceptibility locus
for autoimmune arthritis. J Immunol. 194:2539–2550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klimiuk PA, Domysławska I, Sierakowski S
and Chwiećko J: Regulation of serum matrix metalloproteinases and
tissue inhibitor of metalloproteinases-1 following rituximab
therapy in patients with rheumatoid arthritis refractory to
anti-tumor necrosis factor blockers. Rheumatol Int. 35:749–755.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mc Ardle A, Flatley B, Pennington SR and
FitzGerald O: Early biomarkers of joint damage in rheumatoid and
psoriatic arthritis. Arthritis Res Ther. 17:1412015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue B: Biology of the extracellular
matrix: An overview. J Glaucoma. 23 8 Suppl 1:S20–S23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giannandrea M and Parks WC: Diverse
functions of matrix metalloproteinases during fibrosis. Dis Model
Mech. 7:193–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pap T, Shigeyama Y, Kuchen S, Fernihough
JK, Simmen B, Gay RE, Billingham M and Gay S: Differential
expression pattern of membrane-type matrix metalloproteinases in
rheumatoid arthritis. Arthritis Rheum. 43:1226–1232. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elshabrawy HA, Chen Z, Volin MV, Ravella
S, Virupannavar S and Shahrara S: The pathogenic role of
angiogenesis in rheumatoid arthritis. Angiogenesis. 18:433–448.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP,
Han T, Peng W and Zheng CJ: Matrine exerts a strong anti-arthritic
effect on type II collagen-induced arthritis in rats by inhibiting
inflammatory responses. Int J Mol Sci. 17(pii): E14102016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou M, Qin S, Chu Y, Wang F, Chen L and
Lu Y: Immunolocalization of MMP-2 and MMP-9 in human rheumatoid
synovium. Int J Clin Exp Pathol. 7:3048–3056. 2014.PubMed/NCBI
|
|
31
|
Xue M, McKelvey K, Shen K, Minhas N, March
L, Park SY and Jackson CJ: Endogenous MMP-9 and not MMP-2 promotes
rheumatoid synovial fibroblast survival, inflammation and cartilage
degradation. Rheumatology (Oxford). 53:2270–2079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Itoh Y: Metalloproteinases: Potential
therapeutic targets for rheumatoid arthritis. Endocr Metab Immune
Disord Drug Targets. 15:216–222. 2015. View Article : Google Scholar : PubMed/NCBI
|