Introduction
Dendritic cells (DCs), which differentiate to highly
competent antigen-presenting cells, are pivotal elements of immune
response in processing and presenting antigens (1). DCs present immunogenic or tolerogenic
effects in subsequent immune reactions according to their
maturation and functional and molecular expression (2). The current method of DC generation is
based on bone marrow (BM) culture and granulocyte-macrophage
colony-stimulating factor (GM-CSF) supplementation, and is used
worldwide. However, the DC lineage is complex due to the DC cell
derivation and differentiation, and there are many similarities and
diversities compared with other hematopoietic cells, including
monocytes and macrophages (3,4).
A previous study has demonstrated that BM cells are
able to differentiate to classical (or conventional) DCs (cDCs) in
an in vitro mouse models (5). cDCs are considered the most important
and distinct lineage for stimulating naive T cell activation
(3), although the identification
of other DC subsets, such as plasmacytoid DCs, Langerhans cells and
monocyte-derived DCs, has markedly improved. However, cultured DCs
have been demonstrated to be a heterogeneous group of cells
resulting in variations in the usage of DCs (6,7).
This heterogeneous state may be explained as follows: i) The source
of DCs is highly variable, including from BM, peripheral blood
mononuclear cells or monocytes, or from rodents or humans; and ii)
DCs may be modulated by cultural environments and stimulating
factors, and the differentiation of stem cells or progeny DCs is
extremely complex, which results in numerous subsets. Another
problem is that at the end of the culture process, different DC
subsets are selected for subsequent experiments, including
non-adherent mature DCs, all non-adherent cells, loosely adherent
clusters, both non-adherent and loosely adherent cells or all cells
(8–11). Several previous studies have not
provided information on the DC subsets that were examined (12,13).
This phenomenon reflects a widespread lack of information regarding
the heterogeneity of cultured DCs, which has resulted in a lack of
clear understanding of the findings related to their usage
(14–16). Therefore, efforts are still
required to optimize DC culture systems and to discriminate the
heterogeneity of DC culture subsets.
In the present study, DCs were divided into three
subsets: i) Non-adherent; ii) adherent; and iii) mixed. Cytokine
secretion from progeny DCs and DCs was evaluated on culture days 3,
6 and 8. In addition, the maturation state of the three subsets in
the presence of lipopolysaccharide (LPS) stimulation was detected.
Accordingly, at the end of the culture process, mixed lymphocyte
reaction (MLR) was used to analyze the ability of each subset to
stimulate T cell proliferation by alloantigen presentation. This
study provided a promising BM-derived DC culture system in regards
to the quantity and quality of the final DC products. Notably, to
the best of our knowledge, this was the first study to divide
cultured DCs into three subsets to observe their heterogenic
immunological properties based on their adherent status. These
aspects may be emphasized in immunological investigations when
using cultured DCs.
Materials and methods
Animals
The commonly used mouse strains C57BL/6
(H2b) (n=8) and BALB/c (H2d) (n=32) were used
in the present study. A total of 40 male mice (age, 6–8 weeks;
weight, 20±1 g) were obtained from Beijing HFK Bioscience Co. Ltd.
(Beijing, China), and kept under specific pathogen-free conditions,
at 25°C in 55% humidity and under 12-h light/dark cycles, with free
access to food and water. All experiments in this protocol were
approved by the Institutional Animal Care and Use Committee at
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China).
Bone marrow preparation and DC culture
system
Balb/c were euthanized and rinsed liberally in
ethanol for 5 min. The hindlimbs were severed and the attached soft
tissues were rubbed from the femurs and tibias with sterile gauze.
Both ends of the epiphyses were cut from the marrow cavity and the
marrow was flushed out with RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) into a dish. The medium was
filtered through a 74-µm aperture nylon mesh to a 15 ml centrifuge
tube in order to remove small pieces of bone and debris. The tube
was centrifuged at 300 × g at room temperature for 5 min and the
supernatant was discarded. Red blood cells were collected and lysed
with 1 ml Red Blood Cell Lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) for 2 min to obtain
BM-derived mononuclear cells (BMDMCs). A total of 5 ml RPMI-1640
medium containing 10% FBS was added to dilute the lysis buffer at
room temperature and the mixture was centrifuged at 300 × g at room
temperature for 5 min. Cells (1×106 cells/ml) were
cultured (5% CO2, 37°C) in 10 ml fresh RPMI-1640 medium
containing 10% FBS. Subsequently, recombinant murine (rm) GM-CSF
(20 ng/ml) and rm-interleukin (IL)-4 (10 ng/ml; both from
PeproTech, Inc., Rocky Hill, NJ, USA) were added and the culture
dish was incubated in a humidified 5% CO2 incubator at
37°C.
Following 3 days incubation, the medium (including
non-adherent cells) was aspirated and discarded. Fresh complete
RPMI-640 medium (10 ml) containing rmGM-CSF (20 ng/ml) and rmIL-4
(10 ng/ml) was added and the cells were returned to the incubator.
On day 6, the medium was collected, centrifuged at 300 × g at room
temperature for 5 min, and the cell pellet was resuspended in 10 ml
complete RPMI-1640 medium containing 20 ng/ml rmGM-CSF and 10 ng/ml
rmIL-4. The resuspended cells (~2.5×106 cells) were
returned to the incubator for further culture. On day 7, LPS (500
ng/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
the culture dish to stimulate DC maturation for 24 h, and on day 8,
the different subsets of cultured cells were collected, as
described below, for further investigation.
Isolation of different DC subsets
Cultured cells were divided according to their
different adherent features: Non-adherent, loosely adherent,
dislodgeable adherent and firmly adherent. Non-adherent cells were
the cells floating in the medium following a gently swirling the
dish. Loosely adherent cells were obtained following 3 min
digestion with 0.25% trypsin-EDTA (Gibco; Thermo Fisher Scientific,
Inc.) and gentle pipetting at 37°C. Dislodgeable adherent cells
were defined as the cells that fell to the bottom of the culture
dish following 0.25% trypsin-EDTA digestion for 5 min with vigorous
pipetting at 37°C. Firmly adherent cells were the cells that
remained on the wall following hard trypsin-EDTA digestion and
vigorous pipetting, as have been confirmed as vacuolated
macrophages, as previously described (17). In the present study, DCs were
collected and analyzed in the following subsets: i) Non-adherent
cells; ii) adherent cells, which comprised loosely adherent +
dislodgeable adherent cells; and iii) mixed cells, which comprised
non-adherent cells + adherent cells. As firmly adherent cells do
not contribute to cDC population, they were not used in subsequent
investigations. Cells were harvested, centrifuged at 300 × g for 5
min at room temperature and a single-subset suspension of the
purified cells was prepared for further experiments.
Phenotypic analysis
To analyze phenotypic markers of the different DC
subsets, the cells were stained in the dark at 4°C for 30 min with
the following monoclonal antibodies from eBioscience (Thermo Fisher
Scientific, Inc.): Allophycocyanin (APC)-conjugated anti-CD11c (cat
no. 17-0114, 1:800), phycoerythrin (PE)/cyanine (Cy)5-conjugated
anti-CD40 (cat no. 15-0401, 1:800), fluorescein isothiocyanate
(FITC)-conjugated anti-CD80 (cat no. 11-0801, 1:2,000),
PE/Cy5-conjugated anti-CD86 (cat no. 15-0862, 1:3,333);
PE-conjugated anti-major histocompatibility complex class II
(MHC-II; act no. 12-5321, 1:10,000), APC-conjugated anti-F4/80 (cat
no. 17-4801, 1:100) and PE-conjugated anti-CD11b (cat no. 12-0118,
1:1). Corresponding isotype-matched irrelevant specificity controls
were performed in parallel, using Armenian hamster immunoglobulin
(Ig) G isotype control (cat no. 11-4888; eBioscience; Thermo Fisher
Scientific, Inc.) in the same dilutions as the target antibodies. A
total of 10,000 events were collected for each test by BD
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and cells with large forward scattering and side scattering
were analyzed by FlowJo software version 7.6 (FlowJo LLC, Ashland,
OR, USA).
Cytokine profiling in different
subsets of DCs
During the process of differentiation and
maturation, progeny DCs and parent DCs develop the ability to
secrete cytokines based on their heterogenic immunological features
(18). To explore their immune
function, cytokine secretion profiles were studied on day 3, 6 and
8. At each time point the adherent, non-adherent and mixed cells
were harvested at a density of 7×106. Following 2 washes
with PBS (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA),
the cells were seeded in a 100 mm plastic tissue culture dish in 10
ml complete RPMI-1640 medium for an overnight incubation at 37°C in
a humidified atmosphere containing 5% CO2. Subsequently,
the medium was collected to measure the levels of IL-2 (cat no.
BMS601), IL-12p70 (cat no. BMS6004), interferon (IFN)-γ (cat no.
BMS606), IL-4 (cat no. BMS613) and IL-10 (cat no. BMS614/2)
production by sandwich ELISA kits (Platinum ELISA, all by
eBioscience; Thermo Fisher Scientific, Inc.). Cytokine levels were
measured as pg/ml and quantified by reference, according to the
manufacturer's protocol.
CD3+ T cell isolation and
culture
Male C57BL/6 (H2b) mice (n=8) were
euthanized and their armpits, groin and mesenteric lymph nodes were
harvested. The lymph nodes were placed in a 60×15 mm2
culture dish with 5 ml serum-free PBS. Lymph nodes were minced and
the mixture was filtered through a 74-µm aperture nylon mesh to
obtain allogeneic responder T lymphocytes. The freshly isolated
lymphocytes were centrifuged for 5 min at 300 × g at room
temperature and lymphocytes were purified using a Mouse
CD3+ T Cell Enrichment Column (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Allogeneic T cell activation
MLR was performed to measure the proliferative
response of allogeneic T cells that were stimulated by the cultured
DCs. On day 8 of incubation, the adherent cells, non-adherent cells
and mixed cells were collected and served as stimulators, and the
CD3+ T cells isolated from lymph nodes of allogeneic
C57BL/6 mice served as responders. The responder T cells were 92%
CD3+ cells, and were labeled with a carboxyfluorescein
succinimidyl amino ester tracer at 37°C for 10 min (CFSE; 1 µmol/l
in PBS; Invitrogen; Thermo Fisher Scientific, Inc.), as previously
described (19). The labeling
process was quenched by adding 800 µl FBS on ice for 5 min. The
CFSE-labeled T cells were centrifuged at 300 × g at room
temperature for 5 min. Stimulator DCs (non-adherent cells, adherent
cells or mixed cells) at a density of 1×105 cells/well
were co-cultured with CFSE-labeled T cells (5×105
cells/well) at a 1:5 ratio of DCs to T cells in 96-well
rounded-bottom microtiter plates (Corning Inc., Corning, NY, USA)
at a final volume of 200 µl of RPMI-1640 medium with 10% FBS, and
incubated at 37°C for 4 days. CFSE-labeled T cells cultured alone
served as the negative control. T cells treated with anti-mouse
CD3e (cat no. 16-0031; 1:2,000, eBioscience, Thermo Fisher
Scientific, Inc.) and anti-mouse CD28 (cat no. 16-0281; 1:2,000,
eBioscience, Thermo Fisher Scientific, Inc.) antibodies served as
positive controls. A total of 5 groups of cells (negative control,
positive control, T cells co-cultured with non-adherent cells, T
cells co-cultured with adherent cells and T cells co-cultured with
mixed cells) were collected separately and centrifuged twice for 5
min at 300 × g at room temperature with 2 ml cold FACS buffer (PBS
supplemented with 5% FBS and 0.05% NaN3). Cells were
stained with APC-labeled anti-CD4 (cat no. 17-0042; 1:600,
eBioscience, Thermo Fisher Scientific, Inc.) for 30 min at 4°C in
the dark and analyzed by flow cytometry (FlowJo software version
7.6; FlowJo LLC, Ashland, OR, USA). MLR was evaluated as follows:
i) CD4+ T cells gathering to the y-axis with clear
proliferation stripes following stimulation, and presenting a
strong proliferative response to the stimulator (20); ii) the proliferative index, which
was defined as the sum of the cells in all generations divided by
the calculated number of original cells (21); and iii) the cell division index,
which was defined as the average number of divisions undertaken by
all T cells in the parent generation (22).
Statistical analysis
Data are presented as the mean ± standard deviation.
All experiments were conducted four to eight times. Repeated
measures analysis of variance was used to assess the statistical
significance of the differences between groups, and the Bonferroni
method was used to correct for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were conducted with SPSS software, version
19.0 (IBM Corp., Armonk, NY, USA).
Results
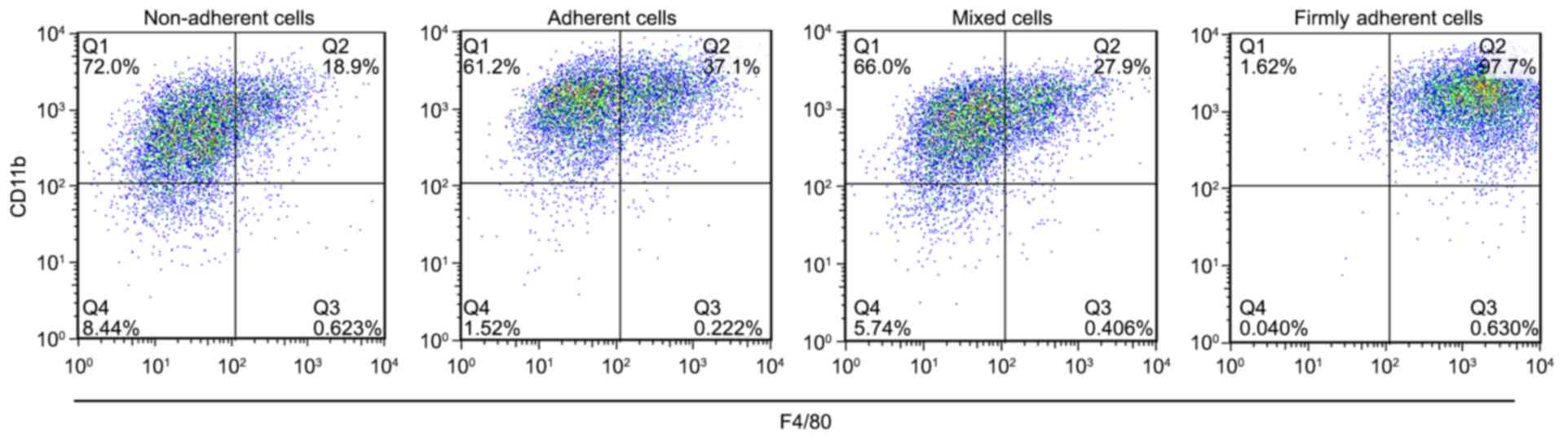
F4/80 and CD11b expression on the cell
surface classifies firmly adherent cells as macrophages
GM-CSF was able to generate populations of
macrophages and DCs in cell culture. The expressions of F4/80 and
CD11b on the cell surface were used as specific markers to identify
macrophages. The percent of F4/80+CD11b+
cells in each group was 21.00±2.59% in the non-adherent cells,
38.17±0.97% in the adherent cells, 28.60±1.04% in the mixed cells
and 98.20±0.46% in the firmly adherent cells (P<0.0001, firmly
adherent cells vs. non-adherent cells, adherent cells and mixed
cells; Fig. 1), which indicated
that the firmly adherent cells consisted of macrophages. These
results are consistent with those described by Inaba et al
(17) in 1992, which demonstrated
that macrophages firmly adhered to the culture vessel, expressed
high levels of the F4/80 antigen expressed little or no MHC-II and
exhibit no MLR-stimulating activity. Because the firmly adherent
cells did not contribute to the population of cDCs, they were not
used for further investigations.
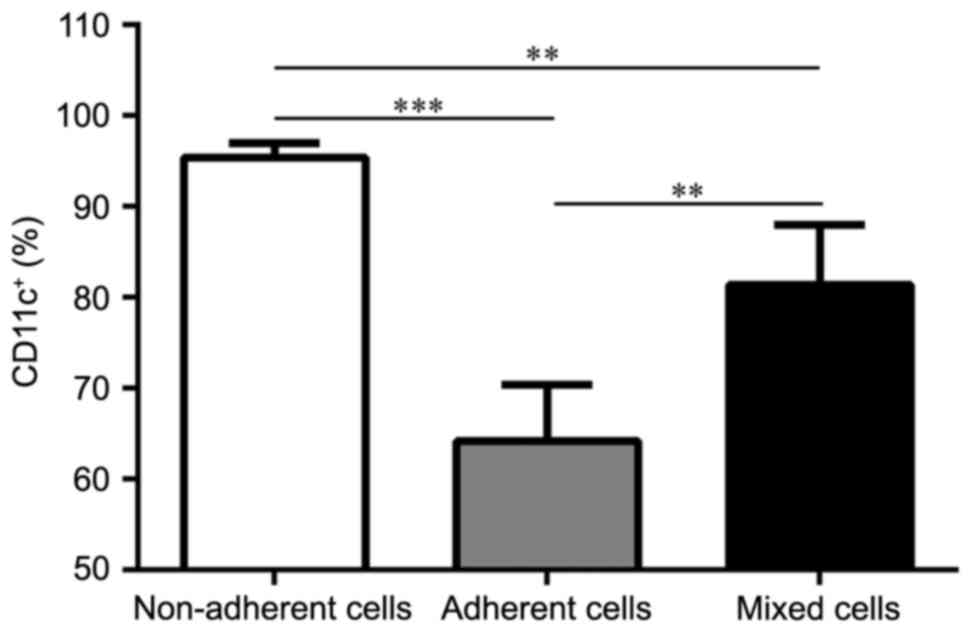
Each DC subset displays different
purity and maturation states
In order to clarify the purity of the three DC
subsets, the remaining three BM-derived DC subsets were probed
CD11c+ expression. Populations of the non-adherent,
adherent and mixed cells were purified on day 8. CD11c+
expression analysis from the cultures revealed that all three DC
subsets were able to expand in GM-CSF and IL-4 cultures, and
generated various levels of CD11c+ cells (Fig. 2): 95.43±1.57% for the non-adherent
cells (P<0.001 vs. adherent cells; P=0.0046 vs. mixed cells);
64.20±6.18% for the adherent cells; and 81.43±6.59% for the mixed
cells (P=0.0026 vs. adherent cells). On day 8, the maturation
phenotype of three subsets was analyzed following LPS stimulation.
The graphs in Fig. 3A and B
demonstrate that the adherent cells expressed the lowest level of
CD11c+CD40+,
CD11c+CD80+,
CD11c+CD86+ and
CD11c+MHC-II+ compared with the non-adherent
cells and mixed cells, which indicated their ability to induce
immune tolerance or suppression. Conversely, the non-adherent cells
displayed the highest expression levels of the costimulatory
molecules, which indicated that the cultured DC population was
homogeneous and that DCs with different degrees of adhesion may
have different maturation status.
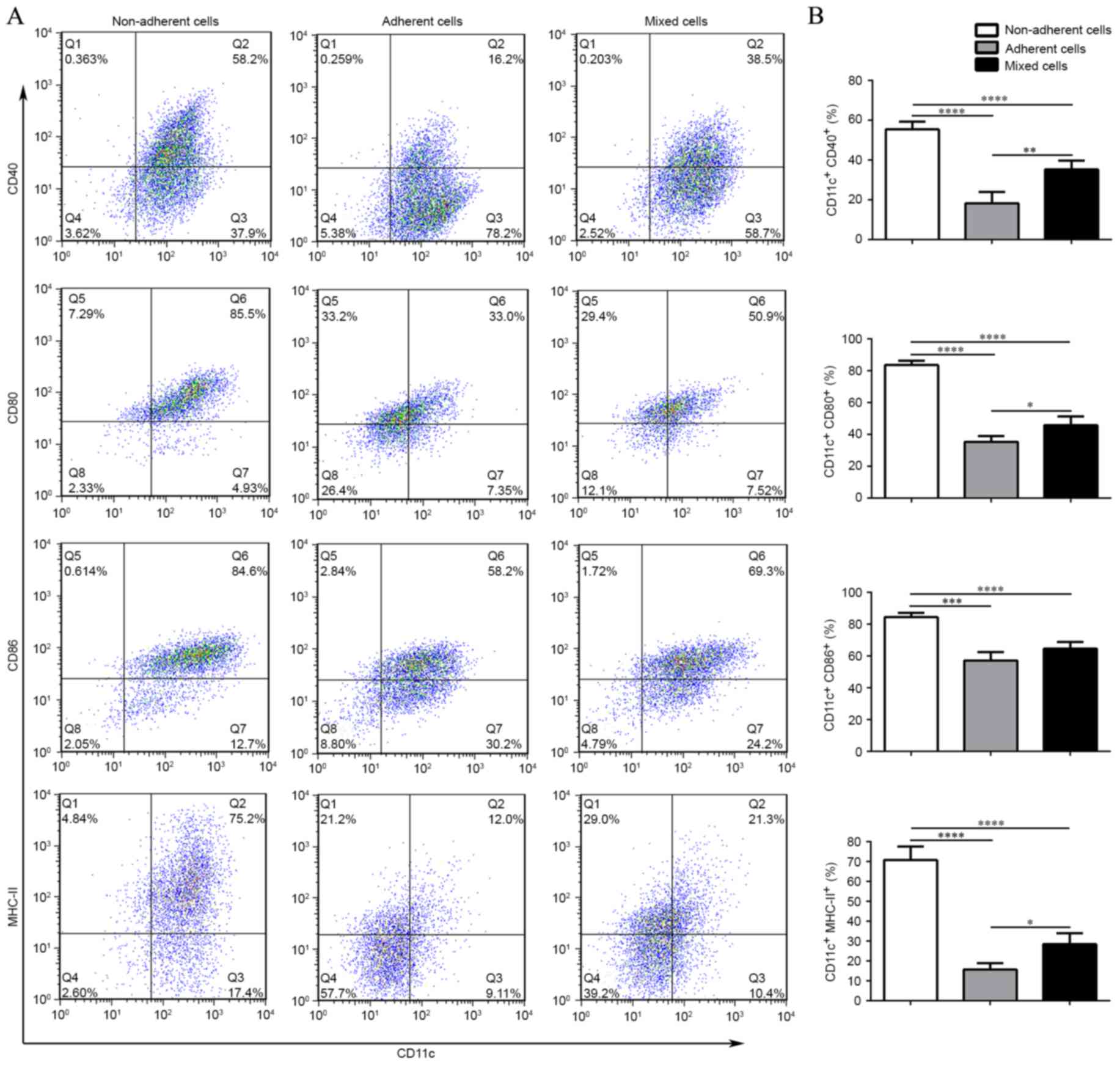 | Figure 3.Different maturation states in
response to LPS stimulation among the non-adherent, adherent and
mixed cells. (A) Representative flow cytometric graphs, obtained
from the cell subsets stained with CD11c, CD40, CD80, CD86, and
MHC-II on day 8. Non-adherent cells expressed the highest levels of
CD11c+CD40+,
CD11c+CD80+,
CD11c+CD86+ and
CD11c+MHC-II+. (B) Flow cytometric analysis
demonstrating the different maturation status in response to LPS
among the non-adherent, adherent and mixed cells. Error bars
indicate the mean ± standard deviation; n=5/group; *P<0.05,
**P<0.005, ***P<0.001 and ****P<0.0001. LPS,
lipopolysaccharide; MHC-II, major histocompatibility complex class
II. |
Each DC subset exhibits different
cytokine secretion levels throughout the DC culture process
Protein expression levels of the secreted cytokines
IL-2, IL-12p70, IFN-γ, IL-4 and IL-10 in the three subsets were
examined on days 3, 6 and 8 (Fig.
4). In addition to IL-4 and IL-12 p70, the expression of IL-2,
IL-10 and IFN-γ in non-adherent cells, adherent cells and mixed
cells was not significantly different between days 3, 6 and 8.
Notably, the cytokine secretion profiles revealed the heterogeneity
of the cultured DCs: IL-4 expression from day 6 appeared to
increase in the non-adherent cells, whereas it appeared to decrease
in the adherent and mixed cells, indicating that DC polarization
occurred and the DC reservoir was not homogenous. On day 8, the
non-adherent cells secreted high levels of both T helper (Th)1-type
(IL-12p70) and Th2-type (IL-4) cytokines, which indicated that the
non-adherent DCs themselves were not homogenous. Furthermore,
following day 6, the curves of the cytokine secretion profiles for
IFN-γ, IL-12 p70 and IL-10 in the three DC subsets became steeper,
suggesting rapid development of the immune function of DCs.
Therefore, day 6 may be a pivotal time point in which to modulate
the immunological characteristics of the cultured DCs.
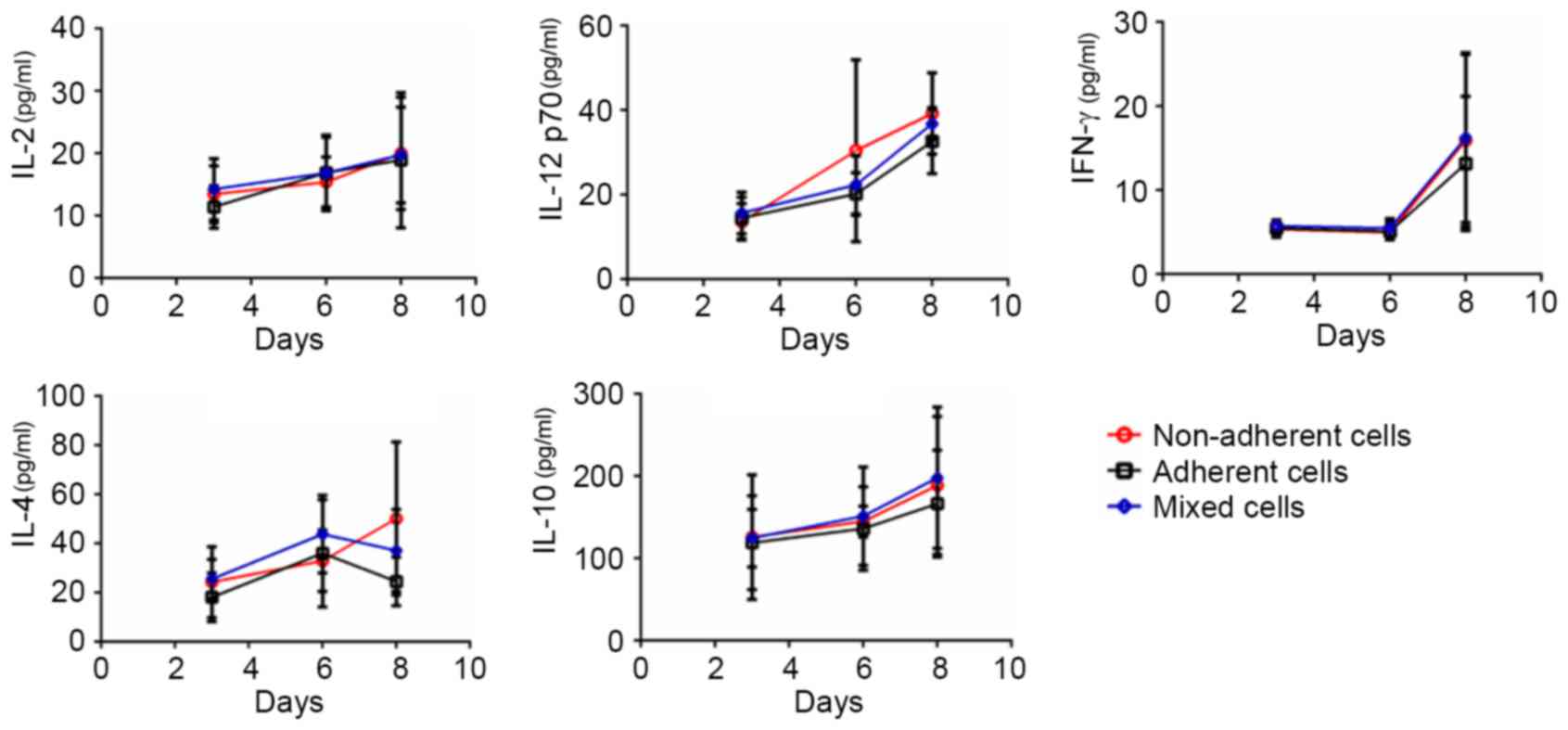 | Figure 4.Cytokine secretion levels in the
non-adherent, adherent and mixed cells throughout the dendritic
cell culture process. On days 3, 6 and 8, cell subsets
(7×106 cells) were harvested, reseeded following an
overnight incubation, and the cell suspension was collected to
quantify the cytokine levels of IL-2, IL-12p70, IFN-γ, IL-4 and
IL-10 using sandwich enzyme-linked immunosorbent assay kits. IFN,
interferon; IL, interleukin. |
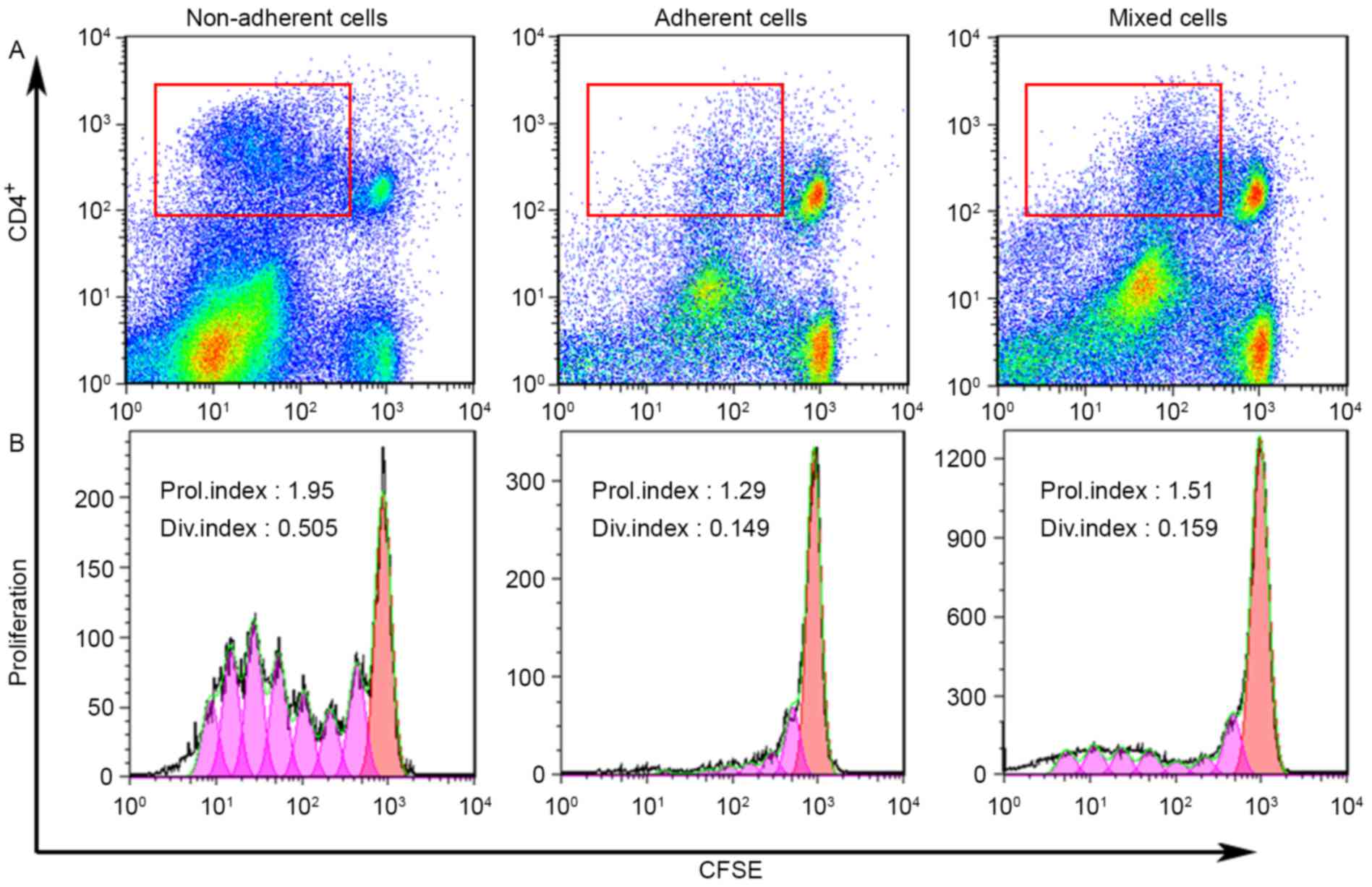
Each DC subset exhibits a different
immunological reaction
The antigen-presenting ability of each DC subset was
examined by MLR. Allogeneic T lymphocytes were stained with CFSE
and cultured alone (negative control), with anti-mouse CD3e and
anti-mouse CD28 (positive control groups), with non-adherent cells,
with adherent cells and with mixed cells. After 4 days of
incubation, cells were harvested and stained with anti-CD4 prior to
analysis by flow cytometry. As presented in Fig. 5A, a bivariate dot-plot of CD4
expression and the level of CFSE fluorescence demonstrated that the
non-adherent cells-treated T cells had undergone division and
gathered on the y-axis, indicating high proliferation (red frame).
The adherent cells-treated T cells exhibited limited division. The
mixed cells-treated T cells demonstrated partial division. The
proliferative index of the gated CD4+ T cells by
stimulation of non-adherent cells, adherent cells and mixed cells
was 1.69±0.33, 1.29±0.09 and 1.30±0.10, respectively, whereas the
index of the negative and positive controls was 1.22±0.24 and
1.67±0.05, respectively. The cell division index of the gated
CD4+ T cells of non-adherent cells, adherent cells and
mixed cells was 0.33±0.21, 0.16±0.02 and 0.11±0.04, respectively,
while the index of the negative and positive controls was 0.13±0.10
and 0.75±0.27, respectively (Fig.
5B). The MLR results demonstrated that the non-adherent DCs
exhibited an strong ability to stimulate allogeneic T cell
proliferation, whereas the ability of adherent DCs to stimulate T
cell proliferation was weak.
Discussion
Heterogeneity of the DC subsets emerges from the
first day during the culture process, owing to complicated DC
differentiation (23). Previous
studies have reportedly observed the presence of: i) BM
non-adherent MHC-II− progenitors attaching to the stroma
or plastic; ii) growing aggregates arising from firmly adherent
MHC-II+ DCs; iii) mature, proliferating
MHC-II+ DCs being released by the loosely attached
aggregate; and iv) non-proliferating, non-adherent
MHC-II+ DCs suspended in the medium, much like many of
the DCs released from the spleen (17,24).
During the permanent differentiation process from BM cells and
progeny DCs to mature DCs, the cultured cells dynamically expressed
a specific molecular form and immunological function (25), which resulted in the co-existence
of various subsets of DCs (26).
To accurately understand the DC reservoir, the immunological
identification of DC subsets is required. To achieve a certain
immunological aim, the investigators should select the
corresponding cultured subset from the DC reservoir based on its
maturity status and immunological features. For example, for
anti-tumor or infectious effects, the mature subset should be used
to prime T cells (27–29), for tolerance induction or
immunosuppressive effects, the immature DCs should be used
(30) and to check the effects of
immune modulation therapy administered in vivo, mixed
cultured DCs should first be tested in vitro (31). Unfortunately, the differences
between the cultured subsets have been largely ignored, leading to
confusion.
In the present study, the three DC subsets were
distinguished from macrophages by detecting the expression of F4/80
and CD11b. CD11c was used to detect the different purity of
non-adherent cells, adherent cells and mixed cells. CD40, CD80,
CD86 and MHC-II were used to detect the different maturation state
of non-adherent cells, adherent cells and mixed cells. The present
results demonstrated that the adherent cells expressed the lowest
levels of CD11c+CD40+,
CD11c+CD80+,
CD11c+CD86+ and
CD11c+MHC-II+ compared with the non-adherent
cells and mixed cells. The non-adherent cells displayed the highest
expression levels of the costimulatory molecules, which indicated
that the cultured DC population was homogeneous.
Given that the discrimination of DC subsets
practically depends on their adherent status, the cells used in the
present study were segregated as non-adherent, adherent, and mixed.
The heterogeneity of the cultured DCs was determined using
phenotypic markers and kinetic analysis of cytokine secretion in
response to LPS and MLR stimulation. Continuous IL-2 secretion by
the three subsets throughout the culture process indicated that the
cultured DCs were functional and that the culture strategy was
promising. The present study also demonstrated DC polarization
through the differences in expression of IL-4 in the adherent and
non-adherent cells. However, the non-adherent cells secreted high
levels of both Th1-type cytokines and Th2-type cytokines on day 8,
and the different secretory function, for IFN-γ, IL-12 p70 and
IL-10, of the adherent cells and non-adherent cells developed
rapidly from day 6. Furthermore, the non-adherent cells appeared to
acquire mature features, whereas the adherent cells exhibited
immature features based on their Th1-type and Th2-type cytokine
secretion and ability to stimulate allogeneic T cell proliferation.
MLR results revealed that non-adherent cells could stimulate the
proliferation and division of allogeneic T lymphocytes, whereas the
ability of adherent DCs to stimulate T cell proliferation was
limited. The mixed cells were demonstrated to partially stimulate
the proliferation and division of T cells. These data indicated
that at the end of DC differentiation and proliferation, the
cultured DC reservoir possessed heterogeneity, and non-adherent
cells were not homogenous.
Limitations of the present study included the small
sample size and the lack of assessments of cell viability and
apoptosis within the experimental groups; further studies are
required to address these issues. In conclusion, the present study
discriminated cultured DCs as subsets of non-adherent, adherent and
mixed cells, and identified their heterogeneous immunological
features. The findings may enhance the knowledge of DC culture
techniques and promote DC research in a more precise manner.
Acknowledgements
This work was supported by grants from The National
Natural Science Foundation of China (grant nos. 81570678 and
81373169 to Nianqiao Gong and Jun Yang, respectively), the National
High-Tech Researching and Developing Program (Program 863) of
Ministry of Science and Technology of China (grant no. 2012AA021010
to Changsheng Ming) and the Clinical Research Physician Program of
Tongji Medical College, HUST (to Nianqiao Gong).
References
|
1
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Zhao LB, Chang S, Ming CS, Yang J
and Gong NQ: Dynamic changes of phenotypes and secretory functions
during the differentiation of pre-DCs to mature DCs. J Huazhong
Univ Sci Technolog Med Sci. 37:191–196. 2017.PubMed/NCBI
|
|
3
|
Satpathy AT, Wu X, Albring JC and Murphy
KM: Re(de)fining the dendritic cell lineage. Nat Immunol.
13:1145–1154. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortez-Retamozo V, Etzrodt M and Pittet
MJ: Regulation of macrophage and dendritic cell responses by their
lineage precursors. J Innate Immun. 4:411–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merad M, Sathe P, Helft J, Miller J and
Mortha A: The dendritic cell lineage: Ontogeny and function of
dendritic cells and their subsets in the steady state and the
inflamed setting. Annu Rev Immunol. 31:563–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shurin MR, Pandharipande PP, Zorina TD,
Haluszczak C, Subbotin VM, Hunter O, Brumfield A, Storkus WJ,
Maraskovsky E and Lotze MT: FLT3 ligand induces the generation of
functionally active dendritic cells in mice. Cell Immunol.
179:174–184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naik SH, Proietto AI, Wilson NS, Dakic A,
Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen
WF, et al: Cutting edge: Generation of splenic CD8+ and
CD8+ dendritic cell equivalents in Fms-like tyrosine
kinase 3 ligand bone marrow cultures. J Immunol. 174:6592–6597.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi HJ and Lu GX: Adherent and non-adherent
dendritic cells are equivalently qualified in GM-CSF, IL-4 and
TNF-α culture system. Cell Immunol. 277:44–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalantari T, Kamali-Sarvestani E, Zhang
GX, Safavi F, Lauretti E, Khedmati ME and Rostami A: Generation of
large numbers of highly purified dendritic cells from bone marrow
progenitor cells after co-culture with syngeneic murine
splenocytes. Exp Mol Pathol. 94:336–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delirezh N and Shojaeefar E: Phenotypic
and functional comparison between flask adherent and magnetic
activated cell sorted monocytes derived dendritic cells. Iran J
Immunol. 9:98–108. 2012.PubMed/NCBI
|
|
11
|
Abdi K, Singh NJ and Matzinger P:
Lipopolysaccharide-activated dendritic cells: ‘Exhausted’ or alert
and waiting? J Immunol. 188:5981–5989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kushwah R, Wu J, Oliver JR, Jiang G, Zhang
J, Siminovitch KA and Hu J: Uptake of apoptotic DC converts
immature DC into tolerogenic DC that induce differentiation of
Foxp3+ Treg. Eur J Immunol. 40:1022–1035. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pantel A, Teixeira A, Haddad E, Wood EG,
Steinman RM and Longhi MP: Direct type I IFN but not MDA5/TLR3
activation of dendritic cells is required for maturation and
metabolic shift to glycolysis after poly IC stimulation. PLoS Biol.
12:e10017592014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Cao Y, Meng Y, You Z, Liu X and
Liu Z: The novel role of thymopentin in induction of maturation of
bone marrow dendritic cells (BMDCs). Int Immunopharmacol.
21:255–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li R, Zheng X, Popov I, Zhang X, Wang H,
Suzuki M, Necochea-Campion RD, French PW, Chen D, Siu L, et al:
Gene silencing of IL-12 in dendritic cells inhibits autoimmune
arthritis. J Transl Med. 10:192012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Q, Meng Y, Wang Y, Li M, Zhang J, Xin
S, Wang L and Shan F: Maturation inside and outside bone marrow
dendritic cells (BMDCs) modulated by interferon-α (IFN-α). Int
Immunopharmacol. 17:843–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inaba K, Inaba M, Romani N, Aya H, Deguchi
M, Ikehara S, Muramatsu S and Steinman RM: Generation of large
numbers of dendritic cells from mouse bone marrow cultures
supplemented with granulocyte/macrophage colony-stimulating factor.
J Exp Med. 176:1693–1702. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmid MA, Kingston D, Boddupalli S and
Manz MG: Instructive cytokine signals in dendritic cell lineage
commitment. Immunol Rev. 234:32–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kunieda Y, Tamura Y, Sasaki H, Kurokawa Y,
Miyagishima T, Ohae Y, Hige S, Gotohda Y, Tanaka J, Higuchi A, et
al: Carcinoid of the papilla of Vater-somatostatinoma-a case
report. Gan No Rinsho. 32:831–836. 1986.(In Japanese). PubMed/NCBI
|
|
20
|
Sela U, Olds P, Park A, Schlesinger SJ and
Steinman RM: Dendritic cells induce antigen-specific regulatory T
cells that prevent graft versus host disease and persist in mice. J
Exp Med. 208:2489–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyons AB and Parish CR: Determination of
lymphocyte division by flow cytometry. J Immunol Methods.
171:131–137. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinto LA, Galvão Castro B, Soares MB and
Grassi MF: An evaluation of the spontaneous proliferation of
peripheral blood mononuclear cells in HTLV-1-infected individuals
using flow cytometry. ISRN Oncol. 2011:3267192011.PubMed/NCBI
|
|
23
|
Haniffa M, Collin M and Ginhoux F:
Ontogeny and functional specialization of dendritic cells in human
and mouse. Adv Immunol. 120:1–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Merad M and Manz MG: Dendritic cell
homeostasis. Blood. 113:3418–3427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weigel BJ, Nath N, Taylor PA,
Panoskaltsis-Mortari A, Chen W, Krieg AM, Brasel K and Blazar BR:
Comparative analysis of murine marrow-derived dendritic cells
generated by Flt3L or GM-CSF/IL-4 and matured with immune
stimulatory agents on the in vivo induction of antileukemia
responses. Blood. 100:4169–4176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dudek AM, Martin S, Garg AD and Agostinis
P: Immature, Semi-Mature, and fully mature dendritic cells: Toward
a DC-cancer cells interface that augments anticancer immunity.
Front Immunol. 4:4382013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strome SE, Voss S, Wilcox R, Wakefield TL,
Tamada K, Flies D, Chapoval A, Lu J, Kasperbauer JL, Padley D, et
al: Strategies for antigen loading of dendritic cells to enhance
the antitumor immune response. Cancer Res. 62:1884–1889.
2002.PubMed/NCBI
|
|
30
|
Beriou G, Moreau A and Cuturi MC:
Tolerogenic dendritic cells: Applications for solid organ
transplantation. Curr Opin Organ Transplant. 17:42–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SJ and Diamond B: Modulation of
tolerogenic dendritic cells and autoimmunity. Semin Cell Dev Biol.
41:49–58. 2015. View Article : Google Scholar : PubMed/NCBI
|