Introduction
Lumbar intervertebral disc degeneration (LIDD) in
humans is a complex chronic disease mainly characterized by low
back pain (1). LIDD and cervical
spondylosis exhibit the highest morbidity among intervertebral
disc-related pathologies. Previous studies have reported that
70–80% of adults suffer from low back pain-related diseases, thus
making LIDD an important health issue (2,3). In
the United States, 1–2% of the population has been reported to
suffer from obvious intervertebral disc protrusion, whereas
~200,000 new cases are reported each year (3,4).
Annular disruption, herniation of the nucleus pulposus, and
oppression of the nerve root and cauda equina syndromeare among the
main manifestations of LIDD (3).
Although an early age of onset for low back pain is not uncommon,
with symptoms sometimes appearing in patients as young as 20 years
old, usually symptoms appear later in life, in patients between 30
and 40 years of age (4).
Low back pain can often be misdiagnosed and believed
to be a result of previous trauma; however, in most cases careful
examination reveals that patients suffer chronic pain in the waist
and lower extremities without traumatic etiology (5). Often, low back pain is transient, and
symptoms can be relieved after rest (6). A previous study reported that low
back pain may be induced by external forces, including excessive
standing, stooping or physical exertion; these causes frequently
interact with each other, accelerating the onset and progression of
back and leg pain (7). In most
cases, pain starts in the lower waist region and gradually extends
to regions innervated by the sciatic nerve, as well as the
buttocks. Lesions in the spinal column can also result in lower
back pain that can spread to the rear side of the thigh (8).
Turmeric (Curcuma longa) is awell-known
plant, commonly used as a food additive and natural dye. It is also
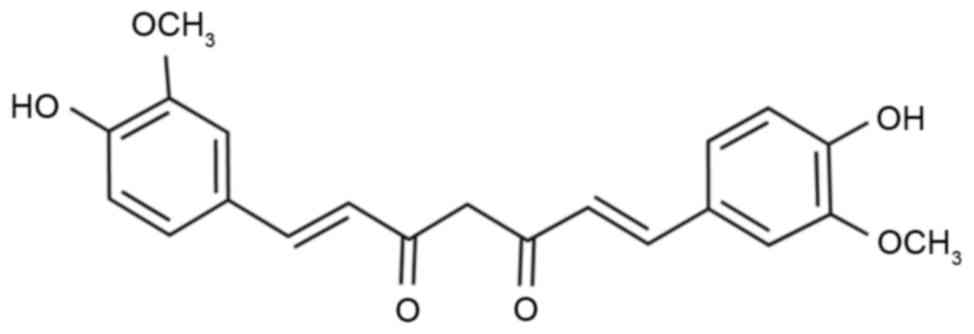
used in Chinese traditional medicine (9). Curcumin (Fig. 1) is the main active compound found
in turmeric, which has previously been reported to possesses
antioxidant properties that can prevent the oxidative damage caused
by free radicals to proteins, lipids, saccharides and nucleic
acids. Free radicals and oxidative stress have been implicated in
inflammation and several diseases, including cardiovascular and
cerebrovascular pathologies, and dermatological conditions
(10,11). The present study investigated the
putative protective effect of curcumin on a rat model of LIDD,
which was revealed to be exerted through regulation of the
expression of inducible nitric oxide synthase (iNOS),
cyclooxygenase (COX)-2, transforming growth factor (TGF)-β1/2,
matrix metalloproteinase (MMP)-9 and brain-derived neurotrophic
factor (BDNF).
Materials and methods
Animals and LIDD model
Sprague-Dawley rats (250–280 g; age, 8–10 weeks;
male) were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) and housed under
temperature-controlled (22±2°C) conditions with a 12/12 h
light/dark cycle and free access to food/water. Rats were
randomized into 3 groups: Control group (n=10) containing healthy
rats, LIDD model group (n=10) containing rats to which LIDD was
induced surgically, and curcumin-treated group (n=10), which
consisted of curcumin-treated LIDD rats. Rats in the control and
LIDD model groups were treated with saline. To induce LIDD, rats
were anesthetized via inhalation of 1.5–3% isoflurane (Shanghai
Jingke Scientific Instrument Co., Ltd., Shanghai, China) with an
oxygen carrier. Subsequently, they were placed in a supine position
on a heated pad, offering anterior access to the lumbar spine. A
gas tight microsyringe (Hamilton Company, Reno, NV, USA) attached
to a custom 33-gauge needle was inserted through the anterior of
the appropriate discs to a controlled depth of 2.5 mm. In the
control group, rats were anesthetized without the induction of
LIDD. All experiments were approved by the Animal Ethics Committee
of The First Affiliated Hospital of Chinese PLA General Hospital
(Beijing, China). Subsequently, rats in the curcumin-treated group
were treated with curcumin (200 mg/kg/day; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 5 weeks.
Histological evaluation
Rats were anesthetized using 35 mg/kg pentobarbital
and then rats were sacrificed using decollation. The intervertebral
discs and adjacent vertebral endplates between lumbar vertebrae L4
and L5 were acquired. The tissues were fixed with 4%
paraformaldehyde for 24–48 h and decalcified with 20% EDTA for 21
days at room temperature. Subsequently, tissue samples were
dehydrated through serial ethanol dilutions (95–100%) at room
temperature and embedded in olefin. Embedded samples were sliced
into 7-µm sections, and tissue morphology was examined using an
image auto-analysis system (CMIAS-99B; Okolab s.r.l., Pozzuoli,
Italy).
Collagen content and interleukin (IL)
levels
Tissue samples from intervertebral discs were
acquired and processed to measure type II collagen content (cat.
no. E-EL-R0234c), and IL-1β (cat. no. E-EL-R0012c) and IL-6 (cat.
no. E-EL-R0015c) levels, using a microplate reader, according to
the manufacturer's protocol (Wuhan Elabscience Biotechnology Co.,
Ltd., Wuhan, China).
Western blot analysis
Intervertebral discs were acquired, weighed and
processed into a fine powder. Tissue samples were homogenized using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) and total protein concentration was
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocol. Equal amounts (80 µg) of extracted protein
samples were separated by 10–12% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked for 1 h with TBS containing Tween
(0.01%) with 5% fat-free milk at room temperature, followed by an
overnight incubation at 4°C with the following primary antibodies:
Rabbit anti-rat iNOS antibody (cat. no. sc-649; 1:3,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-rat MMP-9
antibody (cat. no. sc-10737; 1:2,000; Santa Cruz Biotechnology,
Inc.) and rabbit anti-rat β-actin antibody (cat. no. sc-7210;
1:5,000; Santa Cruz Biotechnology, Inc.) which was used as a
loading control. Subsequently, membranes were probed with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat. no. 14708; 1:5,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1.5 h at room temperature. The bands were
visualized with an Enhanced Chemiluminescence kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and quantified using Quantity
One software (version 3.0; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from intervertebral disc
samples using TRIzol (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Total RNA (1 µg) was reverse
transcribed into cDNA using Advantage RT-for-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR analyses were
performed on cDNA (1 µl) with the Rotor-Gene Q real-time DNA
amplification system (Qiagen China Co., Ltd., Shanghai, China)
using SYBR-Green (Bio-Rad Laboratories, Inc.) according to the
manufacturer's protocol. PCR reactions were performed as follows:
An initial predenaturation step for 1 min at 95°C, followed by 40
cycles of amplification at 95°C for 15 sec, at 60°C for 30 sec and
72°C for 30 sec, and 4°C for 10 min. Sequences of the primers used
for RT-qPCR are presented in Table
I. The experimental results were expressed using
2−ΔΔCq (12).
 | Table I.Sequences of primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| COX-2 |
5′-GGAGCATCCTGAGTGGGATGA-3′ |
5′-AAGCAGGTCTGGGTCGAACTTG-3′ |
| TGF-β1 |
5′-ATTCCTGGCGTTACCTTGG-3′ |
5′-AGCCCTGTATTCCGTCTCCT-3′ |
| TGF-β2 |
5′-GCAGAGTTCAGGGTCTTTCG-3′ |
5′-GCTGGGTTGGAGATGTTAGG-3′ |
| BDNF |
5′-TCTCCCTGCCTCATCCCT-3′ |
5′-CAGAGTCTTCCTTTGCCTAC-3′ |
| GAPDH |
5′-GAGTCAACGGATTTGGTCGT-3′ |
5′-TTGATTTTGGAGGGATCTCG-3′ |
Statistical analysis
Statistical analysis was performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard deviation and all experiments were
repeated three times. Statistical significance was assessed using
one-way analysis of variance, followed by Dunnett's test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
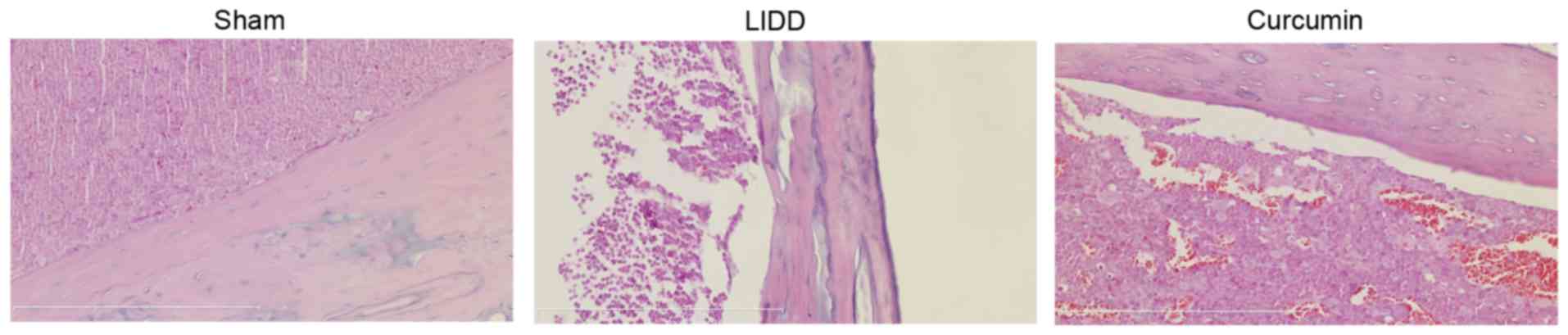
Curcumin improves the histological
profile of rats with LIDD
In order to evaluate the putative protective effects
of curcumin on LIDD, intervertebral disc samples were acquired from
sham-operated rats and from rats with surgically-induced LIDD.
Histological evaluation of the samples revealed extensive
intervertebral tissue injury in rats with surgically-induced LIDD,
which was absent in sham-operated healthy rats. Treatment with
curcumin appeared to prevent tissue injury in rats with
surgically-induced LIDD (Fig.
2).
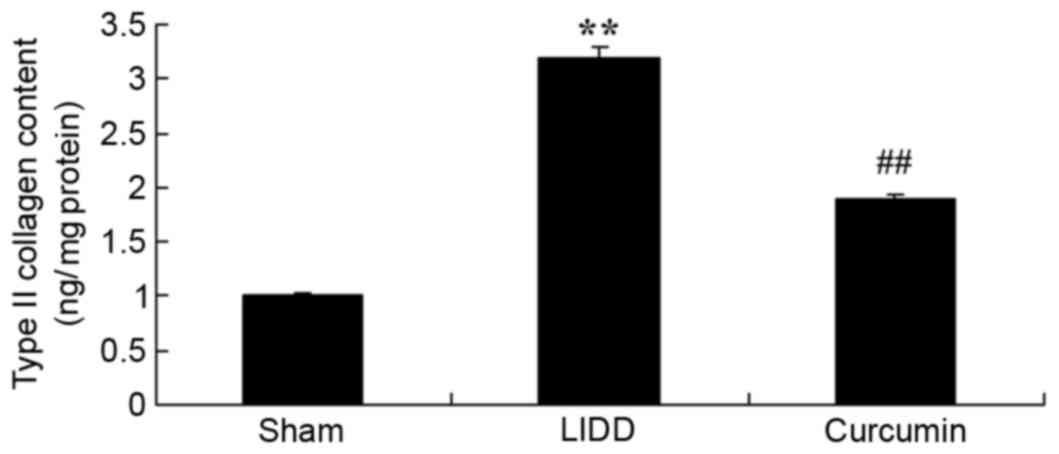
Curcumin reduces type II collagen
content in rats with LIDD
The effects of curcumin on the type II collagen
content of intervertebral disc samples were evaluated in rats with
LIDD. The results revealed that in rats with surgically-induced
LIDD, type II collagen content was significantly increased compared
with in sham-operated normal rats. Treatment with curcumin was
demonstrated to significantly reduce type II collagen content in
rats with LIDD, as compared with in untreated LIDD rats (Fig. 3).
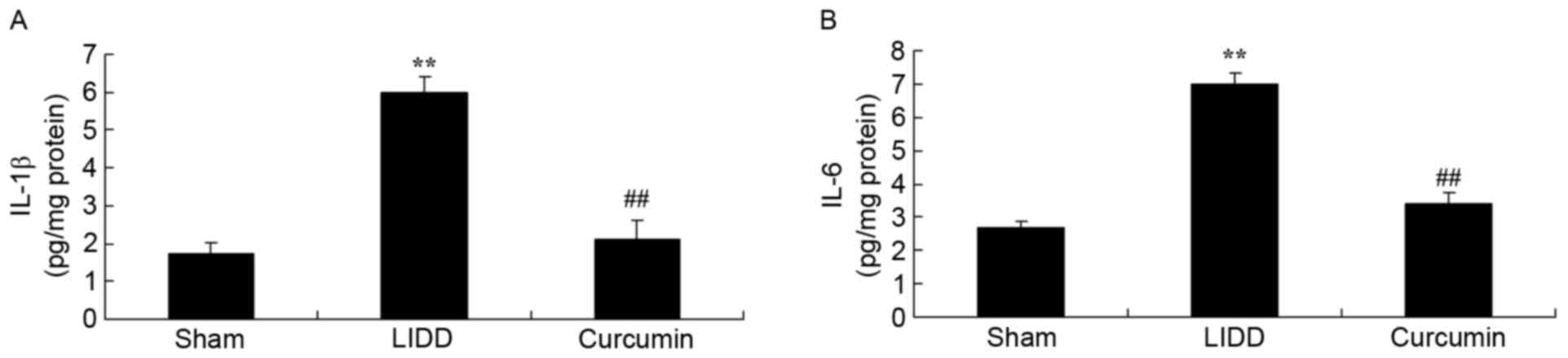
Curcumin reduces IL-1β and IL-6 levels
in rats with LIDD
IL-1β and IL-6 levels were assessed in
intervertebral disc samples, and it was revealed that LIDD induced
a significant increase in IL-1β and IL-6 levels compared with in
the sham group. Treatment with curcumin significantly suppressed
IL-1β and IL-6 levels in rats with LIDD compared with in the
untreated group (Fig. 4).
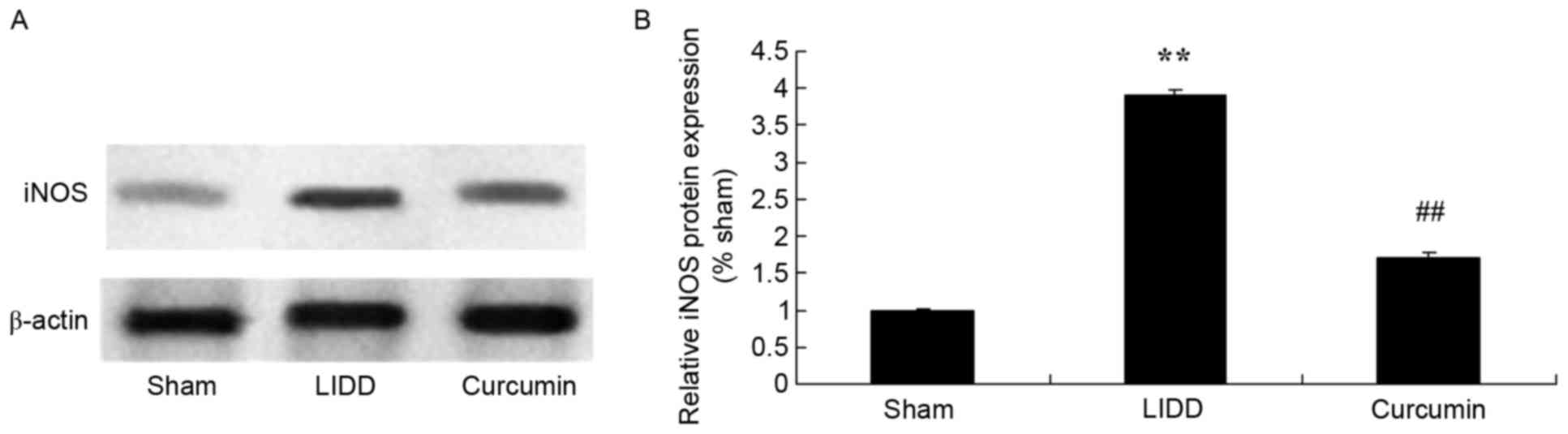
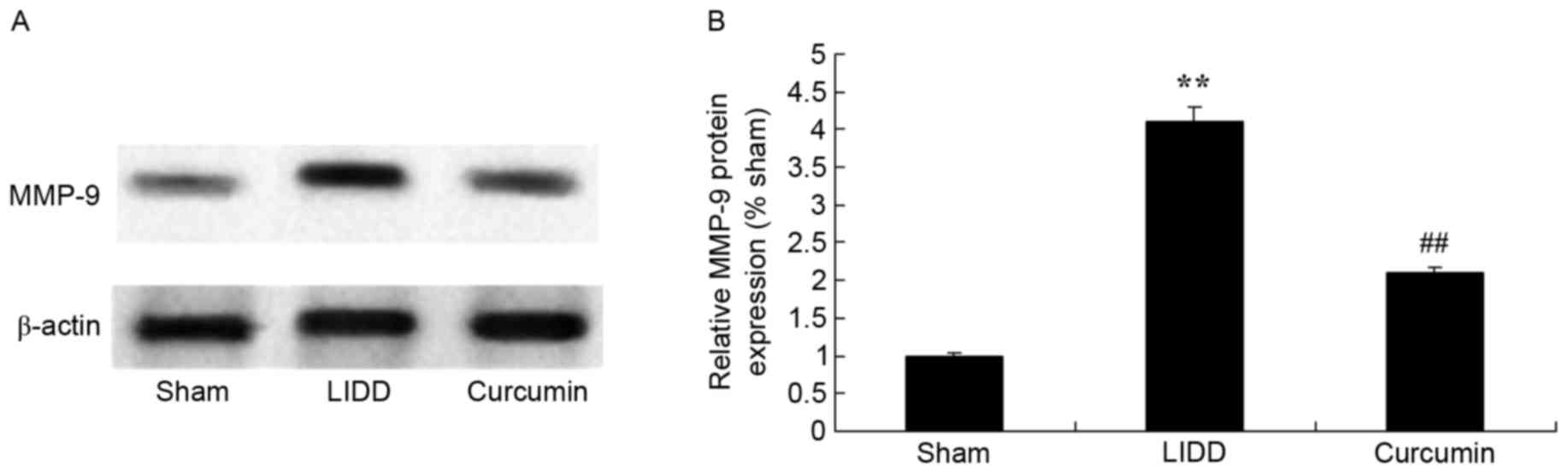
Curcumin reduces the protein
expression of iNOS and MMP-9 in rats with LIDD
The expression levels of iNOS and MMP-9 were
assessed using western blot analysis, and it was revealed that LIDD
induced a significant increase in protein levels compared with in
the sham group. Treatment with curcumin significantly reduced iNOS
and MMP-9 levels in intervertebral disc samples (Figs. 5 and 6).
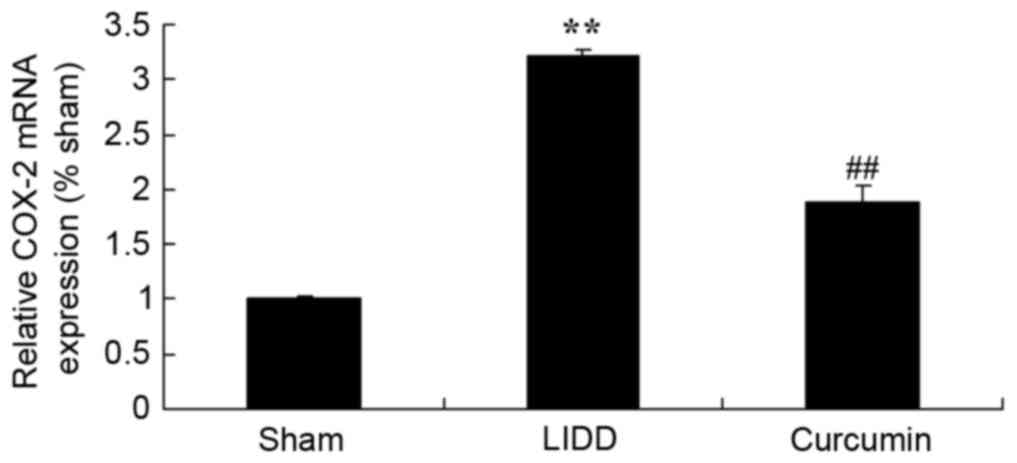
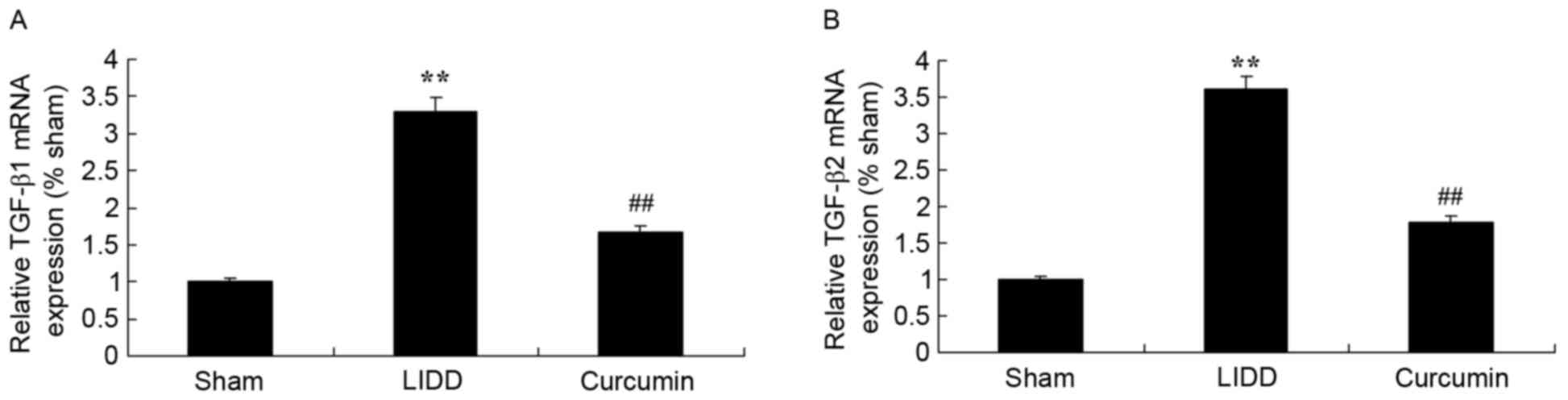
Curcumin reduces mRNA levels of COX-2
and TGF-β1/2 in rats with LIDD
In order to evaluate the mRNA expression levels of
COX-2 and TGF-β1/2, RT-qPCR was employed. Results demonstrated a
significant upregulation in COX-2 and TGF-β1/2 mRNA expression
levels in rats with LIDD compared with in the control group.
Treatment with curcumin appeared to significantly reduce the mRNA
expression levels of COX-2 and TGF-β1/2 in intervertebral disc
samples (Figs. 7 and 8).
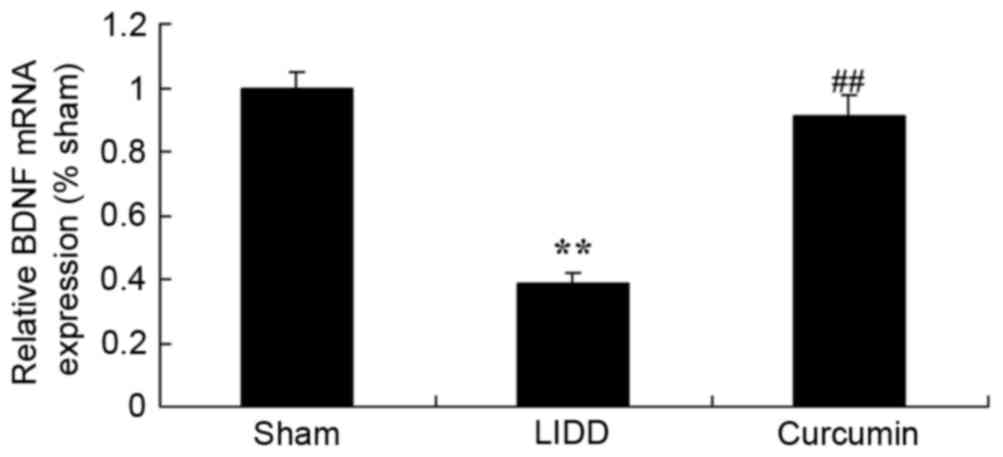
Curcumin increases the mRNA expression
levels of BDNF in rats with LIDD
The present study also examined the effect of
curcumin on the mRNA expression levels of BDNF in rats with
surgically-induced LIDD. In rats with LIDD, BDNF mRNA appeared
significantly downregulated compared with in the control group.
Treatment with curcumin significantly increased BDNF mRNA
expression levels in intervertebral disc samples compared with in
untreated rats (Fig. 9).
Discussion
LIDD is characterized by degeneration, necrosis and
apoptosis of the nucleus pulposus, dehydration and degradation of
the extracellular matrix, as well as changes in collagen content.
These characteristics result in the gradual disappearance of the
nucleus pulposus and its boundaries with the fibrous rings, and in
the progressive development of fibrosis (5). As a consequence, intervertebral disc
tissues gradually lose their normal structure and functionality
(13). In the present study, it
was revealed that curcumin significantly improved tissue injury,
reduced type II collagen content and downregulated IL-1β and IL-6
levels in intervertebral tissue samples from rats with LIDD.
As intervertebral disc degeneration progresses,
mechanical pressure can be exerted on the nerve roots of the spinal
cord. This can result in spinal cord anoxia and ischemia, which
block energy production. Under ischemic conditions, the activity of
the iNOS enzyme is potentiated, whereas the substrate of the
enzyme, required for NO production, is depleted (14). Under these circumstances, iNOS
promotes the production of reactive oxygen species, such as
superoxide anion (O2−) and hydrogen peroxide
(H2O2), which cause tissue damage and cell
necrosis (15). These processes
can have deleterious effects in spinal cord neurons. When motor
neurons become necrotic, NOS activity is reduced and NO levels drop
(15,16). The results of the present study
revealed that curcumin can significantly reduce iNOS levels in
intervertebral disc samples from rats with LIDD. The present
results are in accordance with a previous study, which reported the
anti-inflammatory capabilities of curcumin, exerted through the
downregulation of iNOS (9).
COX-2 is a multi-functional enzyme responsible for
catalyzing the conversion of arachidonic acid into prostaglandins
(PG), which is implicated in several inflammatory processes
(17). It exerts diverse functions
associated with cellular proliferation and apoptosis, and its
expression has been revealed to be upregulated in certain tumors
(17). A previous study reported
that the PG content in degenerating intervertebral discs is
markedly increased (18). PG can
inhibit the synthesis of proteins and polysaccharides in
intervertebral disc cells, whereas this depletion in the nucleus
pulposus is one of the main causes of LIDD (18). The results of the present study
demonstrated that curcumin significantly inhibited COX-2 mRNA
expression in rats with LIDD.
As a key enzyme for metabolic processes of the
extracellular matrix under physiological and pathological states,
MMP-9 has been reported to directly degrade collagen and
polysaccharides, resulting in depolymerization of proteoglycan,
whereas it can also precipitate the activation of other MMPs
(19). The expression of MMP-9 has
been demonstrated to be upregulated in vertebrae of degenerative
processes (20). The present study
revealed that curcumin can significantly suppress MMP-9 expression
in rats with LIDD. In addition, Li et al reported that
curcumin inhibited atherosclerosis in coronary heart disease,
through its inhibitory effects on MMP-9 and tumor necrosis factor-α
(21).
TGF-β1 is a multi-functional regulatory factor
implicated in various processes, including cellular growth and
differentiation. It can have conflicting actions, inducing
apoptosis in some circumstances and triggering anti-apoptotic
mechanisms in others (22). The
present study demonstrated that curcumin can significantly
downregulate TGF-β1 and TGF-β2 mRNA expression levels in rats with
LIDD. The present findings are in accordance with a previous study
reporting that curcumin can improve neural functionality through
suppressing the expression of tumor necrosis factor-α, IL-1β,
TGF-β1 and TGF-β2 following spinal cord injury (10). BDNF can sustain and facilitate the
growth, development and regeneration of sensory, cholinergic,
dopaminergic and GABAergic neurons (23). It has also been reported to prevent
motor neuron death after sciatic nerve transection, and to rescue
nucleus neurons following spinal cord incision (24). The results of the present study
revealed that curcumin can significantly upregulate BDNF mRNA
expression levels in intervertebral disc tissue of rats with LIDD.
Fanaei et al (25) reported
that the effects of curcumin may be mediated through enhancing
serum BDNF levels in women with premenstrual syndrome. The results
of the present study demonstrated that curcumin upregulated BDNF
expression in LIDD.
In conclusion, the present study demonstrated that
curcumin can significantly improve tissue injury, reduce type II
collagen content, and suppress IL-1β and IL-6 levels in a rat model
of LIDD. Curcumin appeared to exert regulatory effects on iNOS,
COX-2, TGF-β1/2, MMP-9 and BDNF in intervertebral disc tissue.
These results suggest that curcumin may have a role as a novel
therapeutic agent for the treatment of LIDD.
References
|
1
|
Vieira LA, De Marchi PL, dos Santos AA,
Christofolini DM, Barbosa CP, Fonseca FL, Bianco B and Rodrigues
LM: Analysis of FokI polymorphism of vitamin D receptor gene in
intervertebral disc degeneration. Genet Test Mol Biomarkers.
18:625–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Høy K, Bunger C, Niederman B, Helmig P,
Hansen ES, Li H and Andersen T: Transforaminal lumbar interbody
fusion (TLIF) versus posterolateral instrumented fusion (PLF) in
degenerative lumbar disorders: A randomized clinical trial with
2-year follow-up. Eur Spine J. 22:2022–2029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brox JI, SØrensen R, Friis A, Nygaard Ø,
Indahl A, Keller A, Ingebrigtsen T, Eriksen HR, Holm I, Koller AK,
et al: Randomized clinical trial of lumbar instrumented fusion and
cognitive intervention and exercises in patients with chronic low
back pain and disc degeneration. Spine (Phila Pa 1976).
28:1913–1921. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delecrin J, Allain J, Beaurain J, Steib
JP, Huppert J, Chataigner H, Ameil M, Aubourg L and Nguyen JM:
Effects of lumbar artificial disc design on intervertebral
mobility: In vivo comparison between mobile-core and fixed-core.
Eur Spine J. 21 Suppl 5:S630–S640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Omair A, Holden M, Lie BA, Reikeras O and
Brox JI: Treatment outcome of chronic low back pain and
radiographic lumbar disc degeneration are associated with
inflammatory and matrix degrading gene variants: A prospective
genetic association study. BMC Musculoskelet Disord. 14:1052013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sedighi M and Haghnegahdar A: Role of
vitamin D3 in treatment of lumbar disc herniation-pain and sensory
aspects: Study protocol for a randomized controlled trial. Trials.
15:3732014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mochida J, Sakai D, Nakamura Y, Watanabe
T, Yamamoto Y and Kato S: Intervertebral disc repair with activated
nucleus pulposus cell transplantation: A three-year, prospective
clinical study of its safety. Eur Cell Mater. 29:202–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Wu W, Li Y, Liu J, Yang K and Chen
Y: Protective effects of preserving the posterior complex on the
development of adjacent-segment degeneration after lumbar fusion:
Clinical article. J Neurosurg Spine. 19:201–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng Y, Lu X, Wang L, Li T, Ding Y, Cao H,
Zhang Y, Guo X and Yu G: Curcumin inhibits the AKT/NF-kB signaling
via CpG demethylation of the promoter and restoration of NEP in the
N2a cell line. AAPS J. 16:649–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan J, Zou M, Xiang X, Zhu H, Chu W, Liu
W, Chen F and Lin J: Curcumin improves neural function after spinal
cord injury by the joint inhibition of the intracellular and
extracellular components of glial scar. J Surg Res. 195:235–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei
X, Cui Y, Sun J, Shang Q, Liu D and Zhu Z: Dietary curcumin
ameliorates aging-related cerebrovascular dysfunction through the
AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem.
32:1167–1177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Liang L, Li L, Han K, Li Q, Peng
Y, Peng X and Zeng K: Increased miR-424-5p expression in peripheral
blood mononuclear cells from patients with pemphigus. Mol Med Rep.
15:3479–3484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siepe CJ, Heider F, Haas E, Hitzl W,
Szeimies U, Stäbler A, Weiler C, Nerlich AG and Mayer MH: Influence
of lumbar intervertebral disc degeneration on the outcome of total
lumbar disc replacement: A prospective clinical, histological,
X-ray and MRI investigation. Eur Spine J. 21:2287–2299. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akeda K, Yamada T, Inoue N, Nishimura A
and Sudo A: Risk factors for lumbar intervertebral disc height
narrowing: A population-based longitudinal study in the elderly.
BMC Musculoskelet Disord. 16:3442015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao B, Chang C, Zhou J, Zhao T, Wang C, Li
C and Gao G: Pycnogenol protects against rotenone-induced
neurotoxicity in PC12 cells through regulating NF-kB-iNOS signaling
pathway. DNA Cell Biol. 34:643–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue Y, Wu J and Sun J: Four types of
inorganic nanoparticles stimulate the inflammatory reaction in
brain microglia and damage neurons in vitro. Toxicol Lett.
214:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jimbo K, Park JS, Yokosuka K, Sato K and
Nagata K: Positive feedback loop of interleukin-1beta upregulating
production of inflammatory mediators in human intervertebral disc
cells in vitro. J Neurosurg Spine. 2:589–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cabraja M, Endres M, Abbushi A, Zenclussen
M, Blechschmidt C, Lemke AJ, Kroppenstedt S, Kaps C and
Woiciechowsky C: Effect of degeneration on gene expression of
chondrogenic and inflammatory marker genes of intervertebral disc
cells: A preliminary study. J Neurosurg Sci. 57:307–316.
2013.PubMed/NCBI
|
|
19
|
Zigouris A, Alexiou GA, Batistatou A,
Voulgaris S and Kyritsis AP: The role of matrix metalloproteinase 9
in intervertebral disc degeneration. J Clin Neurosci. 18:1424–1425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun ZM, Miao L, Zhang YG and Ming L:
Association between the −1562 C/T polymorphism of matrix
metalloproteinase-9 gene and lumbar disc disease in the young adult
population in North China. Connect Tissue Res. 50:181–185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Lu Y, Sun Y and Zhang Q: Effect of
curcumin on permeability of coronary artery and expression of
related proteins in rat coronary atherosclerosis heart disease
model. Int J Clin Exp Pathol. 8:7247–7253. 2015.PubMed/NCBI
|
|
22
|
Zhan Z, Shao Z, Xiong X, Yang S, Du J,
Zheng Q, Wang H, Guo X and Liu Y: Ad/CMV-hTGF-beta1 treats rabbit
intervertebral discs degeneration in vivo. J Huazhong Univ Sci
Technolog Med Sci. 24:599–601, 624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kartha S, Zeeman ME, Baig HA, Guarino BB
and Winkelstein BA: Upregulation of BDNF and NGF in cervical
intervertebral discs exposed to painful whole-body vibration. Spine
(Phila Pa 1976). 39:1542–1548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orita S, Eguchi Y, Kamoda H, Arai G,
Ishikawa T, Miyagi M, Inoue G, Suzuki M, Toyone T, Aoki Y, et al:
Brain-derived neurotrophic factor inhibition at the punctured
intervertebral disc downregulates the production of calcitonin
gene-related peptide in dorsal root ganglia in rats. Spine (Phila
Pa 1976). 36:1737–1743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fanaei H, Khayat S, Kasaeian A and
Javadimehr M: Effect of curcumin on serum brain-derived
neurotrophic factor levels in women with premenstrual syndrome: A
randomized, double-blind, placebo-controlled trial. Neuropeptides.
56:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|