Introduction
Gastric cancer is the third most common cause of
cancer mortality worldwide, accounting for 723,000 deaths in 2012
(1). The low 5-year survival rate
associated with gastric cancer is mainly attributed to the current
lack of appropriate biomarkers for early disease diagnosis
(2).
Hypoxia can induce angiogenesis, which is a critical
step in the generation of tumor vasculature. Therefore, hypoxia in
the tumor microenvironment may be a crucial factor for promoting
the progression of several types of cancer, including gastric
tumors (3). Hypoxia-inducible
factor-1α (HIF1A) is a regulatory factor critical for the
adaptation of tumor cells to a hypoxic environment (4,5), and
its overexpression can promote tumor growth and metastasis through
inducing tumor angiogenesis (6,7). The
expression of HIF1A appears to be potentiated in numerous types of
human cancer, including colorectal (8) and edometrioid carcinomas (9), breast (10), pancreatic (11), prostate (12) and ovarian cancer (13), and many more (14,15).
Prolyl hydroxylases (PHDs) form a small protein
family with a wide distribution across human tissues, and are
considered latent anti-oncogenes (16). PHD3, which is also known as Egl-9
family hypoxia inducible factor 3, is an important member of the
PHD family. PHD3 has been reported to negatively regulate the
expression of HIF1A through the degradation of oxygen-dependent
proteasomes under hypoxic conditions (17). It has previously been suggested
that PHD3 is associated with the local tissue hypoxia that
accompanies tumorigenesis, and may act as a tumor suppressor
(18). Chen et al (19) demonstrated that PHD3 protected the
intestinal epithelial barrier from ulcerative injury, which is one
of the causes leading to the development of gastric cancer.
However, although the actions of PHD3 in the suppression of
angiogenesis, growth and differentiation of tumor cells have
previously been reported (20),
the detailed molecular mechanisms implicating PHD3 in gastric
cancer have yet to be elucidated.
The present study aimed to explore the association
between PHD3 expression and the clinicopathological features of
gastric cancer, and investigate the biological roles of PHD3 in
gastric tumor angiogenesis, invasion and metastasis.
Materials and methods
Tissue specimens
Cancerous and adjacent non-cancerous tissue samples
were obtained from 70 patients (46 male and 24 female; age, 32–76
years) with gastric cancer who underwent radical gastrectomy at the
Zhejiang Provincial People's Hospital (Hangzhou, China) between
January 2008 and December 2009. All tissue samples were resectioned
and immediately stored at −80°C or fixed in 10% buffered formalin
for further use. The tissue samples of 70 cases were used for
immunohistochemistry, 50 cases were used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis and 9 cases were used for western blot analysis. Routine
chemotherapy and radiotherapy were not administered to the patients
prior to surgery. The present study was approved by the Ethics
Committee of the Zhejiang Provincial People's Hospital. Written
informed consent was obtained from the patients.
RT-qPCR
Total RNA was extracted from pulverized tissue
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using the PrimeScript 1st Strand cDNA Synthesis kit (Takara
Bio, Inc., Otsu, Japan). Template RNA solution was heated at 65°C
for 5 min, then immediately cooled on ice. The 20-µl reaction
mixture was incubated at 42°C for 1 h and 70°C for 15 min, and
placed on ice for 2 min. qPCR analysis was performed according to
the manufacturer's protocol of SYBR Premix Ex Taq™ II (Takara Bio,
Inc.). The candidate primers are listed in Table I. GAPDH was used as an internal
control. Initial denaturation (4 min at 95°C) was followed by 40
cycles of amplification at 95°C for 10 sec, annealing at 56°C for
30 sec, and 72°C for 30 sec. Melting curve analysis was performed
at the end of the PCR cycles. The relative expression levels were
calculated using the 2−ΔΔCq method (21).
 | Table I.Primer sequence table. |
Table I.
Primer sequence table.
| Primer | Sequence, 5′-3′ | Temperature, °C |
|---|
| PHD3 | F:
TTGCCAGATGAAGTTATTTGCT | 56 |
|
| R:
TTCCCTCGCTGTGCTCCT |
|
| HIF1A | F:
TCTCCATCTCCTACCCACATACAT | 56 |
|
| R:
TGCTCTGTTTGGTGAGGCTGT |
|
| VEGF | F:
GGCTCTGACCAGGAGTTTG | 56 |
|
| R:
CAACAATGTGTCTCTTCTCTTCG |
|
| GAPDH | F:
TGAAGGTCGGAGTCAACGG | 56 |
|
| R:
CTGGAAGATGGTGATGGGATT |
|
| PHD3 cDNA | F:
CCCAAGCTTGATGCCCCTGGGACACATCAT | 61 |
|
| R:
CCGCTCGAGTCAGTCTTCAGTGAGGGCAGA |
|
Western blot analysis
Pulverized tissue samples were homogenized in lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China).
Suspensions were centrifuged at 14,000 × g for 5 min at 4°C, and
the supernatants were collected. The total protein concentrations
were detected using an Enhanced Bicinchoninic Acid Protein Assay
kit (Beyotime Institute of Biotechnology). Equal amounts of protein
(40 µg) were separated by SDS-PAGE on a 10% gel and transferred
onto polyvinylidene difluoride membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Membranes were blocked with 5% non-fat
dry milk in Tris-buffered saline for 2 h at room temperature.
Subsequently, membranes were incubated with rabbit anti-human PHD3
(cat. no. ab30782) and HIF1A (cat. no. ab51608; both Abcam,
Cambridge, UK), vascular endothelial growth factor (VEGF; cat. no.
19003-1-AP) and GAPDH (cat. no. 10494-1-AP; both ProteinTech Group,
Inc., Chicago, IL, USA) primary antibodies overnight at 4°C.
Following washing with TBS-Tween 20, membranes were incubated with
the secondary antibody (goat anti-rabbit immunoglobulin G,
horseradish peroxidase-conjugated; cat. no. HA1001-100; Hangzhou
HuaAn Biotechnology Co., Ltd., Hangzhou, China) at room temperature
for 2 h, then washed and incubated for 1 min with an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology) to
visualize the bands. GAPDH was used as a loading control. The blots
were quantified using the FluorChem FC2 imaging system
(ProteinSimple, San Jose, CA, USA).
Cell culture
Human gastric epithelial GES-1 cells and the human
gastric cancer cell lines SGC7901, BGC823, MKN45, MKN28, BGC803 and
AGS were purchased from the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China). Cells were
cultured in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal bovine serum (Sijiqing;
Zhejiang Tianhang Biotechnology Co., Ltd., Hangzhou, China), 100
U/ml streptomycin and 100 U/ml penicillin, and maintained at 37°C
in a 5% CO2 atmosphere. Cells were passaged at 80%
confluency using 1 mmol/l EDTA-0.025% trypsin for 1–3 min. The
results of the RT-qPCR analysis demonstrated that AGS cells
exhibited the lowest PHD3 expression and MKN28 cells exhibited the
highest expression among the gastric cancer cells. It is necessary
to note that the MKN28 cell line is known to be mis-identified; it
is derived from the MKN47 cell line.
PHD3 transfection
Gastric cancer AGS and MKN28 cells were plated at a
density of 5.0×105 cells/well in 6-well dishes 24 h
prior to transfection. In order to upregulate the expression of
PHD3, 1 µg total RNA was extracted from GES-1 cell lines with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and was used to synthesize full-length PHD3 CDNAs with Taq
Master Mix (Novoprotein; Sinobio Chemistry Co., Ltd., Dalian,
China), according to the manufacturer's protocol. The primers,
containing HindIII and XhoI restriction enzyme
cutting sites, are listed in Table
I. PCR-amplified full-length human PHD3 cDNA was cloned into
pcDNA3.1 (pcDNA3.1-PHD3), and pcDNA3.1 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used as negative control (pcDNA3.1-NC).
pcDNA3.1-PHD3 and pcDNA3.1-NC were transfected into AGS cells. The
sequence of PHD3 short hairpin (sh)RNA DNA (shRNA-PHD3; Merck KGaA,
Darmstadt, Germany) was CCG GCT ACG TCA AGG AGA GGT CTA ACT CGA GTT
AGA CCT CTC CTT GAC GTA GTT TTT. The negative control (shRNA-NC;
GAC TTC ATA AGG CGC ATGC), whose sequence was not isogeneous with
any human gene, and shRNA-PHD3 were cloned into the pYr-1.1 vector
(Yinrun Biotechnology, Inc., Changsha, China), and then transfected
into MKN28 cells. Transfection was performed using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as the delivery
agent, according to the manufacturer's protocol.
Transwell assays
The migratory and invasive capabilities of
transfected cells were assessed. Transwell migration assay was
carried out in 24-well plates using the Costar Transwell assay kit
(cat no. 3422; Corning Incorporated, Corning, NY, USA). The
invasion assay was carried out using invasion chambers (cat no.
354480; BD Biosciences, San Jose, CA, USA) pre-coated with
Matrigel. Cells were seeded in the upper chamber at a density of
2.0×105 cells/well, and 30% FBS RPMI-1640 culture medium
was added to the lower chamber. Following 48 h of incubation at
37°C in a 5% CO2 atmosphere, unmigrated or noninvasive
cells were removed from the upper surface of the transwell
membranes using a cotton swab, and the migrated or invaded cells on
the lower membrane surface were fixed with ethyl alcohol for 15 min
at room temperature, stained with hematoxylin for 5 min at room
temperature and counted under a light microscope (Olympus
Corporation, Tokyo, Japan).
Immunohistochemistry
Tissues were fixed in 10% neutral buffered formalin
for one day at room temperature, dehydrated by gradient ethanol
(75, 85, 95% and absolute ethyl alcohol), cleared with absolute
xylene and embedded in paraffin. Tissue sections were
deparaffinized and rehydrated. Heat-induced antigen retrieval was
carried out in 0.01 M citrate buffer. Sections were incubated with
3% H2O2 for 20 min at room temperature and
blocked with normal goat serum (Invitrogen; Thermo Fisher
Scientific, Inc.). They were subsequently incubated with rabbit
anti-human PHD3 primary antibody (1:250 in PBS; cat. no. ab30782;
Abcam) overnight at 4°C, followed by incubation with biotinylated
secondary antibody at room temperature for 20 min, and then with
horseradish peroxidase-conjugated polymer (Histostain-Plus kit;
cat. no. 859043; Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature for 20 min according to the manufacturer's
protocol. Finally, the sections were stained with diaminobenzidine
(DAB Substrate kit; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China), lightly counterstained with hematoxylin for 5 min at
room temperature and mounted.
Based on the proportion and the intensity of
positively stained cells, the degree of immunostaining was reviewed
under a light microscope (5 fields; magnification, ×200) and scored
independently by two observers blinded to the clinical outcomes.
The staining intensity was graded on a scale between 0 and 3+ (0,
no staining; 1+, weak immunoreactivity; 2+, moderate
immunoreactivity; 3+, strong immunoreactivity). The percentage of
cells that exhibited positive PHD3 staining was scored as follows:
1, 0–25% positive cells; 2, 26–50% positive; 3, 51–75% positive; 4,
76–100% positive. The staining intensity score and the %
immunoreactivity score were then multiplied to obtain a composite
score. The values of the composite score ranged between 0 and 12,
with 0–5 defined as low expression, and ≥6 defined as high
expression.
Statistical analysis
Comparisons between groups were analyzed using
paired samples t tests. Survival curves were estimated using the
Kaplan-Meier method. Cox regression analysis was used to assess the
prognostic values. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Expression of PHD3 in gastric cancer
and adjacent non-cancerous tissue
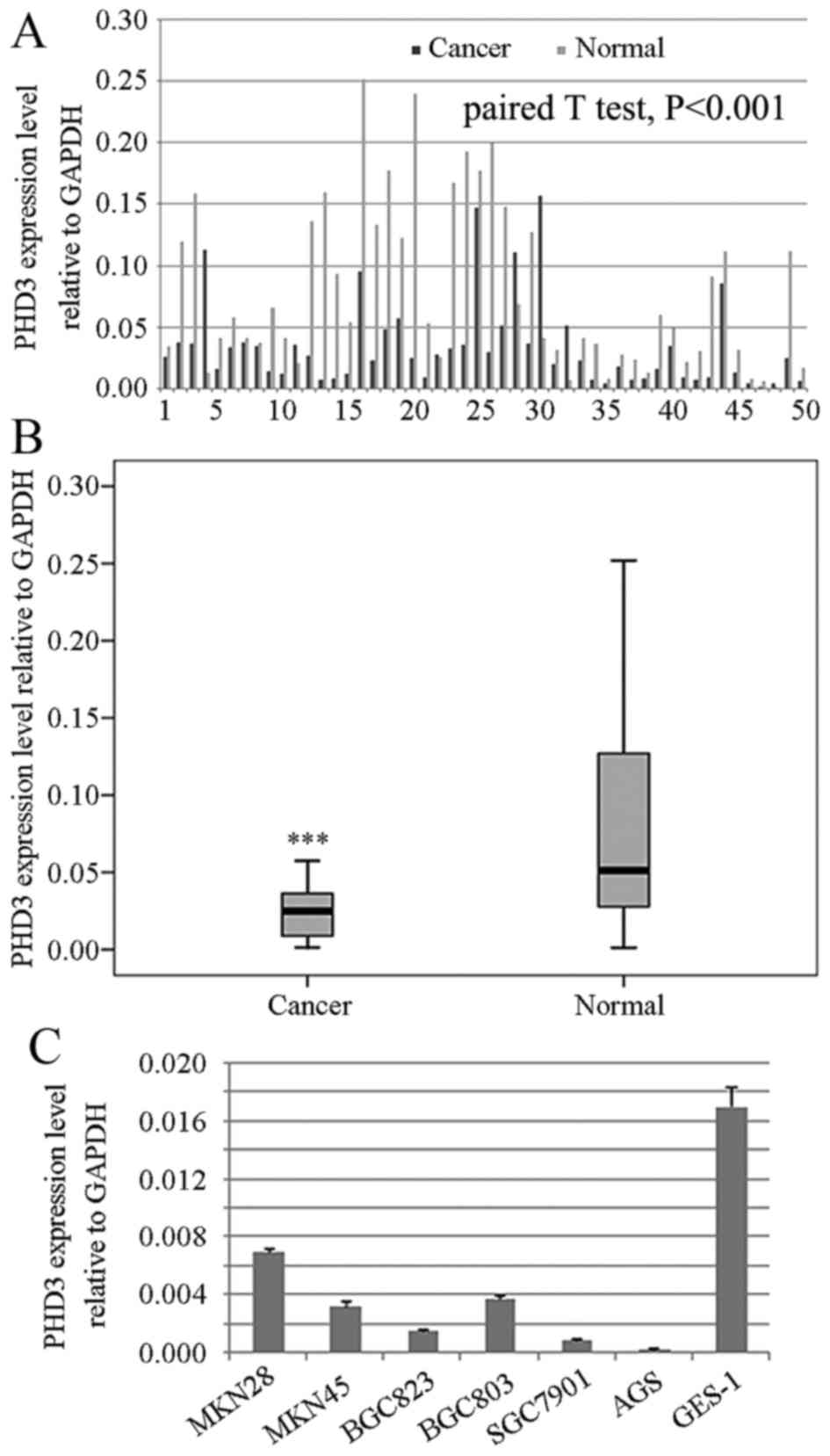
The results of RT-qPCR revealed that 43 out of 50
(86%) paired samples exhibited reduced PHD3 mRNA expression in
cancerous tissue compared with in adjacent non-cancerous tissue, as
analyzed by paired samples t-test (Fig. 1A and B; P<0.001). Results from
western blot analysis and immunohistochemistry were consistent with
RT-qPCR. In addition, PHD3 expression was decreased in gastric
cancer cells, compared with the expression of PHD3 in gastric
epithelial GES-1 cells (Fig. 1C).
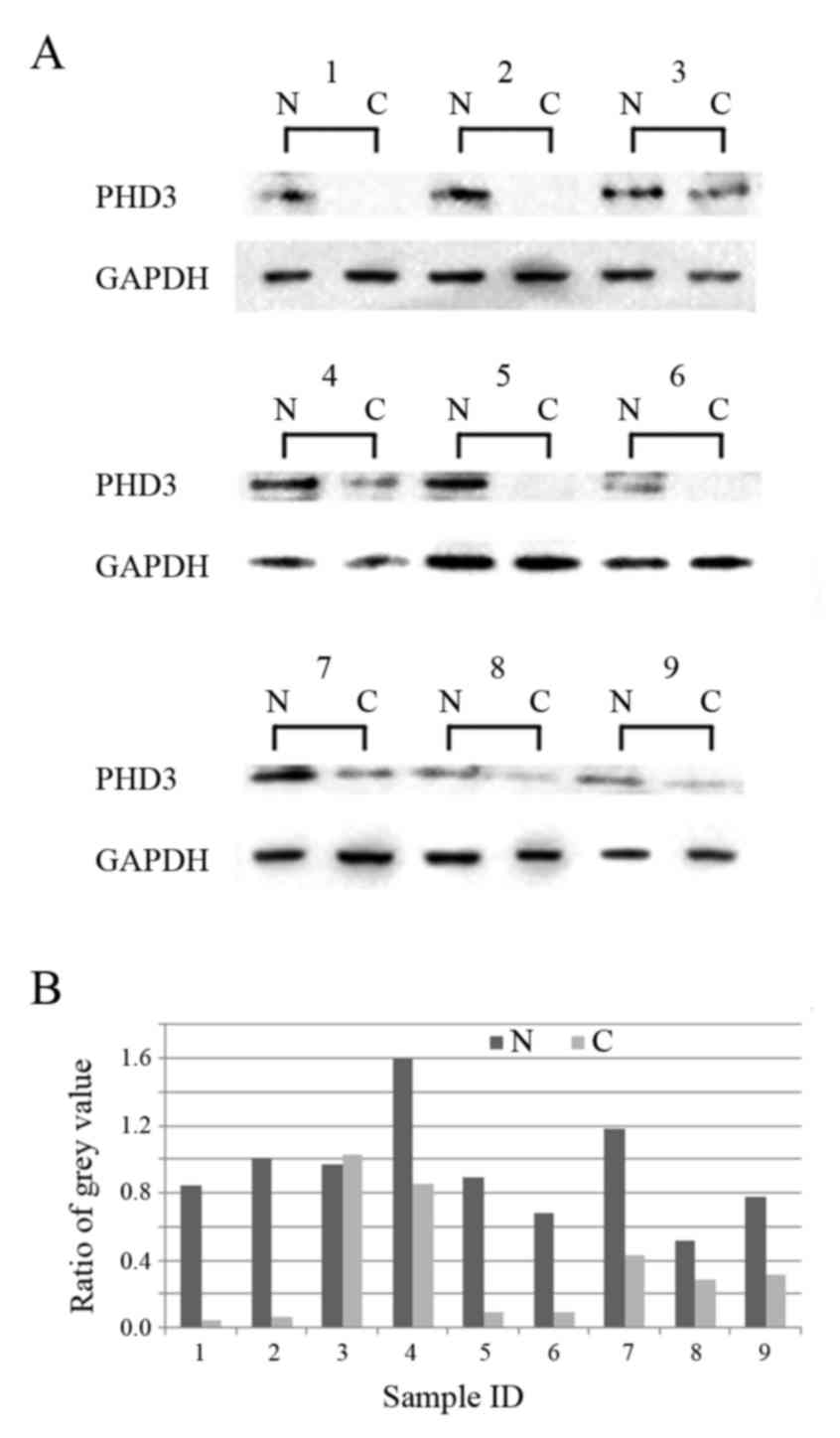
Western blot analysis revealed that 88.9% (8/9 cases) of samples
from adjacent non-cancerous tissue exhibited increased PHD3
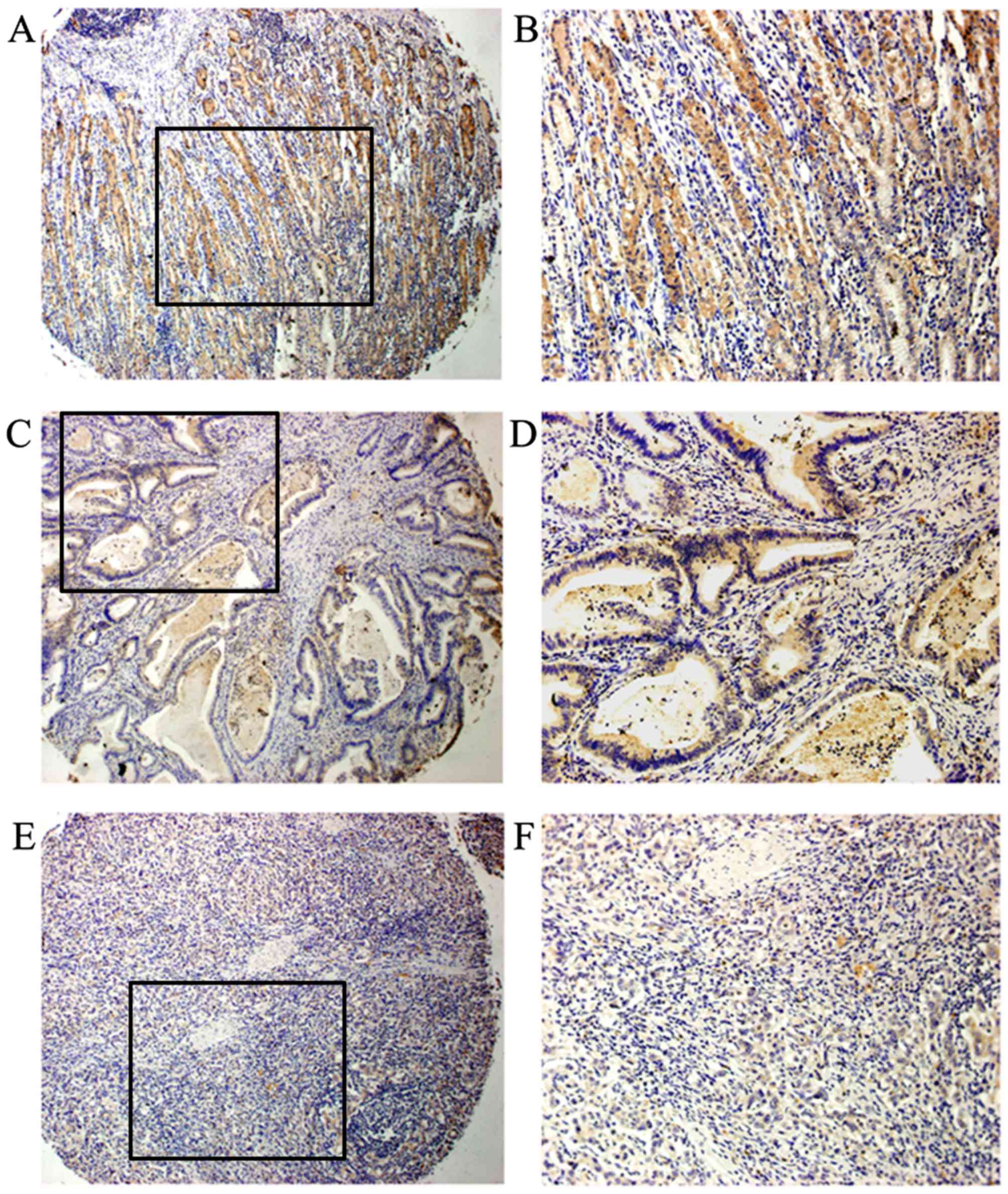
expression compared with in paired cancer tissue samples (Fig. 2). Immunohistochemistry demonstrated
that PHD3 could be detected in all adjacent non-cancerous tissues;
59 out of 70 (84.3%) paired samples exhibited increased PHD3
expression in adjacent non-cancerous tissue compared with in
cancerous tissue (Fig. 3).
Association between PHD3 and
clinicopathological features of gastric cancer
Immunohistochemistry results demonstrated that PHD3
was mainly localized in the cytoplasm and seldom in the nucleus
(Fig. 3). Correlation analysis
revealed PHD3 to be associated with tumor differentiation
(P=0.013), TNM stage (P=0.031) and lymph node metastasis (P=0.014),
but not with age (P=0.853) or gender (P=0.851) of the patients, or
with tumor size (P=0.216) (Table
II).
 | Table II.Association between PHD3 expression
and clinical parameters of gastric cancer. |
Table II.
Association between PHD3 expression
and clinical parameters of gastric cancer.
|
| PHD3 expression
(%) |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | Low | High | P-value
(2-sided) |
|---|
| Age, years |
|
| 0.853 |
|
<61 | 18 (54.5) | 15 (45.5) |
|
|
≥61 | 21 (56.7) | 16 (43.3) |
|
| Gender |
|
| 0.851 |
|
Female | 13 (54.2) | 11 (45.8) |
|
|
Male | 26 (56.5) | 20 (43.5) |
|
| Size, cm |
|
| 0.216 |
|
<5 | 27 (61.3) | 17 (38.7) |
|
| ≥5 | 12 (46.2) | 14 (53.8) |
|
|
Differentiation |
|
| 0.013 |
|
Moderate and well | 9 (36) | 16 (64) |
|
| Poor or
non-differentiated | 30 (66.7) | 15 (33.3) |
|
| TNM stages |
|
| 0.031 |
| <IIb
stage | 19 (45.2) | 23 (54.8) |
|
| ≥IIb
stage | 20 (71.4) | 8 (28.6) |
|
| Lymph node
metastasis |
|
| 0.014 |
| No | 22 (45.8) | 26 (54.2) |
|
|
Yes | 17 (77.3) | 5 (22.7) |
|
The detection rate for increased PHD3 expression was
64% (16/25) in well and moderately differentiated specimens, which
was higher compared with in poorly differentiated or
undifferentiated specimens (33.3%, 15/45). High PHD3 expression was
detected in 22.7% (5/22) of gastric cancer specimens with lymph
node metastasis, which was lower than in specimens without lymph
node metastasis (54.2%, 26/48). The detection rate of high PHD3
expression was also increased in early TNM stages (54.8% in <IIb
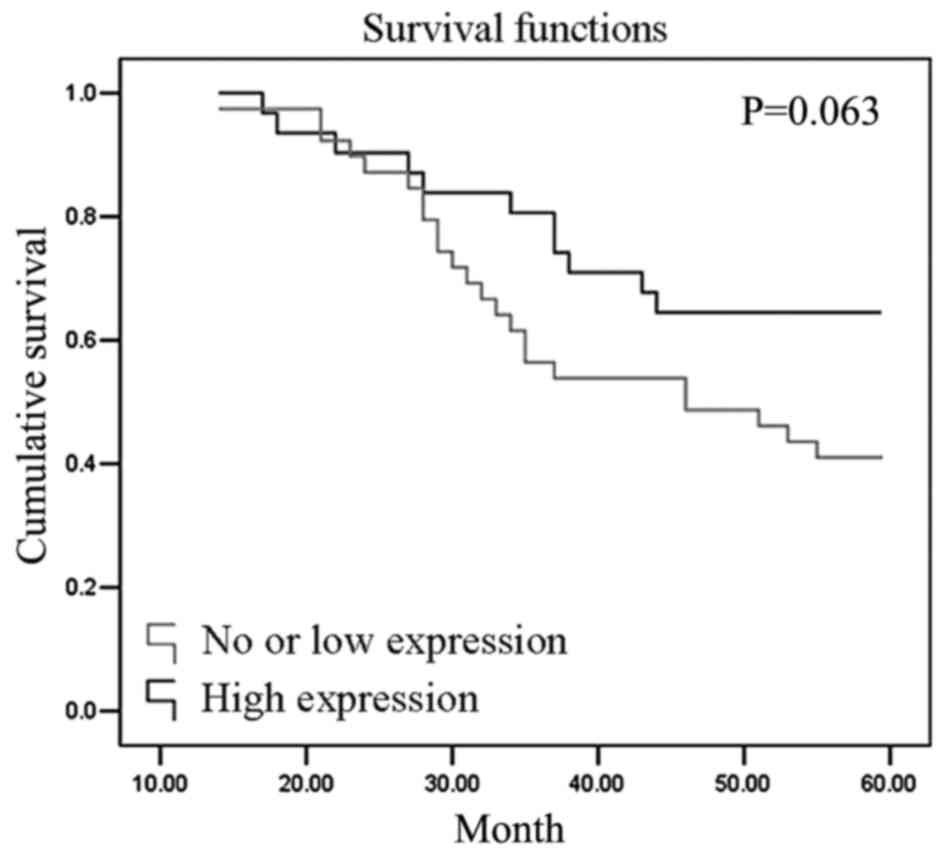
stage vs. 28.6% in ≥IIb stage). The 5-year survival rate of
patients with high PHD3 expression (64.5%) was higher than those
with low PHD3 expression (41.0%). Survival curves were estimated
using the Kaplan-Meier method (Fig.
4, P=0.063).
PHD3 transfection of gastric cancer
cell lines
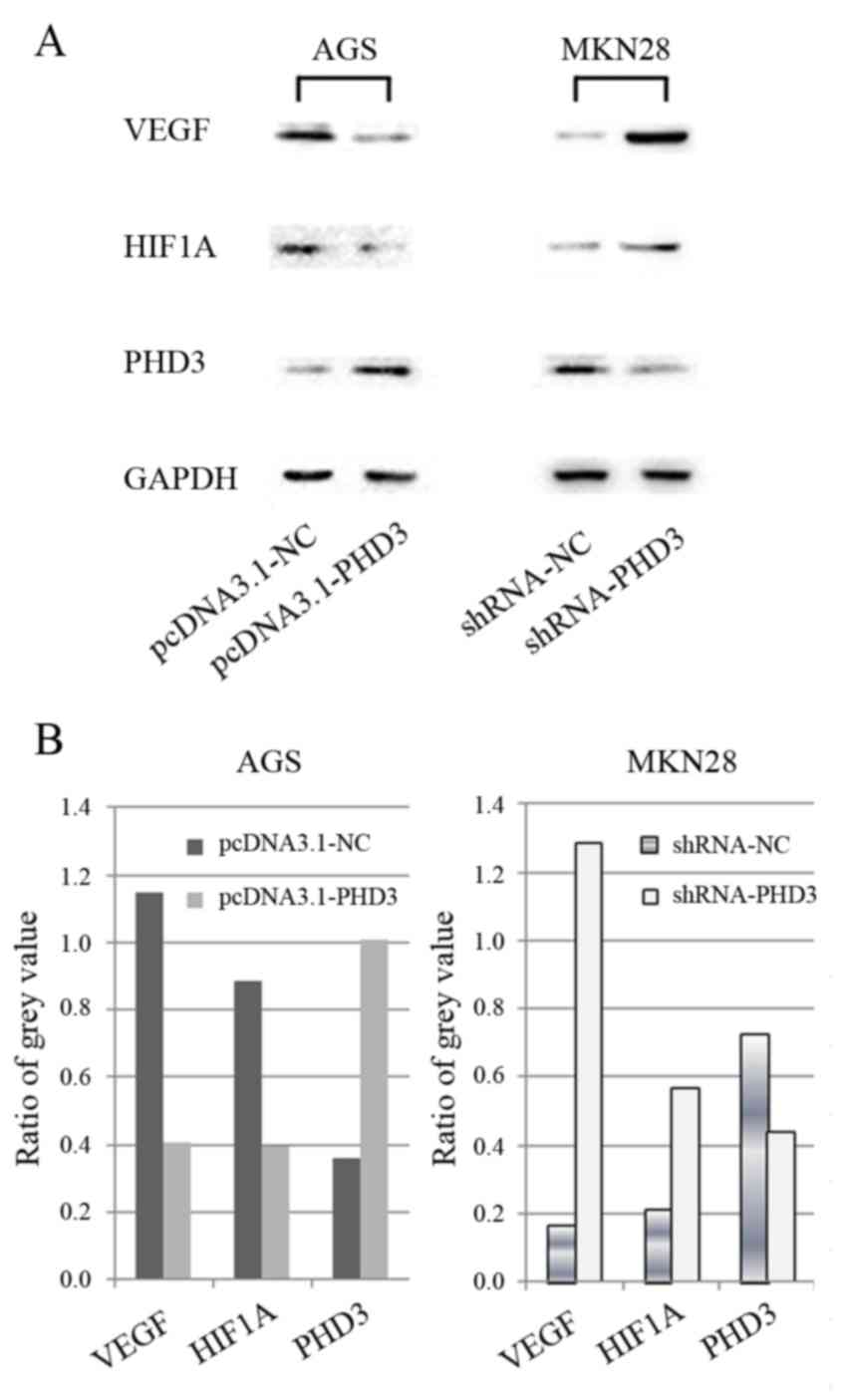
The results of the RT-qPCR analysis demonstrated
that PHD3 protein expression levels appeared lower in AGS cells
compared with in other gastric cancer cell lines (Fig. 1C); however, PHD3 expression
increased after AGS cells were transfected with pcDNA3.1-PHD3
(Fig. 5). Conversely, PHD3
expression levels were decreased in MKN28 cells following
transfection with shRNA-PHD3 (Fig.
5). Furthermore, HIF1A and VEGF expression levels appeared
decreased following PHD3 upregulation in AGS cells, and increased
following PHD3 downregulation in MKN28 cells (Fig. 5).
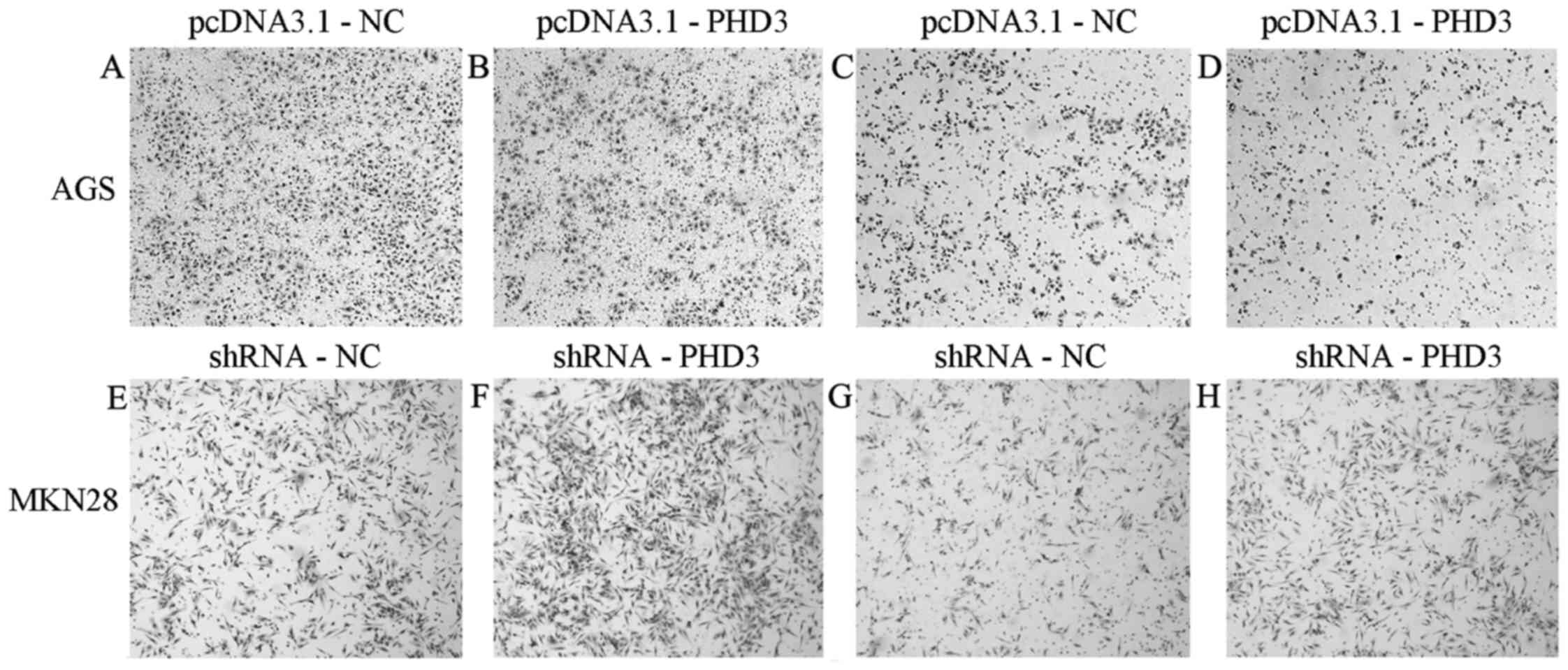
The in vitro transwell migration and invasion
assays revealed that AGS cells transfected with pcDNA3.1-PHD3
exhibited decreased migratory and invasive capabilities compared
with negative control cells. Conversely, MKN28 cells transfected
with shRNA-PHD3 exhibited increased migratory and invasive
capabilities compared with negative control cells (Fig. 6).
Discussion
Previous studies have revealed that the protein
expression levels of PHD3 are low in breast and colon cancer
(22,23), whereas they appear high in lung
(24) and pancreatic cancer
(25). However, the mechanisms
underlying the implication of PHD3 in gastric cancer have yet to be
elucidated. The present study demonstrated that PHD3 levels are
markedly decreased in gastric cancer tissue compared with in
adjacent non-cancerous tissue. These results suggested that PHD3
may act as a tumor suppressor (26). PHD3 expression levels may vary in
different tissue types, and according to the degree of tumor
differentiation.
Correlation analysis of PHD3 expression with regards
to the clinicopathological features of gastric cancer revealed that
increased PHD3 expression was correlated with a high degree of
differentiation of cancer cells, early TNM stage and decreased
lymph node metastasis. These results are consistent with previous
studies (20,27) and suggest that PHD3, or a putative
protein target downstream of PHD3, may participate in tumor
progression. Su et al (25)
reported that PHD3 was upregulated in well-differentiated specimens
of pancreatic cancer, whereas undifferentiated tumors exhibited
reduced PHD3 expression. Although the survival rate of gastric
cancer patients with high PHD3 expression appeared higher than that
of patients with low PHD3 expression, survival analysis revealed
the difference to be insignificant. It has previously been
suggested that PHD3 expression may be used as a prognostic marker
for disease-specific survival in several types of tumors (23,28–30).
Further studies, using larger sample sizes, are required to
elucidate the association between PHD3 expression and the prognosis
of gastric cancer.
To explore the mechanism underlying the implication
of PHD3 in gastric cancer, PHD3 expression was assessed in gastric
cancer cell lines. All gastric cancer cell lines exhibited lower
PHD3 protein expression levels compared with the human gastric
epithelial cell line GES-1. These results are consistent with the
present findings in gastric cancer tissue samples, as well as with
the findings reported by Tanaka et al (31).
To investigate the effects of PHD3 on the migratory
and invasive capabilities of gastric cancer cells, PHD3 expression
was silenced in MKN28 cells, which resulted in increased cellular
migration and invasion. Conversely, upregulation of PHD3 expression
in AGS cells impaired their migratory and invasive capabilities.
PHD3 upregulation has also been reported to inhibit the migration
and invasion of pancreatic cancer cells (25). In order to investigate the
mechanism underlying the actions of PHD3 on cellular migration and
invasion, the expression levels of HIF1A and VEGF were assessed in
AGS and MKN28 gastric cancer cells. Knockdown of PHD3 in MKN28
cells increased HIF1A and VEGF expression, whereas upregulation of
PHD3 expression in AGS cells decreased the expression of HIF1A and
VEGF. These results suggested that PHD3 may negatively regulate the
expression of HIF1A. Furthermore, HIF1A has been reported to
participate in tumor growth and metastasis, through the induction
of VEGF. The present results are consistent with previous studies
(32,33), which reported that decreased HIF1A
expression is associated with impaired angiogenic activity.
Research on the biological functions of HIF1A (34) proposed a similar view. Since the
formation of tumor vasculature is critical for tumor progression,
it may be hypothesized that PHD3 can interfere in the angiogenic
processes of gastric tumors, through the regulation of HIF1A
expression.
In conclusion, the present study suggested that PHD3
may serve a tumor-suppressing role in gastric cancer. PHD3
overexpression may reduce the migratory and invasive capacity of
gastric cancer cells, and inhibit the formation of tumor
vasculature via negatively regulating HIF1A, which has been
revealed to control VEGF transcription. These results demonstrate
the potential for the development of novel therapeutic strategies
for the treatment of gastric cancer, based on the targeted
regulation of PHD3.
Acknowledgements
The present study was supported by the Medicine and
Health Research Foundation of Zhejiang Province (grant nos.
2012KYB016 and 2011KYA069), the National Key Basic Research Program
of China (grant no. 2014CB542101) and the Zhejiang Provincial
Natural Science Foundation of China (grant no. LY14H100003).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baniak N, Senger JL, Ahmed S, Kanthan SC
and Kanthan R: Gastric biomarkers: A global review. World J Surg
Oncol. 14:2122016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muz B, de la Puente P, Azab F and Azab AK:
The role of hypoxia in cancer progression, angiogenesis,
metastasis, and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo X, Li D, Chen Y, An J, Wang K, Xu Z,
Chen Z and Xing J: SNP rs2057482 in HIF1A gene predicts clinical
outcome of aggressive hepatocellular carcinoma patients after
surgery. Sci Rep. 5:118462015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fraga A, Ribeiro R, Principe P, Lobato C,
Pina F, Maurício J, Monteiro C, Sousa H, da Silva F Calais, Lopes C
and Medeiros R: The HIF1A functional genetic polymorphism at locus
+1772 associates with progression to metastatic prostate cancer and
refractoriness to hormonal castration. Eur J Cancer. 50:359–365.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shyu KG, Hsu FL, Wang MJ, Wang BW and Lin
S: Hypoxia-inducible factor 1alpha regulates lung adenocarcinoma
cell invasion. Exp Cell Res. 313:1181–1191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Zhang H and Xing L: Antisense
oligonucleotide of hypoxia-inducible factor-1alpha suppresses
growth and tumorigenicity of lung cancer cells A549. J Huazhong
Univ Sci Technolog Med Sci. 26:448–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baba Y, Nosho K, Shima K, Irahara N, Chan
AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS and Ogino S:
HIF1A overexpression is associated with poor prognosis in a cohort
of 731 colorectal cancers. Am J Pathol. 176:2292–2301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Espinosa I, José Carnicer M, Catasus L,
Canet B, D'angelo E, Zannoni GF and Prat J: Myometrial invasion and
lymph node metastasis in endometrioid carcinomas: Tumor-associated
macrophages, microvessel density, and HIF1A have a crucial role. Am
J Surg Pathol. 34:1708–1714. 2010.PubMed/NCBI
|
|
10
|
Deb S, Johansson I, Byrne D, Nilsson C,
Investigators k Constable L, Fjällskog ML, Dobrovic A, Hedenfalk I
and Fox S: Nuclear HIF1A expression is strongly prognostic in
sporadic but not familial male breast cancer. Mod Pathol.
27:1223–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffmann AC, Mori R, Vallbohmer D,
Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M,
Metzger R, et al: High expression of HIF1a is a predictor of
clinical outcome in patients with pancreatic ductal adenocarcinomas
and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 10:674–679.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vainrib M, Golan M, Amir S, Dang DT, Dang
LH, Bar-Shira A, Orr-Urtreger A, Matzkin H and Mabjeesh NJ: HIF1A
C1772T polymorphism leads to HIF-1α mRNA overexpression in prostate
cancer patients. Cancer Biol Ther. 13:720–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakai H, Watanabe Y, Ueda H and Hoshiai H:
Hypoxia inducible factor 1-alpha expression as a factor predictive
of efficacy of taxane/platinum chemotherapy in advanced primary
epithelial ovarian cancer. Cancer Lett. 251:164–167. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
15
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers, and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minervini G, Quaglia F and Tosatto SC:
Insights into the proline hydroxylase (PHD) family, molecular
evolution and its impact on human health. Biochimie. 116:114–124.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Place TL, Fitzgerald MP, Venkataraman S,
Vorrink SU, Case AJ, Teoh ML and Domann FE: Aberrant promoter CpG
methylation is a mechanism for impaired PHD3 expression in a
diverse set of malignant cells. PLoS One. 6:e146172011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Y, Liang QL, Ou WT, Liu QL, Zhang XN,
Li ZY and Huang X: Effect of stable transfection with PHD3 on
growth and proliferation of HepG2 cells in vitro and in vivo. Int J
Clin Exp Med. 7:2197–2203. 2014.PubMed/NCBI
|
|
19
|
Chen Y, Zhang HS, Fong GH, Xi QL, Wu GH,
Bai CG, Ling ZQ, Fan L, Xu YM, Qin YQ, et al: PHD3 Stabilizes the
Tight Junction Protein Occludin and Protects Intestinal Epithelial
Barrier Function. J Biol Chem. 290:20580–20589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu QL, Liang QL, Li ZY, Zhou Y, Ou WT and
Huang ZG: Function and expression of prolyl hydroxylase 3 in
cancers. Arch Med Sci. 9:589–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rawluszko AA, Bujnicka KE, Horbacka K,
Krokowicz P and Jagodzinski PP: Expression and DNA methylation
levels of prolyl hydroxylases PHD1, PHD2, PHD3 and asparaginyl
hydroxylase FIH in colorectal cancer. BMC Cancer. 13:5262013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peurala E, Koivunen P, Bloigu R,
Haapasaari KM and Jukkola-Vuorinen A: Expressions of individual
PHDs associate with good prognostic factors and increased
proliferation in breast cancer patients. Breast Cancer Res Treat.
133:179–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Zhang J, Li X, Luo X, Fang J and
Chen H: The expression of prolyl hydroxylase domain enzymes are
up-regulated and negatively correlated with Bcl-2 in non-small cell
lung cancer. Mol Cell Biochem. 358:257–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su Y, Loos M, Giese N, Hines OJ, Diebold
I, Görlach A, Metzen E, Pastorekova S, Friess H and Büchler P: PHD3
regulates differentiation, tumour growth and angiogenesis in
pancreatic cancer. Br J Cancer. 103:1571–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tennant DA and Gottlieb E: HIF prolyl
hydroxylase-3 mediates alpha-ketoglutarate-induced apoptosis and
tumor suppression. J Mol Med (Berl). 88:839–849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue J, Li X, Jiao S, Wei Y, Wu G and Fang
J: Prolyl hydroxylase-3 is down-regulated in colorectal cancer
cells and inhibits IKKbeta independent of hydroxylase activity.
Gastroenterology. 138:606–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersen S, Donnem T, Stenvold H, Al-Saad
S, Al-Shibli K, Busund LT and Bremnes RM: Overexpression of the HIF
hydroxylases PHD1, PHD2, PHD3 and FIH are individually and
collectively unfavorable prognosticators for NSCLC survival. PLoS
One. 6:e238472011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Couvelard A, Deschamps L, Rebours V,
Sauvanet A, Gatter K, Pezzella F, Ruszniewski P and Bedossa P:
Overexpression of the oxygen sensors PHD-1, PHD-2, PHD-3, and FIH
Is associated with tumor aggressiveness in pancreatic endocrine
tumors. Clin Cancer Res. 14:6634–6639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gossage L, Zaitoun A, Fareed KR, Turley H,
Aloysius M, Lobo DN, Harris AL and Madhusudan S: Expression of key
hypoxia sensing prolyl-hydroxylases PHD1, −2 and −3 in
pancreaticobiliary cancer. Histopathology. 56:908–920. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka T, Li TS, Urata Y, Goto S, Ono Y,
Kawakatsu M, Matsushima H, Hirabaru M, Adachi T, Kitasato A, et al:
Increased expression of PHD3 represses the HIF-1 signaling pathway
and contributes to poor neovascularization in pancreatic ductal
adenocarcinoma. J Gastroenterol. 50:975–983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rishi MT, Selvaraju V, Thirunavukkarasu M,
Shaikh IA, Takeda K, Fong GH, Palesty JA, Sanchez JA and Maulik N:
Deletion of prolyl hydroxylase domain proteins (PHD1, PHD3)
stabilizes hypoxia inducible factor-1 alpha, promotes
neovascularization and improves perfusion in a murine model of
hind-limb ischemia. Microvasc Res. 97:181–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spanberger T, Berghoff AS, Dinhof C,
Ilhan-Mutlu A, Magerle M, Hutterer M, Pichler J, Wöhrer A, Hackl M,
Widhalm G, et al: Extent of peritumoral brain edema correlates with
prognosis, tumoral growth pattern, HIF1a expression and angiogenic
activity in patients with single brain metastases. Clin Exp
Metastasis. 30:357–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|